Abstract
Acquired hearing loss is caused by complex interactions of multiple environmental risk factors, such as elevated levels of lead and noise, which are prevalent in urban communities. This study delineates the mechanism underlying lead-induced auditory dysfunction and its potential interaction with noise exposure. Young-adult C57BL/6 mice were exposed to: 1) control conditions; 2) 2 mM lead acetate in drinking water for 28 days; 3) 90 dB broadband noise 2 h/day for two weeks; and 4) both lead and noise. Blood lead levels were measured by inductively coupled plasma mass spectrometry analysis (ICP-MS) lead-induced cochlear oxidative stress signaling was assessed using targeted gene arrays, and the hearing thresholds were assessed by recording auditory brainstem responses. Chronic lead exposure downregulated cochlear Sod1, Gpx1, and Gstk1, which encode critical antioxidant enzymes, and upregulated ApoE, Hspa1a, Ercc2, Prnp, Ccl5, and Sqstm1, which are indicative of cellular apoptosis. Isolated exposure to lead or noise induced 8–12 dB and 11–25 dB shifts in hearing thresholds, respectively. Combined exposure induced 18–30 dB shifts, which was significantly higher than that observed with isolated exposures. This study suggests that chronic exposure to lead induces cochlear oxidative stress and potentiates noise-induced hearing impairment, possibly through parallel pathways.
Keywords: Ototoxicity, Lead exposure, Noise-induced hearing loss, Oxidative stress, Cochlea, Auditory dysfunction
1. Introduction
Exposure to lead, a persistent environmental pollutant that is ubiquitous in air, water, and soil, is a major public health hazard. It accumulates in soil and water over a long period of time and is absorbed by humans, predominantly, by inhalation and ingestion. Though regulatory measures prevent environmental exposures above toxic levels, sub-toxic exposures are generally unavoidable as lead is found in lead-based paints in older homes, batteries, solder, pipes, pottery, roofing materials, and some cosmetics. Exposure to even low levels of lead can cause adverse effects in multiple tissues and organs such as blood, liver, brain, and kidney (Ercal et al., 2000; Rio et al., 2001; Sanders et al., 2009; Senut et al., 2012; Song et al., 2017; Witzmann et al., 1999). In addition, exposure to lead has been reported to disrupt the structure as well as the function of the auditory system. Blood lead levels ≥2 μg/dl, which is well below the current action level (5 μg/dl) recommended by the Centers for Disease Control and Prevention (CDC advisory committee report, 2012), were associated with higher odds of high-frequency hearing loss (Shargorodsky et al., 2011). Exposure to low levels of lead during development decreased the expression of voltage-dependent anion channel proteins and disrupted the monoaminergic system in the auditory brainstem (Fortune and Lurie, 2009; Prins et al., 2010a,b). Furthermore, lead exposure induced degeneration of sensory receptor cells in the cochlea, affected auditory nerve conduction velocity, disrupted cochlear blood-labyrinth barrier, and caused vestibular dysfunction (Jones et al., 2008; Klimpel et al., 2017; Lasky et al., 1995; Liu et al., 2013; Yamamura et al., 1989). Despite these overwhelming evidences indicating the ototoxic effects of lead, the underlying mechanism is yet to be fully understood.
Generally, oxidative stress plays a critical role in auditory dysfunction as it activates cochlear cell death pathways in hearing loss associated with aging, exposure to noise, organic solvents, heavy metals such as cadmium, ototoxic drugs such as aminoglycosides and cisplatin, and radiation (Bottger and Schacht, 2013; Huth et al., 2011; Jamesdaniel et al., 2016; Kim et al., 2008; Poirrier et al., 2010; Samson et al., 2008; Warchol, 2010; Wong and Ryan, 2015). Oxidative stress-induced damage has been detected in three discrete regions of the cochlea, namely, the sensory epithelium, the lateral wall, and the modiolus, in acquired hearing loss. Though lead exposure also damages the sensory, vascular, and neuronal components of the cochlea, it is not known whether oxidative stress mediates its ototoxic effects. However, exposure to lead has been reported to cause oxidative damage in other tissues and organs by inducing lipid peroxidation and compromising the antioxidant defense systems (Ahamed and Siddiqui, 2007; Ercal et al., 1996; Roy et al., 2015). Lead-induced oxidative damage has been reported to disrupt the functions of the reproductive, cardiovascular, urinary, and nervous systems (Ding et al., 2001; Hsu et al., 1998; Patra et al., 2001; Patrick, 2006). Therefore, this study sought to investigate whether chronic exposure to lead acetate in drinking water induces oxidative stress in the cochlea.
Environmental exposures to ototoxicants are usually complex and therefore, potential interactions between multiple toxicants at different levels are unavoidable. Combined exposures, even at sub-toxic levels, may have additive or synergistic interactions and can cause auditory dysfunction. For example, simultaneous exposure to even low levels of ototoxic drugs such as cisplatin and noise caused a greater shift in hearing thresholds and histological damage than that caused by isolated exposures (Boettcher et al., 1987). Similarly, simultaneous exposure to organic solvents and noise potentiated the noise-induced permanent threshold shifts (Steyger, 2009).
Although epidemiological studies have indicated an association between hearing loss and simultaneous exposure to lead and noise (Wu et al., 2000) the interaction between lead and noise exposure in inducing hearing loss has not been fully characterized. Combined exposure to lead and noise is expected to have an additive effect in the auditory system because oxidative stress, which plays a pivotal role in mediating noise-induced cochlear cell death, has been reported to facilitate the adverse health effects of lead (Ercal et al., 2001; Henderson et al., 2006; Kopke et al., 2002; Ohlemiller et al., 2000; Samson et al., 2008; Vaziri and Khan, 2007; Yamane et al., 1995). Therefore, we employed a mouse model to test the hypothesis that chronic exposure to lead will induce oxidative stress in the cochlea and potentiate noise-induced hearing loss.
2. Materials and methods
2.1. Animals
Male C57BL/6 mice (4 weeks of age) were purchased from Jackson Laboratories (Jackson Laboratories, Bar Harbor, ME) and allowed to acclimatize for 5 days at the Laboratory Animal Facility of Wayne State University. All animals were housed in a temperature-controlled room with a 12-h light/dark cycle and allowed free access to food and water. The ambient noise in the facility was 50 dB SPL (sound pressure level). Every effort was made to minimize pain and discomfort and all animals were handled and treated according to guidelines established by the National Institutes of Health. Though C57BL/6 strain harbor mutations in Cdh23 and are sensitive to noise, this strain is a good model to study both lead- and noise-induced oxidative stress (Ercal et al., 1996; Samson et al., 2008). The experimental protocol (Fig. 1) was reviewed and approved by the Institutional Animal Care and Use Committee (#16–01-038).
Fig. 1.
Overview of the research design. C57BL/6 mice were exposed to lead (2 mM lead acetate in water for 28 days) and/or noise (2–20 kHz broadband noise at 90 dB sound pressure level, 2 h/day for 2 weeks). Hearing loss was assessed by measuring the shift in auditory thresholds while cochlear oxidative stress was assessed by using targeted gene arrays. Lead load was analyzed by assessing the blood lead levels one day after the last exposure.
2.2. Lead exposure
After the baseline testing twenty four animals were randomly divided into four groups with six animals in each group. Two groups were exposed to lead through drinking water containing 2 mM lead acetate (Cat # 316512, Sigma-Aldrich, St. Louis, MO, USA), for 28 days while animals in the other two groups were given normal drinking water. Three animals were housed in one cage to ensure adequate and similar exposure for all animals. Animal weight, appearance, and behavior were monitored routinely and the mice were provided free access to standard mouse chow.
2.3. Noise exposure
Awake mice were exposed to broadband white noise at 90 dB SPL, 2 h daily for two weeks, in an acoustic chamber (Model RE-121, Acoustic Systems, Austin, TX). The animals were placed on a slowly revolving platform (1 revolution/min) in a custom designed sub-divided cage with one mouse per division. The noise signal was generated by TDT System3 RZ6 Processor (Tucker Davis Technologies, Alachua, FL), amplified by a QSC GX5 power amplifier (QSC LLC, Costa Mesa, CA) and presented to the animals with an overhead 1000 W TW67 3”titanium tweeter (Pyramid, Brooklyn, NY). Noise level was adjusted using a Model 831 sound level meter (Larson Davis, Depew, NY) equipped with a ½ inch free-field microphone (377B02, PCB Piezotronics, Depew, NY), which was calibrated using a Model CAL200 Precision Acoustic Calibrator (Larson Davis, Depew, NY). The noise levels within the cage ranged from 90 to 93 dB SPL.
2.4. Blood and cochleae collection
Animals were euthanized by CO2 inspiration. After ensuring that there is no response to a toe pinch blood was collected by cardiac puncture (~100–150 μl) and stored in 1.5 ml Eppendorf tubes containing 10 μl of 0.5 M EDTA. Then the mice were decapitated and the cochleae were dissected out and immediately frozen in liquid nitrogen.Cochleae were kept at −80 °C degrees until further processing.
2.5. Inductively coupled plasma mass spectrometry (ICP-MS)
Blood samples were initially diluted by adding 75 μl of 0.1% TritionX-100 with 75 μl of blood. The samples were further diluted with 300 μl of 2% nitric acid and incubated between 1 and 2 h. Then the samples were centrifuged and diluted again with 2% nitric acid so that the final dilution was 50 folds. A 13-point standard curve was made with different concentrations of lead ranging from 0.05 to 200 μg/L.The analysis was performed on an Agilent 7700 Series ICP-MS. The Pb and 209Bi (internal) standards were purchased from Inorganic Ventures (Christiansburg, VA).
2.6. Cochlear RNA isolation
Frozen cochleae were immersed in RNAlater-ICE (Catalog # AM7030, Life Technologies, Carlsbad, CA) for at least 16 h and then the cochlear tissue was dissected out of the bulla, under the microscope. Cochlear tissue consisting of the sensory epithelium, lateral wall, and modiolus from 2 cochleae were pooled and homogenized in 500 μl QIAzol Lysis Reagent (Catalog # 79306, Qiagen, Valencia, CA). Total RNA was isolated using the RNeasy Microarray Tissue Mini kit (catalog # 73304, Qiagen, Valencia, CA) following the manufacturer’s instructions. The quality of the RNA and its concentration were verified using NanoDrop 8000 (Thermo Fisher Scientific, Rockford, IL) and the purity of RNA was determined from A260: A230 and A260: A280 ratios.
2.7. Reverse transcription-polymerase chain reaction (PCR) analysis
First strand cDNA was synthesized from 5 μg RNA using the RT2 First Strand kit (catalog # 330401, Qiagen). Then the cDNA was mixed with RT2 SYBR Green qPCR Mastermix (cat. # 330529, Qiagen) and 25 μl of the mixture was loaded in each well of the Oxidative Stress RT2 Profiler PCR Array (Cat. # PAMM-065Z, Qiagen), following the manufacturer’s instructions. Real-time PCR amplification was done using the Step One RT-PCR system (Applied Biosystems, Foster City, CA), which was programed to include a 10 min Hot-Start at 95 °C, followed by 40 cycles of 15 s denaturation at 95 °C and 30 s annealing at 60 °C. Actb, B2m, and Gapdh were used as housekeeping genes and fold changes of mRNA levels were calculated from Ct values of individual genes. Fold regulation of 1.5 or above was considered as upregulation while fold regulation of −1.5 or below was considered as down-regulation.
2.8. Auditory brainstem response (ABR)
Mice were anesthetized with isoflurane (3% induction, 1.5% maintenance with 1 l/min O2) and the hearing thresholds were measured in a sound proof chamber (Model AB-4230, ECKEL, Morrisburg, ON). Acoustic stimuli of 1-ms tone bursts at 4, 8, 16, 24, or 32 kHz, or 25-μs clicks were generated using Tucker-Davis Technologies BioSigRZ software and TDT System3 hardware (TDT, Alachua, FL) and presented to the external auditory meatus. Sub-dermal needle electrodes were used to record the auditory brainstem responses. The intensity of the clicks and tone bursts were gradually reduced in 5 dB decrements from 90 dB SPL. Two hundred stimulus presentations, delivered at 21/s, were averaged to obtain the waveform of the brainstem response. The lowest intensity at which a waveform with an identifiable peak was detected was considered as the hearing threshold. Though isoflurane could alter the ABR levels its potential interference is unlikely to significantly affect the findings of this study because our interpretation of the ABR results and discussion are based on the shifts in hearing thresholds rather than the actual hearing threshold levels. We used the same anesthetic for baseline measurements, which were used for calculating the threshold shifts, and control animals, which were used for comparison, thereby normalizing the effects of isoflurane on the hearing threshold shifts induced by lead and/or noise exposure.
2.9. Data analysis
All data were statistically analyzed using the GraphPad Prism 6 software (La Jolla, CA). Significant differences between the four groups were determined by one-tailed t-tests. Results are expressed as mean ± standard deviation/error.
3. Results
3.1. Exposure of young-adult mice to lead did not alter their weight gain
Body weight was monitored for all animals at the beginning of the study and then on day 14 and day 28 (Fig. 2A). The average weight of the animals in the four groups ranged from 15 to 17 g, at the start of the study. Their body weight increased to 20–21 g on day 14 and was 22–25 g on day 28. Though the lead exposure at the level employed in this study did not alter the normal weight gain of the mice, the blood lead assays confirmed the absorption of lead because the blood lead levels were significantly elevated in the exposed mice (Fig. 2B&C). The average blood lead levels were 293 ± 67 μg/L and 319 ± 44 μg/L in the lead and the combined lead and noise exposed groups, respectively. The average blood lead levels in the control and noise exposed groups were 10 ± 4 μg/L and 12 ± 5 μg/L, respectively. At the time of sacrifice, animals in all four groups were active and did not show any abnormal changes in their appearance or behavior. Taken together, these observations suggested that the level of lead exposure used in this study was either mild or moderate for young-adult mice, and was not capable of inducing severe toxic effects.
Fig. 2.
Effect of lead and noise exposure on body weight and blood lead levels. A) Measurement of body weight on day 14 and day 28 indicated that exposure to lead and/or noise did not alter the normal weight gain of C57BL/6 mice. The results are expressed as mean ± standard deviation, n = 6. B) The concentration of blood lead in mice that consumed water containing 2 mM lead acetate for 28 days (lead and lead + Noise groups) was significantly higher than that of mice which had regular drinking water (control and noise groups). The results are expressed as mean ± standard deviation, n = 6.
3.2. Chronic lead exposure induced significant shift in hearing thresholds
The adverse effects of lead exposure on the auditory system were assessed by measuring the auditory brainstem responses. Recordings done before and after exposure indicated that chronic exposure to lead induced 8–12 dB shifts in hearing thresholds. Comparative analysis of the hearing threshold shifts induced by lead exposure with that of the controls indicated that exposure to lead significantly elevated the hearing thresholds, particularly, when probed with click, 4, 16, 24, and 32 kHz stimuli (Fig. 3). These results suggest that even at moderate levels chronic lead exposure causes significant functional deficits in the auditory system of young-adult mice.
Fig. 3.
Lead-induced hearing loss. A) Auditory brainstem responses of C57BL/6 mice indicate that chronic exposure to lead induced 8–12 dB shift in hearing thresholds. The shifts in the hearing thresholds after exposure to lead were significantly higher than that of the controls. * indicates p = < 0.05, ** indicates p = < 0.01. The results are expressed as mean ± standard error, n = 6. (B) Representative ABR waveform in response to click stimuli illustrates a 10 dB shift in hearing threshold after lead exposure.
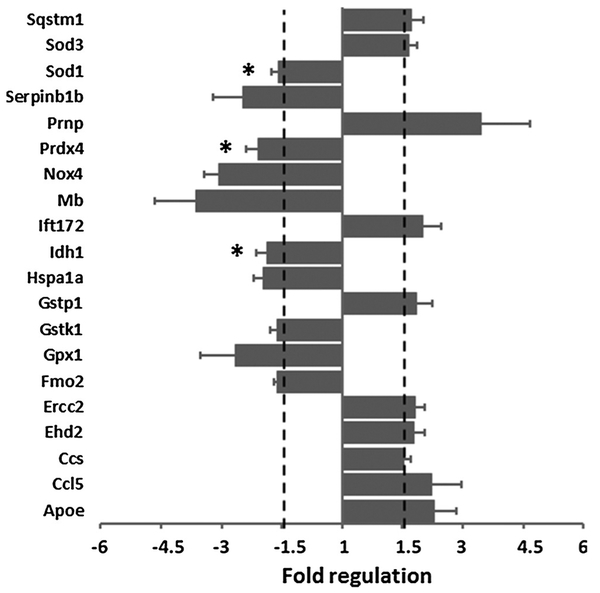
3.3. Lead exposure modulated the expression of oxidative stress genes in the cochlea
Differential expression of oxidative stress genes in the cochlea, analyzed using targeted PCR arrays, indicated that at least 20 of the 84 genes assayed were either upregulated or downregulated in the cochlea by lead exposure (Fig. 4). Genes with high threshold cycle values (> 35) were excluded from the analysis. Only genes that showed a similar trend, either upregulation or downregulation, in all three replicates were included in the analysis. Accordingly, Apoe, Ccl5, Ccs, Ehd2, Ercc2, Gstp1, Ift172, Prnp, Sod3, and Sqstm1 were upregulated while Fmo2, Gpx1, Gstk1, Hspa1a, Idh1, Mb, Nox4, Prdx4, Serpinb1b, and Sod1 were downregulated in the cochlea by lead exposure. These results suggested a potential role of oxidative stress in lead-induced ototoxicity.
Fig. 4.
Differential expression of cochlear oxidative stress genes in lead-exposed mice. Oxidative stress genes that were up or downregulated in the cochlear tissue after exposure to lead are illustrated in the bar graph. The numerical values for negative/down regulation represent the negative inverse of the fold-change. * indicates p = < 0.05. The results are expressed as mean ± standard error, n = 3.
3.4. Lead co-exposure amplified the noise-induced hearing loss
The effect of simultaneous lead exposure on noise-induced hearing loss was assessed by measuring the auditory brainstem responses. The recordings done before and 14 days after chronic exposure to broadband noise at 90 dB SPL, 2 h daily for two weeks, indicated that chronic exposure to low level noise induced 11–25 dB permanent shifts in hearing thresholds in mice. Simultaneous exposure to 2 mM lead acetate in drinking water for four weeks increased the noise-induced hearing threshold shift to 18–30 dB. Comparative analysis of the hearing threshold shifts induced by combined lead and noise exposure with isolated exposure to noise indicated that simultaneous exposure to lead significantly elevated the noise-induced shift in hearing thresholds, particularly, when probed with click, 4, and 32 kHz stimuli (Fig. 5). These results suggested that chronic lead exposure potentiates noise-induced hearing loss.
Fig. 5.
Hearing loss induced by concurrent exposure to lead and noise. A) Auditory brainstem responses of C57BL/6 mice indicate that simultaneous exposure to lead and noise induced 10–25 dB shifts in hearing thresholds. The hearing thresholds after combined exposure to lead and noise were significantly higher than that observed after isolated exposure to noise. * indicates p = < 0.05. The results are expressed as mean ± standard error, n = 6. B) Representative ABR waveform in response to click stimuli illustrates that hearing threshold after combined exposure was 10 dB higher than that observed after isolated noise exposure.
4. Discussion
The link between environmental or occupational exposure to lead and hearing impairment has been well documented (Choi et al., 2012; Forst et al., 1997; Ghiasvand et al., 2016; Pawlas et al., 2015). Though lead induces oxidative damage to mediate its adverse health effects (Bhatti et al., 2009; Dobrakowski et al., 2017) and the inner ear is highly susceptible to oxidative damage (Henderson et al., 2006; Poirrier et al., 2010) the potential role of cochlear oxidative stress in lead-induced auditory dysfunction is yet to be clarified. This study provides the first evidence linking cochlear oxidative stress to lead-induced hearing loss. Chronic exposure to moderate levels of lead acetate in drinking water induced a significant shift in the hearing thresholds and modulated the cochlear expression of 20 genes associated with the oxidative stress pathway. This included genes that regulate the cochlear antioxidant defenses, which were downregulated, and genes that regulate the apoptotic responses, which were upregulated after lead exposure. Moreover, simultaneous exposure to lead enhanced the noise-induced hearing loss, suggesting an additive effect. This is consistent with other reports which indicated that chronic exposure to low levels of lead may be a risk factor for acquired hearing loss from complex exposures (Hwang et al., 2009; Park et al., 2010; Wu et al., 2000) and simultaneous exposure to ototoxicants that induce oxidative stress may have an additive or synergistic effect (Boettcher et al., 1987; Steyger, 2009).
Cochlear oxidative stress pathways are activated by both chemical and non-chemical agents that cause auditory dysfunction (Huth et al., 2011; Poirrier et al., 2010; Wong and Ryan, 2015). The imbalance between the generation of reactive oxygen species and the counteracting defense mechanisms is usually reflected by the changes in the expression of genes that encode free radical scavenging enzymes and endogenous antioxidants. In this study, increased blood lead levels were associated with the downregulation of Sod1, Gpx1, and Gstk1 in the cochlea, which in turn could compromise the ability of the cells to scavenge superoxide radicals, detoxify hydrogen peroxide, and conjugate glutathione to toxicants, respectively. Moreover, lead exposure upregulated Ccs, which encodes copper chaperone for superoxide dismutase, and downregulated Prdx4, which encodes peroxiredoxin 4, and Idh1, which encodes the enzyme that facilitates NADPH- and α-ketoglutarate-related cellular detoxification. These changes collectively suggest that chronic exposure to lead could weaken the cochlear anti-oxidant defense machinery and thereby enhance the susceptibility of the inner ear to oxidative damage. In addition, lead-induced oxidative stress in the cochlea was also indicated by the increase in the expression of Sod3, which encodes for extracellular superoxide dismutase, and Gstp1, which encodes for cytosolic glutathione transferase. Although the levels of Nox4 and Fmo2 were decreased after lead exposure, these changes may not reflect the overall cochlear oxidative stress because Nox4 is just one of the many NADPH oxidase isoforms expressed in the cochlea (Banfi et al., 2004; Vlajkovic et al., 2013) and reactive oxygen species could be generated by other pro-oxidant genes that are more abundantly expressed in the cochlea.
In addition to the changes in the oxidative stress genes, lead exposure altered the expression of several apoptotic genes in the cochlea. Elevated blood lead levels, even at 5 μg/dl concentration, have been correlated with changes in the transcriptional regulation of genes associated with apoptosis (Gillis et al., 2012). Consistent with this report the cochlear expression of ApoE, whose levels are increased in neuronal injury (Elliott et al., 2010), and Hspa1a, which is induced under stressful conditions, were upregulated in lead-exposed animals. Moreover, the levels of Ercc2, which is needed for repairing DNA damage, Prnp, which encodes the prion protein implicated in neurodegeneration, Ccl5, which encodes a chemokine associated with inflammation, and Sqstm1, which encodes an ubiquitin binding protein that regulates autophagy, were increased. The upregulation of these genes in the cochlea are indicative of lead-induced damage to the inner ear. Furthermore, the cochlear expression of genes that encode myoglobin and serine (or cysteine) peptidase inhibitor, clade B, member 1b were decreased while the genes that encode EH-domain containing 2 and intraflagellar transport 172 were increased. Significant changes in expression levels of these genes were detected in the lead treated animals even when this analysis was conducted in just three animals per group. Taken together, the lead-induced changes in the cochlear expression of genes associated with oxidative stress and apoptosis suggest a potential link between elevated blood lead levels and oxidative damage to the cochlea.
Though lead-induced cochlear oxidative stress by itself can cause significant auditory dysfunction, acquired hearing loss is usually a consequence of interaction between multiple environmental risk factors (Boettcher et al., 1987; Steyger, 2009). Noise and lead are among the major risk factors of hearing loss that are prevalent in the urban environment, particularly, in those with older infrastructure. In this study, isolated exposure to lead or noise, for an extended period, induced moderate hearing loss. However, simultaneous exposure to both lead and noise, at similar levels, significantly enhanced the noise-induced shift in hearing thresholds suggesting that chronic lead exposure may have an additive effect on noise-induced hearing loss. This effect could be attributed to the cumulative oxidative damage because both lead and noise exposure induce oxidative stress in the cochlea. This suggests that chronic exposure to even low levels of lead, which otherwise do not have significant toxic effects, may interact with other environmental ototoxicants to cause higher levels of hearing loss.
5. Conclusions
The findings of this study suggest that chronic exposure of young-adult mice to lead activates oxidative stress pathways in the cochlea and induces significant shift in the hearing thresholds. Because lead exposure compromises the cochlear antioxidant defense machinery even moderate elevation of blood lead levels could enhance the susceptibility of the cochlea to oxidative damage. In agreement with this premise, simultaneous exposure to lead amplified the noise-induced hearing loss. Therefore, this study concludes that lead exposure has potential additive effect in the cochlea when co-exposed with other ototoxicants that induce cochlear oxidative stress. However, additional studies with other ototoxicants at different doses are required to verify this hypothesis.
Acknowledgments
Funding
This research was supported by pilot project funding to SJ from the Center for Urban Responses to Environmental Stressors (CURES), an National Institute of Environmental Health Sciences P30 center (P30 ES020957) and K01 grant (K01 ES028750) to SJ from NIH/NIEHS.
Abbreviations:
- SPL
sound pressure level
- ICP-MS
inductively coupled plasma mass spectrometry
- PCR
polymerase chain reaction
- OSHA
occupational safety and health administration
- ABR
auditory brainstem response
Footnotes
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- Ahamed M, Siddiqui MK, 2007. Low level lead exposure and oxidative stress: current opinions. Clin. Chim. Acta 383, 57–64. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH, 2004. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem 279, 46065–46072. [DOI] [PubMed] [Google Scholar]
- Bhatti P, Stewart PA, Hutchinson A, Rothman N, Linet MS, Inskip PD, Rajaraman P, 2009. Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol. Biomarkers Prev 18, 1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA, Henderson D, Gratton MA, Danielson RW, Byrne CD, 1987Synergistic interactions of noise and other ototraumatic agents. Ear Hear 8, 192–212. [DOI] [PubMed] [Google Scholar]
- Bottger EC, Schacht J, 2013. The mitochondrion: a perpetrator of acquired hearing loss. Hear. Res 303, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Hu H, Mukherjee B, Miller J, Park SK, 2012. Environmental cadmium and lead exposures and hearing loss in U.S. adults: the National Health and Nutrition Examination Survey, 1999 to 2004. Environ. Health Perspect 120, 1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Gonick HC, Vaziri ND, Liang K, Wei L, 2001. Lead-induced hypertension:III. Increased hydroxyl radical production. Am. J. Hypertens 14, 169–173. [DOI] [PubMed] [Google Scholar]
- Dobrakowski M, Pawlas N, Kasperczyk A, Kozlowska A, Olewinska E, Machon-Grecka A, Kasperczyk S, 2017. Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum. Exp. Toxicol 36, 744–754. [DOI] [PubMed] [Google Scholar]
- Elliott DA, Weickert CS, Garner B, 2010. Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin. Lipidol 51, 555–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercal N, Treeratphan P, Hammond TC, Matthews RH, Grannemann NH, Spitz DR, 1996. In vivo indices of oxidative stress in lead-exposed C57BL/6 mice are reduced by treatment with meso-2,3-dimercaptosuccinic acid or N-acetylcysteine. Free Radic. Biol. Med 21, 157–161. [DOI] [PubMed] [Google Scholar]
- Ercal N, Neal R, Treeratphan P, Lutz PM, Hammond TC, Dennery PA, Spitz DR, 2000. A role for oxidative stress in suppressing serum immunoglobulin levels in lead-exposed Fisher 344 rats. Arch. Environ. Contam. Toxicol 39, 251–256. [DOI] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N, 2001. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem 1, 529–539. [DOI] [PubMed] [Google Scholar]
- Forst LS, Freels S, Persky V, 1997. Occupational lead exposure and hearing loss. J. Occup. Environ. Med 39, 658–660. [DOI] [PubMed] [Google Scholar]
- Fortune T, Lurie DI, 2009. Chronic low-level lead exposure affects the monoaminergic system in the mouse superior olivary complex. J. Comp. Neurol 513, 542–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasvand M, Mohammadi S, Roth B, Ranjbar M, 2016. The relationship between occupational exposure to lead and hearing loss in a cross-Sectional survey of iranian workers. Front. Public Health 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis BS, Arbieva Z, Gavin IM, 2012. Analysis of lead toxicity in human cells. BMC Genomics 13, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH, 2006. The role of oxidative stress in noise-induced hearing loss. Ear Hear 27, 1–19. [DOI] [PubMed] [Google Scholar]
- Hsu PC, Hsu CC, Liu MY, Chen LY, Guo YL, 1998. Lead-induced changes in spermatozoa function and metabolism. J. Toxicol. Environ. Health A 55, 45–64. [DOI] [PubMed] [Google Scholar]
- Huth ME, Ricci AJ, Cheng AG, 2011. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int. J. Otolaryngol 2011, 937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YH, Chiang HY, Yen-Jean MC, Wang JD, 2009. The association between low levels of lead in blood and occupational noise-induced hearing loss in steel workers. Sci. Total Environ 408, 43–49. [DOI] [PubMed] [Google Scholar]
- Jamesdaniel S, Rathinam R, Neumann WL, 2016. Targeting nitrative stress for attenuating cisplatin-induced downregulation of cochlear LIM domain only 4 and ototoxicity. Redox Biol 10, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LG, Prins J, Park S, Walton JP, Luebke AE, Lurie DI, 2008. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J. Comp. Neurol 506, 1003–1017. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeong HJ, Myung NY, Kim MC, Lee JH, So HS, Park RK, Kim HM, Um JY, Hong SH, 2008. The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ. Health Perspect 116, 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel KE, Lee MY, King WM, Raphael Y, Schacht J, Neitzel RL, 2017. Vestibular dysfunction in the adult CBA/CaJ mouse after lead and cadmium treatment. Environ. Toxicol 32, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke RD, Coleman JK, Liu J, Campbell KC, Riffenburgh RH, 2002. Candidate’s thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope 112, 1515–1532. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Maier MM, Snodgrass EB, Hecox KE, Laughlin NK, 1995. The effects of lead on otoacoustic emissions and auditory evoked potentials in monkeys. Neurotoxicol. Teratol 17, 633–644. [DOI] [PubMed] [Google Scholar]
- Liu X, Zheng G, Wu Y, Shen X, Jing J, Yu T, Song H, Chen J, Luo W, 2013. Lead exposure results in hearing loss and disruption of the cochlear blood-labyrinth barrier and the protective role of iron supplement. Neurotoxicology 39, 173–181. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS, 2000. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J. Assoc. Res. Otolaryngol 1, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Elmarsafawy S, Mukherjee B, Spiro A 3rd., Vokonas PS, Nie H, Weisskopf MG, Schwartz J, Hu H, 2010. Cumulative lead exposure and age-related hearing loss: the VA Normative Aging Study. Hear. Res 269, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra RC, Swarup D, Dwivedi SK, 2001. Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162, 81–88. [DOI] [PubMed] [Google Scholar]
- Patrick L, 2006. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev 11, 114–127. [PubMed] [Google Scholar]
- Pawlas N, Broberg K, Olewinska E, Kozlowska A, Skerfving S, Pawlas K, 2015. Genetic modification of ALAD and VDR on lead-induced impairment of hearing in children. Environ. Toxicol. Pharmacol 39, 1091–1098. [DOI] [PubMed] [Google Scholar]
- Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B, 2010Oxidative stress in the cochlea: an update. Curr. Med. Chem 17, 3591–3604. [DOI] [PubMed] [Google Scholar]
- Prins JM, Brooks DM, Thompson CM, Lurie DI, 2010a. Chronic low-level Pb exposure during development decreases the expression of the voltage-dependent anion channel in auditory neurons of the brainstem. Neurotoxicology 31, 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JM, Brooks DM, Thompson CM, Lurie DI, 2010b. Chronic low-level Pb exposure during development decreases the expression of the voltage-dependent anion channel in auditory neurons of the brainstem. Neurotoxicology 31, 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio B, Froquet R, Parent-Massin D, 2001. In vitro effect of lead acetate on human erythropoietic progenitors. Cell Biol. Toxicol 17, 41–50. [DOI] [PubMed] [Google Scholar]
- Roy A, Queirolo E, Peregalli F, Manay N, Martinez G, Kordas K, 2015. Association of blood lead levels with urinary F(2)-8alpha isoprostane and 8-hydroxy-2-deoxyguanosine concentrations in first-grade Uruguayan children. Environ. Res 140, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson J, Wiktorek-Smagur A, Politanski P, Rajkowska E, Pawlaczyk-Luszczynska M, Dudarewicz A, Sha SH, Schacht J, Sliwinska-Kowalska M, 2008. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by D-methionine. Neuroscience 152, 146–150. [DOI] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, Tchounwou PB, 2009. Neurotoxic effects and bio-markers of lead exposure: a review. Rev. Environ. Health 24, 15–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senut MC, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, Suhr ST, Ruden D, 2012. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 4, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan SG, Henderson E, Eavey R, Curhan GC, 2011. Heavy metals exposure and hearing loss in US adolescents. Arch. Otolaryngol. Head Neck Surg 137, 1183–1189. [DOI] [PubMed] [Google Scholar]
- Song X, Li Z, Liu F, Wang Z, Wang L, 2017. Restoration of autophagy by puerarin in lead-exposed primary rat proximal tubular cells via regulating AMPK-mTOR signaling. J. Biochem. Mol. Toxicol 31. [DOI] [PubMed] [Google Scholar]
- Steyger PS, 2009. Potentiation of chemical ototoxicity by noise. Semin Hear 30, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Khan M, 2007. Interplay of reactive oxygen species and nitric oxide in the pathogenesis of experimental lead-induced hypertension. Clin. Exp. Pharmacol. Physiol 34, 920–925. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Lin SC, Wong AC, Wackrow B, Thorne PR, 2013. Noise-induced changes in expression levels of NADPH oxidases in the cochlea. Hear. Res 304, 145–152. [DOI] [PubMed] [Google Scholar]
- Warchol ME, 2010. Cellular mechanisms of aminoglycoside ototoxicity. Curr. Opin. Otolaryngol. Head Neck Surg 18, 454–458. [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Fultz CD, Grant RA, Wright LS, Kornguth SE, Siegel FL, 1999. Regional protein alterations in rat kidneys induced by lead exposure. Electrophoresis 20, 943–951. [DOI] [PubMed] [Google Scholar]
- Wong AC, Ryan AF, 2015. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TN, Shen CY, Lai JS, Goo CF, Ko KN, Chi HY, Chang PY, Liou SH, 2000. Effects of lead and noise exposures on hearing ability. Arch. Environ. Health 55, 109–114. [DOI] [PubMed] [Google Scholar]
- Yamamura K, Terayama K, Yamamoto N, Kohyama A, Kishi R, 1989. Effects of acute lead acetate exposure on adult guinea pigs: electrophysiological study of the inner ear. Fundam. Appl. Toxicol 13, 509–515. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A, 1995. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol 252, 504–508. [DOI] [PubMed] [Google Scholar]