Abstract
Hypoxic Ischemic Encephalopathy (HIE) is the result of severe anoxic brain injury during the neonatal period and causes life-long morbidity and premature mortality. Currently, therapeutic hypothermia immediately after birth is the standard of care for clinically relevant HIE. However, therapeutic hypothermia alone does not provide complete neuroprotection and there is an urgent need for adjunctive therapies. Ischemic conditioning is an adaptive process of endogenous protection in which small doses of sub-lethal ischemia can provide a protection against a lethal ischemic event. Remote Ischemic Post-conditioning (RIPC), a form of ischemic conditioning, is highly translatable for HIE diagnosed immediately after birth as the conditioned ischemic stimulus is applied at the limb after the lethal ischemic episode. A number of studies in neonatal rats have demonstrated that RIPC is effective at reducing injury in focal cerebral ischemia models and improves neurological outcomes. In this review, we focus on the available data on HIE and its current treatment, models in HIE studies, ischemic conditioning/RIPC and its mechanism. We discuss in particular the effect of RIPC on neonatal brain with HIE. We postulate that combining RIPC with standard therapeutic hypothermia can be an attractive therapeutic approach for HIE.
Keywords: hypoxic ischemic encephalopathy, remote ischemic post-conditioning, hypothermia
Introduction
Therapeutic hypothermia or total body cooling in treating hypoxic ischemic encephalopathy (HIE) is effective in many randomized controlled trials (Gluckman, et al., 2005; Shankaran, et al., 2005; Azzopardi, et al., 2009; Simbruner, Mittal, Rohlmann, & Muche, 2010; Zhou, et al., 2010; Jacobs, et al., 2011). It is the standard of care for infants with HIE. (Higgins, et al., 2011) However, as it does not provide complete protection, the search for adjunctive therapies continues. Remote limb Ischemic post-conditioning (RIPC) is the simple, inexpensive, and safe use of repetitive inflation-deflation procedure of a blood pressure (BP) cuff on the arm or leg to protect distant organs such as the brain, heart, and kidney from ischemic injury. Many preclinical studies demonstrated that RIPC is effective at reducing cerebral, myocardial or retinal injuries resulting from ischemia. In this review, we conducted a literature search to evaluate the rationale for the use of RIPC as an adjuvant treatment for hypoxic ischemic encephalopathy to standard therapeutic hypothermia.
Hypoxic Ischemic Encephalopathy
Hypoxic-ischemia (HI) is a term that is used to describe the constellation of complex physiological, cellular, and molecular changes that are induced by lack of oxygen supply to the brain (Liu, Li, & Gu, 2007; Busl & Greer., 2010). Hypoxic ischemic encephalopathy (HIE) is the result of severe anoxic brain injury during the neonatal period and accompanies life-long morbidity, including cerebral palsy, and premature mortality (Volpe, 2001). The incidence of HIE is between 1 and 8 per 1000 live births in developed countries with rates as high as 26 per 1000 live births in the developing world (Kurinczuk, White-Koning, & Badawi, 2010). Its pathophysiology involves oxidative stress, mitochondrial energy production failure, glutaminergic excito-toxicity and cell death (Marcelino, et al., 2015). The clinical symptoms of HIE include acute symptoms such as seizures, alteration of consciousness, weak breathing, poor muscle tons or metabolic derangement as well as chronic conditions such as cerebral palsy, mental retardation, learning disabilities, and epilepsy (Vannucci & Perlman, 1997; Vannucci R. , 1997; Shah & Perlman, 2009).
Current treatment of HIE
As the consequences of HIE are extensive and cause significant challenges to the affected individual, their family, and society, many potential treatments have been studied (Vannucci & Perlman, 1997; Vannucci R. , 1997; Johnston, Fatemi, Wilson, & Northington, 2011). Therapeutic hypothermia immediately after birth is the standard of care for clinically relevant HIE and appears to have a temporal relationship with outcome (Edwards, et al., 2010). Many studies have shown that therapeutic hypothermia inhibits key steps in the excito-oxidative cascade including energy failure and increases lactic acid, glutamate, and nitric oxide concentrations in the brain (Thoresen, et al., 1995; Thoresen, et al., 1997; Amess, et al., 1997). Based on the information from many studies and workshops presented by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the current standard of care for HIE is the whole body cooling to a core temperature of 33.5°C for 72 hours, starting within 6 hours of birth (Higgins, et al., 2011). Initiation of therapeutic hypothermia within 6 hours after birth in neonates with moderate to severe HIE leads to significant improvement in neurocognitive outcomes and reduced mortality (Shankaran, et al., 2005; Azzopardi, et al., 2009; Gluckman, et al., 2005; Jacobs, et al., 2011; Tagin, Woolcott, Vincer, Whyte, & Stinson, 2012). Surprisingly, longer duration or lower core body temperature does not confer additional benefit and may pose some risk especially in those infants with low birth weight (Higgins, et al., 2011; Thoresen, Bågenholm, Løberg, Apricena, & Kjellmer, 1996; Shankaran, et al., 2012; Shankaran, et al., 2014). These findings are similar to animal models of HIE, wherein the optimal temperature for the P7 rat is likely between 32 °C and 33.5 °C, with no significant additional neuroprotection for rat pups cooled below 33.5 °C (Bona, Hagberg, Loberg, Bagenholm, & Thoresen, 1998; Hobbs, et al., 2008; Wood, et al., 2016). Similar to human studies, intermediate to long duration (5 hours) of therapeutic hypothermia is more effective than 3 hours in rat pups with HI (Hoque, Chakkarapani, Liu, Hobbs, & Thoresen, 2009). However, there was no difference in neuroprotection in rat pups receiving therapeutic hypothermia from 5 hours to 10 hours (Sabir, Scull-Brown, Liu, & Thoresen, 2012).
Therapeutic hypothermia alone does not provide complete neuroprotection. The high prevalence of neurologic morbidity and mortality, even after therapeutic hypothermia, persists with death or severe neurological complications resulting in 40–50% of neonates who underwent therapeutic hypothermia (Edwards, et al., 2010). Due to the high morbidity and mortality, the development of new interventions for patents with HIE is imperative (Tagin, Woolcott, Vincer, Whyte, & Stinson, 2012; Shankaran, et al., 2014; Perlman, et al., 2010). The most likely next advance in care for these infants is in the form of an adjuvant therapy to therapeutic hypothermia (Peliowski-Davidovich, 2012).
Models of hypoxic ischemic encephalopathy
Many models of intrauterine, perinatal, and neonatal hypoxic ischemia have been reported (Northington, 2006). Maternal hypoxia or umbilical cord occlusion in fetal sheep produces a robust hypoxia-ischemia phenotype, but the high cost of lambs and large body size make this model impractical for widespread use (Gleason, Hamm, & Jones, 1990; Harris, Helou, Gleason, Traystman, & Koehler, 2001; Gonzalez, Hunter, Bennet, Power, & AJ., 2005; Lotgering, et al., 2004). The development of rodent models have provided mechanistic insight into the pathophysiology and clinical implications of neonatal HIE. The HIE model in neonatal brain was first validated by Vannucci and colleagues. Based on the Levine preparation in adult, it consists of unilateral common carotid artery ligation followed by systemic hypoxia produced by the inhalation of 8% oxygen –balance nitrogen. This model has been the most used for HIE (Rice, 1981;Vannucci & Vannucci, 1997). Importantly, studies showed that the brain of postnatal day 7 rat is phenotypically similar to an early third trimester human fetus (Clancy, 2001). Neuroprotection in less mature rats requires a more extensive hypoxic insult to reproduce the same histological findings observed at postnatal day 7 (Sheldon, Chuai, & Ferriero, 1996). Vannuci’s model demonstrates a reproducible brain damage model in a spontaneously breathing rat with low mortality. No seizures or cardiopulmonary complications were noted for at least the first 50 hours of survival (Rice, 1981). Hypothermia is also efficacious in animal models of HIE including the Vannuci model (Young, Olenginski, Yagel, & Towfighi, 1983; Bona, Hagberg, Loberg, Bagenholm, & Thoresen, 1998; Mishima, et al., 2004). Brief periods of moderate hypothermia to 32°C after injury delay cell death in 7-day-old rat pups by as much as a week, after which brain atrophy occurs (Trescher, Ishiwa, & Johnston, 1997).
Ischemic Conditioning
Ischemic conditioning is an adaptive process of endogenous protection in which small doses of sub-lethal ischemia protect the organism against a lethal ischemic event. Ischemic conditioning can be performed at a distance or remote from the ischemic target organ (Murry, Jennings, & Reimer, 1986; Gho, Schoemaker, van den Doel, Duncker, & Verdouw, 1996; Schmidt, et al., 2007). Similarly, tumor growth and progression has been noted to utilize these processes. Intermittent hypoxia has been reported within the tumors and is thought to increase tumors’ resistance to major ischemia and anti-cancer therapy (Muscari, et al., 2013). Depending on the temporal relation of the sub-lethal ischemia to the lethal ischemic event, the conditioned stimulus can be applied prior to ischemia and vessel occlusion (pre- conditioning), during ischemia and before reperfusion (per-conditioning), or after the lethal ischemic episode and through reperfusion (post-conditioning). A study found that combining limb remote per- and post- conditioning significantly reduced cerebral ischemia/reperfusion injury (Ren, et al., 2015).
There have been several studies that focus on the neurochemical basis for the neuroprotective role of these ischemic conditioning regimens. Similar pathways and molecules play a role in these conditionings but the roles and timing can be different (Alkan, 2009). Several studies focused on the consequences of the conditioning or simulated the model of pre- conditioning. There are two windows of protection; an early and late window. The mediators for the early window include phosphorylation, transporter regulation, interfering RNA, metabolic pathways such as nitric oxide (NO), increased glucose metabolism, inflammatory responses, and angiogenesis. For the late window, they include regulation of gene, regulator of programmed cell death (stimulate cell survival mechanisms and down regulated apoptotic pathways), receptor modulator like NMDA receptor activation, antioxidant capacity, and suppression of immune system (Alkan T. , 2009; Dornbus & Ding, 2012; Dornbos, et al., 2013; Heyman, et al., 2016; Liu, et al., 2016; Li, et al., 2017). In addition, adaptation of glial cells after pre-conditioning including early differentiation of astrocytes may play a role in neuroprotection as well (Sen, et al., 2011).
Apart from remote conditioning with a repetitive inflation-deflation procedure of a blood pressure (BP) cuff, some studies had utilized isoflurane for post-conditioning. Isoflurane is a volatile anesthetic agent that can mimic ischemic-conditioning in both adult and neonatal brain (Kapinya, et al., 2002; Xiong, et al., 2003; Zhao, Peng, Li, Xu, & Zuo, 2007). A recent study shows that isoflurane post-conditioning can improve survival and neurological outcomes in an adult rat model of cardiac arrest (Zhang, Wu, Yu, & Liu, 2017).
The induction of neuroprotective effect is likely region-specific. A hypoxic pre-conditioning study shows a metabolic adaptation in hippocampus, parietal cortex and cerebellum of the brains of neonatal rats but not the striatum (Marcelino, et al., 2015). This coincides with the result from a study in adult rats that confirms the effect of hypoxic preconditioning including change in gene expression in the frontal cortex but not in the caudate, putamen and thalamus (Omata, et al., 2006).
Neonatal HIE and Post-conditioning
Several studies have shown that immature neonatal brain responds to hypoxic ischemic event differently from adult brain. Infants exposed to hypoxic ischemia tend to present with neonatal encephalopathy leading to difficulty in maintaining respiration, depression of tone and reflexes decreased consciousness, feeding issue and seizures (Nelson & Leviton, 1991; Hassell, Ezzati, Alonso-Alconada, Hausenloy, & Robetson, 2015). Hypoxic ischemic injury usually causes a prominent oxidative stress environment and accumulation of reactive species (Alkan, Goren, Vatansever, & Sarandol, 2008; Fatemi, Wilson, & Johnston, 2009; Penna, et al., 2014). The brain of newborns especially hippocampal cells are more vulnerable to oxidative stress and free radical oxidative damage than the brain of adult leading to the described detrimental consequences (Drunalini-Perera, et al., 2014). In acute hypoxic ischemic event, after the falling of Na+/K+ ATP dependent pump, glutamate overflows and cytoplasmic Ca2+ concentration arises. Ca2+ triggers many downstream toxic cascades and generates high levels of reactive oxygen species. A study showed that Edaravone, a free radical scavenger could reduce the brain damage from hypoxic ischemic injury in neonatal rats (Ikeda, Xia, Kaneko, Sameshima, & Ikenoue, 2002). Hypoxic ischemia also causes changes in the regulation of genes, transcription factors and molecules relating to cell death signaling and immune responses (Guglielmotto, et al., 2009; Sameshima & Ikenoue, 2013). Mitochondrial membrane permeabilization was noted to play a major role in cell death. In adult, the mechanism of this permeabilization transition in hypoxic ischemic event is predominantly secondary to the cyclophilin D-dependent opening while Bax-dependent mitochondrial outer membrane permeabilization is the key mechanism in immature neonatal brain. Bax-inhibiting peptide (BIP) was found to be associated with a reduction of Bax activation, mitochondrial permeabilization and downstream caspase activation (Wang, et al., 2010).
Post-conditioning is appropriate for HIE diagnosed immediately after birth. Remote ischemic post- conditioning (RIPC) has been well established. It is a simple, inexpensive, and safe use of repetitive inflation-deflation procedure of a blood pressure (BP) cuff on the arm or leg to protect distant organs such as the brain, heart, and retina from ischemic injury (Schmidt, et al., 2007; Gho, Schoemaker, van den Doel, Duncker, & Verdouw, 1996; Hess, Hoda, & Bhatia, 2013; Liu, Sha, & Cho, 2013; Zhang, et al., 2014; Yamaguchi, et al., 2015;). RIPC provides protection in the adult and neonatal brain against subsequent lethal ischemia. (Zhou, et al., 2011; Hess, Hoda, & Bhatia, 2013). A randomized crossover study of healthy individuals showed no difference in blood flow, tissue perfusion, concentration of nitrite and platelet mitochondrial function between ischemic conditioning at thigh or arm. Thus, localization does not seem to affect the cyto-protection property of ischemic conditioning (Dezfulian, et al., 2017).
Effects of RIPC
Remote ischemic post-conditioning can reduce infarct size in both neonatal and adult brain. In neonatal brain, a study in rats shows that RIPC alone can significantly reduce the infarct volume after the hypoxic-ischemic brain injury with possibilities of involving opioid receptor/AKT pathway (Zhou, et al., 2011). For adult brain, RIPC can significantly reduce infarct size in both animal and human models (Ren, et al., 2012; Zhao, Ren, Chen, & Shen, 2012; Liu, et al., 2014). RIPC reduced the infarct volume at 48 hours after hypoxic ischemia. It also significantly improved the long-term neurological functional outcomes. (Zhou, et al., 2011). However, RIPC shows no long-term improvement in brain weight (Zhou, et al., 2011; Drunalini-Perera, et al., 2014)
An application of RIPC after the neonatal hypoxic ischemic injury can improve long-term sensory, motor (coordination and strength) and cognitive impairments. These cognitive impairments include short-term memory and spatial learning and memory (Drunalini-Perera, et al., 2014). Isoflurane post-conditioning might have minimal effect on motor function but could significantly alleviate the spatial learning and memory impairments (Xu, et al., 2016). Another study also showed that physical training for 2 weeks after a hypoxic-ischemic insult in neonatal brain could reduce brain damage and decrease learning and memory impairments (Chen & Jiang, 2010).
Many studies have shown that post-conditioning should be done within 6–8 hours after hypoxic ischemic event for favorable outcome. This is likely the therapeutic window and post-conditioning might be able to reverse neuronal apoptosis (Hassell, Ezzati, Alonso-Alconada, Hausenloy, & Robetson, 2015; Xu, et al., 2016). However, a study shows that RIPC up to 24 hours after hypoxic ischemic event could provide a long-term neuroprotection and 3-day consecutive treatment could possibly give greater benefit (Drunalini-Perera, et al., 2014).
Mechanism of RIPC in neonates
The mechanisms of neonatal neuroprotection from RIPC is not well-understood but seems to involve nerve activation and limb ischemia that trigger humoral protective factors that circulate and provide protective effect throughout the body (Hassell, Ezzati, Alonso-Alconada, Hausenloy, & Robetson, 2015). Its neuroprotection can be via the transferring of protective factors such as opioids, receptor stimulation, mitochondrial permeability pore, KATP, pro-survival kinase (phosphatidylinositol-3-kinase [PI3K]/AKT, ERK pathways) (Zhou, et al., 2011; Xu, et al., 2016). This leads to increased cerebral blood flow, decrease in inflammation and upregulation of pro-survival signaling cascades. Plasma nitrite concentration seems to play an important role in increasing the blood flow. Although, there has been no study that specifically assessed neonatal NO, an adult human study shows that, after the first ischemic conditioning, plasma nitrite concentration peaks and remains elevated along with the ongoing conditioning (Dezfulian, et al., 2017). This elevated nitrite involves in cyto-protection from hypoxic/re-oxygenation injuries, mainly secondary to its reduction to NO by deoxygenated heme proteins (Kamga Pride, et al., 2014).
Apart from plasma nitrite, phosphor calmodulin-dependent protein kinase II (Phosphor-CaMKII) and Brain derived neurotrophic factor (BDNF) may play a role in neuroprotection of RIPC as a 2-week physical training after a hypoxic-ischemic insult in neonatal brain increased their expressions (Chen & Jiang, 2010). In addition, isoflurane post-conditioning could induce neuroprotection after hypoxic ischemic event partly by mediating GluR2 subunit of AMPA receptor in neonatal rats (Xu, et al., 2016).
There is a lack of studies in gene regulation after RIPC in neonatal brain. A study in adult rats shows that local cerebral blood flow and increases in angiogenesis in the ischemic brain correlate with upregulated expressions of Notch 1 and Notch intracellular domain (NICD) surrounding the ischemic area (Ren, et al., 2016). Another adult rat study shows involvement of alteration of the expression of hypoxia-inducible factor (HIF)-related genes including vascular endothelial growth factor (VEGF) and erythropoietin (EPO) after remote ischemic pre-conditioning (Heyman, et al., 2016).
Post-conditioning may have significant role in alleviating the surge in of oxidative stress in vulnerable neonatal brain after hypoxic ischemic injury. Post-conditioning in adult rat shows the effect of modulating reactive oxygen species. It exerts this via upregulation of anti-oxidative stress proteins such as nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase −1 (HO-1) (Zhang, et al., 2014).
With evidences from adult studies, ischemic conditioining might also involve many other molecular mechanism including neuroglobin, glial fibrillary acidic protein, lipid peroxidation level, lactic dehydrogenase, creatine kinase activities, JAK-STAT pathway, Protein kinase C pathway, antioxidant enzyme as well as other mRNA expressions (Alkan, 2009; Zhan, et al., 2010; Wang, et al., 2010; Liu, et al., 2012; Zhang, et al., 2014; Liu Y. , Li, Li, & Zou, 2015).
Conclusion
HIE is the result of severe anoxic brain injury during the neonatal period and accompanies life-long morbidity, including cerebral palsy, and premature mortality. Therapeutic hypothermia immediately after birth is the standard of care for HIE. However, this hypothermia alone does not provide complete neuroprotection. Even after therapeutic hypothermia, high prevalence of neurologic morbidity and mortality persists. Due to that, an adjuvant therapy for patents with HIE is imperative.
Ischemic conditioning is an adaptive process of endogenous protection in which small doses of sub-lethal ischemia protect the organism against a lethal ischemic event. Post-conditioning is appropriate for HIE diagnosed immediately after birth as the conditioned stimulus is applied after the lethal ischemic episode. Remote ischemic post- conditioning (RIPC) is well established and is a safe use of repetitive inflation-deflation procedure of a BP cuff on the limb to protect distant organs from ischemic injury. A number of studies in neonatal rats have demonstrated that RIPC is effective at reducing injury in focal cerebral ischemia models. Combining this RIPC with standard therapeutic hypothermia to treat HIE is an attractive therapeutic approach.
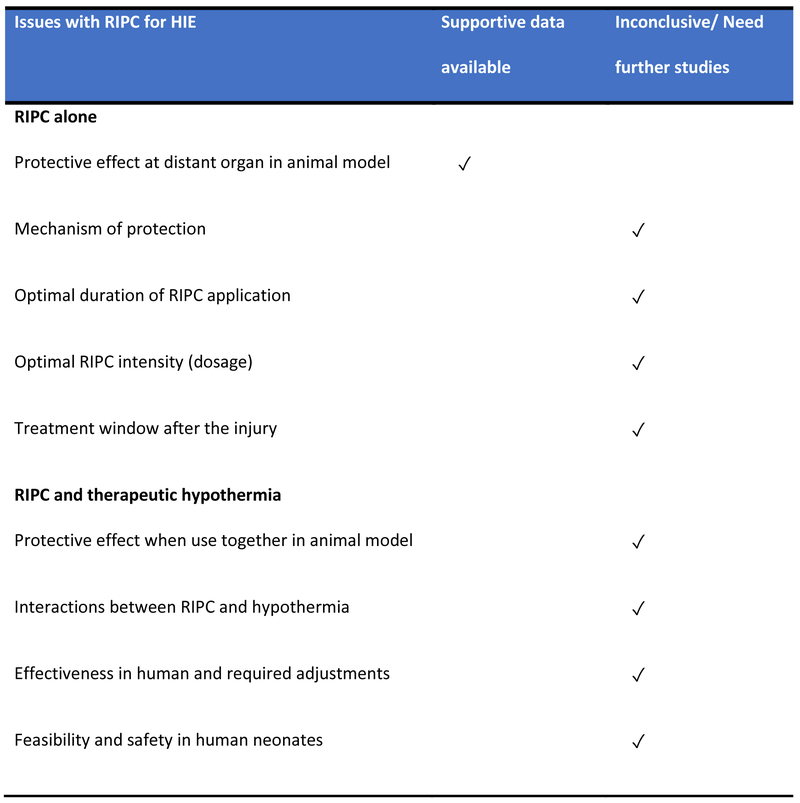
RIPC alone can provide long-term neuroprotective effect. It is easy to use and can be started as late as 24 hours after the initial hypoxic ischemic injury in a rat model. However, beyond 24 hours, it is still unclear how long we can wait to start RIPC. Consecutive treatments may be beneficial as shown in many studies but the optimal regimen including the duration and dose is still inconclusive. Combining this RIPC with standard therapeutic hypothermia to treat HIE has the potential to be an effective synergistic treatment. However, it needs further studies to elucidate the effectiveness of this combination and their interactions as well as its feasibility and safety. In addition, another important question is whether this combination will offer the similar protection and improve neurological outcomes when we extrapolate it to human studies. Figure 1 summarizes the current available data and the issues that require further attention.
Figure 1.
Issues with remote ischemic post-conditioning and hypoxic ischemic encephalopathy
References
- Alka T (2009, October). Neuroprotective effects of ischemic tolerance (preconditioning) and postconditioning. Turk Neurosurg, 19(4), 406–12. [PubMed] [Google Scholar]
- Alkan T, Goren B, Vatansever E, & Sarandol E (2008). Effects of hypoxic preconditioning in antioxidant enzyme activities in hypoxic-ischemic brain damage in immature rats. Turk. Neurosurg, 18, 165–171. [PubMed] [Google Scholar]
- Amess P, Penrice J, Cady E, Lorek A, Wylezinska M, Cooper C, … Reynolds E (1997). Mild hypothermia after severe transient hypoxia-ischemia reduces the delayed rise in cerebral lactate in the newborn piglet. Pediatr Res, 41, 803–808. [DOI] [PubMed] [Google Scholar]
- Azzopardi D, Strohm B, Edwards A, Dyet L, Halliday H, Juszczak E, … Brocklehurst P (2009). Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med, 361, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Bona E, Hagberg H, Loberg E, Bagenholm R. a., & Thoresen M (1998). Protective effects of moderate hypothermia after neonatal hypoxiaischemia: Short- and long-term outcome. Pediatr Res, 43, 738–745. [DOI] [PubMed] [Google Scholar]
- Busl KM, & Greer DM (2010). Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation, 26(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Chen X, & Jiang L (2010). Physical training improves spatial learning and memory impairments following hypoxic ischemic brain damage in neonatal rats. Zhongguo Dang Dai Er Ke Za Zhi, 12(5), 363–7. [PubMed] [Google Scholar]
- Clancy B, Darlington R. a., & Finlay B (2001). Translating developmental time across mammalian species. Neuroscience, 105, 7–17. [DOI] [PubMed] [Google Scholar]
- Dezfulian C, Taft M, Corey C,HG, Krehel N, Rittenberger J, … Shiva S (2017, August). Biochemical signaling by remote ischemic conditioning of the arm versus thigh: Is one raise of the cff enough? Redox Biol, 12, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbos D 3., Zwagerman N, Guo M, Ding J, Peng C, Esmail F, … Ding Y (2013, June). Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J Neurosci Res, 91(6), 818–27. [DOI] [PubMed] [Google Scholar]
- Dornbus D 3., & Ding Y (2012). Mechanisms of neuronal damage and neuroprotection underying ischemia/reperfusion injury after physical exercise. Curr Drug Targets, 13(2), 247–62. [DOI] [PubMed] [Google Scholar]
- Drunalini-Perera P, Hu Q, Tang J, Li L, Barnhart M, Doycheva D, … Tang J (2014). Delayed remote ischemic postconditioning improves long term sensory motor deficits in a neonatal hypoxic ischemic rat model. PLoS One, 9(2), e90258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, Brocklehurst P, Gunn A, Halliday H, Juszczak E, Levene M, … Azzopardi D (2010). Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ, 340, c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang A, Gonzalez F, Sheldon R. a., & Ferriero D (2013). Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia–ischemia. Pediatric research, 73(1), 12–17. doi: 10.1038/pr.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi A, Wilson M, & Johnston M (2009). Hypoxic-ischemic encephalopathy in term infant. Clin. Perinatol, 36, 835–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho B, Schoemaker R, van den Doel M, Duncker D, & Verdouw P (1996). Myocardial protection by brief ischemia in noncardiac tissue. Circulation, 94, 2193–2200. [DOI] [PubMed] [Google Scholar]
- Gleason C, Hamm C. a., & Jones MJ (1990). Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol, 258(H), 1064–1069. [DOI] [PubMed] [Google Scholar]
- Gluckman P, Wyatt J, Azzopardi D, Ballard R, Edwards A, Ferriero D, … Gunn A (2005). Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet, 365, 663–670. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Hunter C, Bennet L, Power G. a., & AJ G (2005, July). Cerebral oxygenation during postasphyxial seizures in near-term fetal sheep. J Cereb Blood Flow Metab, 25(7), 911–918. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Aragno M, Autelli R, Gilberto L, Novo E, Colombatto S, … Tabaton M (2009). The up-regulation of BACE1 mediated by hypoxia and ischemia injury: role of oxidative stress and HIF1 alpha. J. Neurochem, 108, 1045–1056. [DOI] [PubMed] [Google Scholar]
- Harris A, Helou S, Gleason C, Traystman R. a., & Koehler R (2001). Fetal cerebral and peripheral circulatory responses to hypoxia after nitric oxide synthase inhibition. Am J Physiol Regul Integr Comp Physiol, 281, R381–R390. [DOI] [PubMed] [Google Scholar]
- Hassell K, Ezzati M, Alonso-Alconada D, Hausenloy D, & Robetson N (2015). New Horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal, 100, F541–F552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D, Hoda M, & Bhatia K (2013). Remote limb perconditioning and postconditioning: will it translate into a promising treatment for acute stroke? . Stroke, 44(4), 1191–1197. [DOI] [PubMed] [Google Scholar]
- Heyman S, Leibowitz D, Mor-Yosef Levi I, Liberman A, Eisenkraft AR, Khamaisi M, & Rosenberger C (2016, April). Adaptive response to hypoxia and remote ischaemia pre-conditioning: a new hypoxia-inducible factors era in clinical medicine. Acta Physiol (Oxf), 216(4), 395–406. [DOI] [PubMed] [Google Scholar]
- Higgins R, Raju T, Edwards A, Azzopardi D, Bose C, Clark R, & NICHD Hypothermia Workshop Speakers and Moderators. (2011). Hypothermia and Other Treatment Options for Neonatal Encephalopathy: An Executive Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Workshop. The Journal of Pediatrics, 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, & Dingley J (2008). Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. . Stroke, 39, 1307–1313. [DOI] [PubMed] [Google Scholar]
- Hoda M, Bhatia K, Hafez S, Johnson M, Siddiqui S, Ergul A, … Hess D (2014). Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl Stroke Res, 5(4), 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque N, Chakkarapani E, Liu X, Hobbs C, & Thoresen M (2009). Cooling for longer improves neuroprotection after hypoxic-ischaemic insult in a neonatal rat model Pediatric Academic Society Meeting, (p. Poster Presentation). Baltimore, MD. [Google Scholar]
- Ikeda T, Xia X, Kaneko M, Sameshima H, & Ikenoue T (2002). Effect of the free radical scavenger, 3-methyl-1-phenyl-2-pyrazolin-5-one (MCI-186), on hypoxia-ischemia-induced brain injury in neonatal rats. Neuroscience Letters, 329, 33–36. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Morley C, Inder T, Stewart M, Smith K, McNamara P, … Collaboration IC (2011). Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med, 165, 692–700. [DOI] [PubMed] [Google Scholar]
- Johnston M, Fatemi A, Wilson M. a., & Northington F (2011). Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol, 10, 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamga Pride C, Mo L, Quesnelle K, Dagda R, Murillo D, Geary L, … Shiva S (2014, January 1). Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc Res, 101(1), 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinya K, Lowl D, Futterer C, MauMl Waschke K, Isaev N, & Dirnagl U (2002). Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke, 33(7), 1889–98. [DOI] [PubMed] [Google Scholar]
- Kurinczuk J, White-Koning M. a., & Badawi N (2010). Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev, 86(6), 329–338. [DOI] [PubMed] [Google Scholar]
- Leconte C, Tixier E, Freret T, Toutain J, Saulnier R, Boulouard M, … Bernaudin M (2009). Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke, 40, 3349–3355. [DOI] [PubMed] [Google Scholar]
- Li S, Hafeez A, Noorulla F, Geng X, Shao G, Ren C, … Ji X (2017, October). Preconditioning in neuroprotection: From hypoxia to ischemia. Prog Neurobiol, 157, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Peng Z, Zhang N, Yu L, Han S, Li D, & Li J (2012, March). Identification of differentially expressed microRNAs and their PKC isoform specific gene network prediction during hypoxic pre-conditioning and focal cerebral ischemia of mice. J Neurochem, 120(5), 830–41. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J. a., & Gu M (2007, February). The correlation between myocardial function and cerebral hemodynamics in term infants with hypoxic-ischemic encephalopathy. J Trop Pediatr, 53(1), 44–48. [DOI] [PubMed] [Google Scholar]
- Liu X, Sha O, & Cho E (2013, November). Remote ischemic postconditioning promotes the survival of retinal ganglion cells after optic nerve injury. J Mol Neurosci, 51(3), 639–46. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao S,F,L, Kang J, Xiao A, Li F, … Ji X (2014). Remote ischemic postconditioning alleviates cerebral ischemic injury by attenuating endoplasmic reticulum stress-mediated apoptosis. Transl Stroke Res, 5(6), 692–700. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li B, Li Q, & Zou L (2015). Neuroglobin up-regulation after ischemic pre-conditioning in a rat model of middle cerebral artery occlusion. Brain Inj, 29(5), 651–7. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen C, Li X, Ran Y, Xu T, Zhang Y, … Hu X (2016, January). Remote Ischemic Preconditioning-Mediated Neuroprotection against Stroke is Associated with Significant Alterations in PImmune Responses. CNS Neurosci Ther, 22(1), 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotgering F, Bishai J, Struijk P, Blood A, Hunter C, Oberg K, … Longo L (2004). Absence of robust ischemic preconditioning by five 1-minute total umbilical cord occlusions in fetal sheep. J Soc Gynecol Investig, 11, 449–456. [DOI] [PubMed] [Google Scholar]
- Marcelino T, de Lemos Rodrigues P, Miguel P, Netto C, Pereira Silva L, & Matte C (2015, October 5). Effect of matermal exercise on biochemical parameters in rats submitted to neonatal hypoxia-ischemia. Brain Res, 1622, 91–101. [DOI] [PubMed] [Google Scholar]
- Mishima K, Ikeda T, Yoshikawa T, Aoo N, Egashira N, Xia Y, … Fujiwara M (2004). Effects of hypothermia and hyperthermia on attentional and spatial learning deficits following neonatal hypoxia-ischemic insult in rats. Behav Brain Res, 151. [DOI] [PubMed] [Google Scholar]
- Murry C, Jennings R, & Reimer K (1986). Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation, 74(5), 1124–36. [DOI] [PubMed] [Google Scholar]
- Muscari C, Giordano E, Bonafe F, Govoni M, Pasini A, & Guarnieri C (2013, December). Molecular mechanisms of ischemic preconditioning and postconditioning as putative therapeutic targets to reduce tumor survival and malignancy. Med Hypotheses, 81(6), 1141–5. [DOI] [PubMed] [Google Scholar]
- Nelson K, & Leviton A (1991). How much of neonatal encephalopathy is due to birth asphyxia? Am J Dis Child, 145, 1325–31. [DOI] [PubMed] [Google Scholar]
- Northington F (2006). Brief update on animal models of hypoxic-ischemic encephalopathy and neonatal stroke. ILAR J, 47(1), 32–38. doi: 10.1093/ilar.47.1.32 [DOI] [PubMed] [Google Scholar]
- Omata N, Murata T, Takamatsu S, Maruoka N, Yonekura Y, Y F, & Wada Y (2006). Region-specific induction of hypoxic tolerance by expression of stress proteins and antioxidant enzymes. Neurol. Sci, 27, 74–77. [DOI] [PubMed] [Google Scholar]
- Peliowski-Davidovich A &. (2012). Hypothermia for newborns with hypoxic ischemic encephalopathy. . Paediatrics & Child Health, 17(1), 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Wang D, Yu J, Lecker I, PM B, bOWIE d., & oRSER b. (2014). Hydrogen peroxide increases GABAA receptor-mediated tonic current in hippocampal neurons. J Neurosci, 34, 10624–10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman J, Wyllie J, Kattwinkel J, Atkins D, Chameides L, Goldsmith J, … Velaphi S (2010). Neonatal Resuscitation Chapter Collaborators. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendation. Circulation, 122(16 Suppl2), S516–538. [DOI] [PubMed] [Google Scholar]
- Ren C, Gao M, Doenbus D 3, Ding Y, Zeng X, Luo Y, & Ji X (2012, June). Remote ischemic post-conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol Res, 33(5), 514–9. [DOI] [PubMed] [Google Scholar]
- Ren C, Li S, Wang B, Han R, Li N, Gao J, … Ji X (2016, October 22). Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav Brain Res, S0166–4328(16), 30901–9. [DOI] [PubMed] [Google Scholar]
- Ren C, Wang P, Wang B, Li N, Li W, Zhange C, … Ji X (2015). Limb remote ischemic per-conditioning in combination with post-conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor Neurol Neurosci, 33(3), 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J, Vannucci R. a., & Brierley J (1981). The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol, 9, 131–141. [DOI] [PubMed] [Google Scholar]
- Sabir H, Scull-Brown E, Liu X. a., & Thoresen M (2012). Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke, 43, 3364–3370. [DOI] [PubMed] [Google Scholar]
- Sameshima H, & Ikenoue T (2013). Hypoxic-Ischemix Neonatal Encephalopathy:Animal Experiments for Neuroprotective Therapies. Stroke Research and Treatment, 1–11. doi: 10.1155/2013/659374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaoka N, Kawaguchi M, Kawaraguchi Y, Nakamura M, Konishi N, Patel H, … Furuya H (2009). Isoflurane exerts a short-term but not a long-term preconditioning effect in neonatal rats exposed to a hypoxic-ischemic neuronal injury. Acta Anaesthesiol Scand, 46–54. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Smerup M, Konstantinov I, Shimizu M, Li J, Cheung M, … Kharbanda R (2007). Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism:first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol, 292, H1889–H1890. [DOI] [PubMed] [Google Scholar]
- Sen E, Basu A, Willing L, Uliasz T, Myrkalo J, VanuSJ, … Levison S (2011, July 26). Pre-conditioning induces the precocious differentiation of neonatal astrocytes to enhance their neuroprotective properties. ASN Neuro, 3(3), e00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. a., & Perlman M (2009, January). Time courses of intrapartum asphyxia: neonatal characteristics and outcomes. Am J Perinatol, 26(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook A, Ehrenkranz R, Tyson J, McDonald S, Donovan E, … Jobe A (2005). Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med, 353, 1574–1584. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook A, McDonald S, Higgins R, Tyson J, Ehrenkranz R, … Walsh M (2012). Temperature Profile and Outcomes of Neonates Undergoing Whole Body Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. Pediatric Critical Care Medicine ., 13(1), 53–59. doi: 10.1097/PCC.0b013e31821926bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Laptook A, Pappas A, McDonald S, Das A, & Tyson J (2014). Effect of Depth and Duration of Cooling on Deaths in the NICU Among Neonates With Hypoxic Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA, 312(24), 2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R, Chuai J. a., & Ferriero D (1996). A rat model for hypoxic ischemic brain damage in very premature infants. Biol Neonate, 69, 327–341. [DOI] [PubMed] [Google Scholar]
- Shen Y, Pan S, Ge J, & Hao Z (2012, September). Exercise preconditioning provides early cardioprotection against exhaustive exercise in rats: potential involvement of protein kinase C delta translocation. Mol Cell Biochem, 368(1–2), 89–102. [DOI] [PubMed] [Google Scholar]
- Simbruner G, Mittal R, Rohlmann F, & Muche R (2010, October). Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics, 126(4), e771–8. [DOI] [PubMed] [Google Scholar]
- Tagin M, Woolcott C, Vincer M, Whyte R, & Stinson D (2012). Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis . Arch Pediatr Adolesc Med , 166, 558–566. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Bågenholm R, Løberg E, Apricena F. a., & Kjellmer I (1996). Posthypoxic cooling of neonatal rats provides protection against brain injury. Archives of Disease in Childhood Fetal and Neonatal Edition, 74(1), F3–F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen M, Penrice J, Lorek A, Cady E, Wylezinska M, Kirkbride V, … Reynolds EO (1995). Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res, 37, 667–670. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Satas S, Puka-Sundvall M, Whitelaw A, Hallström A, Løberg E, … Hagberg H (1997). Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport, 8, 3359–3362. [DOI] [PubMed] [Google Scholar]
- Trescher W, Ishiwa S. a., & Johnston M (1997). Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev, 19, 326–338. [DOI] [PubMed] [Google Scholar]
- Vannucci R (1997). Hypoxic–ischemic encephalopathy: clinical aspects In Fanaroff A. a., & Martin R, Neonatal–Perinatal Medicine (pp. 877–891). Philadelphia, PA: Mosby Yearbook Inc. [Google Scholar]
- Vannucci R. a., & Perlman J (1997). Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics, 100(6), 1004–1014. [DOI] [PubMed] [Google Scholar]
- Vannucci R, & Vannucci S (1997). A model of perinatal hypoxic-ischemic brain damage. Annals of the New York Academy of Sciences, 835, 234–249. [DOI] [PubMed] [Google Scholar]
- Volpe J (2001). Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev, 7(1), 56–64. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhou D, Wang C, Gao Y, Q Z, Qian G, & MA D (2010, August). Hypoxic preconditioning suppresses group III secreted phospholipase A2-induced apoptosis via Jak2-Stat3 activation in cortical neurons. J Neurochem, 114(4), 1039–48. [DOI] [PubMed] [Google Scholar]
- Wang X, Han W, Du XZ, Carlsson Y, Mallard C, Jacotot E, & Hagberg H (2010). Neuroprotective Effect of Bax-Inhibiting Peptide on Neonatal Brain Injury. Stroke, 41, 2050–2055. [DOI] [PubMed] [Google Scholar]
- Wood T, Osredkar D, Puchades M, Maes E, Falck M, Flatebø T, & … Thoresen M (2016). Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia. Scientific Reports, 6, 23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Zheng Y, Wu M, Hou L, Zhu ZZ, & Lu Z (2003). Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg, 233–7. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xue H, Zhao P, Yang Y, Ji G, Yu W, … Wang F (2016). Isoflurane postconditioning induces concentration-and timing-dependent neuroprotection partly mediated by the GluR2 AMPA receptor in neonatal rats after brain hypoxia-ischemia. J Anesth, 427–436. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, … Iwao H (2015, January 15). Repeated remote ischemic conditioning atleft ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol, 178, 239–46. [DOI] [PubMed] [Google Scholar]
- Young R, Olenginski T, Yagel S. a., & Towfighi J (1983). The effect of graded hypothermia on hypoxic-ischemic brain damage: A neuropathologic study in the neonatal rat. Stroke, 14, 929–934. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jizhang Y, Kwiecien T, Li N, Zhang Y, Ji X, … Ding Y (2014, May). effects of remote ischemic conditioning against ischemic/reperfusion-induced retinal injury in rats. Vis Neurosci, 31(3), 245–52. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu M, Yu H, & Liu J (2017, July). Emulsified isoflurane postconditioning improves survival and neurological outcomes in a rat model of cardiac arrest. Exp Ther Med, 14(1), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ren C, Chen X, & Shen J (2012). From Rapid to Delayed and Remote Post- conditioning: the Evolving Concept of Ischemic Postconditioning in Brain Ischemia. . Current Drug Targets, 13(2), 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Peng L, Li L, Xu X, & Zuo Z (2007). Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology, 963–970. [DOI] [PubMed] [Google Scholar]
- Zhou W, Cheng G, Shao X, Liu X, Shan R, Zhuang D, … Group CS (2010). Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr, 157(3), 367–72. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fathali N, Lekix T, Ostrowski R, Chen C, Martin R, … Zhang J (2011, February). Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke, 42(2), 439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]