Abstract
Background and Aims:
Among the greatest hurdles to pancreatic cancer (PC) therapy is the limited tissue penetration of systemic chemotherapy because of tumor desmoplasia. The primary study aim was to determine the toxicity profile of EUS-guided fine-needle injection (EUS-FNI) with gemcitabine. Secondary endpoints included the ability to disease downstage leading to an R0 resection and overall survival (OS) at 6 months, 12 months, and 5 years after therapy.
Methods:
In a prospective study from a tertiary referral center, gemcitabine (38 mg/mL) EUS-FNI was performed in patients with PC before conventional therapy. Initial and delayed adverse events (AEs) were assessed within 72 hours and 4 to 14 days after EUS-FNI, respectively. Patients were followed for ≥ years or until death.
Results:
Thirty-six patients with stage II (n = 3), stage III (n = 20), or stage IV (n = 13) disease underwent gemcitabine EUS-FNI with 2.5 mL (.7–7.0 mg) total volume of injectate per patient. There were no initial or delayed AEs reported. Thirty-five patients (97.2%) were deceased at the time of analysis with a median 10.3 months of follow-up (range, 3.1–63.9). OS at 6 months and 12 months was 78% and 44%, respectively. The median OS was 10.4 months (range, 2.7–68). Among patients with stage III unresectable disease, 4 (20%) were downstaged and underwent an R0 resection.
Conclusions:
Our study suggests the feasibility, safety, and potential efficacy of gemcitabine EUS-FNI for PC. Additional data are needed to verify these observations and to determine the potential role relative to conventional multimodality therapy. (Gastrointest Endosc 2017;86:161–9.)
Pancreatic cancer (PC) is the fourth leading cause of cancer-related mortality in the United States. In 2016 an estimated 53,070 patients will be diagnosed, and there will be 41,780 deaths because of PC.1 For most PC patients, surgical resection offers the only hope of long-term survival. However, at diagnosis only 20% of patients are surgical candidates,2 and <10% undergo complete surgical resection.3 Even in this select group, the 5-year overall survival (OS) rate is <20%.4,5 PC is deemed unresectable if it has spread to distant lymph nodes or organs (which is the case in 50% of patients) and is associated with a 5-year survival rate of 2%.2 Another 30% are deemed unresectable because the cancer encases crucial arteries; although minimal venous involvement may be resectable, tumor encasement or occlusion typically indicates unresectability.
Over 40 years ago it was demonstrated that combined 5-fluorouracil and radiation therapy prolonged median survival to 9 to 10 months for patients with locally advanced PC (LAPC).6 Despite evaluation of more than 50 new agents designed to enhance the efficacy, there has been little advancement, with each drug failing to produce a meaningful improvement in resectability rates or survival.2,7–9 Although many agents have demonstrated initial promise for PC therapy, further investigation almost uniformly reveals no survival benefit.9–17 Most consider that a chemotherapy regimen provides a clinically significant improvement when extending survival by only weeks.12 Thus, the 4-month improved survival associated with FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin) is considered unparalleled and one of the most widely regarded advances in PC management.18
Because PC is associated with significant desmoplastic response, chemoresistance may be partially because of inadequate tumor cell penetration of systemically administered cytotoxics. EUS allows fine-needle injection (FNI) that may overcome problems associated with tumor-related desmoplasia. Increasing intratumoral chemo-therapy levels may provide the potential to increase the local effects of chemotherapy while boosting the radiation effect within the tumor bed. We report findings of a study designed to investigate the safety and efficacy of such an approach.
We hypothesized that gemcitabine could be safely administered by EUS-FNI and potentially enhance the efficacy of conventional multimodality therapy for patients with PC when administered as a 1-time induction therapy before any planned conventional multimodality therapy. The primary endpoint was the observation of initial or delayed toxicity. Secondary endpoints included the ability to downstage LAPC leading to an R0 resection and OS at specified time points: 6 months, 12 months, and at 5 years of follow-up.
METHODS
Patient eligibility required fulfillment of all the following criteria: (1) cytohistologic confirmation of PC; (2) resection not attempted because of unresectability, poor operability, or patient refusal; (3) age ≥ 18 years; (4) Eastern Cooperative Oncology Group performance status of 0 to 1; (5) measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines19; and (6) the laboratory parameters absolute neutrophil count ≥ 1500/mm3, hemoglobin > 9.0 g/dL, platelets ≥ 100,000/mm3, total bilirubin ≤ 2 × upper limit of normal, aspartate aminotransferase ≤ 3 × upper limit of normal, and creatinine ≤1.5 × upper limit of normal.
Contraindications to enrollment included any 1 of the following: (1) prior pancreatic surgery or chemotherapy or overlapping radiation fields; (2) significant infection or coexistent medical condition precluding protocol therapy; (3) concurrent or prior malignancy unless disease-free ≥3 years, except for nonmelanoma skin cancer, carcinoma in situ of the cervix or bladder, Gleason Grade < 7 organ-confined prostate cancer; (4) grade ≥ 1 nausea or vomiting; (5) pregnant or nursing women and men or women of childbearing potential who were unwilling to use contraception, with a pregnancy test obtained if appropriate; or (6) presence of intervening structures prohibiting EUS tumor access.
Patients were evaluated in the Pancreas Clinic by a medical pancreatologist, pancreatic surgeon, medical oncologist, radiation oncologist, radiologist, and endosonographer who each deemed the patient appropriate for study participation. This determination was made at baseline within 14 days before EUS-FNI. During this time helical CT, hematology parameters, and hepatic and renal chemistries were obtained. Patients were followed for ≥5 years after EUS-FNI or until death. The study was approved by the Institutional Review Board (IRB no. 08–006055 00), and patients provided written informed consent.
EUS-FNI technique
The technique of EUS-FNI was adopted from our pre-study experience when we performed EUS-FNI on a compassionate care basis on 5 occasions for patients with PC using a gemcitabine concentration identical to that used for systemic administration (38 mg/mL). This pre-study cohort of patients was informed as to the uncertain and potential life-threatening risks and lack of existing data and written informed consent was obtained. Diagnostic EUS was performed using a curvilinear echoendo-scope (GF-UC160P-AT8 or GF-UC140P-AL5; Olympus Medical Systems, Center Valley, Pa) for staging and to obtain a tissue diagnosis and/or celiac neurolysis, when indicated. Gemcitabine was injected using a 22-gauge needle (Wilson-Cook Medical Inc, Winston-Salem, NC) by 1 of 2 endosonographers (M.J.L., F.C.G.). With each injection pass, we placed the needle tip .5 to 1.0 cm from the distal tumor edge (further from the transducer) with injection as the needle was retracted toward the proximal (closer to the transducer) tumor border in an effort to confine the injectate within the tumor. The general goal was to inject approximately 50% of the dose in a uniform distribution along the perimeter of the lesion at sites of local infiltration (eg, blood vessels) and 50% within the remainder of the tumor. The volume and pattern of injection were modified based on the initial drug dispersal. We continued to inject gemcitabine until the drug was no longer confined within the tumor and instead began to preferentially infiltrate along the needle tract or via other routes to peritumoral sites instead of remaining within the tumor. Additional passes were performed using the same technique. The point at which additional needle passes no longer resulted in intratumoral spread the procedure was terminated, leading to varied injection volumes. The amount of gemcitabine administered was determined by the degree and pattern of spread and the volume could not automatically be determined without risking unintended spread and patient safety.
Although varied, in our prestudy experience we determined that intratumorally injected gemcitabine, which is easily seen because of its hyperechoic appearance, typically spread from its initial site of injection throughout the tumor over a period of 5 to 10 minutes at which time no further diffusion could be appreciated at EUS. We assigned a score based on the subjective ultrasonographic assessment of the final 3-dimensional extent (determined no earlier than 10 minutes after the last injection, at which point no further diffusion was detected). The visual assessment of the completeness of therapy was defined in terms of 3 features: (1) extent of intratumoral spread; (2) pattern of spread; and (3) homogeneity of spread. A 4-point scale was used, with 1 representing the least dispersed pattern and 4 representing a more complete pattern and extent of intratumoral gemcitabine spread.
Conventional multimodality therapy
The use of conventional multimodality therapy (chemo-therapy with or without radiation) was delayed until the EUS-FNI toxicity assessment was completed, as defined below. Administration of conventional multimodality therapy was determined by each patient’s medical oncologist and radiation oncologist. The specific regimen used, dose, dose modifications, and timing of delivery of both chemotherapy and/or radiation therapy were not influenced by study participation. Patients were typically treated with 50.4 Gy in 28 fractions using 3-dimensional techniques with concomitant continuous infusion 5-fluorouracil.
Safety assessment
The study was reviewed periodically by a data and safety monitoring board to assess the toxicity, in cohorts of 9 patients, in the following manner. First, the initial adverse event (AE; attributed to the EUS procedure) was assessed during a clinic visit conducted within 72 hours of EUSFNI. EUS-FNA has a reported risk of 2% or less for causing pancreatitis, perforation, bleeding, or infection.20–27 We expected an incremental risk of pancreatitis when performing EUS-FNI to be similar to that for FNA. If at any time 2 patients within a 9-patient cohort experienced any of these AEs of such severity requiring hospitalization, the study was suspended to allow for full data review. After consideration by the Data and Safety Monitoring Board and the Institutional Review Board, a decision was made as to whether and how to resume the study.
Scond, a delayed AE (attributed to intratumoral gemcitabine injection) was assessed at 4 to 14 days after EUS-FNI and before initiating conventional multimodality therapy, if administered. Patients underwent a clinical evaluation and a repeat CT with hematology testing, hepatic and renal chemistries. The maximum grade for each type of toxicity was recorded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0, for AE monitoring and reporting. AEs were evaluated in cohorts containing 3 patients. If at any time 2 patients within a 9-patient cohort experienced an unacceptable AE (grade 3 or 4 toxicity in any of the 3 patients), the study was suspended to allow for full data review. Any grade 5 event within 30 days of EUS-FNI required an immediate study pause and full review.
Assessment of efficacy
Any potential benefit in terms of efficacy was based on the ability to downstage LAPC leading to a, R0 resection and OS at 6 months, 12 months, and 5 years of follow-up from the date of EUS-FNI. Based on RECIST guidelines, the overall response rate was also determined by independent and blind review and calculated based on the number of patients who had a complete or partial response divided by the total number of eligible patients who had started treatment and could be assessed via RECIST criteria. Patients were followed for a minimum of 5 years after EUSFNI or until death.
Statistical analysis
Continuous variables were summarized using median and range with differences between groups compared using a Wilcoxon rank sum test. Categorical variables were summarized using frequency and percentage, with differences between groups compared using a Pearson χ2 test. OS duration was defined as time to death or last follow-up after date of diagnosis at the Mayo Clinic. Kaplan-Meier methods were used to calculate median survival by group. Cox proportional hazard regression models were used to estimate hazard ratio and 95% confidence intervals. Log rank tests were used to test for significant differences between groups. Variables related to the intratumoral chemotherapy administration (number of injections, dosage, spread) were also described. All tests were 2-sided, with P ≤ .05 as the criterion standard for determining significance. The SAS volume 9.3 for LINUX statistical software package (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
RESULTS
From April 26, 2009 to June 29, 2010, 36 patients with PC (median age, 65 years; range, 42–86; 19 men and 17 women) with stage II (n = 3), stage III (n = 20), or stage IV (n = 13) disease underwent gemcitabine (38 mg/mL) EUS-FNI (Table 1). At baseline patients were deemed to have unresectable disease (n = 33), to be a poor operative candidate (n = 2), or to decline resection (n = 1). A designation of unresectability was established by members within the Multidisciplinary Pancreas Clinic and did not include any patients with borderline resectable tumors but was limited to those with more advanced disease (20 locally advanced and 13 metastatic). Although practice patterns slightly vary among surgeons, in our center the criteria and definitions for unresectability that are most reflective of those adopted during the study timeframe were from the National Comprehensive Cancer Network V.1.2010.28
TABLE 1.
Characteristics of study patients (n = 36) and characteristics of survival
| Characteristic | Description |
|---|---|
| Age at start of treatment, y | |
| Mean (SD) | 65 (12.4) |
| Range | (42−86) |
| Body mass index | |
| Mean (SD) | 26.7 (4.8) |
| Range | (19.7−38.5) |
| Stage, n | |
| I | 0 |
| II | 3 |
| III | 20 |
| IV | 13 |
| Follow-up, mo | |
| Completed (deceased), n | 35 |
| Median (range) | 10.4 (3.2−63.9) |
| OS, estimate | |
| Median, mo (range) | 10.4 (7.5−13.9) |
| 6 months (95% CI) | 77.8% (62.9%−89.6%) |
| 2 months (95% CI) | 44.4% (28.7%−60.6%) |
SD, Standard deviation; CI, confidence interval; OS, overall survival.
The pancreatic tumors were located in the pancreatic head, body, and tail in 18, 14, and 4 patients, respectively. For the 18 patients with a tumor located in the pancreatic head, 16 had an indwelling stent at the time of EUS-FNI. Gemcitabine EUS-FNI was administered via 3 (range, 1–4) needle passes and 2.5 mL (corresponding gemcitabine dose range, .7–7.0 mL) total volume of injectate per patient, corresponding to an intratumoral gemcitabine dose of 95.0 mg (range, 26.6–266 mg). Subsequent conventional chemoradiotherapy (n = 22), chemotherapy alone (n = 10), no therapy (n = 1), or indeterminate therapy (n = 3) was administered.
Toxicity
No initial or delayed AEs were associated with intratumoral therapy when assessed before initiating conventional chemoradiotherapy.
Overall survival
Thirty-five patients (97.2%) were deceased at the time of analysis, with a median of 10.3 months follow-up (3.1–63.9). OS at 6 months, 12 months, and 5 years was 78%, 44%, and 3%, respectively. The median OS was 10.4 months (95% confidence interval, 2.7–68).
Confirmed response, disease downstaging, and resected patients
The confirmed response was determined in 28 of 36 patients enrolled. We limited this assessment to the 28 patients in whom we had access to all necessary medical records and imaging studies to allow us to accurately assess the response using the RECIST guidelines. Patients were classified as having a complete response (n = 0), partial response (n = 7, 25%), stable disease (n = 16, 57%), and progressive disease (n = 5, 17.8%).
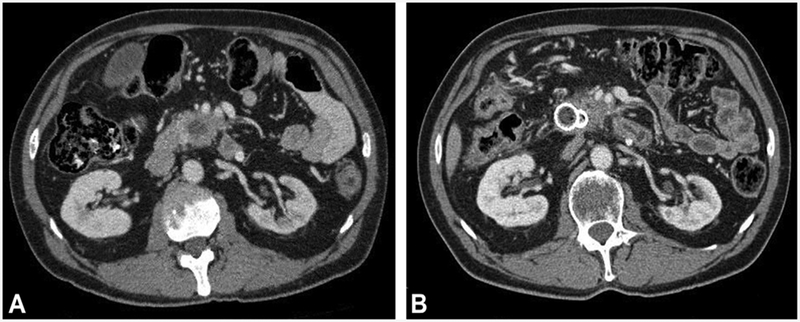
Among the 20 patients with stage III unresectable disease, 4 (20.0%) were downstaged and each underwent an R0 resection. They include patients initially staged as T4N1M0 (n = 3) and T3N1M1 (n = 1). The latter patient had several liver metastases with EUS-FNA cytology of the primary lesion interpreted as highly suspicious for adeno-carcinoma based on the presence of malignant groups but fewer than 10 such groups, which at our institution is the threshold for interpreting a sample as “positive for malignancy.” All other EUS-FNA cytologic interpretations of malignancy for the entire study cohort were based on the finding of positive for malignancy. Among the 4 resected patients, 2 died from cancer-related deaths at 32.4 months and 13.4 months, a third patient died at 42.1 months because of intractable Clostridium difficile infection without evidence of cancer, and the fourth patient remains alive at 72.1 months without clinical evidence of residual or recurrent cancer (Fig. 1).
Figure 1.
CT from a 54-year-old man with PC. A, Initial CT before therapy reveals a 32 × 25 mm, T4N1M0 pancreatic head tumor. B, After EUS-FNI of 76 mg gemcitabine followed by conventional chemoradiation, the CT revealed tumor regression and downstaging. Surgical pathology after open pancreaticoduodenectomy with en-bloc segmental superior mesenteric vein resection and segmental superior mesenteric artery resection revealed a 2.3 × 2.3 × .8 cm fibrotic mass with multiple foci of microscopic adenocarcinoma without any vascular invasion and an R0 resection and T2N0M0 disease. The patient remains alive 72.1 months after therapy without evidence of malignancy. PC, pancreatic cancer; FNI, fine-needle injection.
Pattern and extent of intratumoral spread
A visual assessment of the completeness of therapy as defined in Methods using a 4-point scale based on the extent of intratumoral spread, the pattern of spread, and the homogeneity of spread was correlated with patient outcomes (Table 2, Figs. 2 and 3). A score of 1 (least complete), 2, 3, and 4 (most complete) was recorded in 8 (22.2%), 9 (25.0%), 12 (33.0%), and 7 (19.4%) patients, respectively. Patients who experienced a more complete therapy based on the score developed the greatest benefit with regard to increase in median survival (P < .0001) with a consistent trend of increasing survival as completeness increased.
TABLE 2.
Median OS relative to the pattern and extent of intratumoral spread
| Spread | No. of patients | No. of patients with metastatic disease | Median survival (mo)* Months (95% CI) | P value† |
|---|---|---|---|---|
| 2 | 9 (25.0%) | 3 (33.3%) | 7.1 (3.7−10.6) | |
| 3 | 12 (33.0%) | 3 (25.0%) | 14.1 (10.2−16.0) | |
| 4 | 7 (19.4%) | 1 (14.3%) | 32.0 (21.4−42.2) |
Visual assessment of the completeness of therapy, in terms of the pattern and extent of intratumoral spread, using a 4-point scale, with 1 representing the least dispersed pattern and 4 representing a more complete pattern and extent of intratumoral spread. The score was based on 3 features: (1) the final visually assessed ultrasonographic 3dimensional extent (determined no earlier than 10 minutes after the last injection, at which point no further diffusion was detected), (2) pattern, and (3) homogeneity of intratumoral gemcitabine spread.
OS, Overall survival.
Value in parentheses is 95% confidence interval.
Log-rank test.
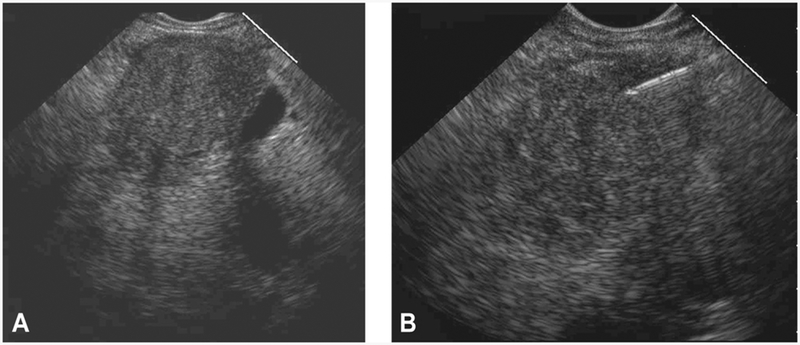
Figure 2.
EUS from a 53-year-old woman with PC demonstrating an ideal pattern and extent of intratumoral spread of gemcitabine that was assigned a score of 4 out of 4. A, EUS appearance before EUS-FNI reveals a hypoechoic, homogenous, well-defined mass. B, After EUS-FNI, the hyperechoic-appearing gemcitabine infiltrated throughout the mass with a uniform pattern of spread that resulted in a more hyperechoic-appearing mass secondarily resulting in a more inhomogeneous echotexture and less well-defined borders as compared with the pretreatment appearance. PC, pancreatic cancer; FNI, fine-needle injection.
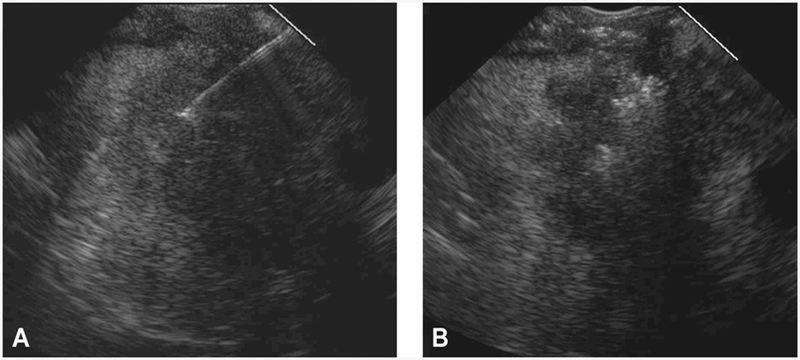
Figure 3.
EUS from a 73-year-old man with PC demonstrating more of a less complete pattern and extent of intratumoral gemcitabine spread assigned a score of 1 out of 4. A, EUS appearance before EUS-FNI reveals a hypoechoic, homogenous mass with an irregular border. B, After EUS-FNI, the hyperechoic-appearing gemcitabine infiltrated only a small portion of the mass and in a nonuniform pattern of spread. The hyperechoic-appearing region is restricted to the central portion of the mass. Most of the gemcitabine extruded from the tumor and can be seen within the peripancreatic space. PC, pancreatic cancer; FNI, fine-needle injection.
DISCUSSION
For over 40 years chemoradiotherapy has been used to treat PC. Despite great optimism for new regimens, the median survival for LAPC remains only 9 to 11 months with chemoradiotherapy and 6 months for those with metastatic disease receiving gemcitabine.2,6,29 Neoadjuvant therapy is often given in an attempt to downstage LAPC and permit resection. However, for patients with truly unresectable tumors (ie, excluding borderline resectable), chemoradiotherapy seldom provides effective downstaging with an R0 resection, and prolonged survival rarely occurs.
A challenge for delivering chemoradiotherapy for PC is the lack of penetration of therapeutic agents into the tumor, secondary to desmoplasia,30–32 and variations in the biophysical properties associated with PC, producing islands of neoplastic tissue interspersed among dense stroma, thereby altering the pattern and limiting the accessibility of chemotherapeutic agents.30–33 This has led some to explore the utility of stromal-disrupting agents.34,35 Diffusion of biologic agents after EUS-FNI may be based on different principles from systemically administered chemotherapy. As the interstitial space is injected, drug concentrations can likely be reached that are greater than systemically administered medications, thereby overwhelming any protective transport systems developed by neoplastic cells. By increasing intratumoral chemotherapy levels, we sought to increase the local tumor toxicity from chemotherapy and to boost the radiation sensitivity within the tumor bed. In addition, most PC-related AEs and symptoms arise from local disease, and therefore focal therapy may be of clinical benefit even if survival is only marginally improved. In addition, locally delivered agents may have a dissemination pattern that follows the vascular and lymphatic drainage of the pancreas, thereby providing opportunity for tumor destruction along these sites.
Since 1997 gemcitabine, used either alone or in combination with other drugs, has been a standard first-line systemic therapy for LAPC or metastatic PC.36 Gemcitabine acts as a radiosensitizer through nucleotide pool alterations, cell cycle redistribution, induction of apoptosis, inhibition of DNA synthesis, and altered DNA repair.37–39 Although different agents and regimens are often used today, we elected to administer gemcitabine given the long track record of use and safety and relative benefit when delivered systemically. To our knowledge, this study represents the first investigation using the use of EUS-FNI with standard chemotherapeutic agents for the management of PC.
Only limited data have explored the use of intratumoral therapy for PC. EUS-FNI was used for intratumoral injection of T lymphocytes (Cytoimplant) in 8 patients with unresectable PC in a phase I trial.40 Patients had stage II (n = 4), stage III (n = 3), or stage IV (n = 1) disease. Cytoimplant was injected in escalating doses of 3, 6, or 9 billion cells by EUS-FNI. Two partial responses and 1 minor response were noted. The median survival was 13.2 months for the group as a whole. Three grade 3 GI toxicities and 3 elevations of bilirubin reversed with biliary stenting were identified. Seven patients had a low-grade fever responsive to acetaminophen. A subsequent phase II/III trial was prematurely terminated because response rates and survival were greater for patients randomized to gemcitabine versus those randomized to Cytoimplant. Cytoimplant is no longer administered because of subsequent studies, printed in abstract form, reporting severe drug-related sepsis.
EUS was used to guide 8 injections over 8 weeks of ONYX-015, a gene-deleted replication-selective adenovirus that preferentially kills malignant cells, in 21 patients with LAPC or metastatic disease who also underwent systemic chemoradiation therapy.41 Eight weekly EUS-FNI treatments session were performed. Two patients had partial regression, 2 had a minor response, and 11 patients had disease progression or toxicity, prohibiting study completion. Pancreatitis did not develop; however, sepsis occurred in 2 patients before the eventual implementation of antibiotic prophylaxis. Two patients developed a duodenal perforation with transduodenal injection and in no patients undergoing transgastric delivery. In a pilot study dendritic cells were injected under EUS guidance in 7 patients with LAPC or metastatic PC after unsuccessful gemcitabine therapy.42 No procedure or drug-related AEs were reported, and the median survival was 9.9 months.
More recently, EUS or CT was used to inject TNFerade in patients with LAPC.43 TNFerade is a replication-deficient adenoviral vector containing the human TNF-α gene that expresses tumor necrosis factor-α under the control of the Egr-1 promoter, which is inducible by chemo-therapy and radiation. TNFerade was injected weekly for 5 weeks along with systemic therapy. TNFerade was injected by a single percutaneous needle pass or up to 4 EUS injections. Dose-limiting toxicities developed in 4 of 50 patients, including pancreatitis in 2 patients and hypo-tension and biliary obstruction in 1 patient each. No severe AEs were noted. At the maximum tolerate dose 4 of 5 patients reassessed as surgically resectable achieved pathologically negative margins, and 3 survived 24 months. Intratumoral therapy did not interfere with subsequent surgical resection. For the 42 patients who had an objective response measured, 1 patient experienced a complete response, 3 had partial responses, 4 had a minor response, 12 had stable disease, and 19 had progressive disease. Seven patients ultimately underwent resection and 6 had clear margins, 3 of whom survived longer than 24 months.
In our study the presence of an indwelling stent had no apparent impact on intratumoral drug delivery and assessment of spread. Minor adjustments in echoendoscope positioning and rotation allowed targeting throughout the tumor and assessment of treatment adequacy. However, EUS-FNI should be performed before stent placement whenever clinically feasible. Although the completeness of therapy corresponded to prolonged survival, the significance of this finding is potentially limited by the subjective nature of this assessment, and the reproducibility must be clearly established.
There are other limitations of our study, including the inability to determine if EUS-FNI provided a survival advantage. Most of our patients had unresectable LAPC (stage III) or metastatic (stage IV) disease for whom the reported median OS wass 6 to 12 months and 3 to 8 months, respectively.12,14,16,36,44–49 Although a more prolonged survival has been reported with FOLFIRINOX, our study was conducted before the introduction of this treatment option.18,50–53 Although the median OS for our stage III and IV patients was 9.8 and 8.7 months, respectively, the lack of randomization to a matched control cohort does not allow us to determine the impact of EUS-FNI on survival. In our experience, for patients with truly unresectable tumors (ie, excluding borderline resectable) we seldom able achieve downstaging with an R0 resection using standard chemoradiotherapy, yet 4 of 20 patients (20%) with LAPC were effectively downstaged with an R0 resection. Although these findings may support our hypothesis and suggest that the intratumoral therapy enhanced chemo-therapy delivery while avoiding local and secondary tumor toxicity, sources of potential bias must be considered given the nonrandomization. It would be premature to attribute the downstaging and any potential survival advantage to EUS-FNI therapy.
Other limitations include the use of a single treatment arm and nonprotocolized delivery of standard multimodality therapy that was not regulated by the study. Even if EUS-FNI and other local therapies are proven safe and effective, challenges remain when attempting to determine the potential role in the treatment algorithm of patients with PC, including the need and impact of multiple treatment sessions, the effect of escalating volumes and/or concentration of chemotherapy, the shifting pattern in use of chemotherapy and radiation therapy, and associated financial impact. The challenge is to determine whether EUS-FNI and intratumoral therapy provides a clinical advantage in a safe and cost-effective manner.
The success of EUS-FNI may largely depend on efforts to optimize the pattern and completeness of therapy. Such efforts may benefit from the development of needles that permit a more homogenous and evenly dispersed injection throughout the distal aspect of the needle and not solely from the needle tip. The use of a porous membrane and/or multiside-hole needle must be designed to account for differences that exist among different chemotherapy particle sizes and viscosities. In addition, the combined use of antistromal agents that preferentially target the desmoplastic region may be considered. The future use and role of EUS-FNI depends not only on efficacy but also on the shifting pattern in use of chemotherapy agents and radiation therapy. One cannot extrapolate the findings of gemcitabine EUS-FNI to other agents, and analogous studies will be needed to evaluate other emerging cytotoxic or targeted agents.
In conclusion, our study suggests the feasibility, safety, and potential efficacy of EUS-FNI of gemcitabine for PC. These data offer promise in terms of tumor downstaging and impact on survival. The ultimate aim of any such therapy is to improve the success of tumor downstaging, to decrease disease recurrence, improve quality of life, prolong survival, and increase the cure rate. Prospective randomized and sufficiently powered studies are needed to verify the clinical success of EUS-FNI for tumor downstaging, decreasing disease recurrence, improving quality of life, prolonging survival, and increasing the cure rate. Additional data are also needed to understand the potential impact of tumor location, presence of an indwelling stent, pattern and extent of intratumoral injectate extent, and the role in patients with a borderline resectable tumor.
Abbreviations:
- AE
adverse event
- FNI
fine-needle injection
- LAPC
locally advanced pancreatic cancer
- OS
overall survival
- PC
pancreatic cancer
Footnotes
DISCLOSURE:
All authors disclosed no financial relationships relevant to this publication. Research support for this study (M.J.L.) was provided by a Career Development Award of the Mayo Clinic Specialized Program of Research Excellence in Pancreatic Cancer (P50CA102701).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ujiki MB, Talamonti MS. Guidelines for the surgical management of pancreatic adenocarcinoma. Semin Oncol 2007;34:311–20. [DOI] [PubMed] [Google Scholar]
- 3.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database [see comment]. J Am Coll Surg 1999;189:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995;221:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators [see comment]. J Gastrointest Surg 2000;4:567–79. [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Childs DS Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 1969;2:865–7. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology 2005;128:1642–54. [DOI] [PubMed] [Google Scholar]
- 8.Taieb J, Lecomte T, Aparicio T, et al. FOLFIRI.3, a new regimen combining 5-fluorouracil, folinic acid and irinotecan, for advanced pancreatic cancer: results of an Association des Gastro-Enterologues Oncologues (Gastroenterologist Oncologist Association) multicenter phase II study. Ann Oncol 2007;18:498–503. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513–8. [DOI] [PubMed] [Google Scholar]
- 10.Novarino AMT, Satolli MA, Chiappino I, et al. FOLFOX-4 regimen or single-agent gemcitabine as first-line chemotherapy in advanced biliary tract cancer. Am J Clin Oncol 2013;36:466–71. [DOI] [PubMed] [Google Scholar]
- 11.Colucci G, Labianca R, Di Costanzo F, et al. Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato D, Gruppo Oncologico Italiano di Ricerca C. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 2010;28:1645–51. [DOI] [PubMed] [Google Scholar]
- 12.Moore MJ, Goldstein D, Hamm J, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group [see comment]. J Clin Oncol 2007;25:1960–6. [DOI] [PubMed] [Google Scholar]
- 13.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2005;23:8033–40. [DOI] [PubMed] [Google Scholar]
- 14.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009;27:3778–85. [Erratum: J Clin Oncol 2009 Dec 127(34):5859.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol 2006;24:4441–7. [DOI] [PubMed] [Google Scholar]
- 16.Stathopoulos GP, Syrigos K, Aravantinos G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer 2006;95:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conroy T, Desseigne F, Ychou M, et al. Groupe Tumeurs Digestives of U, Intergroup P.FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 20.O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc 2001;53:470–4. [DOI] [PubMed] [Google Scholar]
- 21.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087–95. [DOI] [PubMed] [Google Scholar]
- 22.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut 1999;44:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiersema MJ, Levy MJ, Harewood GC, et al. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc 2002;56:275–8. [DOI] [PubMed] [Google Scholar]
- 24.Levy MJ, Jondal ML, Clain JE, et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc 2003;57:101–6. [DOI] [PubMed] [Google Scholar]
- 25.Erickson RA. EUS-guided FNA. Gastrointest Endosc 2004;60:267–79. [DOI] [PubMed] [Google Scholar]
- 26.Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc 2004;60:385–9. [DOI] [PubMed] [Google Scholar]
- 27.Gress F, Michael H, Gelrud D, et al. EUS-guided fine-needle aspiration of the pancreas: evaluation of pancreatitis as a complication. Gastrointest Endosc 2002;56:864–7. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. Practice guidelines for pancreatic cancer. Available at: http://www.nccn.org V.1.2010. Accessed December 1, 2010.
- 29.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 30.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut 2011;60:861–8. [DOI] [PubMed] [Google Scholar]
- 31.Koay EJ, Baio FE, Ondari A, et al. Intra-tumoral heterogeneity of gemcitabine delivery and mass transport in human pancreatic cancer. Phys Biol 2014;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koay EJ, Truty MJ, Cristini V, et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest 2014;124:1525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 2009;136:187–95. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. British Journal of Cancer 2013;109:926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. [see comment]. J Clinical Oncology 1997;15:2403–13. [DOI] [PubMed] [Google Scholar]
- 37.Heinemann V, Hertel LW, Grindey GB, et al. Comparison of the cellular pharmacokinetics and toxicity of 2’,2’-difluorodeoxycyti-dine and 1-beta-D-arabinofuranosylcytosine. Cancer Research 1988;48:4024–31. [PubMed] [Google Scholar]
- 38.van Moorsel CJ, Bergman AM, Veerman G, et al. Differential effects of gemcitabine on ribonucleotide pools of twenty-one solid tumour and leukaemia cell lines. Biochimica et Biophysica Acta 2000;1474: 5–12. [DOI] [PubMed] [Google Scholar]
- 39.Huang P Plunkett W Fludarabine- and gemcitabine-induced apoptosis: incorporation of analogs into DNA is a critical event. Cancer Chemotherapy & Pharmacology 1995;36:181–8. [DOI] [PubMed] [Google Scholar]
- 40.Chang KJ, Nguyen PT, Thompson JA, et al. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer 2000;88:1325–35. [DOI] [PubMed] [Google Scholar]
- 41.Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratu-moral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res 2003;9:555–61. [PubMed] [Google Scholar]
- 42.Irisawa A, Takagi T, Kanazawa M, et al. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: a pilot study. Pancreas 2007;35:189–90. [DOI] [PubMed] [Google Scholar]
- 43.Hecht JR, Farrell JJ, Senzer N, et al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc 2012;75:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hidalgo M Pancreatic cancer. N Engl J Med 2010;362:1605–17. [DOI] [PubMed] [Google Scholar]
- 45.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu J, Zhao G, Wang H-X, et al. A meta-analysis of gemcitabine containing chemotherapy for locally advanced and metastatic pancreatic adenocarcinoma. J Hematol Oncol 2011;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sultana A, Tudur Smith C, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer 2008;99:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640–8. [DOI] [PubMed] [Google Scholar]
- 49.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaccaro V, Sperduti I, Milella M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;365:768–9; author reply 769. [DOI] [PubMed] [Google Scholar]
- 51.Moorcraft SY, Khan K, Peckitt C, et al. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin Colorect Cancer 2014;13:232–8. [DOI] [PubMed] [Google Scholar]
- 52.Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18: 543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22: 1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]