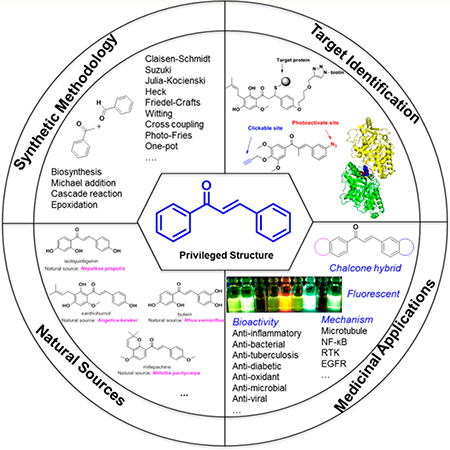
Abstract
Privileged structures have been widely used as an effective template in medicinal chemistry for drug discovery. Chalcone is a common simple scaffold found in many naturally occurring compounds. Many chalcone derivatives have also been prepared due to their convenient synthesis. These natural products and synthetic compounds have shown numerous interesting biological activities with clinical potentials against various diseases. This review aims to highlight the recent evidence of chalcone as a privileged scaffold in medicinal chemistry. Multiple aspects of chalcone will be summarized herein, including the isolation of novel chalcone derivatives, the development of new synthetic methodologies, the evaluation of their biological properties, and the exploration of the mechanisms of action as well as target identification. This review is expected to be a comprehensive, authoritative, and critical review of the chalcone template to the chemistry community.
Graphic abstract
1. INTRODUCTION
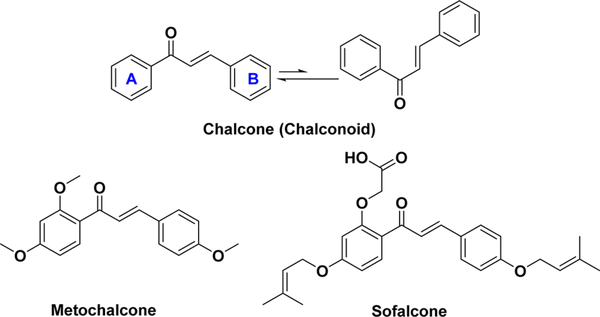
A chalcone is a simple chemical scaffold of many naturally occurring compounds and has a widespread distribution in vegetables, fruits, teas, and other plants.1–5 The word “chalcone” is derived from the Greek word “chalcos”, meaning “bronze”, which results from the colors of most natural chalcones.3 Chalcone compounds have a common chemical scaffold of 1,3-diaryl-2-propen-1-one, also known as chalconoid, that exists as trans and cis isomers, with the trans isomer being thermodynamically more stable (Figure 1).3,6 In this article, the phenyl ring attached to the carbonyl group is defined to be the A ring and the other benzene ring is named as the B ring (Figure 1).
Figure 1.
Structures of chalcone and two clinically approved chalconebased drugs.
The chalcone family has attracted much interest not only from the synthetic and biosynthetic perspectives but also due to its broad interesting biological activities. Therapeutic applications of chalcones trace back thousands of years through the use of plants and herbs for the treatment of different medical disorders, such as cancer, inflammation, and diabetes.1–5 Several chalcone-based compounds have been approved for clinical use. For example, metochalcone was once marketed as a choleretic drug, while sofalcone was previously used as an antiulcer and mucoprotective drug (Figure 1).2,3
Chalcones have been extensively studied, with many minireviews published1,3,4,7–22. However, the accurate mechanisms of action for the wide-ranging biological activities of chalcones are still not well understood. This review aims to highlight the recent advances in using chalcone as a privileged scaffold in medicinal chemistry, focusing on research articles published in the past 10 years (with a few exceptions). Several aspects of chalcone use will be summarized, including biosynthesis, synthetic methodologies and applications, biological activities, and target exploration.
2. CHALCONES FROM NATURAL SOURCES
Chalcones are the core of many biologically interesting compounds from natural sources and have attracted substantial research attention for decades. How many natural chalcones have been isolated and structurally elucidated? The answer to this question depends on how broadly the net is cast. As in many articles, the term “chalcone” refers generically to chemicals with an α,β-unsaturated ketone system. Thus, the chalcone family has extensive structural diversity and can be roughly classified into two categories: simple/classical chalcones and hybrid chalcones with the core scaffold of 1,3-diaryl2-propen-1-one. Bichalcones, such as rhuschalcone from Rhus pyroides, contain two chalcone moieties in a single structure.23 Dihydrochalcones, such as the fleminchalcones from Flemingia philippinensis, are a class of compounds with a reduced α,β-unsaturated double bond.24 Chalcone mimics (e.g., piperlongumines25) and fused chalcones (e.g., oxyfadichalcones26) are not structurally traditional chalcones, although they have a similar α,β-unsaturated ketone system or fused forms derived from chalcones by special biosynthesis pathways. When searching for the classical chalcone shown in Figure 1 in the well-known chemical databases, more than 92 000 chalcones can be found in SciFinder and over 1000 of them have biological data reported in PubChem (accessed August 2016). Therefore, the number of natural chalcones may ultimately not be countable with certainty. Representative classical chalcones, bichalcones, dihydrochalcones, chalcone mimics, and fused chalcones isolated from natural sources in recent years and their potential biological activities are summarized in Table 1. Some of their biological activities and applications will be discussed in the following sections.
Table 1.
| Entry | Name | Structure | Activity | Natural Source |
|---|---|---|---|---|
| Classical chalcones | ||||
| 1 | isoliquiritigenin27-29 |
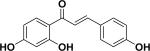
|
Anti-cancer Cancer chemopreventive Anti-oxidant Anti-inflammation |
Nepalese propolis |
| 2 | butein30,31 |
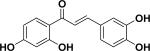
|
Anti-cancer Anti-inflammation |
Rhus verniciflua |
| 3 | cardamonin32 |
|
Schistosoma mansoni ATP diphosphohydrolase |
Piper aduncum L. |
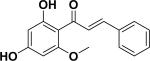
| 4 | sappanchalcone33,34 |
|
Anti-inflammation | Sappan Lignum |
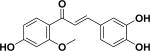
| 5 | 3-deoxysappanchalcone33 |
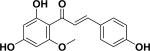
|
||
| 6 | isobavachalcone35,36 |
|
Anti-oxidant α-Glucosidase inhibitory activity Cancer chemopreventive Anti-cancer Anti-bacterial Anti-fungal |
Psoralea corylifolia (Leguminosae)
Kadsura ananosma |
| 7 | bavachalcone37 |
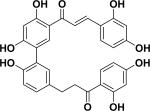
|
Anti-bacterial | Psoralea corylifolia |
| 8 | ajejuchalcone A38 |
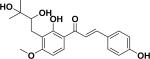
|
NDb | Apis mellifera |
| 9 | ajejuchalcone B38 |
|
||
| 10 | ajejuchalcone C38 |
|
||
| 11 | ajejuchalcone D38 |
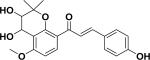
|
||
| 12 | ajejuchalcone E38 |
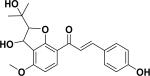
|
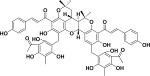
||
| 13 | ajejuchalcone F38 |
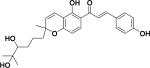
|
||
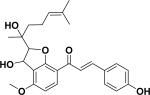
| 14 | a(±)-jejuchalcone G38 |
|
||
| 15 | morachalcone A39 |
|
Cytotoxicity | Maclura pomifera |
| 16 | broussochalcone A40 |
|
Protein kinase C inhibitor | Broussonetia papyrifera |
| 17 | xanthohumol41-43 |
|
Anti-HIV-1 Anti-bacterial Anti-cancer |
Hops Humulus lupulus/Angelica keiskei/Koidzumi |
| 18 | xanthoangelol44,45 |
|
Anti-cancer | Angelica keiskei/Psorala corylifolia |
| 19 | gemichalcone C46 |
|
ND | Hypericum geminiflorum |
| 20 | isogemichalcones B47 |
|
Cytotoxicity |
Hypericum
Geminiflorum |
| 21 | isogemichalcones C48 |
|
Cytotoxicity | Broussonetia papyrifera |
| 22 | spinochalcone A49 |
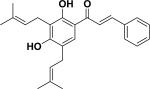
|
Cytotoxicity | Aeschynomene fascicularis |
| 23 | spinochalcone C49 |
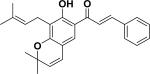
|
ND | Aeschynomene fascicularis |
| 24 | candidachalcone50 |
|
Estrogenic activity | Tephrosia Candida |
| 25 | 4’-O-methylbavachalcone35 |
|
α-Glucosidase inhibitory activity | Psoralea corylifolia |
| 26 | isobavachromene35 |
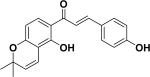
|
α- Glucosidase inhibitory activity | Psoralea corylifolia |
| 27 | licochalcone A51,52 |
|
Anti-inflammation Anti-cancer |
Glycyrrhiza inflata |
| 28 | oxyphyllumchalcone A26 |
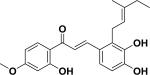
|
Cytotoxicity | Desmodium oxyphyllum |
| 29 | oxyphyllumchalcone B26 |
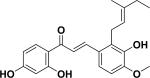
|
||
| 30 | oxyphyllumchalcone c26 |
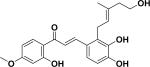
|
||
| 31 | pinostrobin chalcone53 |
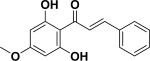
|
Cytotoxicity | Alpinia mutica |
| 32 | amillepachine54 |
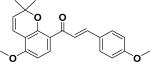
|
Anticancer | Millettia pachycarpa |
| 33 | a4,2’,4’-trihydroxy-3′-[(2E,5E)-7-methoxy-3,7-dimethyl-2,5-octadienyl]chalcone55 |
|
Heat shock protein promoter activity |
Angelica keiskei
Koidzumi (aerial parts) |
| 34 | a(±)-4,2′,4′-trihydroxy-3′-[(2E)-6-hydroxy-7-methoxy-3,7-dimethyl-2-octenyl]chalcone55 |
|
||
| 35 | a4,2′, 4′ -trihydroxy-3′ -[(2E)-3-methyl-5-(1,3-dioxolan-2-yl)-2-pentenyl]chalcone55 |
|
||
| 36 | a2′,3′-furano-4-hydroxy-4′-methoxychalcone55 |
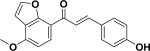
|
||
| 37 | a(±)-4-hydroxy-2±,3± -(2,3-dihydro-2-methoxyfurano)-4± -methoxychalcone55 |
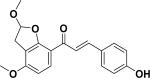
|
||
| 38 | a(-)-rubrichalcolactone56 |
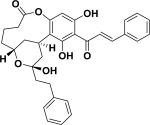
|
ND | Cryptocarya rubra |
| 39 | rhuschalcone II23 |
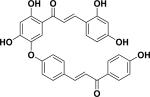
|
Cytotoxicity | Rhus pyroides |
| 40 | rhuschalcone III23 |
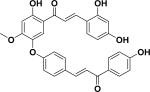
|
||
| 41 | rhuschalcone IV23 |
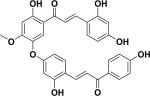
|
||
| 42 | rhuschalcone V23 |
|
||
| 43 | verbenachalcone57 |
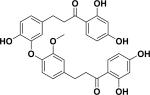
|
Enhancement of nerve growth factor-mediated neurite outgrowth | Verbena littoralis |
| 44 | afleminchalcone A24 |
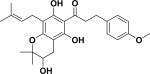
|
Tyrosinase inhibitor | Flemingia philippinensis |
| 45 | afleminchalcone B24 |
|
||
| 46 | aflemiphilippinone B58 |
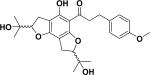
|
Anti-proliferative activity Apoptosis-inducing property |
Flemingia philippinensis |
| 47 | aflemiphilippinone C58 |
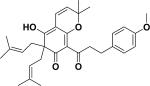
|
Anti-proliferative activity Apoptosis-inducing property |
Flemingia philippinensis |
| 48 | elastichalcone A59 |
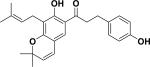
|
ND | Artocarpus elasticus |
| 49 | elastichalcone B59 |
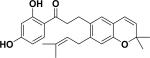
|
Free radical scavenging inhibitory activity | Artocarpus elasticus |
| 50 | elatadihydrochalcone60 |
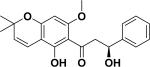
|
Anti-plasmodial | Tephrosia elata |
| 51 | chinendihydrochalcone61 |
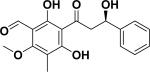
|
Anti-fungal and cytotoxicity | Desmos chinensis (stem barks) |
| Chalcone mimics/Fused chalcones | ||||
| 52 |
a4-cinnamoyl-4-hydroxy-3-methoxycyclohex-2-enone62 |
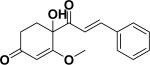
|
Nitric oxide production inhibitory activity | Nepalese propolis |
| 53 | schefflerichalcone63 |
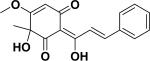
|
Non-toxic | Uvaria scheffleri |
| 54 | atunicatachalcone64 |
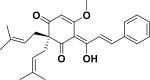
|
ND | Tephrosia tunicate (roots) |
| 55 | akamalachalcone E65 |
|
Anti-fungal | Mallotus philippinensis |
| 56 | aoxyfadichalcone A26 |
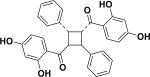
|
ND | Oxytropis falcata |
| 57 | aoxyfadichalcone B26 |
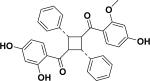
|
||
| 58 | aoxyfadichalcone C26 |
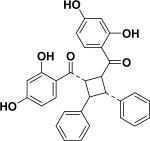
|
||
| 59 | anardoaristolone A66 |
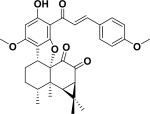
|
Protective effects on H2O2-induced myocardial injury | Nardostachys chinensis |
First report of the isolation of chalcone from a natural source.
The biological activities have not been determined in the references (ND = not determined).
3. FLUORESCENT PROPERTIES OF CHALCONES
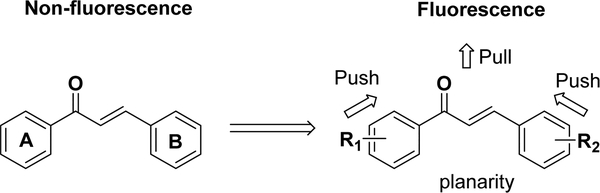
Because of its conjugated system, chalcones with proper electron-pulling and electron-pushing functional groups on the benzene ring(s) can be fluorescent (Figure 2),67–73 making them potential chemical probes for mechanistic investigations and imaging/diagnosis.
Figure 2.
Electron push–pull pairs for fluorescent chalcones.
As a fluorescent compound, the photophysical parameters, which include the absorption (Abs λm) and emission (Emi λm) wavelengths, extinction coefficient (ε), and quantum yield (ϕ), are critical for biological applications. The dynamic range of detection is determined by the Abs λm and Emi λm values. The fluorescence brightness, which is the product of ε and ϕ at the maximum absorption wavelength, is associated with the detection sensitivity. Some nonstructural factors are also critical to the fluorescent intensity, such as the solvents and the biological components/additives.69,73
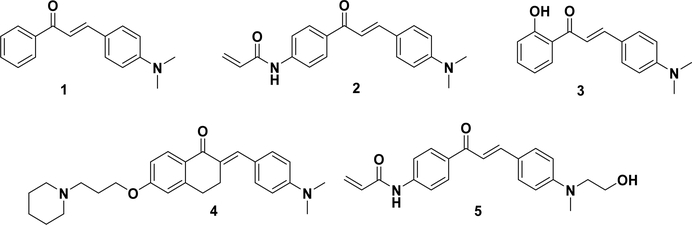
The dimethylamino group is a widely used substituent in fluorescent probes and has also been introduced into fluorescent chalcone compounds (Figure 3). 4-Dimethylaminochalcone (1) was first reported by Jiang et al. as a fluorescent probe for detecting micelle formation.74,75 Very recently, the authors have synthesized a small library of fluorescent chalcones to systematically characterize the structural effects on their intrinsic fluorescence and evaluate the influence of several biologically relevant environmental factors.76 The 4-dimethylaminochalcone compounds exhibited similar-absorptions, with an Abs λm between 390 and 460 nm and an Emi λm between 450 and 620 nm. The ε and ϕ values were between 28 000 and 38 000 and 0 and 0.40, respectively. Several compounds showed good fluorescence brightness, with values exceeding 6000 M−1 cm−1, which is comparable to that of commercial fluorophores (e.g., Cy 3.18–6000 M−1 cm−1). A structure–fluorescence relationship (SFR) study demonstrated the following: (1) for the chalcone moiety, the molecular planarity played a critical role in the fluorescence, e.g., introducing a methyl group to the α-position of the unsaturated ketone resulted in the loss of fluorescence; (2) for the A ring, weak electron-donating groups (e.g., a methoxyl group) were favorable to the quantum yield, while electron-withdrawing (e.g., a nitro group) or strong electron-donating (e.g., a dimethylamino group) substituents significantly decreased it; (3) for the B ring, disubstituted amino groups were essential for fluorescence, such as piperidine, piperazine, dimethylamino, and diethylamino groups; and (4) for the α,β-unsaturated ketone system, the extension of the double bond decreased the fluorescence and caused a red shift of the maximum emission wavelength. The fluorescence–environment relationship (FER) showed that the fluorescent intensity of chalcone-based compounds depends highly on the solvent polarity, the pH, and the interactions with proteins or detergents. In aprotic solvents, chalcone’s fluorescence decreases as the solvent polarity decreases, although the fluorescence is completely lost in protic solvents, such as water or EtOH, at neutral pH. However, it could be partially recovered by the addition of BSA, Triton-X100, or Tween-20. A similar result was obtained by another study, in which 4′-N,N-dimethylamino-4-methylacryloylamino chalcone (2) containing both electron-withdrawing and electron-donating groups was synthesized as a fluorescent sensor for determining the water content in organic solvents.69 The fluorescent intensity of compound 2 decreased with an increase in the water concentration in acetone, ethanol, and acetonitrile solutions. Such a sensor was useful for water determination with a low detection limit (<0.01%).69 The loss of fluorescence in a protonic solvent is potentially due to the formation of hydrogen bonds between the solvent and the nitrogen of chalcone’s dialkylamino group, keeping the nitrogen lone pair electrons out of the conjugate system and leading to the nonfluorescent property.76
Figure 3.
Representative fluorescent chalcones.
Nevertheless, fluorescent chalcones have been explored for their potential to detect different diseases. Compound 3 was designed based on a phosphorylated chalcone hybridized from 4-dimethylaminochalcone to visualize alkaline phosphatase through emission color changes in living cells.77 Stark et al. reported a series of chalcone analogues (4) that could be used to image human histamine H3 receptors (hH3R) in stably transfected HEK-293 cells.78 Fluorescent chalcones have also been applied to image stem cells.54,79 For example, Lee et al. designed a novel library of 160 fluorescent chalcone amide compounds.70 Two amides were introduced into these chalcones on each side of the scaffold to enhance the fluorescence emission intensity. Interestingly, CDg4 (5) was applied as a novel green fluorescent probe for detecting mouse embryonic stem cells (mESCs), where its molecular binding target was identified as the glycogen of the stem cell colony surface. These investigations have provided new possibilities for using nonradioactive alternatives in novel binding assays and as visualization tools.
4. SYNTHESIS
4.1. Biosynthesis
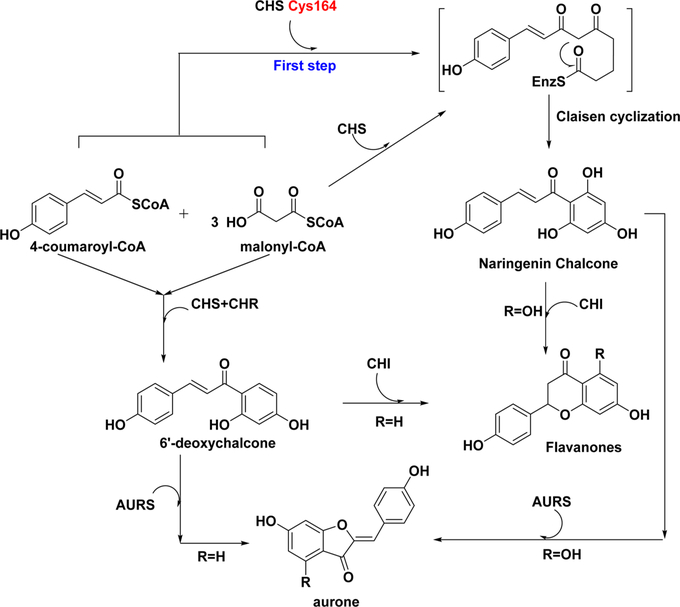
Chalcone synthase (CHS), the first type III polyketide synthase (PKS) superfamily discovered in the 1970s,80 is a ubiquitous enzyme in higher plants. CHS has also been detected in several lower plants, such as the liverwort Marchantia polymorpha.81 All other members in this family are labeled “CHS-like” enzymes. CHS is responsible for the biosynthesis of different chalcones. The CHS superfamily enzymes are associated with the biosynthesis of diverse secondary metabolites, including flavonoids, stilbenes, and aurones.82 Joseph P. Noel and coworkers developed an important framework for the biosynthetic mechanism by crystallizing CHS from the legume Medicago sativa, a process that provided clear structural information about chalcone biosynthesis.83 CHS exists as a homodimer, and the size of each monomer is approximately 42–45 kDa.81 Cys164, Phe215, His303, and Asn336 are the key residues of the active site and have been conserved among all CHS members and CHS-like enzymes. CHS produces chalcones by transferring a coumaroyl moiety from one 4coumaroyl-coenzyme A (CoA) to Cys164 as the first step. Subsequently, three malonyl-CoA thioesters form an intermediate via a polyketide reaction (Scheme 1). After the generation of a thioester-linked tetraketide, a regiospecific Claisen-type cyclization occurs and forms a new ring system to generate naringenin chalcone (Scheme 1).81,84,85 Naringenin chalcone is converted into 6′-deoxynaringenin chalcone (isoliquiritigenin, Table 1, entry 1) in the presence of chalcone reductase (CHR) and CHS. Other plant secondary metabolites, such as stilbenes, phloroglucinols, resorcinols, and benzophenones, could be biosynthesized in a similar manner with the corresponding catalytic enzymes.80,81,85–89 Flavonoids and isoflavonoids are produced by CHS and chalcone isomerase (CHI), respectively, using naringenin chalcones as the substrates.81 Naringenin chalcones are also the building blocks for the biosynthesis of aurone compounds by a plant catechol oxidase, aurone synthase (AURS).90 These conversions from chalcones to flavanones or aurones could also be realized by chemical reactions, such as the Algar–Flynn–Oyamada reaction.91
Scheme 1.
Biosynthesis of Chalcones and Downstream Pathways
Chalcones serving as precursors have generated a range of plant metabolites, revealing interesting biological activities, which will be discussed in the following sections. Taking such experience from nature, simple chalcones have been synthetically hybridized with other templates, such as stilbenes (see section 6.3.2.5).
4.2. Chemical Synthesis
Chalcones are generally prepared by condensation reactions via base or acid catalysis. Although chalcones are one type of easily synthesizable α,β-unsaturated ketone, a growing number of new techniques and procedures have recently been reported because of their interesting biological activities and the development of various catalysts or reaction conditions. The synthetic strategies, general methodologies, catalysts, and conditions used in the synthesis of chalcone scaffolds are summarized below.
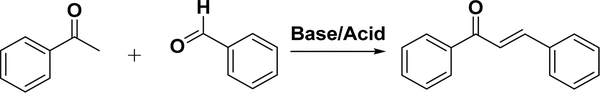
4.2.1. Claisen–Schmidt Condensation.
4.2.1.1. Overview.
The Claisen–Schmidt reaction is named after two pioneering investigators, R. L. Claisen92 and J. G. Schmidt,93 and describes a process in which a benzaldehyde and a methyl ketone are condensed in the presence of catalysts (Scheme 2).
Scheme 2.
Claisen–Schmidt Condensation of Chalcone
This reaction is deemed one of the most classical reactions in organic chemistry.94 The catalysts are either strong bases or acids. In the case of base catalysis, the chalcone is generated from the aldol product via dehydration in an enolate mechanism, while in the case of acid catalysis, it is generated via an enol mechanism.95 The main drawback of this reaction is the slow reaction rate; the reaction typically needs several days for completion. The reaction could also result in a complex mixture containing the desirable product, byproducts, and sometimes starting materials. The yield therefore could vary dramatically, depending on the reactants and catalysts, ranging from <10% to near 100% conversion.95,96 Nevertheless, this reaction has been employed in most publications because of its experimental simplicity and highly efficient formation of the carbon–carbon double bond with little restriction to the complexity of the molecules. Széll and co-workers synthesized á series of nitrochalcones and showed that the presence of electron-donating groups in the aldehyde favored condensation by acids, while electron-withdrawing substituents favored condensation by base conditions.97 Generally, the base condition is more common in chalcone synthesis.
4.2.1.2. Applications.
4.2.1.2.1. Base Condition (Table 2, Entries 1–6).
Table 2.
Cases of Claisen–Schmidt Condensation for the Synthesis of Chalcones
| Entry | Catalyst | Solvent | Reaction | Ref |
|---|---|---|---|---|
| 1 | NaOH | ethanol |
|
98 |
| 2 | NaOH KOH |
methanol ethanol |
|
55,56, 99 |
| 3 | Piperazine | methanol ethanol |
|
100 |
| 4 | Ca(OH)2 Ba(OH)2 Sr(OH)2 |
aprotic solvent |
|
101 |
| 5 | LiHDMS | THF |
|
102 |
| 6 | NaNO3 (LiNO3)/natural phosphates |
methanol | 6,103 | |
| 7 | HCl | ethanol | 107 | |
| 8 | AlCl3 | carbon disulfide |
107 | |
| 9 | BF3–Et2O | dioxane | 105,110 | |
| 10 | SOCl2 | ethanol |
|
454 |
| 11 | p-TsOH | acetic acid |
|
109 |
| 12 | NaOH | / |
|
113 |
| 13 | KF-Al2O3 | / |
|
115 |
| 14 | CaO | / |
|
114 |
| 15 | TBAB | inorganic alkaline solution |
|
119 |
| 16 | hydrotalcite | [dbmin]BF4 |
|
120 |
The classical Claisen–Schmidt condensation is base-catalyzed with potassium tert-butoxide, sodium hydroxide, or potassium hydroxide in methanol or ethanol at room temperature. This reaction has been widely used for the synthesis of hydroxyl-substituted chalcones, typically with good to excellent yields (60–90%). A group of ferrocenylchalcones has been synthesized under the common base (sodium hydroxide) condition (Table 2, entry 1).98 In some cases, an increase in temperature is required, and the base condition also requires modification. For example, the α-carbon of a ketone is difficult to dehydrate when the ketone is electrophilesubstituted (Table 2, entry 2). Such a reaction requires reflux at an elevated temperature or takes a longer time at room temperature.55,56,99 However, with a nucleophile substitution on the α-position, mild conditions are generally sufficient (Table 2, entry 3).100
In some cases, the classical Claisen–Schmidt condensation process has been performed with slight modifications of the catalyst or the solvent system. For example, β-trifluoromethylated chalcones (Table 2, entry 4) can be prepared with alkaline earth metal hydroxides of calcium, barium, or strontium in aprotic solvents for facile water removal.101 Removing the water generated is favorable to the reaction. Thus, the Dean–Stark condition using toluene as the solvent to remove water could accelerate the reaction and has been confirmed to obtain chalcones over a shorter period and at higher yields (data unpublished). Lithium bis(trimethylsilyl)amide (LiHDMS) has also been previously used as a base to catalyze the condensation and obtained chalcones with less than 50% yields (Table 2, entry 5).102 The NaNO3 or LiNO3/natural phosphate/ methanol system is another extremely efficient basic catalyst for the Claisen–Schmidt condensation process (Table 2, entry 6) from which chalcones are easily obtained in high yields at room temperature.6,103
4.2.1.2.2. Acid Condition (Table 2, Entries 7–11).
Although base catalysts are generally used for the synthesis of chalcones, Brønsted acids,97,104 Lewis acids,105–108 and solid acids109 have also been utilized as acid catalysts. The most common application using ethanol saturated with the Brønsted acid HCl is marginally successful, with only a 10–40% yield (Table 2, entry 7).90 Dry HCl gas has been shown to be more favorable to the reaction because it acts as not only a catalyst but also a water absorbent.97,104
Aluminum chloride (AlCl3) has also been used as a Lewis acid for chalcone synthesis (Table 2, entry 8). Two moles of acetophenone per mole of AlCl3 provide the chalcone product in a high yield (73%).107 The mechanism of the reaction is not simply the result of the hydrogen chloride evolved by the heating of acetophenone and AlCl3; an intermediate of the general type of R–O–Al–R2 actually promotes the condensation.
The application of boron trifluoride–etherate (BF3−Et2O) in the condensation was first reported in a study describing the use of BF3 gas in chalcone synthesis.110 In 2007, Narender et al. reported the use of BF3−Et2O to obtain 15 chalcones with 75–96% yields with reaction times of less than 3 h (Table 2, entry 9).105
Petrov et al. reported that 16 chalcones, including 4hydroxyl-chalcone (Table 2, entry 10), were synthesized in a SOCl2/ethanol system in high yields (73–96%).454 SOCl2 was used as a convenient alternative to the gaseous HCl in the condensation, where HCl was generated in situ by the reaction of SOCl2 with absolute ethanol.
Chalcones have also been obtained with p-toluenesulfonic acid (p-TsOH) as the catalyst and acetic acid as the solvent at 70 °C with yields greater than 70% (Table 2, entry 11).109 Zn(bpy)(OAc)2, TiCl4, Cp2ZrH2/NiCl2, and RuCl3 have also been utilized as acid catalysts in the condensation.106,108,111,112
4.2.1.2.3. Nonsolvent Condition (Table 2, Entries 12–14).
Solvent-free conditions have also been applied for chalcone synthesis, such as grinding113 or microwave irradiation.114 Rateb and co-workers reported a method using sodium hydroxide ground with an aldehyde and ketone to obtain a chalcone in an 80% yield (Table 2, entry 12).113 A nitrochalcone was synthesized under the grinding conditions with a novel base catalyst KF–Al2O3 with over a 94% yield in 5 min (Table 2, entry 13).115 Microwave irradiation has also been applied in nonsolvent systems to construct the chalcone core.116 A facile, solvent-free ecofriendly method using calcium oxide as a solid base catalyst for the synthesis of chalcones under microwave conditions has been reported.114 Fifteen chalcones with electron-donating as well as electron-withdrawing groups on both the ketone and aldehyde moieties have been produced in 57–88% yields using this method (Table 2, entry 14).114 A green catalyst system of solid sulfonic acid from bamboo was discovered by Xu et al. and catalyzed the condensation under a nonsolvent condition with isolated yields ranging from 60 to 82%.117 The nonsolvent microwave methodology has the following advantages: (1) it avoids the influence of the solvent and reduces the byproducts; (2) it enables greater flexibility for the reaction temperature because it is unconstrained by the boiling point and volatility of the solvents; (3) it dramatically reduces the reaction time; (4) it significantly improves the reaction yield over those of common solvent systems.
4.2.1.2.4. Other Conditions (Table 2, Entries 15 and 16).
Green chemistry, which has attracted considerable interest in organic synthesis,73,101 has recently been applied for chalcone synthesis. Water is an ideal solvent for organic reactions, where the chalcone moiety is prepared in a water system in the presence of a phase-transfer catalyst (PTC). Duan et al. reported a modification of the Claisen–Schmidt condensation using water as the solvent with cetyltrimethylammonium bromide (CTMAB) as the PTC and potassium carbonate as the base catalyst. High yields of the desired chalcones were obtained under these mild conditions.118 In 2013, heterocyclic ring-containing chalcones were obtained using tetrabutylammonium bromide (TBAB) as the PTC with an inorganic alkaline solution as the base under microwave conditions (Table 2, entry 15).119 Ionic liquids have also been utilized. Wu et al. reported the use of 1,3-dibutyl-2-methylimidazolium tetrafluoroborate ([dbmin]BF4) as the ionic liquid and hydrotalcite as a solid acidic catalyst to give chalcones with a yield of 98.5% (Table 2, entry 16).120 This method has several advantages, such as the low use of catalyst, convenient product separation, and catalyst recycling without reduced activity.
4.3. Synthesis of Chalcones Using Other Well-Known Strategies
Because the Claisen–Schmidt condensation process sometimes leads to a complex mixture that is difficult to purify for the desired chalcone compound, other well-known reactions have been explored for the synthesis of chalcones, including crosscoupling (e.g., Suzuki reaction, Heck reaction, Julia–Kocienski reaction, and Wittig reaction), Friedel–Crafts acylation, photoFries rearrangement, and one-pot synthesis from alcohols.
4.3.1. Cross-Coupling.
4.3.1.1. Suzuki Coupling.
Suzuki coupling121,122 was first reported in 1979 by Akira Suzuki, who shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for palladium-catalyzed crosscouplings. Suzuki coupling is a powerful palladium-catalyzed carbon–carbon bond forming reaction. It was first applied for chalcone synthesis in 2003.123
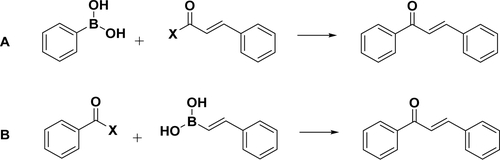
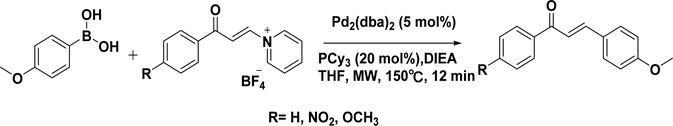
Based on the retrosynthetic analysis, two approaches123 are conceivable for the synthesis of chalcones: coupling cinnamoyl chloride with phenylboronic acid (Scheme 3A) and coupling benzoyl chloride with phenylvinylboronic acid (Scheme 3B). As expected, the reaction conditions affect the reaction yield. Bumagin’s conditions (solvent, acetone/water = 3/1; catalyst, PdCl2, 3%; base, Na2CO3) give a moderate yield (23–37%) for coupling between cinnamoyl chloride and phenylboronic acids,124 while McCarthy’s conditions (solvent, anhydrous toluene; catalyst, tetrakis(triphenylphosphine)palladium(0); base, CeCO3) provide ∼50 and ∼90% isolated yields for the above two approaches, respectively.125 The Suzuki coupling reaction has been extended to synthesize chalcones with electron-withdrawing substituents or electron-donating substituents, indicating that the electronic property of the substituents on the benzene rings has a minimal effect. Buszek et al. first reported an interesting example of Suzuki–Miyaura coupling for the synthesis of chalcones from N-vinylpyridinium tetrafluoroborate salt with yields of 60–81% (Scheme 4).126 These salts represent a novel class of palladium-catalyzed electrophilic coupling partners reacting with a wide range of boronic acids. Additionally, the salts are air-stable and nonhygroscopic crystals that can be easily prepared quantitatively in one step from the activated acetylenes and either pyridinium or trialkylammonium tetrafluoroborates.
Scheme 3.
Suzuki Coupling for Chalcone Synthesis
Scheme 4.
Suzuki–Miyaura Coupling for Chalcone Synthesis
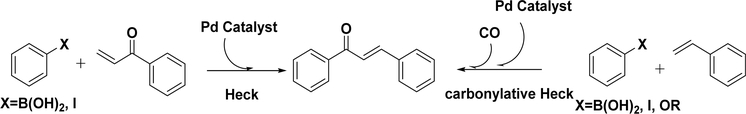
4.3.1.2. Heck Reaction.
Structurally, chalcone is also a stilbene, which can be obtained by the classical Heck reaction of an arylboronic acid or aryl iodide and an unsaturated ketone in the presence of a base and a palladium catalyst (Scheme 5).127–134 Cavarischia et al. first reported the synthesis of aryl vinyl ketones and direct coupling with aryl iodides to rapidly provide chalcone derivatives in excellent yields (75–96%) under catalytic conditions [Pd(OAc)2, Ph3P, CH3CN, TEA].135 Heck coupling can also be achieved via rhodium-catalyzed carbon–carbon bond formation, which is a competitive side reaction of the conjugate addition of the phosphine–rhodium catalyzed reaction. Zou et al. reported an approach using this competition between Heck coupling and the conjugate addition reaction to synthesize the chalcone moiety.129 In their work, phenylboronic acid reacted with phenyl vinyl ketone using (PPh 3) 3RhCl (3%) or RhCl3 (3%)−(±)2,2-bis- (diphenylphosphino)-1,1-binaphthalene (15%) as the catalyst in a toluene–water biphasic system. The resulting chalcones and dihydrochalcones were obtained in a ratio of approximately 50:50 using (PPh3)3RhCl, in contrast to a >99:1 selectivity of the conjugate adduct if the other catalyst was used.
Scheme 5.
Heck Coupling and Carbonylative Heck Coupling for Chalcone Synthesis
Carbonylative Heck coupling has also been developed to make chalcones, which was first reported by Beller et al. in 2010 (Scheme 5).133 The desired chalcone was initially obtained under palladium-catalyzed conditions with a yield of only 8%, prompting them to improve the methodology. As expected, phenyl triflate with carbon monoxide and different styrene derivatives under optimized conditions ([(cinnamyl)PdCl]2, dppp, NEt3, toluene, 100 °C, 20 h) gave the corresponding chalcones in fair to excellent yields (71–95%). They also extended this methodology to arylboronic acid132 and aryl iodide131 and synthesized chalcones in satisfactory yields. Skrydstrup and co-workers134 further expanded this work and reported a two-chamber system to synthesize chalcones and dihydrochalcones using the carbonylative Heck reaction. In their study, a system was used that enables a measured amount of carbon monoxide generated ex situ in chamber A to be a reagent for a parallel Heck reaction in chamber B. Twenty-two chalcones were synthesized, and a 13C isotope labeled chalcone was also attained using 13CO gas. This is a new application in chalcone and carbonylative isotope chemistry.
Although the metal-catalyzed Heck reaction is considered a highly efficient approach for chalcone synthesis, its application is limited because of the limited availability of aryl vinyl ketones and the need for pressurized carbon monoxide.131–134 However, the work was still notable for several reasons: (1) it represents a connection between the already established carbonylative Suzuki and carbonylative Sonogashira reactions; (2) the change in CO as a function of oxygen, while readily explicable, had not been previously established; (3) it is a powerful tool for the isotope labeling of chalcone-derived compounds by a facile isotope-labeling gas.
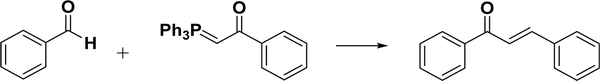
4.3.1.3. Wittig Reaction.
The Wittig reaction or Wittig olefination is another easy method to create alkene compounds. Chalcone is a reasonable alkene template for the Wittig reaction strategy (Scheme 6). The initial attempt used triphenylbenzoylmethylene phosphorane and benzaldehyde and required 3 days of reflux in benzene or 30 h in THF with a fair yield of 70%.136,137 Further development has indicated that the reaction rate could be significantly enhanced via microwave irradiation. For example, Huang et al. reported the synthesis of various chalcones using eight aromatic aldehydes. Good yields (>80%) were obtained for all of the substrates studied, and the reaction could be finished in 5–6 min using microwave irradiation methods.138
Scheme 6.
Wittig Reaction for Chalcone Synthesis
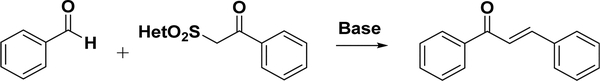
4.3.1.4. Julia–Kocienski Olefination.
The Julia–Kocienski olefination (Scheme 7), a modification of the Julia olefination reaction, was first applied to synthesize chalcones by Kumar and co-workers in 2010.139 Heteroaryl-sulfonyl phenylethanone was prepared following Jorgensen s method140. Thirty-one chalcones were obtained by the olefination of the Julia reagents and benzaldehyde in basic media. This reaction was influenced by several factors, such as the base, temperature, Julia reagent, and solvent. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) was the most efficient base, while the Julia reagent with benzothiazole being the heteroaryl was the best. Nonpolar solvents were more favorable than polar solvents, generally decreasing the yields along the series THF > DCM > CHCl3 > CH3CN > MeOH. The yield dramatically decreased when the temperature was at −78 °C.139 However, the trans-isomer was still the main product under such a low-temperature condition, where the stereoselectivity could be explained by the Newman projection.139,141
Scheme 7.
Julia–Kocienski Olefination for Chalcone Synthesis
4.3.1.5. Other Cross-Couplings.
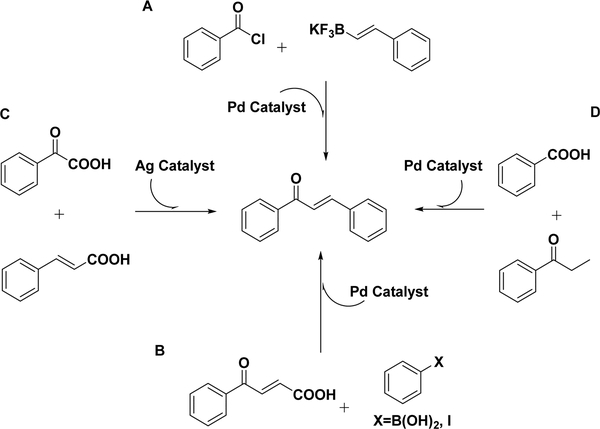
In addition to the above cross-couplings, metal-catalyzed direct cross-coupling (using, for example, silver142 or palladium143–145) has been thoroughly investigated in recent years as a method for forming chalcones.126 As shown in Scheme 8A, Al-Al-Masum and coworkers developed a direct cross-coupling reaction of benzoyl chlorides and potassium styryltrifluoroborates to obtain the corresponding chalcones in the presence of PdCl2(dtbpf) as the catalyst and K2CO3, taking advantage of microwave irradiation. Eight chalcones were synthesized using this condition, providing good to excellent yields (56–96%).144
Scheme 8.
Other Cross-Couplings for Chalcone Synthesis
Palladium-catalyzed decarboxylative coupling using 3-benzoylacrylic acids (Scheme 8B) is another recently developed strategy for chalcone synthesis.146 It has been reported that 3benzoylacrylic acids react with arylboronic acids or aryl halides in the presence of a palladium catalyst and a copper salt oxidant [Cu(OAc)2·H2O] to produce chalcone derivatives. A mechanistic investigation found that chalcone was generated by ArPdX-mediated decarboxylation or a Heck-type reaction and demonstrated the limitation of the Heck coupling: the direct use of 3-acylacrylic acids is much better than the use of the corresponding vinyl ketones due to their availabilities and stabilities.143 The silver-catalyzed double-decarboxylative protocol (Scheme 8C) is another method to build chalcone scaffolds. Chalcones have been synthesized from α-keto acids and cinnamic acids, which are readily available, in the presence of silver nitrate (AgNO3), sodium thiosulfate (Na2S2O8), and potassium carbonate (K2CO3) under mild aqueous conditions with good yields. A mechanism has been tentatively proposed. Ag(II) from Ag(I) by peroxodisulfate oxidation is used to generate an aryl radical from α-keto acid, releasing one molecular carbon dioxide and Ag(I) cation. The aryl radical is added to the cinnamate anion at the α-position, leading to the formation of chalcone with another molecular loss of carbon dioxide and Ag(I).142 The strategy of using saturated propiophenones to produce unsaturated chalcones has also been demonstrated using a combination of decarboxylation and dehydrogenation (Scheme 8D).147 The researchers crosscoupled aryl carboxylic acids using a PCy3-supported palladium catalyst to obtain chalcones in fair yields, which is an extension of the Heck reaction that overcomes the limits of its starting materials.
In summary, the above classical cross-coupling strategies have been developed for the construction of carbon–carbon double bonds under mild conditions with good yields, providing diverse and useful chalcone derivatives in the fields of synthetic chemistry and pharmaceutical chemistry. These strategies have their own advantages and disadvantages and can be selected and utilized depending on the specific circumstances, such as the starting materials, solvents, and catalyst conditions as well as the simplicity of purification.
4.3.2. Other Strategies.
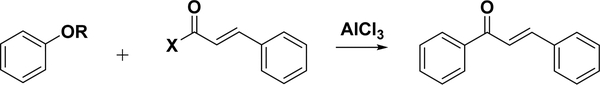
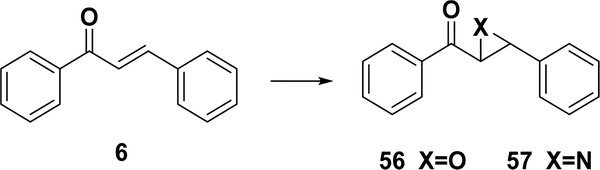
4.3.2.1. Friedel–Crafts Acylation with Cinnamoyl Chloride.
With the use of a strong Lewis acid catalyst, such as aluminum trichloride, chalcones can be synthesized by the Friedel–Crafts acylation of an aromatic ether and cinnamoyl chloride (Scheme 9). This method was reported by Shotter et al. in 1978, with four chalcones made in fair yields.148 Nevertheless, this reaction has not been widely used for chalcone synthesis.
Scheme 9.
Friedel–Crafts Acylation for Chalcone Synthesis
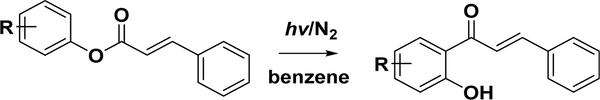
4.3.2.2. Photo-Fries Rearrangement of Phenyl Cinnamates.
The photochemistry of chalcones has attracted considerable interest since the early 1970s because 2′hydroxychalcones are the key intermediates of flavonoid biosyntheses in nature.149 The Fries rearrangement (Scheme 10) is commonly applied to the photosynthesis of these chalcones, where a convenient rearrangement reaction of a phenyl cinnamate to a hydroxy aryl ketone occurs in the presence of Lewis acids. This is an ortho- or para-selective reaction, which is affected by the reaction conditions, such as temperature and solvents. Obara et al.150 first reported that phenyl cinnamates could be irradiated using benzene as the solvent under nitrogen with a high-pressure mercury-arc lamp (450 W) to obtain 10% 2′-hydroxychalcone and 2% 4′-hydroxychalcone. Subsequent research has extended this reaction to different substituted chalcones, including dihydroxychalcones,151 O-methoxymethyl-protected dihydroxychalcones,152 and multisubstituted chalcones from natural sources.153 Ramakrishnan and Kagan found that methanol, ethanol, and chloroform are also suitable for this rearrangement.154 Although the yield could be improved to approximately 50% by changing the solvent, complete conversion of the phenyl cinnamates has not been achieved to date. Considering the limits of the reaction, long reaction time, low yield, and difficult operation, this procedure is not widely employed.
Scheme 10.
Photo-Fries Rearrangement for Chalcone Synthesis
4.3.2.3. One-Pot Synthesis of Chalcones.
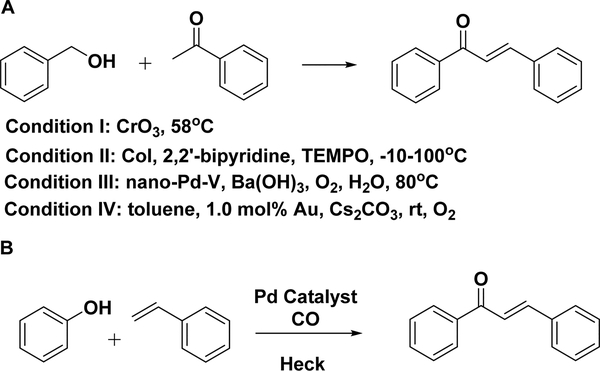
One-pot synthesis is a methodology to improve the efficiency of a reaction and avoid the purification of intermediates to save time and increase the overall yield. The chalcone scaffold has recently been synthesized by a one-pot synthesis from alcohol and ketone.
As illustrated in Scheme 11A, chromium(VI) oxide is slowly added to a mixture of a primary alcohol and a ketone, constructing chalcone in moderate to high yields (65–98%). It is apparent that the alcohol is oxidized to the corresponding aldehyde in situ and reacts with the ketone to obtain the final product.155,156 Xu et al. reported the one-pot reaction of an alcohol and different ketones by changing the reaction temperature from −10 to 100 °C for 10–96 h with a catalyst consisting of copper iodide, 2,2′-bipyridine, and 2,2,6,6tetramethylpiperidine-1-oxyl (TEMPO) (Scheme 11A, condition II).157 Uozumi and co-workers reported that a novel water-soluble nanopalladium (nano-Pd-V) prepared from PdCl2 effectively catalyzed the reaction and made chalcones from α-ketones and alcohols in 92% yield (Scheme 11A, condition III).455 In 2005, a heterogeneous and recyclable palladium catalyst [Pd/AlO(OH)] was reported to catalyze the alkylation of ketones with primary alcohols (Scheme 11A, condition IV). This catalyst was active without ligands or additives. Enones, such as chalcones, can be selectively produced in the presence of oxygen (1 atm O2). However, under an argon atmosphere, ketones were the major product.158 This strategy has been developed with a recyclable gold catalyst that catalyzed the one-pot reaction and obtained chalcones in high yields and selectivity under an oxygen balloon.159 Mechanically, the alcohol is oxidized to an aldehyde and then reacted with a ketone by condensation. The strength of this application is to extend the classical Claisen–Schmidt condensation using benzyl alcohols instead of aldehydes as starting materials.
Scheme 11.
One-Pot Synthesis of Chalcones
In addition to benzyl alcohols, phenols have also been used in one-pot synthesis. An example was published involving the synthesis of chalcones from phenols, which was an extension of the above carbonylative Heck coupling with the activation of phenol in a one-pot manner (Scheme 11B).133
4.4. Synthesis of cis-Chalcones
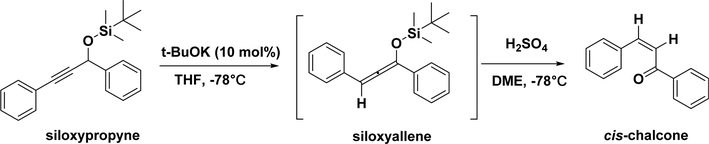
Very few studies have reported the synthesis of cis-chalcones, a thermodynamically less stable isomer, compared with the more stable trans-chalcones. Yoshizawa et al. (Scheme 12) used siloxypropynes and potassium tert-butoxide (t-BuOK) at −78 °C to obtain important intermediates, namely, siloxyallenes, which were treated with a strong acid (H2SO4) and 1,2dimethoxyethane (DME) to produce cis-chalcones. The cis-chalcones were prepared in a high yield (up to 85%) and with an excellent geometry preference (up to 99:1 cis/trans ratio).456
Scheme 12.
Synthesis of cis-Chalcones
5. CHEMICAL REACTIONS RELATED TO MICHAEL ACCEPTORS
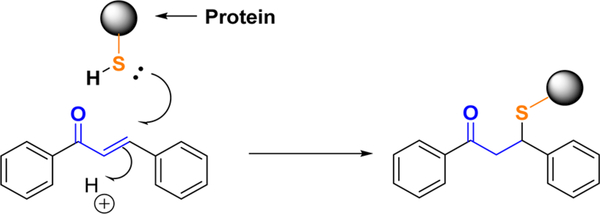
Michael acceptors, containing an electrophile, are generally biologically active. They are involved in the regulation of many signaling pathways in cells and are important tools in chemical biology research. The chalcone scaffold contains an α,β-unsaturated ketone functional group perceived as a potential Michael acceptor. Biologically, the Michael acceptor of the chalcones can readily form covalent bonds with the sulfhydryl of cysteine or other thiols to obtain the Michael adduct (Scheme 13), which may play an important role in their biological activities.160–164 For example, chalcones could modulate the Keap1-Nrf2-ARE pathway through the covalent modification of the cysteines of Keap1 and release Nrf2 to induce phase II enzymes (see section 6.2.1.3). Chemically, the Michael reaction is one of the most efficient methods for carbon–carbon bond formation and is widely applied in synthetic chemistry. The electron density on the two aromatic rings affects the enone’s electrophilicity for the reaction.164 The Michael-related addition, cascade reaction, and epoxidation will be discussed in this section as well.
Scheme 13.
Michael Addition of a Chalcone with Cysteine
5.1. Michael Addition
Chalcone has been widely used in organic synthesis to establish highly enantioselective Michael adducts. The asymmetric catalytic conjugate addition of a stabilized carbanion nucleophile to α,β-unsaturated carbonyl compounds represents one of the most important carbon–carbon bond forming reactions in organic chemistry because the adducts are interesting intermediates for further optimization, such as aminocarbonyls, pyrrolidines, and aminoalkanes.165–169
The first attempt to achieve the enantioselective Michael addition was reported in 1978. Wynberg and co-workers employed N-dodecyl-N-methylephedrinium fluoride as a catalyst for the 1,4-addition of nitromethane to chalcone to form slightly enantioenriched adducts (enantiomeric excess [ee] 23%).170 Although the enantioselectivity was not very high, this pioneering work encouraged people to explore more strategies and catalysts. The enantioselective addition to the α,β-unsaturated ketone of chalcones has recently resulted in great successes with different reagents and catalysts (Schemes 14–18 and Table 3).
Scheme 14.
Sulfa-Michael Addition of Chalcones
Scheme 18.
Michael–Mannich Domino Reaction
Table 3.
Michael Addition of Nitroalkanes to Chalcones
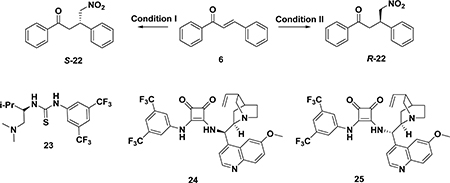
| |||||||
|---|---|---|---|---|---|---|---|
| condition I |
condition II |
||||||
| entry | catalyst | temp, time | catalyst | temp, time | ee (%) | yield (%) | ref |
| 1 | 10 mol % cat. 23 | 50 °C, 72 h | – | – | 95 | 100 | 195 |
| 2 | – | – | 10 mol % cat. 12 | toluene, 25 °C, 110 h | 96 | 94 | 194 |
| 3 | 10 mol % cat. 24 | ClCH2CH2Cl, 80 °C, 72 h | – | – | 94 | 97 | 196 |
| 4 | – | 5 mol % cat. 25 | ClCH2CH2Cl, 80 °C, 72 h | 95 | 99 | 196 | |
| 5 | – | 100 mol % cat. per-6-ABCD | H2O, room temp, 24 h | 68.5 | 100 | 174 | |
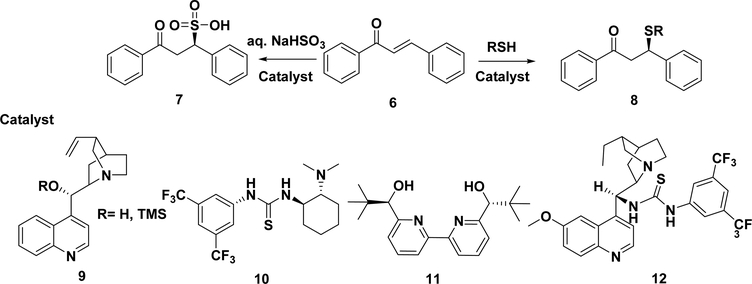
5.1.1. Sulfa-Michael Addition of Chalcones.
The enantioselective sulfa-Michael addition (Scheme 14) has been recognized as one of the most important methodologies to construct carbon–sulfur (C–S) bonds, mimicking the reaction of chalcones with cysteines in the biological systems.171,172 In 2001, the Michael addition of thiophenol to trans-chalcone was catalyzed by (+)-cinchonine (9), resulting in nearly optically pure adducts 8 with an ee of 91–95% in multigram quantities.173 Wang et al. reported that a chiral bifunctional amine thiourea (10) catalyzed asymmetric Michael addition reactions between chalcones and thioacetic acid and gave the adducts in excellent yields (75–100%) with moderate enantioselectivities (33–65%).190 The asymmetric Michael addition of thiols to chalcones was also attempted in water. Pitchumani et al. used heptakis(6-amino-6-deoxy)-β-cyclodextrin (per-6-ABCD) as the catalyst, and the adduct was achieved in an enantiomeric excess of up to 61%.174 In 2011, Vaccaro and co-workers reported the sulfa-addition in water using a highly efficient Sc(OTf)3/bipyridine (11) catalytic system to produce compound 8 with an ee of 96%.175 Both the aqueous medium and catalytic system can be recovered and recycled with no loss in enantioselectivity.
Adamo et al. reported the first example of the catalytic enantioselective addition of sodium bisulfite to α,β-unsaturated ketones by the selection of an appropriate aminothiourea bifunctional catalyst (12). The desired sulfonic acids (7) were synthesized in high yields (87–97%) and with excellent enantioselectivity (84–99%) (Scheme 14).176
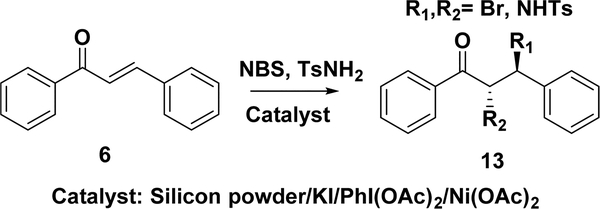
5.1.2. Aminohalogenations of Chalcones.
The aminohalogenations of α,β-unsaturated ketones have also attracted great research interest, and several methods have recently been developed for the aminobromination (13) of chalcones (Scheme 15). Wei et al. reported the aminobromination (13) of chalcones with p-toluenesulfonamide (4-TsNH2) and N-bromosuccinimide (NBS) in the presence of 1 mol % silicon powder as the catalyst in high yields (up to 98%) and with excellent regio- and stereoselectivities (anti:syn > 95%).177 They also reported the aminobromination of olefins catalyzed by KI with the TsNH2–NBS combination. This metal-free condition gave the adduct in good to excellent yields (45– 98%) and with high regio- and stereoselectivities (no syn-products were detected).178 Li and co-workers reported a method for the aminochlorination of chalcones with 2-NsNCl2 in an ionic liquid.179 Hypervalent iodine compounds, such as PhI(OAc)2, are usually used as the clean and efficient oxidants.180,181 Wang et al. reported the aminohalogenation of chalcone promoted by hypervalent iodine compounds.182 They also reported the first PhI(OAc)2-catalyzed aminobromination of chalcones in water with TsNH2 and NBS.183 Ni(OAc)2 can also be used as the catalyst in the aminobromination of chalcones.184 These methods have significant strengths, although limitations still exist, such as procedural difficulties, unsuitability for one-pot operation, and high catalyst loading.
Scheme 15.
Aminohalogenation of Chalcones
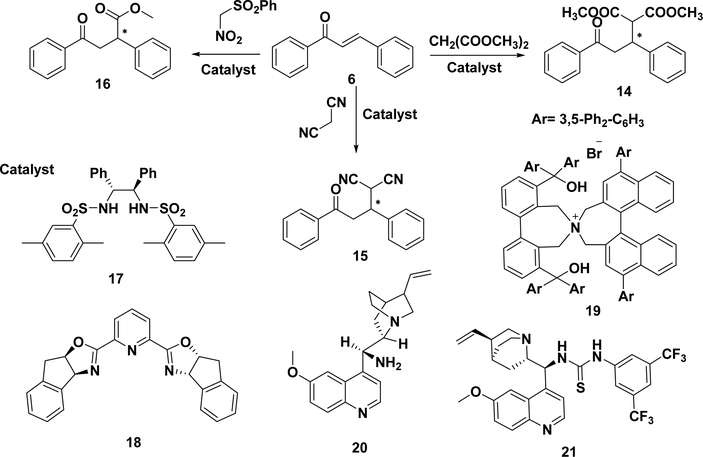
5.1.3. Asymmetric Michael Addition of Malonates or Malononitriles to Chalcones.
The asymmetric Michael addition of malonates to chalcone is another type of carbon– carbon bond formation. A complex named lanthanum–sodiumBINOL, prepared from La(O-i-Pr)3, (R)-BINOL (3 mol equiv), and NaO-t-Bu (3 mol equiv), has been reported to asymmetrically catalyze the addition of malonates to chalcone and give a product with a 77% ee and 93% yield (Scheme 16).185 A simple bis-sulfonamide type ligand (17) and Sr(O-iPr)2 as the catalyst have been used to obtain the product (14) in good yield (91%) and ee (97%).186 A calcium-BINOL catalyst has also been developed for asymmetric Michael addition, but only 42% ee has been obtained.187 Recently, Lippur et al. reported a mild condition using CaCl2, bisoxazoline (18), and dimethyl malonate for the asymmetric Michael addition. The condition was suitable for most α,β-unsaturated ketones. However, using chalcones as the starting materials, a low yield (14%) and low enantioselectivity (9%) of the products (14) were obtained, likely due to the effect of steric hindrance.188 Asymmetric phase-transfer catalysis has also been utilized. Maruoka et al. reported that the phase-transfercatalyzed Michael addition of diethyl malonate in the presence of an N-spiro quaternary ammonium salt catalyst 19 (3 mol %) and K2CO3 (10 mol %) in toluene tolerated both electronwithdrawing and electron-donating groups on the benzyl rings. The corresponding Michael adducts were obtained in excellent yields (>97%) and high ee (85–94%). This catalyst system was also used to afford another Michael adduct 15 in a 98% yield with 81% ee.189 Wang et al. reported the use of a cinchonine catalyst (12) in this reaction and obtained the adduct in 85– 95% yields and 87–93% ee.190 Most of the above strategies have achieved great success with high yields and excellent selectivities, although there are still some areas to be further improved, such as the need for an excess of malonate (4–5.6 equiv) with a long reaction time (from 72 to 144 h).
Scheme 16.
Asymmetric Michael Addition of Malonates/Malononitriles to Chalcones
In addition to compound 14 with two ester groups, an α-stereogenic γ-keto ester (16) has also been produced by employing nitro(phenylsulfonyl)-methane in the presence of catalyst 20 and Cl2CHCOOH.188,191 The addition of malononitrile to a chalcone derivative in the presence of a quinine-derived bifunctional thiourea tertiary amine (21) as a catalyst results in compound 15. The mechanism of the Michael addition of malononitrile to chalcones has also been examined using theoretical calculations.192
5.1.4. Asymmetric Michael Addition of Nitroalkanes to Chalcones.
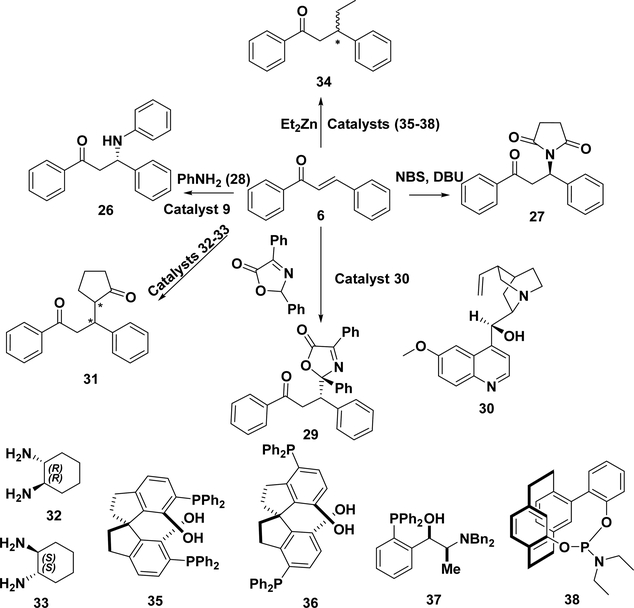
The asymmetric Michael addition of nitroalkanes to chalcones was first reported by Sundararajan and coworkers in 2001.193 Using a chiral polymer and LiAl as the catalyst system, they obtained R-22 with a good yield (90%) but only 51% ee. Subsequent studies have developed several different catalysts for this reaction and provided various chiral products with high yields and ee values (Table 3). For example, in 2005, a cinchona alkaloid derived chiral bifunctional thiourea organocatalyst (12, Scheme 14) was used in the Michael addition of nitroalkanes to chalcones, and R-22 was obtained,194 while catalyst 23 was used to give S-22.195 In 2010, Du and co-workers reported a class of squaramide-based organocatalysts, among which squaramides 24 and 25 showed excellent catalytic activity to obtain the desired R or S enantiomers, respectively, with high yields and excellent enantioselectivities (94–95% ee).196 The above conditions could achieve high ee and yields, although the reaction time was long. R-22 could also be obtained by per-6-ABCD (mentioned in section 5.1.1) with 100% conversion in 24 h but only 68.5% ee.174
5.1.5. Other Michael Additions of Chalcones.
Xu and co-workers prepared a series of R-aromatic amine–chalcones (26, Scheme 17) using phenylamine (28) and silicon-based Lewis acid (TMSX)–cinchonine (9) cocatalysts under solventfree conditions with high enantioselectivity (>99%) and conversion (>99%).197 In 2013, Liang et al. reported another example of β-aminoketones (27), which were prepared by the reaction of chalcones with a combination of NBS and DBU with high yields (>80%).198 Cinchona alkaloids are commonly used in the aza-Michael addition reaction. For example, the addition of azlactones to chalcone derivatives have been achieved (29) using malononitrile and a cinchona alkaloid (30).199 Wang and co-workers developed a method for the addition of cyclopentanone to chalcone by a simple and commercially available system of chiral 1,2-diaminocyclohexanes (32 and 33) and hexanedioic acid to obtain 31. Their method exhibited good yields (up to 92%) and excellent enantioselectivities (up to 99% ee). It also solved the problem of the low reactivity and high steric hindrance of chalcone substrates in the organocatalytic asymmetric Michael addition reactions in comparison to simple ketones.199
Scheme 17.
Other Michael Additions of Malonates to Chalcones
Shibata and co-workers developed a method of using Cu/Zn complex catalyzed alkylation at the α,β-unsaturated position (compound 34). S-34 was obtained by (S)-6,6′-SPINOLPHOS (35), while R-34 was prepared with (S)-4,4′-SPINOLPHOS (36), both of which had over 90% ee.200 The R-isomer was also synthesized using Cu(OTf)2 and aminohydroxyphosphine (37), with 98% ee.201 The copper-catalyzed asymmetric conjugate addition of diethylzinc to chalcones has been extended using [2.2]paracyclophane-derived monodentate phosphoramidite (38) as a ligand, with a 98% yield and 95% ee.202 Compared with those required under other conditions,201 the loadings of the catalyst (1 mol %) and ligand (1.2 mol %) have been found to be extremely low.202
5.2. Cases of Chalcone-Involving Cascade Reactions
It is becoming popular to construct multiple stereocenters in a single step using organocatalyzed cascade reactions.203–207 Chalcone is a very important type of starting material that could undergo enantioselective Michael addition involving cascade reactions, such as the Michael–Mannich reaction or Michael–Michael addition.208,209
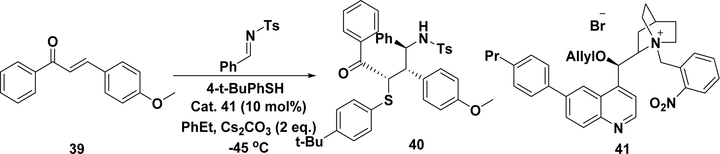
As mentioned in section 5.1, C–S bond formation is important in organic chemistry for chiral sulfur-containing bioactive compounds. A three-component intermolecular Michael–Mannich domino reaction (Scheme 18) using chalcone (39) as the Michael acceptor with catalyst 41 and Cs2CO3 has been shown to result in the formation of 40 in high yield (>74%), diastereoselectivity (dr, >95:5), and ee (>95%).210
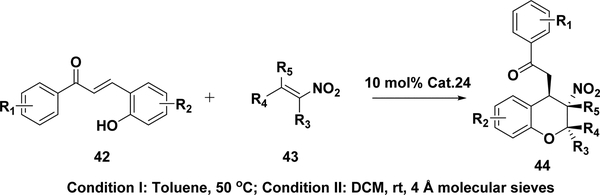
An asymmetric oxa-Michael–Michael cascade reaction (Scheme 19) between trans-nitrostyrene (43) and 2-hydroxychalcones (42) with the use of the same catalyst (24) as in the nitroalkanes’ Michael addition (Table 4) has recently been employed to obtain chiral chroman derivatives with excellent enantioselectivities (up to 99%) and good yields (up to 95%) and diastereoselectivities (up to 5:1).211 This reaction has also been used to synthesize 2-CF3 chromanes. β-CF3-nitroalkenes instead of trans-nitrostyrene (43) have been used for the squaramide (24) catalyzed cascade reaction with 42 to yield CF3-containing heterocyclic compounds (44) bearing three contiguous stereogenic centers with satisfactory qualities.212
Scheme 19.
Asymmetric Oxa-Michael–Michael Addition of trans-Nitrostyrene (43) to 2-Hydroxychalcones
Table 4.
Asymmetric Epoxidation of Chalcones
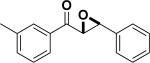
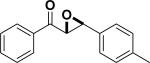
| Entry | Structure | Catalyst/ Solvent | Yield % | ee (Major) % | Refs |
|---|---|---|---|---|---|
| 1 |
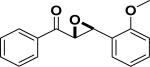
|
|
/ | 25% | 223 |
| 2 |
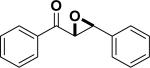
|
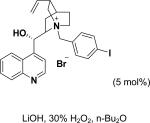
|
97% | 84% | 224, 225 |
| 3 |
|
100% | 92% | ||
| 4 |
|
95% | 89% | ||
| 5 |
|
|
94% | 98.5% | 226 |
| 6 |
|
Cat. 14 (3 mol%), aq NaOCl, Toluene, 0°C | 99% | 96% | 227 |
| 7 |
|
|
99% | 94% | 228 |
| 8 |
|
|
90% | 91% | 229 |
| 9 |
|
|
75% | 69.2% | 231 |
| 10 |
|
62% | 84% | ||
| 11 |
|
69% | 71.4% | ||
| 12 |
|
|
96% | 96% | 233 |
| 13 |
|
poly-L-leucine, NaOH, 30% H202, Toluene | 85% | 93% | 232 |
| 14 |
|
poly-D-leucine, NaOH, 30% H202, CH2Cl2 | 98% | 93% | 234 |
| 15 |
|
|
85% | 95% | 235 |
| 16 |
|
|
99% | 98% | 236 |
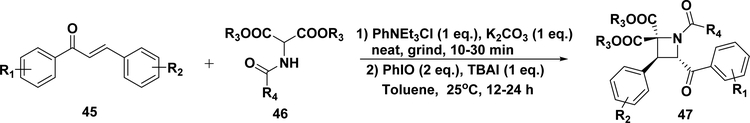
Azetidines are a class of important frameworks because of their remarkable medicinal and biological activities.213,214 In 2010, Fan and co-workers reported a facile stereoselective synthesis (Scheme 20) of highly functionalized azetidines (47) from a novel [2 + 2]-cycloaddition of 2-aminomalonates (46) to chalcones (45) under grind-promoted, solvent-free, and PhIO/Bu4NI mediated oxidative cyclization conditions. Twenty-two derivatives were obtained in good yields (46– 75%).215
Scheme 20.
[2 + 2]-Cycloaddition of 2-Aminomalonates to Chalcones
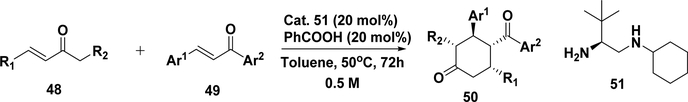
As shown in Scheme 21, an asymmetric cross-cascade reaction of different α,β-unsaturated ketones can be catalyzed by a bulky primary amine salt (51). Twenty-one compounds (50) have been formed with excellent enantioselectivity (92– 99% ee) and diastereoselectivity (>30:1 dr).216 The method has also been extended to construct spirocyclic compounds using cyclic enones containing exocyclic double bonds (49).216
Scheme 21.
Enantioselective Cross-Reactions of Different Enones: Synthesis of Cyclohexanone
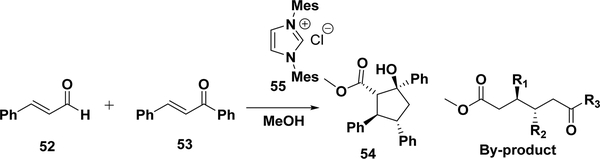
Nair and co-workers reported another case of the construction of four contiguous stereocenters in a stereoselective manner (Scheme 22).217 Methyl-hydroxycyclopentanecarboxylate (54) was prepared in a one-pot operation by the nucleophilic heterocyclic carbene (NHC) (55) catalyzed annulation of an enal (52) with chalcones (53) in methanol. Although the reaction yields were decent (59–70%), there were considerable amounts of byproducts (17–33%).
Scheme 22.
Annulation of p-Methoxycinnamaldehyde with Chalcones
5.3. Epoxidation and Aziridination of Chalcones
Epoxides and aziridines are extremely important intermediates existing in many natural products.218–222 Great efforts have been put forth for chalcones’ epoxidation (56) and aziridination (57) (Scheme 23) for the enhancement of not only the yield but also the ee value. Several types of catalysts, such as PTC and peptide-type catalysts, have been used to obtain the highly enantioselective compounds.
Scheme 23.
Epoxidation and Aziridination of Chalcones
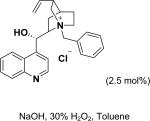
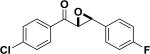
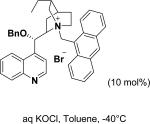
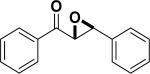
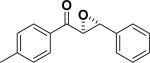
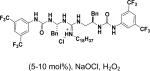
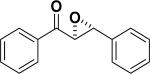
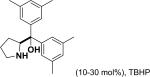
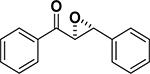
In 1976, Wynberg and co-workers first utilized the cinchona alkaloid derived quaternary ammonium salt as a PTC to catalyze the epoxidation of chalcone (Table 4, entry 1).223 The ee value was initially not very good (25% ee), but it was a great success in that period of time, encouraging scientists to explore better conditions for epoxidation. In 1998, Arai and co-workers discovered that the substituents on the phenyl ring of the N-benzyl unit of quaternary ammonium salt catalysts were of great importance in the asymmetric induction, among which p-iodophenyl gave the products with the highest yields (>95%) and ee (>84%) (Table 4, entry 2–4).224,225 In 1999, Corey and co-workers further developed a PTC catalyst, where the C-9 hydroxyl was substituted by benzyl ethers. The new catalyst was demonstrated to give a remarkably high enantioselective (98.5% ee) product using KOCl as the oxidant (Table 4, entry 5).226 In addition to cinchona alkaloid derived quaternary ammonium salts, binaphthyl has also been applied as a PTC. For example, Maruoka and co-workers used binaphthyl-based spiro quaternary ammonium salts (19) to catalyze the epoxidation of chalcones (Table 4, entry 6).227 The epoxidation product was obtained in a 99% yield and 96% ee using catalyst 19 and NaOCl as the oxidant reagent. A urea–guanidinium salt was shown to be an effective catalyst for epoxidation, where the functional groups were suggested to contribute cooperatively by interacting with guanidine–hydrogen peroxide (H2O2) and urea–enones, giving the epoxidation products in a 99% yield and 91% ee (Table 4, entry 7).228 β-Amino alcohol catalysts, such as α,α-diphenyl-L-prolinol, are effective for the epoxidation, giving epoxidated chalcones under tert-butyl hydroperoxide (TBHP) conditions at a 90% yield and 91% ee (Table 4, entry 8).229 In addition to prolinols, several other types of amino alcohols have also been examined, demonstrating that four- and six-membered ring catalysts are less effective than the corresponding prolinol catalysts (structures not shown).230 Chiral amine salt catalysts are another class of catalysts for the epoxidation of chalcones. Although such catalysts are very useful for ketones, the epoxidation of chalcones has only been achieved for up to 84% yield using TBHP (Table 4, entries 9–11).231
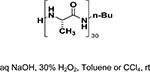
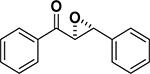
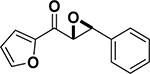
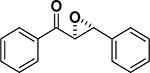
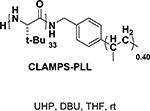
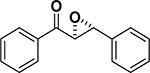
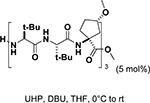
Peptide-type catalysts are another type of reagents for chalcone’s epoxidation. A polypeptide-catalyzed asymmetric epoxidation of (E)-chalcone using H2O2−NaOH in a toluene– water system was first reported by Juliá and co-workers in 1980.232 Using a poly-L-alanine after the further optimization of the length from 5 to 30, the epoxidation of chalcone was highly effective, and the product was provided in a 96% yield and 96% ee (Table 4, entry 12).233 Subsequently, the reaction solvent and the catalyst can be replaced by CCl4 and poly-L-leucine, respectively. Comparable results have been achieved for chalcone with poly-L-leucine (85% yield and 93% ee) (Table 4, entry 13). Moreover, poly-D-leucine results in the optical isomer of the epoxide at a 98% yield and 93% ee (Table 5, entry 14).234 Considering the insolubility of the polypeptide catalyst in toluene and water, a triphasic reaction system has also been used. Roberts and co-workers developed a nonaqueous phase method, where the aqueous H2O2−NaOH was replaced by urea−H2O2 (UHP) and DBU. The chalcones were efficiently epoxidized with immobilized poly-L-leucine (CLAMPS-PLL) under this biphasic condition with a 85% yield and 95% ee (Table 4, entry 15).235 Polypeptides containing unnatural amino acids have also been applied for the epoxidation of chalcones. For example, an epoxide has been obtained in a 99% yield and 98% ee using a cyclic α,α-disubstituted amino acid catalyst (Table 4, entry 16) with a UHP–DBU–THF system.236
Table 5.
Asymmetric Aziridination of Chalcones
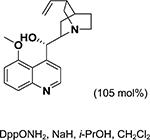
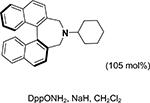
For the asymmetric aziridination of chalcones, the progress is relatively slow. As shown in Table 5, a base and O-mesitylenesulfonylhydroxylamine (MSH) or O- (diphenylphosphinyl)hydroxylamine (DppONH2) as the NHtransfer agents are effective for the aziridination of chalcones, giving products with good yields (64–90%) and moderate ee values (37–56%).237–239
In this section, some examples of the epoxidation and aziridination of chalcones are provided. For details on this topic, it is advised to refer to a well-reviewed paper by Shi et al. in 2014.240
6. MEDICINAL ASPECTS OF CHALCONES
6.1. Overview of Biological Activities
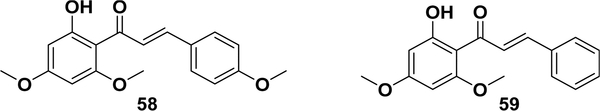
Chalcones exhibit a broad spectrum of biological activities, probably due to their small structures and Michael acceptor features, which make them tolerant to different biological molecules and allow them to readily or reactively bind with them. The biological activities of chalcones include anticancer activity, cancer-preventative effects, anti-inflammatory activity, antibacterial activity, antituberculosis activity, antidiabetic activity, antioxidant activity, antimicrobial activity, antiviral activity, antimalarial activity, neuroprotective effects, and others.1–5,8,10,12–14,18,21,22,241–251 As presented in Table 1, even a single chalcone compound can exhibit several types of bioactivities. For example, isoliquiritigenin (Table 1, entry 1) has at least anticancer, cancer-chemopreventive, antioxidant, and anti-inflammatory activities. Xanthohumol (Table 1, entry 17) also exhibits anti-HIV-1, antibacterial, and anticancer activities. In addition to these therapeutic potentials, the side effects have also been evaluated. Recently, Xing et al. found a hepatotoxic risk for one type of chalcone, which needs to be more thoroughly investigated.252 Flavokawains A (58) and B (59) (Figure 4), two chalcone derivatives isolated from kava kava, a natural source of great human health relevance, exhibit hepatotoxic synergism with aceta6minophen, demonstrating and characterizing the hepatotoxic risk of kava. Another study reported that flavokawain A could significantly inhibit cytochrome P450 isotypes (CYP1A2,CYP2D1,CYP2C6, and CYP3A2), providing the mechanistic insights of the hepatic adverse side effect of flavokawain A and kava extracts.253 These studies have provided valuable information for the future development of in vivo chalcone studies and have contributed to the progress of chalcone-based drug discovery.
Figure 4.
Flavokawains A (58) and B (59).
6.2. Representative Mechanisms of Action of Chalcones
Tremendous effort has been devoted to characterizing the mechanisms of action of these chalcone compounds. Their multitarget and broad-spectrum biological activities have been reviewed in previous papers.1–5,7–22,241 Nevertheless, there is not enough convincing evidence to support some of these molecular targets.1,254 In this section, the representative mechanisms of action of chalcones reported in recent years are summarized, and the targets predicted by computational modeling are detailed in section 7.1.
6.2.1. Michael Acceptor Related Mechanisms.
6.2.1.1. IκB Kinases (IKKs).
IκB kinases (IKKs) are one of the key regulators of the nuclear factor kappa B (NF-κB) pathway, which is recognized as the central mediator of immune responses and inflammation.255 Intervening with the NF-κB through IKK inhibition is expected to suppress the NF-κB protein translocation to the nucleus, which is considered to be a promising strategy for disease treatment, especially against inflammation and inflammation-related cancer.256,257 Mechanistically, cysteine 179 of IKKβ has been shown to be of great importance to IKK inhibition, indicating a covalent-reactive site for biological processes.
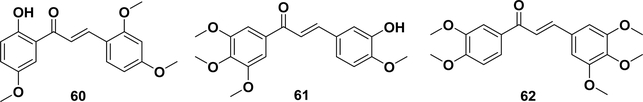
As mentioned in section 5.1, the Michael acceptors of chalcones could be covalently modified by proteins, which is one of the major mechanisms of their therapeutic potentials.85 The chalcones demonstrate NF-κB inhibitory activity by the covalent modification of the IKK proteins via the α,β-unsaturated ketone with the Michael-type activity.173 For example, Pandey et al. reported that butein (Table 1, entry 2)258 inhibited IKKβ in biochemical- and cell-based assays. Additionally, isoliquiritigenin (Table 1, entry 1),259,260 flavokawain A (58) and B (59),261 licochalcone A (Table 1, entry 27),52 and xanthohumol (Table 1, entry 17)43 all have anti-inflammatory and anticancer activities, where the dual activities might result from inhibiting IKKβ by the covalent modification of cysteine 179.174,175 DK-139 (60, Figure 5) has been found to induce an anti-inflammatory effect on microglial cells by inhibiting the Akt/IκB kinase (IKK) and nuclear translocation of the NF-κB signaling pathway.262 In 2009, 3hydroxy-4,3′,4′,5′-tetramethoxychalcone (61) was reported to exhibit potent anticancer activity in vitro and in vivo that correlated with its NF-κB inhibitory activity. Compound 61 has also been proposed to react with the cysteines of IKKβ, with an inhibition of 46% at 10 μM.164 A subsequent study of its mechanism of action showed that this compound killed different cancer cells through a JNK-mediated autophagy pathway that triggers c-IAP.263 The combination of this compound with TNF-related apoptosis-inducing ligand (TRAIL) or cisplatin significantly increases its cytotoxicity in lung cancer cells. It has been demonstrated that the synergistic effect is the result of the suppression of the expression of the cellular FLICE (FADD-like IL-1b-converting enzyme)-inhibitory protein large (c-FLIPL) and cellular inhibitor of apoptosis proteins (c-IAPs), which cooperatively activate autophagy.264,265 Toll-like receptor 4 (TLR4) and a coreceptor, myeloid differentiation 2 (MD2), have been reported to regulate the downstream signal transduction, such as MAPK phosphorylation and NF-κB activation. Compound 62 has been demonstrated to have MD2 inhibitory activity leading to antiinflammatory effects in an LPS-induced acute lung injury (ALI) model.266
Figure 5.
Structures of chalcones as NF-κB inhibitors.
6.2.1.2. Thioredoxin Reductase (TrxR).
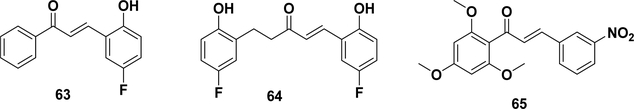
Thioredoxin (Trx) is one of the major biological antioxidants regulating the cellular redox balance. This enzyme system, consisting of thioredoxin reductase (TrxR) with selenocysteine, is overexpressed in many human tumors and is recognized as a potential target for cancer therapy.267,268 Gan et al. reported that chalcones 63 and 64 showed cellular TrxR inhibitory activity in a panel of Michael acceptor-type pharmacophores (Figure 6). MS analysis demonstrated that the most potent chalcone derivative (64) covalently modified TrxR at the selenocysteine residue U498.162 In 2015, Zhang et al. reported a series of chalcone analogues based on xanthohumol (Table 1, entry 17). Among them, compound 65 displayed good cytotoxicity against HeLa cells (IC50 = 1.4 μM), selective inhibition of TrxR, and induction of cell apoptosis. Mechanistically, the U498C mutation of TrxR was performed to support the covalent mechanism. As a result, this compound could significantly decrease the cellular thiol level and induce the expression of reactive oxygen species (ROS).269
Figure 6.
Structures of chalcones as TrxR inhibitors
6.2.1.3. Keap1-Nrf2-ARE Pathway.
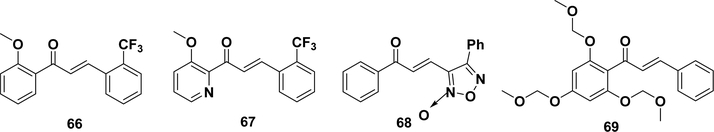
Nuclear factor erythroid 2 related factor 2 (Nrf2) is key to inducing the phase II enzymes and antioxidant enzymes that prevent the oxidative stress,270 which could cause cancer, diabetes, Alzheimer’s disease, arteriosclerosis, and inflammation.271–279 Under unstressed conditions, Nrf2 remains at a low cellular concentration and is negatively regulated by another cellular component, namely, Kelch-like ECH-associated protein 1 (Keap1).280 Upon exposure to oxidative stress, Keap1 is deactivated such that Nrf2 escapes from the Keap1-mediated degradation and translocates into the nucleus to transcriptionally activate the ARE-dependent antioxidant genes. Electrophilic agents have been reported to induce Nrf2 through the covalent modification of the Keap1 cysteines, resulting in a conformational change that facilitates the process of Nrf2 escaping from the Keap1–Nrf2 interaction.281,282 Kumar et al. reported a novel trifluoromethylchalcone (66, Figure 7) as a potent activator of Nrf2 using in vitro and in vivo models.283 The potency of the chalcone in human lung epithelial cells was measured by the expression of Nrf2-dependent antioxidant genes, such as glutamate–cysteine ligase modifier subunit (GCLM), NADPH:quinone oxidoreductase 1 (NQO1), and heme oxygenase 1 (HO-1). In the small intestine of mice, the GCLM and NQO1 after treatment were 6-fold and 10-fold higher, respectively, compared with the vehicle. A subsequent study developed a similar series of heterocyclic chalcone-based Nrf2 activators (e.g., 67) with increased aqueous solubility and oral bioavailability and enhanced in vivo efficacy.284 However, whether these chalcones covalently modify the cysteines of Keap1 has not been characterized. Furoxanyl chalcone (68) is a heterocycle containing compound that translocated Nrf2 and significantly induced the activities of phase II enzymes in the liver.285 2′,4′,6′-Tris(methoxymethoxy) chalcone (69) has been reported to induce the expression of heme oxygenase 1 (HO1).286 Natural products, e.g., licochalcone287 and xanthohumol,288 can also induce phase II enzymes and activate Nrf2 in cells. A recent study with a chalcone-based probe confirmed that the probe was covalently bound with the cysteines of Keap1 in AREc32 reporter cells (for mdetails, see section 7.2.2.1).289
Figure 7.
Structures of chalcones as Nrf2 activators
6.2.2. Other Mechanisms or Targets of Chalcones Validated by in Vivo Models.
6.2.2.1. Microtubule Formation.
Microtubules, ubiquitous dynamic polymers of α- and β-tubulin heterodimers, are in a highly dynamic polymerization–depolymerization process in cells.1,290–292 Numerous synthetic (Figure 8) and natural chalcones (Table 1) have been reported to exhibit antimicrotubule activities.
Figure 8.
Representative antimicrotubule chalcones.
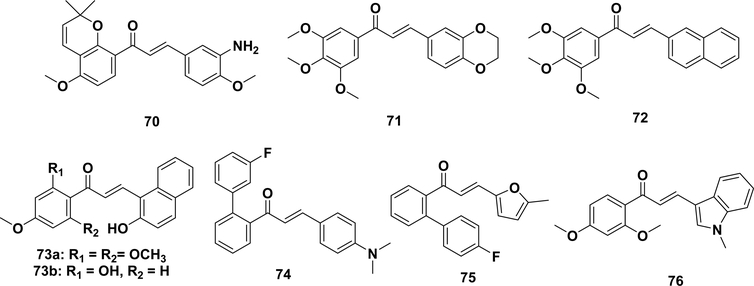
Millepachine (Table 1, entry 32), first isolated from Millettia pachycarpa, induces cell cycle arrest and apoptosis in human hepatocarcinoma cells in vitro and in vivo.54 With the aim of enhancing the antiproliferative activity of mellepachine, Yang and co-workers developed an amino-substituent millepachine derivative (70) exhibiting excellent anticancer activity against a panel of drug sensitive cancer cell lines and multidrug-resistant cancer cells. Several studies using microtubule dynamics and competitive assays have provided support that this compound inhibits tubulin polymerization by binding at the colchicine site.293–295 In addition to simple chalcones, chalcones with fused structures (71–73, 76) have also exhibited antimicrotubule and cytotoxicity effects. Bu et al. synthesized a novel o-aryl chalcone (74) by the Suzuki–Claisen–Schmidt reaction. This chalcone showed potent cytotoxicity against several multidrugresistant cancer cell lines (paclitaxel-resistant human ovarian carcinoma cells, vincristine-resistant human ileocecum carcinoma cells, and doxorubicin-resistant human breast carcinoma cells) in an extremely low nanomolar range. A target determination assay indicated that the chalcone was bound to tubulin at the colchicine site and induced mitosis and arrested cells at the G2/M phase,296 which is a key feature of antimicrotubule agents.297 Compound 75 suppressed approximately 50% of the growth of A549 tumor xenografts without an apparent loss of body weight in nude mice.296,290 An indole–chalcone (76), namely, IPP51, induced prometaphase arrest and the subsequent apoptosis of bladder cancer cells and showed a significant inhibition of tumor growth without a great loss in body weight. Biochemically, this compound inhibited tubulin polymerization and competed for colchicine binding to soluble tubulin.298
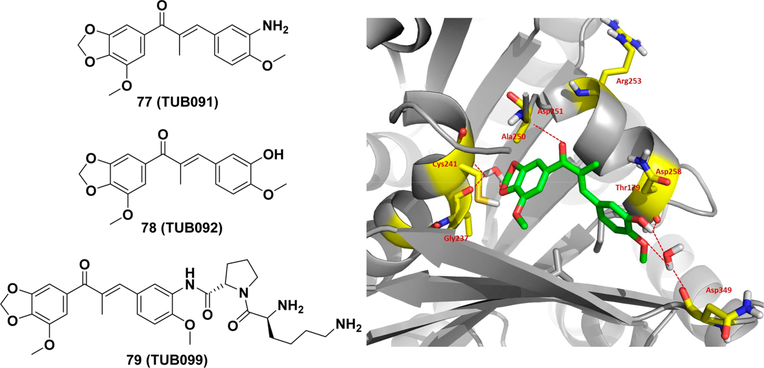
Very recently, Liekens et al. reported a landmark investigation of a series of newly designed chalcones, namely, TUB091 (77) and TUB092 (78).299 TUB092 was soaked in the crystals of a protein complex comprising αβ-tubulin (T2) dimers, a stathmin-like protein RB3 (R), and tubulin tyrosine ligase (TTL). The high-resolution cocrystal structure (2.4 Å) provided the first insight into a chalcone compound binding with tubulin (PDB entry 5JVD). The chalcone bound with tubulin at the colchicine binding site (Figure 9): (a) the 1,3benzodioxole ring of TUB092 located in the hydrophobic pocket formed by the side chains of β-tubulin residues; (b) a water-mediated hydrogen bond to the backbone carbonyl and amide of Gly237 and Cys241 was formed; (c) the carbonyl of the α,β-unsaturated ketone generated a hydrogen bond with Asp251; (d) another two water-mediated hydrogen bonds were formed by the backbone carbonyls of Thr179 and Asn349 with the hydroxyl and methoxy groups. A subsequent solubility optimization identified a prodrug (TUB099, 79) with an L-LysL-Pro dipeptide, showing a 1954-fold better solubility (31 mg/ mL in PBS) than TUB091 (0.016 mg/mL in PBS). TUB099 also inhibited primary tumor growth and spontaneous metastasis in mice (iv injection, 10 mg/kg, 5 days) with 90% or higher inhibition.
Figure 9.
Structures of TUB chalcones and cocrystal structure of TUB092 with tubulin [generated using PyMol (http://www.pymol.org/)].
6.2.2.2. Receptor Tyrosine Kinase Inhibitory Activities.
Receptor tyrosine kinases (RTKs), including the epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor receptor (VEGFR), are cell-signaling effectors responsible for cancer development.300 Isoliquiritigenin (Table 1, entry 1) exhibits EGFR inhibitory activity and in vivo antitumor efficacy against a mutant EGFR-expressing xenograft mouse model.28 This compound has also been found to inhibit the VEGFR enzymatic activity and VGEF-induced angiogenesis in vivo.29 Yang et al. reported that natural butein (Table 1, entry 2) inhibited EGFR in the micromolar range. A competitive biochemical assay showed that the compound potentially bound to the ATP-binding pocket.30,31 In 2013, Limper et al. reported that xanthoangelol (Table 1, entry 18) could inhibit EGFR in enzymatic assays.45
6.2.2.3. Aldose Reductase Inhibitory Activities.
Aldose reductase (ALR2) catalyzes the conversion of glucose to sorbitol, which is the first step in the polyol pathway of glucose metabolism.301,302 Isoliquiritigenin and butein have also exhibited ALR2 inhibitory activities in biochemical assays and inhibited sorbitol accumulation in vivo.303–305 Iwata et al. optimized isoliquiritigenin to obtain compound 80 (Figure 10), which showed nanomolar inhibitory activity and 100-fold potency compared to that of isoliquiritigenin.306
Figure 10.
Structures of chalcones as ALR2 and COX inhibitors.
6.2.2.4. Cyclooxygenase Inhibitory Activities.
Cyclooxygenase (COX), a target gene of NF-κB, is thought to be involved in the pathology of cancer and inflammation.307,308 Broussochalcone A (Table 1, entry 16) has been demonstrated to inhibit COX activity. Synthetic compound 81 has been designed as a dual inhibitor of COX and 5-lipoxygenase (5LOX), exhibiting both mechanisms of action in vivo.309 Ozdemir and co-workers reported a class of indole–chalcones (82) showing potent COX inhibitory activity as well as in vivo anti-inflammatory activity.310 The N-arylpyrazole of celecoxib has been hybridized into chalcone, where compound 83 selectively inhibits COX-2 activity with good in vivo efficacy.311
6.2.3. Other Mechanisms of Action.
Chalcones have also been characterized to act on many other cell signaling pathways, such as JAK/STAT,312 ROS/MAPK,313 and p38.314 However, these topics are not covered in this review because not enough evidence has been provided to support them as the direct target(s) for chalcones. More discussion about the chalcones’ target identification strategies will be presented in section 7.
6.3. Hybrid Chalcones
Molecular hybridization is a strategy used for the design of new chemical entities by the fusion of two different chemotypes. This is an alternative to combination chemotherapy, where two or more drugs of different mechanisms of action are combined for the treatment.315,316 However, the simple combination chemotherapy has a high risk of drug–drug interaction.15,317 Chalcones are recognized as a privileged scaffold for the incorporation of different molecules or pharmacophores with various activities. The synthesis of these hybrids or conjugations typically uses the classical condensation or the synthesis methods discussed above (section 4) to build the chalcone core. In addition to the biological activities for multitargeting mechanisms, hybrid molecules are also selected for other reasons, such as improving the solubility and oral bioavailability. Two approaches, the construction of ketone or aldehyde hybrids and linkage with chalcones, are detailed below.
6.3.1. Fused Hybrids.
Fused chalcones are easily achieved by the Claisen–Schmidt condensation process using fused aldehydes or ketones (Scheme 24). Based on this strategy, several fused chalcones (Figures 11–15) have been developed with various biological activities.
Scheme 24.
Synthetic Strategy of Fused Chalcone Hybrids
Figure 11.
Structures of boron-containing chalcones.
Figure 15.
Other representative fused chalcone hybrids
6.3.1.1. Boron-Containing Chalcones.
Chalcone–benzoxaborole (84), prepared from the intermediates 6-formylbenzoxaborole and the corresponding ketone, has recently been found to inhibit Trypanosoma brucei growth and possess antitrypanosomal activity (Figure 11).318 Boronic–chalcone (85) was described early in 2002 as a fluorescent probe for the detection of fluorides.319 Boronic–chalcone exhibits not only fluorescent properties but also other biological activity. Compound 85 has been reported as an antitumor agent targeting MDM2 oncoprotein.320 Compound 86 can induce antitumor activity against malignant glioma cell lines both in vitro and in vivo.321 Compound 87 exhibits potent anticancer activity (HCT116 cells, IC50 = 3.9 μM) together with proteasome inhibitory activity.322
6.3.1.2. Coumarin–Chalcones.
Coumarin–chalcones are another interesting class of hybrids (Figure 12), which hybridize a six-member ring at the 1,6-positions (88),323–325 4,5-positions (89), or 2,3-positions (90)326 of the chalcone’s aldehyde side or the 2′,3′-positions (91)327 of the chalcone’s ketone side. The hybrids (89–91) are easy to synthesize through the traditional Claisen–Schmidt condensation using fused aldehydes or ketones. The preparation is completely different for the 1,6-position fused coumarin–chalcone (88), where a one-pot Knoevenagel condensation can be applied using a substituted salicylaldehyde, a β-ketoester, and piperidine in ethanol.323,328,329
Figure 12.
Structures of coumarin–chalcones
Compound 88 has been reported to possess antioxidant activity.323 The electrochemical properties of coumarin– chalcones are better than those of the reference compounds quercetin and catechin, and they also exhibit a high radical scavenging capacity. The compounds have also presented good cytoprotective effects against H2O2- and ONOO–-induced cell death but low cytotoxicity against BAEC cells. Another study provided more evidence on the radical-scavenging property of the hybrids.330 Chalcone–coumarin derivatives also possess antitumor activity that significantly inhibited in vitro and in vivo tumor growth.325,326 Compound 88 has been reported to show antibacterial activity for the treatment of tenacibaculosis in fish.324 A biscoumarin–chalcone hybrid (90) exhibited good in vitro anti-inflammatory and moderate protective activity in carrageenan-induced paw edema in albino rats (33%).331 Compound 91 has been demonstrated to have excellent cytotoxic activity against paclitaxel-resistant cancer cells.327 The Hence, derivatives containing the π-conjugated potential chromophore system and spectroscopic and photophysical properties of a coumarinyl chalcone (92) have also been evaluated.332 Similar to the fluorescent properties of chalcones, these properties are extremely sensitive to the polarity of the solvent (see section 3).
6.3.1.3. Indole–Chalcones.
Indole is a scaffold that commonly appears in natural products and synthesized compounds due to its broad spectrum of biological activity.333,334 To date, at least two types of indole–chalcone hybrids (93–95, Figure 13) have been developed with their biological activities evaluated.295,310,335–337 Sashidhara et al. obtained indole–chalcone fibrates based on indole and coumarin–chalcone fibrate, and they exhibited potent in vitro antioxidant and significant in vivo antidyslipidemic effects.336 The architecture of compound 93 has also been evaluated in cancer cells. 3-(5-Methoxy-2-methyl-1H-indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one (94, MOMIPP) exhibits methuosisinducing activity at submicromolar concentrations. A 6-azido MOMIPP was designed by a photolabeling strategy for target identification. However, no pull-down experiment has been performed, and the exact binding target(s) is not well understood.337 This scaffold has been found to possess in vitro and in vivo anti-inflammatory activity nonselectively targeting COX-1 and COX-2.336 Unlike the scaffold of compound 93, compound 95, another type of indole–chalcone, has been synthesized from indole-5-carboxaldehyde.295,335 This indole–chalcone labeled with 125I can be used in β-amyloid imaging probes for detecting Alzheimer’s disease (AD), targeting Aβ1–42 aggregates with high affinity (Ki = 1.97 antiglioma activity by the activation of the Bax/mitochondrial/caspase-9 pathway and the inhibition of the p53-MDM2 pathway.338,339 Loch-Neckel et al. reported a further investigation of the mechanism of an analogue (97).338 In vitro and in vivo, it could inhibit glioma cell growth and induce mitochondrial apoptosis in U87-MG glioma cells via the inhibition of MDM2.
Figure 13.
Structures of indole–chalcones.
6.3.1.5. Other Fused Hybrid Chalcones.
Several hybrids besides those discussed above have also been reported to exhibit potent anticancer activities (Figure 15). For example, β-carboline–chalcone (98) exhibits significant DNA binding interaction and DNA stabilization.340 Imidazothiazole–chalcone (99) exhibits promising cytotoxicity with a microtubuledestabilizing mechanism and could compete with colchicine.15,341 Anthraquinone–chalcone (100) shows high cytotoxicity in HeLa cells.342,343 The compound induces the activity of caspase-3 and caspase-8 in HeLa cells and has shown potent inhibition of MMP-2 secretion.
6.3.2. Hybrids Using Linkages.
Using linkers is another common method to connect chalcones with other active compounds (Figures 16–21). The use of a direct connection like an amide, diol, or ester linkage or the use of a triazole via click chemistry are the most convenient strategies. These hybrids typically retain or enhance the biological properties of the parent chalcones. In this section, representative hybrids using a linker are discussed.
Figure 16.
Amide-linked chalcones
Figure 21.
Phthalocyanine–chalcone conjugates
6.3.2.1. Using an Amide as a Linker.
A series of α-bromoacryloylamido chalcones (Figure 16) have been obtained by the hybridization of an α-bromoacryloyl moiety and the α,β-unsaturated ketone system of the chalcone framework, where they might covalently react with the targets.344 Compounds 101 and 102 exhibit the highest activity against tumor cell growth (IC50 < 1 μM) and 10–100-fold increases in potency compared with the corresponding amide derivatives. The mechanism of action has only been preliminarily studied, and the compounds appear to induce apoptosis mediated by the involvement of the mitochondria and the activation of caspase- 3. Dithiocarbamate–chalcones using an amide as the linker (103) exhibited excellent growth inhibition against SK-N-SH cells, with an IC50 value of 2.03 μM, but are nontoxic to normal cells (GES-1, >64 μM), mechanistically inducing apoptosis and arresting the cell cycle at the G0/G1 phase.345
6.3.2.2. Using a Diol as a Linker.
A diol linker is one of the most widely utilized strategies to connect pharmacophores in medicinal chemistry.346 Several chalcone hybrids have been successfully designed and synthesized using this method (Figure 17). The 1,4-dihydropyridyl group is an important pharmacophore that has been introduced into hybrid molecules.81 The 1,4-dihydropyridyl–chalcones (104) can be synthesized in glycol and showed significant vasorelaxant activities, but no mechanism of action or binding target(s) has been reported.347 Pyrrolobenzodiazepine–chalcone (105) can be prepared by employing a solid-phase synthetic protocol via an intramolecular aza-Wittig reductive cyclization. This compound shows promising anticancer activities in an NCI-60 panel and exhibited a significant DNA-binding affinity as determined by thermal denaturation studies.348,349 Recently, a series of novel dihydroartemisinin (DHA)–chalcones connected by a diol have been reported.350 Compound 106 exhibits an IC50 value of 0.3 μM in cytotoxicity, which is similar to that of the standard therapy (doxorubicin, IC50 = 0.3 μM), and a 6-fold increase in activity compared to that of DHA.
Figure 17.
Diol-linked chalcones
6.3.2.3. Using an Ester or Ether as a Linker.
Using an ester or ether is a simple strategy to connect different pharmacophores by directly reacting with the hydroxyl groups of the chalcone (Figure 18). For example, chalcone–amidobenzothiazole conjugates (107 and 108) have been shown to exhibit potent activities against different cancer cell lines in the range 0.85–3.3 μM and induced cell cycle arrest at the G2/M phase.351 A chalcone–platinum(II) complex (109) has been found to exhibit an excellent antitumor effect against a panel of 21 cancer cell lines, similar to the activity of its parent chalcone but different in long-term treatment and slightly different in the mechanism of apoptosis induction.352 This hybrid containing a cisplatin moiety shows promising activity, although no in vivo data has been published. Metronidazole is an FDA-approved drug for trichomoniasis, and resistance usually occurs in metronidazole treated Trichomonas vaginalis.353 A metronidazole–chalcone hybrid (110) has been reported to be active against not only metronidazole-susceptible but also metronidazole-resistant T. vaginalis.354
Figure 18.
Ester- or ether-linked chalcones
6.3.2.4. Using 1,2,3-Triazole as a Linker.
Using a click reaction to achieve 1,2,3-triazole-linked chalcone hybrids (Figure 19) is an efficient strategy to connect two pharmacophores due to the mild reaction conditions and the triazole’s varied biological activities.355 This type of chalcone has typically been synthesized using an azido-chalcone and an alkyne compound, enabling the use of either an ether linker or a propargyloxychalcone and an azide compound.
Figure 19.
1,2,3-Triazole linked chalcones
Coumarin–chalcone can be hybridized by a 1,2,3-triazole ring at different positions (111a and 111b), exhibiting both anticancer and antimalarial activities.356 The hybrids exhibit better cytotoxicity against HepG2 cells than etoposide and extremely low toxicity toward the normal cells. The binding targets have been predicted to be tubulin and falcipain-2 by molecular docking, although no validation has been published. Chibale et al. reported a series of chalcone–dienone hybrid compounds containing aminoquinoline or nucleoside templates.357 They were hybridized into chalcone molecules to improve the biological activities, solubility, and/or oral bioavailability. Among them, chloroquine–chalcone (112) was found to be the most potent, showing antimalarial activities with submicromolar IC50 values against the D10, Dd2, and W2 strains of Plasmodium falciparum. A preliminary mechanistic study showed that the compounds decently inhibited β-hematin formation. However, the β-hematin inhibition and in vitro antimalarial potency are not well-correlated (see section 6.3.2.5).
Liu et al. designed and synthesized 24 matrine–1H-1,2,3triazole–chalcone conjugates using propargyloxychalcone and a 13-azido matrine with an excellent yield by a Michael addition reaction between sophocarpine and excess trimethylsilyl azide in the presence of acetic acid at ambient temperature. The conjugated compound 113 exhibited more potent anticancer activity than 5-fluorouracil against four human cancer cell lines and low cytotoxicity to NIH3T3 normal cells. Moreover, a synergistic effect was observed after hybridization, as compound 113 showed better antiproliferative activity against A549 compared with matrine alone or a simple combination of chalcone and matrine. Nevertheless, the most important characteristic of the hybrid was its favorable safety profile in vivo. The compound was found to have an excellent antitumor efficacy (89.6% tumor growth inhibition) in the A549 xenograft nude mouse model (10.0 mg/kg/day, 20 days, iv) without any apparent loss of body weight.358
β-Lactam has been recognized to be an evergreen bioactive scaffold with not only the antimicrobial activity of naturally occurring bicyclic penicillin and cephalosporin but also various other biological activities.359 Kumar et al. hybridized the lactam scaffold into chalcones with a click reaction.360 Thirteen β-lactam–triazole–chalcones were designed and synthesized. The most potent compound (114) exhibited high cytotoxicity against lung (A549) and leukemia (THP-1) cancer cells, with IC50 values < 1 μM. However, the anticancer activities of the other hybrids were not remarkable.
6.3.2.5. Other Linkers.
Chalcone–benzothiazole (115)361 and chloroquine–chalcone (116)362 have been conjugated to hydrazine at different sites of chalcones (Figure 20). Compound 115 shows antifilarial activity selectively targeting Brugia malayi thymidylate kinase (BnTMK). Compound 116 is a hybrid connecting on the α-position of the chalcone and exhibiting antimalarial activity. Notably, it has high efficacy against both chloroquine-susceptible (3D7) and chloroquineresistant (K1) strains of P. falciparum in the nanomolar range, which is probably due to the inhibition of β-hematin formation.
Figure 20.
Other representative linked chalcones.
Another chloroquine–chalcone hybrid connected by piperazine can be optimized from the chloroquine–triazole–chalcone (112) to address the poor solubility.363 The chloroquine– piperazine–chalcone (117) was designed by the in silico and in vitro prediction of the physical chemistry and ADME properties using different linkers. The in vitro antiplasmodial activities against the D10, Dd2, and W2 strains of P. falciparum of compound 117 have been demonstrated to be 10-fold lower than that of the triazole hybrid (112). The strength of this piperazine–chalcone is its excellent solubility under acidic conditions (pH 2.0, >100 μg/mL), although there is no such increase for the triazole compound 112 under the same conditions (pH 2.0, <1.6 μg/mL). Mechanistically, this compound exhibits greater potency in the β-hematin inhibition assay than compound 112, which is identified to be a primary in vitro mechanism of action.
Combretastatin A-4 is an attractive microtubule-targeting natural product from the bark of Combretum caffrum.364 Kamal et al. reported a series of imidazolone–chalcone hybrids to mimic the combretastatin A-4 scaffold and evaluated them for anticancer activities against a panel of 53 human tumor cell lines derived from nine different cancer types (leukemia, nonsmall cell lung, colon, CNS, renal, prostate, ovarian, breast, and melanoma).365 Compound 118 showed good anticancer activity, with GI50 values ranging from 1.26 to 10.5 μM and arresting cells at the G2/M phase.
Stilbene derivatives, such as stilbenoids and chalcones, are naturally present in plants and share most of their biosynthetic pathways (see section 4.1).366 These two compounds have been hybridized (119) using a Claisen–Schmidt–Knoevenagel–Heck approach and evaluated for their antiplasmodial activity, inhibiting 50% of P. falciparum at a concentration of 2.2 μM, in contrast to the 32.5 μM concentration required in the case of the simple mixing of equimolar stilbene and chalcone.367 The mechanism is not well understood, although it has only been characterized to cause chromatin condensation, DNA fragmentation, and the loss of mitochondrial membrane potentials in P. falciparum.
Several other hybrids have also been reported to possess promising biological activities. Recently, anthraquinone– chalcone (120) has been shown to have high cytotoxicity toward HeLa cells but low toxicity to normal cells.368 Mondhe and co-workers reported a novel quinazolinone chalcone derivative (121) as a potential anticancer agent inducing mitochondrial-dependent apoptosis and inhibiting the PI3K/ Akt/mTOR signaling pathway.369 Hybrid 122, in which indole is directly connected to the chalcone scaffold, showed potent inhibitory activity against vascular cell adhesion molecule-1 and significant anti-inflammatory effects in a mouse model.370 Sulfonamide chalcones (123) exhibited good in vitro antifilarial activities against the human lymphatic filarial parasite B. malayi by affecting the folate pathway.371
A combination of photodynamic therapy (PDT) and vascular-disrupting agents (VDAs) is a creative approach that attempts to mitigate their limits (for their respective limits, refer to ref 372). It has been reported that chalcones with VDA properties can covalently bind to phthalocyanine with PDT properties to achieve phthalocyanine–chalcone conjugates (Figure 21, 124 and 125).372,373 Compound 124 was first designed and obtained by the condensation of tetrahydroxylated nonperipherally substituted Zn(II) phthalocyanine and isocyanate chalcone.372 Compound 125 has been designed to address the deficiency of being too hydrophobic for biological explorations.373 This monochalcone–phthalocyanine has been synthesized and characterized to show an improved vascular targeting activity due to the incorporation of a cleavable bond (highlighted in red) selectively releasing chalcone at the tumor tissue and the capability to generate singlet oxygen. This example provides a promising strategy for the treatment of solid tumors by combining a photosensitizer and a VDA into one molecule.
7. TARGET IDENTIFICATION
The identification and confirmation of the molecular target(s) of bioactive compounds, especially natural products, is often a decisive step in pharmaceutical research.374 Researchers have devoted tremendous efforts in exploring the direct binding target(s) using different strategies. This section presents the most frequently employed experimental approaches for target identification, such as in silico methods and activity-based protein profiling (ABPP). Several representative examples illustrating the state of the art will be provided.
7.1. Computational Strategy
In silico methods such as quantitative structure–activity relationships (QSARs), molecular docking, and virtual screening have been widely used in the target research of natural and synthetic chalcones (Figures 22 and 23).
Figure 22.
Chalcones targeting tubulin predicted by computational strategy.
Figure 23.
Other representative chalcones for target identification by computational strategy.
Ducki et al. predicted tubulin to be the target of chalcone due to the similarity between chalcone and the β-tubulin inhibitor combretastatin A4 (CA4). A 5D-QSAR model was used to conclude that the methyl group at the α-position made a sizable difference in the preferred conformation from s-cis (126) to strans (127) for tubulin binding. This theory explains the high potency of α-methyl chalcone (K562, IC50 = 0.21 nM; tubulin, IC50 = 0.46 μM).375,376 In 1992, MDL-27048 (128) was the first chalcone found to have antimitotic activity.377 This compound was bound to tubulin at the colchicine-binding site and inhibited tubulin polymerization.378 Based on a proposed binding model for MDL-27048,377,378 a virtual screening of 9720 natural compounds was carried out. Compound 129 has been found to show good inhibitory activity of tubulin polymerization.379 Compounds 107 and 130–136 (Figure 22) have been designed and synthesized in later medicinal chemistry work. These compounds were originally predicted to bind to tubulin at the colchicine-binding site, which has been confirmed via in vitro competition binding assays.298,351,380–384 Very recently, a series of novel indole– chalcone derivatives were synthesized and evaluated for their antiproliferative activity.76 Among these indole–chalcones, compound 133 has exhibited IC50 values of 3–9 nM against six cancer cell lines, similar activities against resistant cancer cells, and low toxicity toward normal human cells. Molecular docking and mechanistic studies have demonstrated that this compound could bind to the colchicine-binding site, inhibit tubulin polymerization with an IC50 of 2.68 μM, arrest the cell cycle at the G2/M phase, induce apoptosis, and decrease the mitochondrial membrane potential (MMP). Moreover, this compound and its phosphate salt 134 with better water solubility have been shown to exhibit 66 and 70% in vivo antitumor inhibitory rates (ip, 30 mg/kg), respectively, without any apparent loss of body weight.
Molecular docking has also been applied to predict the binding mode and explain the phenotypic activity of EGFR,385 aurora kinase,386 anaplastic lymphoma kinase (ALK) enzyme,387 the estrogen receptor (ERβ),387 and Torpedo californica AChE (TcAChE)388 with their corresponding chalcones (137–141, Figure 23). The advantage of the computational strategy is the convenience of predicting the binding target(s) of chalcones before the biological validation. However, the binding site and binding specificity need validation.
The technique of activity-based proteome profiling (ABPP) in the direct target identification of bioactive chalcones is discussed in section 7.2.389–395
7.2. Activity-Based Protein Profiling Strategy
Although the ABPP strategy has been widely used for natural products396,397 and synthetic molecules,398,399 its application to chalcone and its analogues has not been widely published. In this review, representative cases including the probe design reported recently are summarized.
7.2.1. Overview of the Activity-Based Probes.
An activity-based probe (ABP) is the prerequisite to perform ABPP. The probe possesses the specific reactivity and structural characteristics of the active sites, which can be covalently bound with the receptor through its tag. Thus, the ABPs require three basic components to achieve this goal: a reactive group, a binding or affinity core, and a receptor tag, such as a fluorophore for the detection, enrichment, and identification of the target protein(s).399 Additionally, a linker is typically designed by the incorporation of a functional group to avoid steric hindrance between the ABPs and their target proteins.374 Currently, ABPs are generally classified into three types: (a) electrophilic probes, such as an α,β-unsaturated moiety that could selectively modify the nucleophilic residues in the active sites of the target protein(s);400–411 (b) photoaffinity probes that create a covalent bond with the protein through activation by UV irradiation;393,412–418 and (c) clickable probes that could introduce different receptor tags in situ.419–422 For the chalcone’s target identification, the design of the chalcone probes adopts one of the above strategies or a combination of them to obtain a multifunctional probe to identify their targets successfully.25,101,289,374,423 For more information on the development of small-molecule probes, several recent reviews are available.374,399
7.2.2. Case Studies.
7.2.2.1. Target Identification by Click Chemistry.
4-Hydroxyderricin (142), isolated from Angelica keiskei,424 has attracted great interest due to its antibacterial activity.42,425–427 It is hypothesized that the α,β-unsaturated double bond in the compound is a Michael acceptor that potentially captures nucleophiles, such as cysteines. This is likely the main mechanism for its high activity against Staphylococcus aureus. After establishing the structure–activity relationship (SAR) of 4-hydroxyderricin,425,426 an ABP (143) was designed and synthesized for the target identification via a chemical proteomics approach (Scheme 25).423 S. aureus NCTC 8325 cell lysates were incubated with 143 in a concentration gradient ranging from 5 to 100 μM and subsequently reacted with Rh-biotin-N3 through click chemistry. The bound proteins were enriched and isolated with avidin–agarose beads, followed by SDS–PAGE and Coomassie staining. Although more than one band was observed, the most prominent hit exhibiting the strongest labeling intensities in a dose–response manner was a protein with a molecular mass of approximately 50 kDa. Mass spectrometry revealed that this bound protein was seryl-tRNA synthetase (STS), with five cysteines. Probe 143 also effectively inhibited the catalytic activity of STS in a dose–response manner, and the STS protein was competitively labeled by the parent compound 142. A further special-site mutation experiment showed that more than one cysteine was involved in the binding. Unfortunately, the exact binding details are still unclear and cocrystallization could shed more light on this.
Scheme 25.
Structural Formula of 4-Hydroxyderricin and the ABPP
Recently, a similar target study on the natural product xanthohumol (Scheme 26) has been published.289 Structurally, xanthohumol (Table 1, entry 17) is very similar to 4hydroxyderricin (142). Interestingly, this compound has attracted much attention due to its potential as a cancer chemopreventive agent.428–430 Based on the same principles as the Michael addition and click chemistry, xanthohumol was designed with an alkyne handle connecting propylene glycol as a short linker to obtain its ABP (145). Through copper(I)catalyzed azide–alkyne cycloaddition, the direct target of 145 in AREc32 reporter cells was confirmed to be the Keap1 protein and several newly identified target proteins, including glucose-6-phosphate dehydrogenase (G6PDH), which were demonstrated to be inhibited by the xanthohumol-based ABPP at low micromolar concentrations.
Scheme 26.
Structural Formula of Xanthohumol and the ABPP
7.2.2.2. Target Identification by Photoaffinity Labeling (PAL).
The above two cases used the Michael acceptor of chalcones that is a weak electrophile and may not reach all the thiols.431 Based on this theory, these two probes might selectively target specific proteins due to their steric hindrance and noncovalent binding feature. Nevertheless, the nonspecific binding is a key problem that cannot be ignored in target research using the ABPP approach. The cells or lysates are generally exposed to a high concentration of the immobilized probe that might label the specific target(s) and the nonspecific ones. These binders will increase the complexity of the mass spectrometry. Thus, the high affinity of the probe with some negative controls is deemed more desirable for successful target identification. Studies conducted in the authors’ lab have recently involved the development of a library of chalcones (data unpublished), showing a sharp SAR in their cellular cytotoxicity and leading to the design of structurally similar cytotoxic and noncytotoxic chalcones as chemical probes. Among them, compound 147 with an α-methyl group exhibited excellent antitumor activity (IC50 = 0.4 μM). The high potency due to its conformational change can be explained by the theory of Ducki et al.101,375 However, the electron-donating methyl group and the induced conformational changes decrease the activity of the intrinsic electrophile. For the consideration of this question, three trifunctional probes101 (148–150, Scheme 27) with an azide that could be photoactivated by UV irradiation432–435 and an alkyne coupled by copper(I)-catalyzed Huisgen [3 + 2] cycloaddition (click chemistry)355,436 to a biotin-azide were designed. A significant cytotoxicity SAR was obtained, and chemical proteomics was performed. One 52-kDa protein was clearly labeled by compound 148, but not the noncytotoxic chalcones (149 and 150). The other nonspecific binders were also eliminated by the negative controls. The 52kDa protein was isolated by the subsequent streptavidin– biotin-based target enrichment. A mass spectrometry analysis unambiguously identified the target protein as β-tubulin. This finding was confirmed by biochemical and cell cycle assays. MSbased peptide quantitation revealed that compound 148 might modify the peptide N337–K350 (NSSYFVEWIPNNVK) near the colchicine-binding site. Although this study did not discover new targets for chalcone’s cytotoxicity, the findings provided solid evidence for the computational study predicting tubulin as chalcone’s target (see section 7.1) and matched well with the first cocrystallization of chalcone derivatives with tubulin (see section 6.2.2.1). To the best of our knowledge, this is the first time PAL chalcone probes have been used to explore chalcone’s direct cellular targets. More importantly, chalcone can be developed as a privileged structure instead of a promiscuous one.101
Scheme 27.
Structural Formula of PAL Chalcones
7.2.2.3. Target Identification by Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC).
For SILAC-based target identification,437,438 the cells are cultured under either normal conditions or conditions to incorporate isotope-labeled, such as 13C and 15N, essential amino acids.438,439 Piperlongumine (152, Scheme 28A), a natural product from Piper longum L., is structurally similar to chalcone in that it can selectively kill cancer cells with increasing cellular ROS levels.25,440–442 Specific piperlongumine–protein interactions have been identified using the SILAC methodology and a piperlongumine bead (153). Glutathione S-transferase 1 (GSTP1) or carbonyl reductase 1 (CBR1) has been identified to be the cellular target contributing to piperlongumine-induced ROS. Some other indirect targets have also been proposed.25 However, the knockdown of GSTP1 or CBR1 does not affect the ROS levels or apoptosis in cancer cells, raising the uncertainty of them being the essential targets.25 Recently, Liang et al. reported that the selenocysteine-containing antioxidant enzyme TrxR1 might be a primary binding target of the piperlongumine.443 Subsequent studies have revealed that the lactam is critical for the cytotoxicity due to its much stronger electrophile property than that of the α,β-unsaturated double bond,441 allowing it to react with thiols of the target protein(s). Thus, using ABPP and the design of ABPs (154 and 155) might be a promising future direction as the strategy for target identification (Scheme 28B).
Scheme 28.
Structural Formula of Piperlongumine and Proposed Strategy
8. CONCLUSIONS AND PERSPECTIVES
Privileged structures have been widely used as an effective strategy in medicinal chemistry for drug discovery. The strengths of this strategy are as follows: (1) they can quickly provide structurally novel chemotypes by modifying the central core structure and/or introducing the side chains of the existing active compounds; (2) synthetically, well-established protocols are typically established to allow quick expansion of the amount of derivatives for biological uses; (3) they are the core molecular frameworks, providing useful ligands for multiple targets by rational structural optimization.444–453 Chalcone is a common scaffold found in many naturally occurring compounds, especially plant-derived natural products. In addition, many chalcone derivatives have been prepared due to their convenient synthesis. Chalcone is regarded as a privileged structure of great practical interests because these natural and synthetic chalcone derivatives have shown numerous interesting biological activities with clinical potential against various diseases. Chalcone has attracted considerable research interest in multiple disciplines, with over 1300 peer-reviewed publications during the past five years. The research topics include the isolation of novel chalcone derivatives, the development of new synthetic methodologies, the evaluation of biological properties, and the exploration of the mechanism of action including target identification. New evidence has emerged to support chalcone being a privileged template instead of a promiscuous structure. Research on the development of an activity-based photoactive probe approach to identify the direct cellular targets of chalcone-based compounds conducted in the authors’ lab has provided unambiguous evidence for tubulin being the direct cellular target responsible for chalcone’s cytotoxicity. Moreover, the first cocrystallization (PDB entry 5JVD) of a chalcone derivative with tubulin has recently been published. In the future, with these promising advances, chalcone-based science is expected to be applied in drug discovery. Nevertheless many challenges remain, with two listed as follows:
In organic chemistry, numerous efforts have been made in the methodology development of chalcones’ synthesis and Michael and olefin moiety applications. However, the synthesis needs to be optimized because the reaction conditions and yields using the commonly accepted approach vary depending on the starting materials. The chemical methodologies of chalcone-based natural products with multiple chiral centers and diversity-oriented organic synthesis using a chalcone scaffold to make a biological compound library need to be extended.
In medicinal chemistry, various chalcones and chalconemimics have been isolated for biological uses. Because chalcones represent a very simple scaffold, it is easy to elucidate the structure, although novel structures are not easy to identify. Nevertheless, the natural chalcones as well as their biological uses will still attract great interest in the future. Synthetic analogues need to be designed for further SAR study and to determine their particular properties (e.g., fluorescence) in biological applications. Given the poor solubility of most chalcone compounds, the in vivo efficacy has not reached the expected levels in preclinical evaluations. Thus, the optimization of the physicochemical properties will be one of the most important research directions of chalcone-based compounds. For the targets of chalcone compounds, several proposed targets must be verified. Activity-based protein profiling is a powerful approach for target identification that must be determined on a case-by-case basis due to the properties of chalcone molecules. It is encouraging that the first cocrystal structure of chalcone with the cytotoxicity target tubulin has been published, providing the insights of structure-based information for further optimization. More cocrystallizations are also needed in the structural biology of chalcone analogues with their direct binding target(s), which will guide the further optimization for medicinal chemists to rationalize the bioactivity of interest and the side effects such as hepatotoxicity in vivo.
These questions will no doubt attract further interest in the coming years. It is also expected that novel chalcone-based drugs will be discovered using modern drug discovery strategies and new chemical sources. In addition, more studies on the chalcone moiety in other areas not mentioned in this review are also expected.
Figure 14.
Structures of chalcone–quinoxalines
ACKNOWLEDGMENTS
This research was funded by grants from the National Natural Science Foundation of China (81502978 to C.Z. and 81673352 to Z.M.), the Bio-Pharmaceutical Project of Science and Technology of Shanghai (15431901700 to Z.M.), the Shanghai “ChenGuang” project (16CG42 to C.Z.) and the National Cancer Institute, National Institutes of Health (USA, R01CA193278 to C.X.). We thank Dr. Binghe Gu from Dow Chemical for critically reading and editing the manuscript.
ABBREVIATIONS
- 4-TsNH2
p-toluenesulfonamide
- 5-LOX
5-lipoxygenase
- 99mTc
technetium-99m
- ABP
activity-based probe
- ABPP
activity-based proteome profiling
- AD
Alzheimer’s disease
- AgNO3,
silver nitrate
- ALK
anaplastic lymphoma kinase
- ALR2
aldose reductase
- AlCl3
aluminum chloride
- ARE
antioxidant response element
- BnTMK
Brugia malayi thymidylate kinase
- BF3–Et2O
boron trifluoride–etherate
- CA4
combretastatin A4
- c-FLIPL
cellular FLICE-inhibitory protein large
- CHI
chalcone isomerase
- CHR
chalcone reductase
- CHS
chalcone synthase
- c-IAP
cellular inhibitor of apoptosis protein
- CO
carbon monoxide
- COX
cyclooxygenase
- CTMAB
cetyltrimethylammonium bromide
- DBU
1,8-diazabicyclo[5.4.0]undec-7-ene
- DHA
dihydroartemisinin
- DME
1,2-dimethoxyethane
- Dr
diastereoselectivity
- ee
enantiomeric excess
- EGFR
epidermal growth factor receptor
- ERβ
estrogen receptor
- FER
fluorescence–environment relationship
- FLICE
FADD-like IL-1b-converting enzyme
- G6PDH
glucose-6-phosphate dehydrogenase
- GCLM
glutamate–cysteine ligase modifier subunit
- GSTP1
glutathione S-transferase 1
- hH3R
human histamine H3 receptors
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- IKK
IκB kinase
- K2CO3
potassium carbonate
- LiHDMS
lithium bis(trimethylsilyl)amide
- mESC
mouse embryonic stem cells
- MMP
mitochondrial membrane potential
- MOMIPP
3-(5-methoxy-2-methyl-1H-indol-3-yl)-1-(4-pyridinyl)-2-propen-1-one
- Na2S2O8
sodium thiosulfate
- NBS
N-bromosuccinimide
- NF-κB
nuclear factor kappa B
- NHC
nucleophilic heterocyclic carbene
- NQO1
NADPH:quinone oxidoreductase 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- PAL
photoaffinity labeling
- PDT
photodynamic therapy
- per-6-ABCD
heptakis(6-amino-6-deoxy)-β-cyclodextrin
- PKS
polyketide synthase
- PSC
pluripotent stem cells
- PTC
phase-transfer catalyst
- p-TsOH
p-toluenesulfonic acid
- QSAR
quantitative structure–activity relationship
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- TrxR
thioredoxin reductase
- SAR
structure–activity relationship
- SFR
structure–fluorescence relationship
- SILAC
stable isotope labeling with amino acids in cell culture
- STS
seryl-tRNA synthetase
- TBAB
tetrabutylammonium bromide
- t-BuOK
potassium tert-butoxide
- TcAChE
Torpedo californica AChE
- TEA
triethylamine
- TEMPO
2,2,6,6-tetramethylpiperidine-1-oxyl
- THF
tetrahydrofuran
- TRAIL
TNF-related apoptosis-inducing ligand
- UHP
urea–H2O2
- VDA
vascular-disrupting agent
Biographies
Chunlin Zhuang is currently a lecturer at the School of Pharmacy, Second Military Medical University, China. He received his Ph.D. in medicinal chemistry from the research group of Prof. Wannian Zhang in 2014. In 2012, he was sponsored by the China Scholarship Council’s Ph.D. Abroad Training Plan to work under the supervision of Prof. Chengguo Xing at the University of Minnesota. His research interests are mainly focused on the target identification of natural products and structure-based drug design.
Wen Zhang received his Ph.D. from the Institute of Materia Medica, Chinese Academy of Sciences, in 2005. He moved to Istituto di Chimica Biomolecolare—CNR (Italy) as a visiting researcher in 2006 and completed postdoctoral work at Universität Paderborn (Germany) in 2007. He joined the faculty of the Second Military Medical University and was nominated as a full professor in 2008. He has held a joint professorship with Tongji University since 2013. His research interests focus on the discovery and potential target screening of functional molecules from marine invertebrates and associated microorganisms, which have resulted in more than 60 scientific articles.
Chunquan Sheng received his bachelor’s degree in pharmacy (2000) and Ph.D. in medicinal chemistry (2005) from the Second Military Medical University and then joined the Department of Medicinal Chemistry, School of Pharmacy, in 2005 as a faculty member. Currently, he is a professor and Director of the Department of Medicinal Chemistry. His research interests include the structurebased design and synthesis of drug-like molecules with an emphasis on the small molecule inhibitors of protein–protein interactions and multitargeting drugs.
Wannian Zhang received his bachelor’s degree in pharmacy (1968) and M.S. degree in medicinal chemistry (1981) from the Second Military Medical University. He worked as a professor of Medicinal Chemistry from 1992 and was Director of the Department of Medicinal Chemistry, School of Pharmacy, Second Military Medical University, from 1992 to 1994. From 1994 to 2001, he served as Dean of the School of Pharmacy. Currently, Prof. Zhang is Chief of the State’s Key Discipline of Medicinal Chemistry, Second Military Medical University. He has held a joint professorship with Ningxia Medical University and served as Dean of the School of Pharmacy since 2011. His research interests are mainly focused on antifungal and antitumor drug design and development. He has published more than 100 scientific articles and developed four compounds that have been certificated by the CFDA.
Chengguo Xing received his bachelor’s degree from Dalian University of Technology (1996) and Ph.D. in organic chemistry from Arizona State University (2001). He joined Harvard University working with Prof. Andrew G. Myers as a postdoctoral associate in 2001 and the University of Minnesota as a faculty member in 2003. Currently, he is a professor and the Frank A. Duckworth Eminent Scholar Chair in Drug Research and Development at the University of Florida. His research interests are mainly in isolating, identifying, designing, and synthesizing biologically active small molecules, employing such candidates as probes to tackle fundamental health-related biological questions and diseases and evaluating their clinical potentials in clinically relevant animal models.
Zhenyuan Miao is currently an associate professor at the School of Pharmacy, Second Military Medical University, China. He received his Ph.D. degree in medicinal chemistry from the research group of Prof. Wannian Zhang in 2006. In 2015, he joined the research group of Prof. Gunda I. Georg as a visiting scholar. His research interests are mainly focused on the medicinal chemistry aspects of natural products, small molecule inhibitors of protein–protein interactions, and fluorinecontaining drug discovery.
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Zhou B; Xing C Diverse Molecular Targets for Chalcones with Varied Bioactivities. Med. Chem 2015, 5, 388–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Batovska DI; Todorova IT Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol 2010, 5, 1–29. [DOI] [PubMed] [Google Scholar]
- (3).Sahu NK; Balbhadra SS; Choudhary J; Kohli DV Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem 2012, 19, 209–225. [DOI] [PubMed] [Google Scholar]
- (4).Singh P; Anand A; Kumar V Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem 2014, 85, 758–777. [DOI] [PubMed] [Google Scholar]
- (5).Karthikeyan C; Moorthy NS; Ramasamy S; Vanam U; Manivannan E; Karunagaran D; Trivedi P Advances in chalcones with anticancer activities. Recent Pat. Anti-Cancer Drug Discovery 2015, 10, 97–115. [DOI] [PubMed] [Google Scholar]
- (6).Sebti S; Solhy A; Smahi A; Kossir A; Oumimoun H Dramatic activity enhancement of natural phosphate catalyst by lithium nitrate. An efficient synthesis of chalcones. Catal. Commun 2002, 3, 335–339. [Google Scholar]
- (7).Sharma V; Kumar V; Kumar P Heterocyclic chalcone analogues as potential anticancer agents. Anti-Cancer Agents Med. Chem 2013, 13, 422–432. [PubMed] [Google Scholar]
- (8).Boumendjel A; Ronot X; Boutonnat J Chalcones derivatives acting as cell cycle blockers: potential anti cancer drugs? Curr. Drug Targets 2009, 10, 363–371. [DOI] [PubMed] [Google Scholar]
- (9).Dimmock JR; Elias DW; Beazely MA; Kandepu NM Bioactivities of chalcones. Curr. Med. Chem 1999, 6, 1125–1149. [PubMed] [Google Scholar]
- (10).Go ML; Wu X; Liu XL Chalcones: an update on cytotoxic and chemoprotective properties. Curr. Med. Chem 2005, 12, 483–499. [DOI] [PubMed] [Google Scholar]
- (11).Leon-Gonzalez AJ; Acero N; Munoz-Mingarro D; Navarro I; Martin-Cordero C Chalcones as Promising Lead Compounds on Cancer Therapy. Curr. Med. Chem 2015, 22, 3407–3425. [DOI] [PubMed] [Google Scholar]
- (12).Mahapatra DK; Asati V; Bharti SK Chalcones and their therapeutic targets for the management of diabetes: structural and pharmacological perspectives. Eur. J. Med. Chem 2015, 92, 839–865. [DOI] [PubMed] [Google Scholar]
- (13).Mahapatra DK; Bharti SK; Asati V Chalcone scaffolds as anti-infective agents: structural and molecular target perspectives. Eur. J. Med. Chem 2015, 101, 496–524. [DOI] [PubMed] [Google Scholar]
- (14).Mahapatra DK; Bharti SK; Asati V Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem 2015, 98, 69–114. [DOI] [PubMed] [Google Scholar]
- (15).Kamal A; Kashi Reddy M; Viswanath A The design and development of imidazothiazole-chalcone derivatives as potential anticancer drugs. Expert Opin. Drug Discovery 2013, 8, 289–304. [DOI] [PubMed] [Google Scholar]
- (16).Matos MJ; Vazquez-Rodriguez S; Uriarte E; Santana L Potential pharmacological uses of chalcones: a patent review (from June 2011 – 2014). Expert Opin. Ther. Pat 2015, 25, 351–366. [DOI] [PubMed] [Google Scholar]
- (17).Das M; Manna K Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight. J. Toxicol 2016, 2016, 7651047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Mahapatra DK; Bharti SK Therapeutic potential of chalcones as cardiovascular agents. Life Sci 2016, 148, 154–172. [DOI] [PubMed] [Google Scholar]
- (19).Bukhari SN; Franzblau SG; Jantan I; Jasamai M Current prospects of synthetic curcumin analogs and chalcone derivatives against mycobacterium tuberculosis. Med. Chem 2013, 9, 897–903. [DOI] [PubMed] [Google Scholar]
- (20).Nasir Abbas Bukhari SN; Jasamai M; Jantan I Synthesis and biological evaluation of chalcone derivatives (mini review). Mini-Rev. Med. Chem 2012, 12, 1394–1403. [DOI] [PubMed] [Google Scholar]
- (21).Kontogiorgis C; Mantzanidou M; Hadjipavlou-Litina D Chalcones and their potential role in inflammation. Mini-Rev. Med. Chem 2008, 8, 1224–1242. [DOI] [PubMed] [Google Scholar]
- (22).Kumar D; Kumar M; Kumar A; Singh SK Chalcone and curcumin derivatives: a way ahead for malarial treatment. Mini-Rev. Med. Chem 2013, 13, 2116–2133. [DOI] [PubMed] [Google Scholar]
- (23).Mdee LK; Yeboah SO; Abegaz BM Rhuschalcones II-VI, five new bichalcones from the root bark of Rhus pyroides. J. Nat. Prod 2003, 66, 599–604. [DOI] [PubMed] [Google Scholar]
- (24).Wang Y; Curtis-Long MJ; Lee BW; Yuk HJ; Kim DW; Tan XF; Park KH Inhibition of tyrosinase activity by polyphenol compounds from Flemingia philippinensis roots. Bioorg. Med. Chem 2014, 22, 1115–1120. [DOI] [PubMed] [Google Scholar]
- (25).Raj L; Ide T; Gurkar AU; Foley M; Schenone M; Li X; Tolliday NJ; Golub TR; Carr SA; Shamji AF; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (26).Zhang XJ; Li LY; Wang SS; Que S; Yang WZ; Zhang FY; Gong NB; Cheng W; Liang H; Ye M; et al. Oxyfadichalcones A-C: three chalcone dimers fused through a cyclobutane ring from Tibetan medicine Oxytropis falcata Bunge. Tetrahedron 2013, 69, 11074–11079. [Google Scholar]
- (27).Funakoshi-Tago M; Okamoto K; Izumi R; Tago K; Yanagisawa K; Narukawa Y; Kiuchi F; Kasahara T; Tamura H Anti-inflammatory activity of flavonoids in Nepalese propolis is attributed to inhibition of the IL-33 signaling pathway. Int. Immunopharmacol 2015, 25, 189–198. [DOI] [PubMed] [Google Scholar]
- (28).Jung SK; Lee MH; Lim DY; Kim JE; Singh P; Lee SY; Jeong CH; Lim TG; Chen H; Chi YI; et al. Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J. Biol. Chem 2014, 289, 35839–35848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wang Z; Wang N; Han S; Wang D; Mo S; Yu L; Huang H; Tsui K; Shen J; Chen J Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One 2013, 8, e68566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yang EB; Zhang K; Cheng LY; Mack P Butein, a specific protein tyrosine kinase inhibitor. Biochem. Biophys. Res. Commun 1998, 245, 435–438. [DOI] [PubMed] [Google Scholar]
- (31).Yang EB; Guo YJ; Zhang K; Chen YZ; Mack P Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol 2001, 1550, 144–152. [DOI] [PubMed] [Google Scholar]
- (32).de Castro CC; Costa PS; Laktin GT; de Carvalho PH; Geraldo RB; de Moraes J; Pinto PL; Couri MR; Pinto PF; Da Silva Filho AA Cardamonin, a schistosomicidal chalcone from Piper aduncum L. (Piperaceae) that inhibits Schistosoma mansoni ATP diphosphohydrolase. Phytomedicine 2015, 22, 921–928. [DOI] [PubMed] [Google Scholar]
- (33).Washiyama M; Sasaki Y; Hosokawa T; Nagumo S Antiinflammatory constituents of Sappan Lignum. Biol. Pharm. Bull 2009, 32, 941–944. [DOI] [PubMed] [Google Scholar]
- (34).Fu LC; Huang XA; Lai ZY; Hu YJ; Liu HJ; Cai XL A new 3-benzylchroman derivative from Sappan Lignum (Caesalpinia sappan). Molecules 2008, 13, 1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Oh KY; Lee JH; Curtis-Long MJ; Cho JK; Kim JY; Lee WS; Park KH Glycosidase inhibitory phenolic compounds from the seed of Psoralea corylifolia. Food Chem 2010, 121, 940–945. [Google Scholar]
- (36).Haraguchi H; Inoue J; Tamura Y; Mizutani K Antioxidative components of Psoralea corylifolia (Leguminosae). Phytother. Res 2002, 16, 539–544. [DOI] [PubMed] [Google Scholar]
- (37).Qiu RL; Li L; Zhu MH; Liu J Study on the chemical constituents of Psoralea corylifolia. Zhong Yao Cai 2011, 34, 1211–1213. [PubMed] [Google Scholar]
- (38).Shimomura K; Sugiyama Y; Nakamura J; Ahn MR; Kumazawa S Component analysis of propolis collected on Jeju Island, Korea. Phytochemistry 2013, 93, 222–229. [DOI] [PubMed] [Google Scholar]
- (39).Chung MI; Lai MH; Yen MH; Wu RR; Lin CN Phenolics from Hypericum geminiflorum. Phytochemistry 1997, 44, 943–947. [Google Scholar]
- (40).Wang JP; Tsao LT; Raung SL; Lin CN Investigation of the inhibitory effect of broussochalcone A on respiratory burst in neutrophils. Eur. J. Pharmacol 1997, 320, 201–208. [DOI] [PubMed] [Google Scholar]
- (41).Wang Q; Ding ZH; Liu JK; Zheng YT Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antiviral Res 2004, 64, 189–194. [DOI] [PubMed] [Google Scholar]
- (42).Inamori Y; Baba K; Tsujibo H; Taniguchi M; Nakata K; Kozawa M Antibacterial activity of two chalcones, xanthoangelol and 4-hydroxyderricin, isolated from the root of Angelica keiskei KOIDZUMI. Chem. Pharm. Bull 1991, 39, 1604–1605. [DOI] [PubMed] [Google Scholar]
- (43).Harikumar KB; Kunnumakkara AB; Ahn KS; Anand P; Krishnan S; Guha S; Aggarwal BB Modification of the cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by xanthohumol leads to suppression of NF-kappaB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood 2009, 113, 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Tabata K; Motani K; Takayanagi N; Nishimura R; Asami S; Kimura Y; Ukiya M; Hasegawa D; Akihisa T; Suzuki T Xanthoangelol, a major chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma and leukemia cells. Biol. Pharm. Bull 2005, 28, 1404–1407. [DOI] [PubMed] [Google Scholar]
- (45).Limper C; Wang Y; Ruhl S; Wang Z; Lou Y; Totzke F; Kubbutat MH; Chovolou Y; Proksch P; Watjen W Compounds isolated from Psoralea corylifolia seeds inhibit protein kinase activity and induce apoptotic cell death in mammalian cells. J. Pharm. Pharmacol 2013, 65, 1393–1408. [DOI] [PubMed] [Google Scholar]
- (46).Chung MI; Weng JR; Lai MH; Yen MH; Lin CN A new chalcone, xanthones, and a xanthonolignoid from Hypericum geminiflorum. J. Nat. Prod 1999, 62, 1033–1035. [DOI] [PubMed] [Google Scholar]
- (47).Lee D; Bhat KP; Fong HH; Farnsworth NR; Pezzuto JM; Kinghorn AD Aromatase inhibitors from Broussonetia papyrifera. J. Nat. Prod 2001, 64, 1286–1293. [DOI] [PubMed] [Google Scholar]
- (48).Balunas MJ; Su B; Brueggemeier RW; Kinghorn AD Natural products as aromatase inhibitors. Anti-Cancer Agents Med. Chem 2008, 8, 646–682. [PMC free article] [PubMed] [Google Scholar]
- (49).Caamal-Fuentes EE; Peraza-Sanchez SR; Torres-Tapia LW; Moo-Puc RE Isolation and Identification of Cytotoxic Compounds from Aeschynomene fascicularis, a Mayan Medicinal Plant. Molecules 2015, 20, 13563–13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Hegazy ME; El-Hamd HMA; El-Halawany AM; Djemgou PC; Shahat AA; Pare PW Estrogenic activity of chemical constituents from Tephrosia candida. J. Nat. Prod 2011, 74, 937–942. [DOI] [PubMed] [Google Scholar]
- (51).Cui Y; Ao M; Hu J; Yu L Anti-inflammatory activity of licochalcone A isolated from Glycyrrhiza inflata. Z. Naturforsch., C: J. Biosci 2008, 63, 361–365. [DOI] [PubMed] [Google Scholar]
- (52).Funakoshi-Tago M; Tanabe S; Tago K; Itoh H; Mashino T; Sonoda Y; Kasahara T Licochalcone A potently inhibits tumor necrosis factor alpha-induced nuclear factor-kappaB activation through the direct inhibition of IkappaB kinase complex activation. Mol. Pharmacol 2009, 76, 745–753. [DOI] [PubMed] [Google Scholar]
- (53).Malek SN; Phang CW; Ibrahim H; Wahab NA; Sim KS Phytochemical and cytotoxic investigations of Alpinia mutica rhizomes. Molecules 2011, 16, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Wu W; Ye H; Wan L; Han X; Wang G; Hu J; Tang M; Duan X; Fan Y; He S; et al. Millepachine, a novel chalcone, induces G2/M arrest by inhibiting CDK1 activity and causing apoptosis via ROS-mitochondrial apoptotic pathway in human hepatocarcinoma cells in vitro and in vivo. Carcinogenesis 2013, 34, 1636–1643. [DOI] [PubMed] [Google Scholar]
- (55).Kil YS; Choi SK; Lee YS; Jafari M; Seo EK Chalcones from Angelica keiskei: Evaluation of Their Heat Shock Protein Inducing Activities. J. Nat. Prod 2015, 78, 2481–2487. [DOI] [PubMed] [Google Scholar]
- (56).Ren Y; Yuan C; Qian Y; Chai HB; Chen X; Goetz M; Kinghorn AD Constituents of an extract of Cryptocarya rubra housed in a repository with cytotoxic and glucose transport inhibitory effects. J. Nat. Prod 2014, 77, 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Li YS; Matsunaga K; Kato R; Ohizumi Y Verbenachalcone, a novel dimeric dihydrochalcone with potentiating activity on nerve growth factor-action from Verbena littoralis. J. Nat. Prod 2001, 64, 806–808. [DOI] [PubMed] [Google Scholar]
- (58).Kang WJ; Li DH; Han T; Sun L; Fu YB; Sai CM; Li ZL; Hua HM New chalcone and pterocarpoid derivatives from the roots of Flemingia philippinensis with antiproliferative activity and apoptosis-inducing property. Fitoterapia 2016, 112, 222–228. [DOI] [PubMed] [Google Scholar]
- (59).Ramli F; Rahmani M; Kassim NK; Hashim NM; Sukari MA; Akim AM; Go R New diprenylated dihyrochalcones from leaves of Artocarpus elasticus. Phytochem. Lett 2013, 6, 582–585. [Google Scholar]
- (60).Mcrae JM; Yang Q; Crawford RJ; Palombo EA Acylated flavonoid tetraglycoside from Planchonia careya leaves. Phytochem. Lett 2008, 1, 99–102. [Google Scholar]
- (61).Tuntipaleepun M; Chakthong S; Ponglimanont C; Plodpai P; Voravuthikunchai SP Antifungal and cytotoxic substances from the stem barks of Desmos chinensis. Chin. Chem. Lett 2012, 23, 587–590. [Google Scholar]
- (62).Awale S; Shrestha SP; Tezuka Y; Ueda JY; Matsushige K; Kadota S Neoflavonoids and related constituents from Nepalese propolis and their nitric oxide production inhibitory activity. J. Nat. Prod 2005, 68, 858–864. [DOI] [PubMed] [Google Scholar]
- (63).Ichimaru M; Nakatani N; Moriyasu M; Nishiyama Y; Kato A; Mathenge SG; Juma FD; ChaloMutiso PB Hydroxyespintanol and schefflerichalcone: two new compounds from Uvaria scheffleri. J. Nat. Med 2010, 64, 75–79. [DOI] [PubMed] [Google Scholar]
- (64).Andrei CC; Ferreira DT; Faccione M; de Moraes LA; de Carvalho MG; Braz-Filho R C-prenylflavonoids from roots of Tephrosia tunicata. Phytochemistry 2000, 55, 799–804. [DOI] [PubMed] [Google Scholar]
- (65).Kulkarni RR; Tupe SG; Gample SP; Chandgude MG; Sarkar D; Deshpande MV; Joshi SP Antifungal dimeric chalcone derivative kamalachalcone E from Mallotus philippinensis. Nat. Prod. Res 2014, 28, 245–250. [DOI] [PubMed] [Google Scholar]
- (66).Liu ML; Duan YH; Hou YL; Li C; Gao H; Dai Y; Yao XS Nardoaristolones A and B, Two Terpenoids with Unusual Skeletons from Nardostachys chinensis Batal. Org. Lett 2013, 15, 1000–1003. [DOI] [PubMed] [Google Scholar]
- (67).Ullah A; Ansari FL; Ihsan-ul-Haq; Nazir S; Mirza B Combinatorial synthesis, lead identification, and antitumor study of a chalcone-based positional-scanning library. Chem. Biodiversity 2007, 4, 203–214. [DOI] [PubMed] [Google Scholar]
- (68).Ono M; Ikeoka R; Watanabe H; Kimura H; Fuchigami T; Haratake M; Saji H; Nakayama M Synthesis and evaluation of novel chalcone derivatives with (99m)Tc/Re complexes as potential probes for detection of beta-amyloid plaques. ACS Chem. Neurosci 2010, 1, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Niu CG; Guan AL; Zeng GM; Liu YG; Li ZW Fluorescence water sensor based on covalent immobilization of chalcone derivative. Anal. Chim. Acta 2006, 577, 264–270. [DOI] [PubMed] [Google Scholar]
- (70).Lee SC; Kang NY; Park SJ; Yun SW; Chandran Y; Chang YT Development of a fluorescent chalcone library and its application in the discovery of a mouse embryonic stem cell probe. Chem. Commun. (Cambridge, U. K.) 2012, 48, 6681–6683. [DOI] [PubMed] [Google Scholar]
- (71).Gaber M; Fayed TA; El-Daly SA; El-Sayed YS Spectral properties and inclusion of a hetero-chalcone analogue in organized media of micellar solutions and beta-cyclodextrin. Photochem. Photobiol. Sci 2008, 7, 257–262. [DOI] [PubMed] [Google Scholar]
- (72).Organero JA; Moreno M; Santos L; Lluch JM; Douhal A Photoinduced Proton Transfer and Rotational Motion of 1-Hydroxy2-acetonaphthone in the S1 State: A Theoretical Insight into Its Photophysics. J. Phys. Chem. A 2000, 104, 8424–8431. [Google Scholar]
- (73).Amde M; Liu JF; Pang L Environmental Application, Fate, Effects, and Concerns of Ionic Liquids: A Review. Environ. Sci. Technol 2015, 49, 12611–12627. [DOI] [PubMed] [Google Scholar]
- (74).Jiang YB; Wang XJ Stoichiometric-dependent intramolecular charge transfer flourescence of p-dimethylaminochalcone in β-cyclodextrin host-guest systems. J. Photochem. Photobiol., A 1994, 81, 205–209. [Google Scholar]
- (75).Jiang YB; Wang XJ; Lin L Fluorescent Probing of the Restriction by Aqueous Micelles of the Formation of the Photoinduced Biradical State P* of 4-(Dimethy1amino)chalcone. J. Phys. Chem 1994, 98, 12367–12372. [Google Scholar]
- (76).Zhou B; Jiang P; Lu J; Xing C Characterization of the Fluorescence Properties of 4-Dialkylaminochalcones and Investigation of the Cytotoxic Mechanism of Chalcones. Arch. Pharm (Weinheim, Ger.) 2016, 349, 539–552. [DOI] [PubMed] [Google Scholar]
- (77).Song Z; Kwok RT; Zhao E; He Z; Hong Y; Lam JW; Liu B; Tang BZ A ratiometric fluorescent probe based on ESIPT and AIE processes for alkaline phosphatase activity assay and visualization in living cells. ACS Appl. Mater. Interfaces 2014, 6, 17245–17254. [DOI] [PubMed] [Google Scholar]
- (78).Tomasch M; Schwed JS; Weizel L; Stark H Novel chalcone-based fluorescent human histamine h(3) receptor ligands as pharmacological tools. Front. Syst. Neurosci 2012, DOI: 10.3389/fnsys.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Yun SW; Kang NY; Park SJ; Ha HH; Kim YK; Lee JS; Chang YT Diversity oriented fluorescence library approach (DOFLA) for live cell imaging probe development. Acc. Chem. Res 2014, 47, 1277–1286. [DOI] [PubMed] [Google Scholar]
- (80).Kreuzaler F; Hahlbrock K Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin (5,7,4′trihydroxyflavanone) from p-coumaroyl coenzyme A and malonyl coenzyme A. FEBS Lett 1972, 28, 69–72. [DOI] [PubMed] [Google Scholar]
- (81).Austin MB; Noel JP The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep 2003, 20, 79–110. [DOI] [PubMed] [Google Scholar]
- (82).Yamazaki Y; Suh DY; Sitthithaworn W; Ishiguro K; Kobayashi Y; Shibuya M; Ebizuka Y; Sankawa U Diverse chalcone synthase superfamily enzymes from the most primitive vascular plant, Psilotum nudum. Planta 2001, 214, 75–84. [DOI] [PubMed] [Google Scholar]
- (83).Ferrer JL; Jez JM; Bowman ME; Dixon RA; Noel JP Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol 1999, 6, 775–784. [DOI] [PubMed] [Google Scholar]
- (84).Abe I; Watanabe T; Morita H; Kohno T; Noguchi H Engineered biosynthesis of plant polyketides: manipulation of chalcone synthase. Org. Lett 2006, 8, 499–502. [DOI] [PubMed] [Google Scholar]
- (85).Abe I; Morita H Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat. Prod. Rep 2010, 27, 809–838. [DOI] [PubMed] [Google Scholar]
- (86).Flores-Sanchez IJ; Verpoorte R PKS activities and biosynthesis of cannabinoids and flavonoids in Cannabis sativa L. plants. Plant Cell Physiol 2008, 49, 1767–1782. [DOI] [PubMed] [Google Scholar]
- (87).Flores-Sanchez IJ; Verpoorte R Plant polyketide synthases: a fascinating group of enzymes. Plant Physiol. Biochem 2009, 47, 167–174. [DOI] [PubMed] [Google Scholar]
- (88).Jez JM; Ferrer JL; Bowman ME; Austin MB; Schroder J; Dixon RA; Noel JP Structure and mechanism of chalcone synthase-like polyketide synthases. J. Ind. Microbiol. Biotechnol 2001, 27, 393–398. [DOI] [PubMed] [Google Scholar]
- (89).Shi SP; Wanibuchi K; Morita H; Endo K; Noguchi H; Abe I Enzymatic formation of unnatural novel chalcone, stilbene, and benzophenone scaffolds by plant type III polyketide synthase. Org. Lett 2009, 11, 551–554. [DOI] [PubMed] [Google Scholar]
- (90).Molitor C; Mauracher SG; Rompel A Aurone synthase is a catechol oxidase with hydroxylase activity and provides insights into the mechanism of plant polyphenol oxidases. Proc. Natl. Acad. Sci. U. S. A 2016, 113, E1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Bennett M; Burke AJ; O’Sullivan WI Aspects of the AlgarFlynn-Oyamada (AFO) reaction. Tetrahedron 1996, 52, 7163–7178. [Google Scholar]
- (92).Claisen L; Claparede A Condensationen von Ketonen mit̀ Aldehyden. Ber. Dtsch. Chem. Ges 1881, 14, 2460–2468. [Google Scholar]
- (93).Schmidt JG Ueber die Einwirkung von Aceton auf Furfurol und auf Bittermandelöl in Gegenwart von Alkalilauge. Ber. Dtsch. Chem. Ges 1881, 14, 1459–1461. [Google Scholar]
- (94).Powers DG; Casebier DS; Fokas D; Ryan WJ; Troth JR; Coffen DL Automated parallel synthesis of chalcone-based screening libraries. Tetrahedron 1998, 54, 4085–4096. [Google Scholar]
- (95).Nielsen AT; Houlihan WJ The Aldol Condensation. Org. React 2011, 16, 1–438. [Google Scholar]
- (96).Mukaiyama T The Directed Aldol Reaction. Org. React 1982, 28, 203–331. [Google Scholar]
- (97).Szell T; Sohár I New nitrochalcones. IX. Can. J. Chem 1969, 47, 1254–1258. [Google Scholar]
- (98).Song QB; Li XN; Shen TH; Yang SD; Qiang GR; Wu XL; Ma YX Synthesis of novel chalcone analogues of ferrocene biarenes. Synth. Commun 2003, 33, 3935–3941. [Google Scholar]
- (99).Dhar DN; Barton D The Chemistry of Chalcones and Related Compounds; Wiley-VCH: Weinheim, Germany, 1981; p 285. [Google Scholar]
- (100).Armesto D; Horspool WM; Martin N; Ramos A; Seoane C Synthesis of cyclobutenes by the novel photochemical ring contraction of 4-substituted 2-amino-3,5-dicyano-6-phenyl-4H-pyrans. J. Org. Chem 1989, 54, 3069–3072. [Google Scholar]
- (101).Patel DD; Lee JM Applications of ionic liquids. Chem. Rec 2012, 12, 329–355. [DOI] [PubMed] [Google Scholar]
- (102).Daskiewicz JB; Comte G; Barron D; Di Pietro A; Thomasson F Organolithium mediated synthesis of prenylchalcones as potential inhibitors of chemoresistance. Tetrahedron Lett 1999, 40, 7095–7098. [Google Scholar]
- (103).Sebti S; Solhy A; Tahir R; Boulaajaj S; Mayoral JA; Fraile JM; Kossir A; Oumimoun H Calcined sodium nitrate/ natural phosphate: an extremely active catalyst for the easy synthesis of chalcones in heterogeneous media. Tetrahedron Lett 2001, 42, 7953–7955. [Google Scholar]
- (104).Golay MJ Note on Gas Chromatographic Injection. Nature 1964, 202, 489–490. [DOI] [PubMed] [Google Scholar]
- (105).Narender T; Reddy KP A simple and highly efficient method for the synthesis of chalcones by using borontrifluorideetherate. Tetrahedron Lett 2007, 48, 3177–3180. [Google Scholar]
- (106).Iranpoor N; Kazemi F RuCl3 catalyses aldol condensations of aldehydes and ketones. Tetrahedron 1998, 54, 9475–9480. [Google Scholar]
- (107).Calloway NO; Green LD Reactions in the Presence of Metallic Halides. I. β-Unsaturated Ketone Formation as a Side Reaction in Friedel-Crafts Acylations. J. Am. Chem. Soc 1937, 59, 809–811. [Google Scholar]
- (108).Mazza LJ; Guarna A An Improved Synthesis of 1,3Diphenyl-2-buten-1-ones (β-Methylchalcones). Synthesis 1980, 1980, 41–44. [Google Scholar]
- (109).Tan CH; Zheng RX; Luo SY; Xin QY Synthesis of Chalcone Catalyzed by P-toluenesulfonic Acid. Contemp. Chem. Ind 2012, 41, 23–25. [Google Scholar]
- (110).Breslow DS; Hauser CR Condensations.1 XI. Condensations of Certain Active Hydrogen Compounds Effected by Boron Trifluoride and Aluminum Chloride2. J. Am. Chem. Soc 1940, 62, 2385–2388. [Google Scholar]
- (111).Hasaninejad A; Zare A; Balooty L; Mehregan H; Shekouhy M Solvent-Free, Cross-Aldol Condensation Reaction Using Silica-Supported, Phosphorus-Containing Reagents Leading to alpha,alpha′-Bis(arylidene)cycloalkanones. Synth. Commun 2010, 40, 3488–3495. [Google Scholar]
- (112).Nakano T; Irifune S; Umano S; Inada A; Ishii Y; Ogawa M Cross-Condensation Reactions Of Cycloalkanones with Aldehydes And Primary Alcohols under the Influence Of Zirconocene Complexes. J. Org. Chem 1987, 52, 2239–2244. [Google Scholar]
- (113).Rateb NM; Zohdi HF Atom-Efficient, Solvent-Free, Green Synthesis of Chalcones by Grinding. Synth. Commun 2009, 39, 2789–2794. [Google Scholar]
- (114).Kulkarni P Calcium Oxide Catalyzed Synthesis of Chalcone Under Microwave Condition. Curr. Microwave Chem 2015, 2, 144–149. [Google Scholar]
- (115).Liu XF; Shi DH Research progress in the synthesis of chalcone. Appl. Chem. Ind 2009, 38, 1210–1213. [Google Scholar]
- (116).Liu XL; Zhao ZG; Zeng BT; Yi FM Microwaveassisted synthesis of chalcones under solvent-free conditions. Chem. Res. Appl 2007, 19, 574–576. [Google Scholar]
- (117).Xu Q; Yang ZG; Yin DL; Zhang F Synthesis of chalcones catalyzed by a novel solid sulfonic acid from bamboo. Catal. Commun 2008, 9, 1579–1582. [Google Scholar]
- (118).Duan HC; Jiang H; Gong H Green Synthesis of Chalcone Derivative. China Pharm 2006, 15, 30–31. [Google Scholar]
- (119) <p/>).Yang J; Ji G; Liu H; Lian Y CN Patent 103408494A, 2013; Chem. Abstr 2013, 160, 33884.
- (120).Wu H; Ye HQ; Chen DC A new method for green synthesis of chalcone. Ind. Catal 2006, 14, 34–37. [Google Scholar]
- (121).Miyaura N; Suzuki A Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc., Chem. Commun 1979, 19, 866–867. [Google Scholar]
- (122).Miyaura N; Yamada K; Suzuki A A new stereospecific crosscoupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett 1979, 20, 3437–3440. [Google Scholar]
- (123).) Eddarir S; Cotelle N; Bakkour Y; Rolando C An efficient synthesis of chalcones based on the Suzuki reaction. Tetrahedron Lett 2003, 44, 5359–5363. [Google Scholar]
- (124).Bumagin NA; Korolev DN Synthesis of unsymmetric ketones via ligandless Pd-catalyzed reaction of acyl chlorides with organoboranes. Tetrahedron Lett 1999, 40, 3057–3060. [Google Scholar]
- (125).Haddach M; McCarthy JR A new method for the synthesis of ketones: The palladium-catalyzed cross-coupling of acid chlorides with arylboronic acids. Tetrahedron Lett 1999, 40, 3109–3112. [Google Scholar]
- (126).Buszek KR; Brown N N-Vinylpyridinium and -ammonium tetrafluoroborate salts: New electrophilic coupling partners for Pd(0)catalyzed Suzuki cross-coupling reactions. Org. Lett 2007, 9, 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Heck RF; Nolley JP Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem 1972, 37, 2320–2322. [Google Scholar]
- (128).Mizoroki T; Mori K; Ozaki A Arylation of Olefin with Aryl Iodide Catalyzed by Palladium. Bull. Chem. Soc. Jpn 1971, 44, 581. [Google Scholar]
- (129).Zou G; Guo JP; Wang ZY; Huang W; Tang J Hecktype coupling vs. conjugate addition in phosphine-rhodium catalyzed reactions of aryl boronic acids with alpha,beta-unsaturated carbonyl compounds: a systematic investigation. Dalton T 2007, 28, 3055–3064. [DOI] [PubMed] [Google Scholar]
- (130).Bianco A; Cavarischia C; Guiso M The Heck coupling reaction using aryl vinyl ketones: Synthesis of flavonoids. Eur. J. Org. Chem 2004, 2004 (13), 2894–2898. [Google Scholar]
- (131).Wu XF; Neumann H; Spannenberg A; Schulz T; Jiao H; Beller M Development of a general palladium-catalyzed carbonylative Heck reaction of aryl halides. J. Am. Chem. Soc 2010, 132, 14596–14602. [DOI] [PubMed] [Google Scholar]
- (132).Wu XF; Neumann H; Beller M Palladium-Catalyzed Oxidative Carbonylative Coupling Reactions of Arylboronic Acids with Styrenes to Chalcones under Mild Aerobic Conditions. Chem. - Asian J 2012, 7, 282–285. [DOI] [PubMed] [Google Scholar]
- (133).Wu XF; Neumann H; Beller M Palladium-Catalyzed Coupling Reactions: Carbonylative Heck Reactions To Give Chalcones. Angew. Chem., Int. Ed 2010, 49, 5284–5288. [DOI] [PubMed] [Google Scholar]
- (134).Hermange P; Gogsig TM; Lindhardt AT; Taaning RH; Skrydstrup T Carbonylative Heck Reactions Using CO Generated ex Situ in a Two-Chamber System. Org. Lett 2011, 13, 2444–2447. [DOI] [PubMed] [Google Scholar]
- (135).Bianco A; Cavarischia C; Farina A; Guiso M; Marra C A new synthesis of flavonoids via Heck reaction. Tetrahedron Lett 2003, 44, 9107–9109. [Google Scholar]
- (136).Bestmann HJ; Arnason B Reaktionen mit Phosphinalkylenen, II. C-Acylierung von Phosphin-alkylenen. Ein neuer Weg zur Synthese von Ketonen. Chem. Ber 1962, 95, 1513–1527. [Google Scholar]
- (137).Ramirez F; Dershowitz S Phosphinemethylenes.1 II. Triphenylphosphineacylmethylenes. J. Org. Chem 1957, 22, 41–45. [Google Scholar]
- (138).Xu C; Chen G; Huang X Chalcones by the wittig reaction of a stable ylide with aldehydes under microwave irradiation. Org. Prep. Proced. Int 1995, 27, 559–561. [Google Scholar]
- (139).Kumar A; Sharma S; Tripathi VD; Srivastava S Synthesis of chalcones and flavanones using Julia-Kocienski olefination. Tetrahedron 2010, 66, 9445–9449. [Google Scholar]
- (140).Nielsen M; Jacobsen CB; Holub N; Paixao MW; Jorgensen KA Asymmetric organocatalysis with sulfones. Angew. Chem., Int. Ed 2010, 49, 2668–2679. [DOI] [PubMed] [Google Scholar]
- (141).Alonso DA; Fuensanta M; Najera C; Varea M 3,5bis(trifluoromethyl)phenyl sulfones in the direct Julia-Kocienski olefination. J. Org. Chem 2005, 70, 6404–6416. [DOI] [PubMed] [Google Scholar]
- (142).Zhang N; Yang DS; Wei W; Yuan L; Nie FF; Tian LJ; Wang H Silver-Catalyzed Double-Decarboxylative Cross-Coupling of alpha-Keto Acids with Cinnamic Acids in Water: A Strategy for the Preparation of Chalcones. J. Org. Chem 2015, 80, 3258–3263. [DOI] [PubMed] [Google Scholar]
- (143).Unoh Y; Hirano K; Satoh T; Miura M PalladiumCatalyzed Decarboxylative Arylation of Benzoylacrylic Acids toward the Synthesis of Chalcones. J. Org. Chem 2013, 78, 5096–5102. [DOI] [PubMed] [Google Scholar]
- (144).Al-Masum M; Ng E; Wai MC Palladium-catalyzed direct cross-coupling of potassium styryltrifluoroborates and benzoyl chlorides-a one step method for chalcone synthesis. Tetrahedron Lett 2011, 52, 1008–1010. [Google Scholar]
- (145).Zhou J; Wu G; Zhang M; Jie XM; Su WP Pd-Catalyzed Cross-Coupling of Aryl Carboxylic Acids with Propiophenones through a Combination of Decarboxylation and Dehydrogenation. Chem. - Eur. J 2012, 18, 8032–8036. [DOI] [PubMed] [Google Scholar]
- (146).Weaver JD; Recio A; Grenning AJ; Tunge JA Transition Metal-Catalyzed Decarboxylative Allylation and Benzylation Reactions. Chem. Rev 2011, 111, 1846–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Zhou J; Wu G; Zhang M; Jie X; Su W Pd-catalyzed crosscoupling of aryl carboxylic acids with propiophenones through a combination of decarboxylation and dehydrogenation. Chem. - Eur. J 2012, 18, 8032–8036. [DOI] [PubMed] [Google Scholar]
- (148).Shotter RG; Johnston KM; Jones JF Reactions of unsaturated acid halides–IV1: Competitive friedel-crafts acylations and alkylations of monohalogenobenzenes by the bifunctional cinnamoyl chloride. Tetrahedron 1978, 34, 741–746. [Google Scholar]
- (149).Sisa M; Bonnet SL; Ferreira D; Van der Westhuizen JH Photochemistry of Flavonoids. Molecules 2010, 15, 5196–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Obara H; Takahashi H The Photochemical Fries Rearrangement of Phenyl Cinnamate. Bull. Chem. Soc. Jpn 1967, 40, 1012. [Google Scholar]
- (151).Obara H; Takahashi H; Hirano H The Photo-Fries Rearrangement of Hydroxyphenyl Cinnamates. Bull. Chem. Soc. Jpn 1969, 42, 560–561. [Google Scholar]
- (152).Onodera J; Obara H The Photo-Fries Rearrangement of Bis(methoxymethoxy)phenyl Cinnamates. Bull. Chem. Soc. Jpn 1974, 47, 240–241. [Google Scholar]
- (153).Bhatia VK; Kagan JA Photochemical Synthesis of 2′,6′Dihydroxy-4′-Methoxy-and 2′,4′-Dihydroxy-6′-Methoxy-Chalcones. Chem. Ind 1970, 1203–1204. [Google Scholar]
- (154).Ramakrishnan VT; Kagan J Photochemical synthesis of 2′hydroxychalcones from phenyl cinnamates. J. Org. Chem 1970, 35, 2901–2904. [Google Scholar]
- (155).Chen D; Li Y CN Patent 102786371A, 2011; Chem. Abstr 2012, 158, 317167.
- (156).Li YA; Chen DY Novel and Efficient One Pot Condensation Reactions between Ketones and Aromatic Alcohols in the Presence of CrO3 Producing alpha,beta-Unsaturated Carbonyl Compounds. Chin. J. Chem 2011, 29, 2086–2090. [Google Scholar]
- (157).Xu Q; Tian H; Jin L CN Patent 102775288A, 2012; Chem. Abstr 2012, 158, 11329.
- (158).Kwon MS; Kim N; Seo SH; Park IS; Cheedrala RK; Park J Recyclable palladium catalyst for highly selective alpha alkylation of ketones with alcohols. Angew. Chem., Int. Ed 2005, 44, 6913–6915. [DOI] [PubMed] [Google Scholar]
- (159).Kim S; Bae SW; Lee JS; Park J Recyclable gold nanoparticle catalyst for the aerobic alcohol oxidation and C-C bond forming reaction between primary alcohols and ketones under ambient conditions. Tetrahedron 2009, 65, 1461–1466. [Google Scholar]
- (160).Dinkova-Kostova AT; Massiah MA; Bozak RE; Hicks RJ; Talalay P Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. U. S. A 2001, 98, 3404–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Dinkova-Kostova AT; Cheah J; Samouilov A; Zweier JL; Bozak RE; Hicks RJ; Talalay P Phenolic Michael reaction acceptors: combined direct and indirect antioxidant defenses against electrophiles and oxidants. Med. Chem 2007, 3, 261–268. [DOI] [PubMed] [Google Scholar]
- (162).Gan FF; Kaminska KK; Yang H; Liew CY; Leow PC; So CL; Tu LN; Roy A; Yap CW; Kang TS; et al. Identification of Michael acceptor-centric pharmacophores with substituents that yield strong thioredoxin reductase inhibitory character correlated to antiproliferative activity. Antioxid. Redox Signaling 2013, 19, 1149–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Dinkova-Kostova AT; Abeygunawardana C; Talalay P Chemoprotective properties of phenylpropenoids, bis(benzylidene)cycloalkanones, and related Michael reaction acceptors: correlation of potencies as phase 2 enzyme inducers and radical scavengers. J. Med. Chem 1998, 41, 5287–5296. [DOI] [PubMed] [Google Scholar]
- (164).Srinivasan B; Johnson TE; Lad R; Xing C Structureactivity relationship studies of chalcone leading to 3-hydroxy-4,3′,4′,5′tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J. Med. Chem 2009, 52, 7228–7235. [DOI] [PubMed] [Google Scholar]
- (165).Dalko PI; Moisan L In the golden age of organocatalysis. Angew. Chem., Int. Ed 2004, 43, 5138–5175. [DOI] [PubMed] [Google Scholar]
- (166).Solladie-Cavallo A; Marsol C; Pescitelli G; Di Bari L; Salvadori P; Huang XF; Fujioka N; Berova N; Cao XL; Freedman TB; et al. (R)-(+)- and (S)-(–)-1-(9-Phenanthryl)ethylamine: Assignment of absolute configuration by CD tweezer and VCD methods, and difficulties encountered with the CD exciton chirality method. Eur. J. Org. Chem 2002, 2002, 1788–1796. [Google Scholar]
- (167).Li H; Song J; Deng L Catalytic enantioselective conjugate additions with alpha,beta-unsaturated sulfones. Tetrahedron 2009, 65, 3139–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Krause N; Hoffmann-Röder A Recent Advances in Catalytic Enantioselective Michael Additions. Synthesis 2001, 2001 (2), 0171–0196. [Google Scholar]
- (169).Ballini R; Bosica G; Fiorini D; Palmieri A; Petrini M Conjugate additions of nitroalkanes to electron-poor alkenes: recent results. Chem. Rev 2005, 105, 933–971. [DOI] [PubMed] [Google Scholar]
- (170).Colonna S; Hiemstra H; Wynberg H Asymmetric Induction in the Base -catalysed Michael Addition of Nitromethane to Chalcone. J. Chem. Soc., Chem. Commun 1978, 6, 238–239. [Google Scholar]
- (171).Tian X; Cassani C; Liu Y; Moran A; Urakawa A; Galzerano P; Arceo E; Melchiorre P Diastereodivergent asymmetric sulfa-Michael additions of alpha-branched enones using a single chiral organic catalyst. J. Am. Chem. Soc 2011, 133, 17934–17941. [DOI] [PubMed] [Google Scholar]
- (172).Enders D; Luttgen K; Narine AA Asymmetric sulfaMichael additions. Synthesis 2007, 2007 (7), 959–980. [Google Scholar]
- (173).Skarzewski J; Zielinska-Blajet M; Turowska-Tyrk I Simple preparation of enantiomeric Michael adducts of thiophenol to chalcones: easily available new chiral building blocks. Tetrahedron: Asymmetry 2001, 12, 1923–1928. [Google Scholar]
- (174).Suresh P; Pitchumani K Per-6-amino-beta-cyclodextrin catalyzed asymmetric Michael addition of nitromethane and thiols to chalcones in water. Tetrahedron: Asymmetry 2008, 19, 2037–2044. [Google Scholar]
- (175).Bonollo S; Lanari D; Pizzo F; Vaccaro L Sc(III)-catalyzed enantioselective addition of thiols to alpha,beta-unsaturated ketones in neutral water. Org. Lett 2011, 13, 2150–2152. [DOI] [PubMed] [Google Scholar]
- (176).Moccia M; Fini F; Scagnetti M; Adamo MF Catalytic enantioselective addition of sodium bisulfite to chalcones. Angew. Chem., Int. Ed 2011, 50, 6893–6895. [DOI] [PubMed] [Google Scholar]
- (177).Wei JF; Chen ZG; Lei W; Zhang LH; Wang MZ; Shi XY; Li RT Silicon powder: the first nonmetal elemental catalyst for aminobromination of olefins with TsNH(2) and NBS. Org. Lett 2009, 11, 4216–4219. [DOI] [PubMed] [Google Scholar]
- (178).Wei JF; Zhang LH; Chen ZG; Shi XY; Cao JJ KIcatalyzed aminobromination of olefins with TsNH(2)-NBS combination. Org. Biomol. Chem 2009, 7, 3280–3284. [DOI] [PubMed] [Google Scholar]
- (179).Wang YN; Ni BK; Headley AD; Li GG Ionic liquid, 1n-butyl-3-methylimidazolium bis(trifluoro-methanesulfonyl)imide, resulted in the first catalyst-free aminohalogenation of electron-deficient alkenes. Adv. Synth. Catal 2007, 349, 319–322. [Google Scholar]
- (180).Zhdankin VV; Stang PJ Recent developments in the chemistry of polyvalent iodine compounds. Chem. Rev 2002, 102, 2523–2584. [DOI] [PubMed] [Google Scholar]
- (181).Moriarty RM Organohypervalent iodine: development, applications, and future directions. J. Org. Chem 2005, 70, 2893–2903. [DOI] [PubMed] [Google Scholar]
- (182).Wu XL; Wang GW Aminohalogenation of ElectronDeficient Olefins Promoted by Hypervalent Iodine Compounds. Eur. J. Org. Chem 2008, 2008, 6239–6246. [Google Scholar]
- (183).Wu XL; Wang GW Hypervalent iodine-mediated aminobromination of olefins in water. Tetrahedron 2009, 65, 8802–8807. [Google Scholar]
- (184).Sun H; Zhi SJ; Han JL; Li G; Pan Y Highly regio- and stereoselective synthesis of alpha-(N-alkyl-N-p-toluenesulfonyl)-betabromo-ketones via Ni(OAc)2-catalyzed aminobromination of chalcones. Chem. Biol. Drug Des 2010, 75, 269–276. [DOI] [PubMed] [Google Scholar]
- (185).Sasai H; Arai T; Satow Y; Houk KN; Shibasaki M The First Heterobimetallic Multifunctional Asymmetric Catalyst. J. Am. Chem. Soc 1995, 117, 6194–6198. [Google Scholar]
- (186).Agostinho M; Kobayashi S Strontium-catalyzed highly enantioselective Michael additions of malonates to enones. J. Am. Chem. Soc 2008, 130, 2430–2431. [DOI] [PubMed] [Google Scholar]
- (187).Kumaraswamy G; Sastry MNV; Jena N Calcium-BINOL: a novel and efficient catalyst for asymmetric Michael reactions. Tetrahedron Lett 2001, 42, 8515–8517. [Google Scholar]
- (188).Lippur K; Kaabel S; Jarving I; Rissanen K; Kanger T CaCl2, Bisoxazoline, and Malonate: A Protocol for an Asymmetric Michael Reaction. J. Org. Chem 2015, 80, 6336–6341. [DOI] [PubMed] [Google Scholar]
- (189).Ooi T; Ohara D; Fukumoto K; Maruoka K Importance of chiral phase-transfer catalysts with dual functions in obtaining high enantioselectivity in the Michael reaction of malonates and chalcone derivatives. Org. Lett 2005, 7, 3195–3197. [DOI] [PubMed] [Google Scholar]
- (190).Wang J; Li H; Zu L; Jiang W; Xie H; Duan W; Wang W Organocatalytic enantioselective conjugate additions to enones. J. Am. Chem. Soc 2006, 128, 12652–12653. [DOI] [PubMed] [Google Scholar]
- (191).Prakash GK; Wang F; Zhang Z; Ni C; Haiges R; Olah GA Enantioselective synthesis of alpha-stereogenic gamma-keto esters via formal umpolung. Org. Lett 2012, 14, 3260–3263. [DOI] [PubMed] [Google Scholar]
- (192).Su Z; Lee HW; Kim CK Theoretical investigation on mechanism of asymmetric Michael addition of malononitrile to chalcones catalyzed by Cinchona alkaloid aluminium(III) complex. Org. Biomol. Chem 2011, 9, 6402–6409. [DOI] [PubMed] [Google Scholar]
- (193).Sundararajan G; Prabagaran N A new polymer-anchored chiral catalyst for asymmetric Michael addition reactions. Org. Lett 2001, 3, 389–392. [DOI] [PubMed] [Google Scholar]
- (194).Vakulya B; Varga S; Csampai A; Soos T Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org. Lett 2005, 7, 1967–1969. [DOI] [PubMed] [Google Scholar]
- (195).Manzano R; Andres JM; Alvarez R; Muruzabal MD; de Lera AR; Pedrosa R Enantioselective conjugate addition of nitro compounds to alpha,beta-unsaturated ketones: an experimental and computational study. Chem. - Eur. J 2011, 17, 5931–5938. [DOI] [PubMed] [Google Scholar]
- (196).Yang W; Du DM Highly enantioselective Michael addition of nitroalkanes to chalcones using chiral squaramides as hydrogen bonding organocatalysts. Org. Lett 2010, 12, 5450–5453. [DOI] [PubMed] [Google Scholar]
- (197).Yang HM; Li L; Li F; Jiang KZ; Shang JY; Lai GQ; Xu LW Silicon-based Lewis acid assisted cinchona alkaloid catalysis: highly enantioselective aza-Michael reaction under solvent-free conditions. Org. Lett 2011, 13, 6508–6511. [DOI] [PubMed] [Google Scholar]
- (198).Wei Y; Lin S; Liang F; Zhang J N-bromosuccinimide/1,8diazabicyclo[5.4.1]undec-7-ene combination: beta-amination of chalcones via a tandem bromoamination/debromination sequence. Org. Lett 2013, 15, 852–855. [DOI] [PubMed] [Google Scholar]
- (199).Wang J; Wang X; Ge Z; Cheng T; Li R Highly enantioselective Michael addition of cyclopentanone with chalcones via novel di-iminium mechanism. Chem. Commun (Cambridge, U. K.) 2010, 46, 1751–1753. [DOI] [PubMed] [Google Scholar]
- (200).Endo K; Hamada D; Yakeishi S; Ogawa M; Shibata T Multinuclear Cu-catalysts based on SPINOL-PHOS in asymmetric conjugate addition of organozinc reagents. Org. Lett 2012, 14, 2342–2345. [DOI] [PubMed] [Google Scholar]
- (201).Hajra A; Yoshikai N; Nakamura E Aminohydroxyphosphine ligand for the copper-catalyzed enantioselective conjugate addition of organozinc reagents. Org. Lett 2006, 8, 4153–4155. [DOI] [PubMed] [Google Scholar]
- (202).Han L; Lei Y; Xing P; Zhao XL; Jiang B [2.2]Paracyclophane-derived monodentate phosphoramidite ligands for copper-catalyzed asymmetric conjugate addition of diethylzinc to substituted chalcones. J. Org. Chem 2015, 80, 3752–3757. [DOI] [PubMed] [Google Scholar]
- (203).Guo HC; Ma JA Catalytic asymmetric tandem transformations triggered by conjugate additions. Angew. Chem., Int. Ed 2006, 45, 354–366. [DOI] [PubMed] [Google Scholar]
- (204).Enders D; Grondal C; Huttl MR Asymmetric organocatalytic domino reactions. Angew. Chem., Int. Ed 2007, 46, 1570–1581. [DOI] [PubMed] [Google Scholar]
- (205).Yu X; Wang W Organocatalysis: asymmetric cascade reactions catalysed by chiral secondary amines. Org. Biomol. Chem 2008, 6, 2037–2046. [DOI] [PubMed] [Google Scholar]
- (206).Grondal C; Jeanty M; Enders D Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem 2010, 2, 167–178. [DOI] [PubMed] [Google Scholar]
- (207).Moyano A; Rios R Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev 2011, 111, 4703–4832. [DOI] [PubMed] [Google Scholar]
- (208).Zhang X; Zhang S; Wang W Iminium-allenamine cascade catalysis: one-pot access to chiral 4H-chromenes by a highly enantioselective Michael-Michael sequence. Angew. Chem., Int. Ed 2010, 49, 1481–1484. [DOI] [PubMed] [Google Scholar]
- (209).Li H; Zu L; Xie H; Wang J; Jiang W; Wang W Enantioselective organocatalytic double Michael addition reactions. Org. Lett 2007, 9, 1833–1835. [DOI] [PubMed] [Google Scholar]
- (210).Ding R; Zheng B; Wang Y; Peng Y A Cation-Directed Enantioselective Sulfur-Mediated Michael/Mannich Three-Component Domino Reaction involving Chalcones as Michael Acceptors. Org. Lett 2015, 17, 4128–4131. [DOI] [PubMed] [Google Scholar]
- (211).Saha P; Biswas A; Molleti N; Singh VK Enantioselective Synthesis of Highly Substituted Chromans via the Oxa-MichaelMichael Cascade Reaction with a Bifunctional Organocatalyst. J. Org. Chem 2015, 80, 11115–11122. [DOI] [PubMed] [Google Scholar]
- (212).Zhu Y; Li X; Chen Q; Su J; Jia F; Qiu S; Ma M; Sun Q; Yan W; Wang K; et al. Highly Enantioselective Cascade Reaction Catalyzed by Squaramides: the Synthesis of CF3-Containing Chromanes. Org. Lett 2015, 17, 3826–3829. [DOI] [PubMed] [Google Scholar]
- (213).Cromwell NH; Phillips B The azetidines. Recent synthetic developments. Chem. Rev 1979, 79, 331–358. [Google Scholar]
- (214).Robin S; Rousseau G Formation of Four-Membered Heterocycles through Electrophilic Heteroatom Cyclization. Eur. J. Org. Chem 2002, 2002, 3099–3144. [Google Scholar]
- (215).Ye Y; Wang H; Fan R Stereoselective construction of highly functionalized azetidines via a [2 + 2]-cycloaddition. Org. Lett 2010, 12, 2802–2805. [DOI] [PubMed] [Google Scholar]
- (216).Huang H; Wu W; Zhu K; Hu J; Ye J Highly diastereoand enantioselective cross-cascade reactions of different enones. Chem. - Eur. J 2013, 19, 3838–3841. [DOI] [PubMed] [Google Scholar]
- (217).Nair V; Babu BP; Vellalath S; Varghese V; Raveendran AE; Suresh E Nucleophilic heterocyclic carbene catalyzed annulation of enals to chalcones in methanol: a stereoselective synthesis of highly functionalized cyclopentanes. Org. Lett 2009, 11, 2507–2510. [DOI] [PubMed] [Google Scholar]
- (218).Nielsen LPC; Jacobsen EN Catalytic Asymmetric Epoxide Ring-Opening Chemistry. In Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006; Chapter 7, p 229. [Google Scholar]
- (219).Olofsson B; Somfai P Vinylepoxides in Organic Synthesis. In Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006; Chapter 9, p 315. [Google Scholar]
- (220).Singh GS; D’Hooghe M; De Kimpe N Synthesis and reactivity of C-heteroatom-substituted aziridines. Chem. Rev 2007, 107, 2080–2135. [DOI] [PubMed] [Google Scholar]
- (221).Zhou ZL; Yang YX; Ding J; Li YC; Miao ZH Triptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep 2012, 29, 457–475. [DOI] [PubMed] [Google Scholar]
- (222).Ismail FM; Levitsky DO; Dembitsky VM Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem 2009, 44, 3373–3387. [DOI] [PubMed] [Google Scholar]
- (223).Helder R; Hummelen JC; Laane RWPM; Wiering JS; Wynberg H Catalytic asymmetric induction in oxidation reactions. The synthesis of optically active epoxides. Tetrahedron Lett 1976, 17, 1831–1834. [Google Scholar]
- (224).Arai S; Tsuge H; Shioiri T Asymmetric epoxidation of alpha,beta-unsaturated ketones under phase-transfer catalyzed conditions. Tetrahedron Lett 1998, 39, 7563–7566. [Google Scholar]
- (225).Arai S; Tsuge H; Oku M; Miura M; Shioiri T Catalytic asymmetric epoxidation of enones under phase-transfer catalyzed conditions. Tetrahedron 2002, 58, 1623–1630. [Google Scholar]
- (226).Corey EJ; Zhang FY Mechanism and conditions for highly enantioselective epoxidation of alpha,beta-enones using chargeaccelerated catalysis by a rigid quaternary ammonium salt. Org. Lett 1999, 1, 1287–1290. [DOI] [PubMed] [Google Scholar]
- (227).Ooi T; Ohara D; Tamura M; Maruoka K Design of new chiral phase-transfer catalysts with dual functions for highly enantioselective epoxidation of alpha,beta-unsaturated ketones. J. Am. Chem. Soc 2004, 126, 6844–6845. [DOI] [PubMed] [Google Scholar]
- (228).Tanaka S; Nagasawa K Guanidine-Urea Bifunctional Organocatalyst for Asymmetric Epoxidation of 1,3-Diarylenones with Hydrogen Peroxide. Synlett 2009, 2009 (04), 667–670. [Google Scholar]
- (229).Lattanzi A Enantioselective epoxidation of alpha,beta-enones promoted by alpha,alpha-diphenyl-L-prolinol as bifunctional organocatalyst. Org. Lett 2005, 7, 2579–2582. [DOI] [PubMed] [Google Scholar]
- (230).Russo A; Lattanzi A Asymmetric epoxidation of transchalcones organocatalyzed by beta-amino alcohols. Eur. J. Org. Chem 2008, 2008, 2767–2773. [Google Scholar]
- (231).Wang QF; Chen H; Liu P; Wang QJ; Wang XG; Zhang SY Asymmetric Epoxidation of alpha,beta-Enones Catalyzed by Chiral Amine Salts. Chin. J. Org. Chem 2009, 29, 1617–1620. [Google Scholar]
- (232).Julia S; Masana J; Vega JC “Synthetic Enzymes”. Highly Stereoselective Epoxidation of Chalcone in a Triphasic TolueneWater-Poly[(S)-alanine] System. Angew. Chem., Int. Ed. Engl 1980, 19, 929–931. [Google Scholar]
- (233).Julia S; Guixer J; Masana J; Rocas J; Colonna S; Annuziata R; Molinari H Synthetic enzymes. Part 2. Catalytic asymmetric epoxidation by means of polyamino-acids in a triphase system. J. Chem. Soc., Perkin Trans 1 1982, 1317–1324. [Google Scholar]
- (234).Kroutil W; Lasterra-Sanchez ME; Maddrell SJ; Mayon P; Morgan P; Roberts SM; Thornton SR; Todd CJ; Tüter M Development of the Juliá asymmetric epoxidation reaction. Part 2. Application of the oxidation to alkyl enones, enediones and unsaturated keto esters. J. Chem. Soc., Perkin Trans 1 1996, 2837–2844. [Google Scholar]
- (235).Bickley JF; Roberts SM; Ye RH; Skidmore J; Smith CB Stereocontrolled conversion of some optically active (4S,5R)dihydroisoxazoles into acyclic 3-acetamido-1,2-diols. Tetrahedron 2003, 59, 5731–5736. [Google Scholar]
- (236).Nagano M; Doi M; Kurihara M; Suemune H; Tanaka M Stabilized alpha-helix-catalyzed enantioselective epoxidation of alpha,beta-unsaturated ketones. Org. Lett 2010, 12, 3564–3566. [DOI] [PubMed] [Google Scholar]
- (237).Shen YM; Zhao MX; Xu J; Shi Y An amine-promoted aziridination of chalcones. Angew. Chem., Int. Ed 2006, 45, 8005–8008. [DOI] [PubMed] [Google Scholar]
- (238).Armstrong A; Baxter CA; Lamont SG; Pape AR; Wincewicz R Amine-promoted, organocatalytic aziridination of enones. Org. Lett 2007, 9, 351–353. [DOI] [PubMed] [Google Scholar]
- (239).Page PCB; Bordogna C; Strutt I; Chan YH; Buckley BR Asymmetric Aziridination of Chalcone Promoted by BinaphthaleneBased Chiral Amines. Synlett 2013, 24, 2067–2072. [Google Scholar]
- (240).Zhu Y; Wang Q; Cornwall RG; Shi Y Organocatalytic asymmetric epoxidation and aziridination of olefins and their synthetic applications. Chem. Rev 2014, 114, 8199–8256. [DOI] [PubMed] [Google Scholar]
- (241).Orlikova B; Tasdemir D; Golais F; Dicato M; Diederich M Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr 2011, 6, 125–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Hameed A; Abdullah MI; Ahmed E; Sharif A; Irfan A; Masood S Anti-HIV cytotoxicity enzyme inhibition and molecular docking studies of quinoline based chalcones as potential nonnucleoside reverse transcriptase inhibitors (NNRT). Bioorg. Chem 2016, 65, 175–182. [DOI] [PubMed] [Google Scholar]
- (243).Casano G; Dumetre A; Pannecouque C; Hutter S; Azas N; Robin M Anti-HIV and antiplasmodial activity of original flavonoid derivatives. Bioorg. Med. Chem 2010, 18, 6012–6023. [DOI] [PubMed] [Google Scholar]
- (244).Cole AL; Hossain S; Cole AM; Phanstiel O t. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg. Med. Chem 2016, 24, 2768–2776. [DOI] [PubMed] [Google Scholar]
- (245).Deng J; Sanchez T; Al-Mawsawi LQ; Dayam R; Yunes RA; Garofalo A; Bolger MB; Neamati N Discovery of structurally diverse HIV-1 integrase inhibitors based on a chalcone pharmacophore. Bioorg. Med. Chem 2007, 15, 4985–5002. [DOI] [PubMed] [Google Scholar]
- (246).Mishra L; Sinha R; Itokawa H; Bastow KF; Tachibana Y; Nakanishi Y; Kilgore N; Lee KH Anti-HIV and cytotoxic activities of Ru(II)/Ru(III) polypyridyl complexes containing 2,6-(2′-benzimidazolyl)-pyridine/chalcone as co-ligand. Bioorg. Med. Chem 2001, 9, 1667–1671. [DOI] [PubMed] [Google Scholar]
- (247).Wu JH; Wang XH; Yi YH; Lee KH Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg. Med. Chem. Lett 2003, 13, 1813–1815. [DOI] [PubMed] [Google Scholar]
- (248).Aboul-Fadl T; El-Shorbagi AN; Hozien ZA; Sarhan AW Investigation of alkylating, antineoplastic and anti-HIV potentials of the chalcones: 2-(3-arylpropenoyl)benzimidazole and their corresponding N1-methyl derivatives. Boll. Chim. Farm 2000, 139, 228–234. [PubMed] [Google Scholar]
- (249).Yang M; Li N; Li F; Zhu Q; Liu X; Han Q; Wang Y; Chen Y; Zeng X; Lv Y; et al. Xanthohumol, a main prenylated chalcone from hops, reduces liver damage and modulates oxidative reaction and apoptosis in hepatitis C virus infected Tupaia belangeri. Int. Immunopharmacol 2013, 16, 466–474. [DOI] [PubMed] [Google Scholar]
- (250).Park JY; Ko JA; Kim DW; Kim YM; Kwon HJ; Jeong HJ; Kim CY; Park KH; Lee WS; Ryu YB Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem 2016, 31, 23–30. [DOI] [PubMed] [Google Scholar]
- (251).Wan Z; Hu D; Li P; Xie D; Gan X Synthesis, Antiviral Bioactivity of Novel 4-Thioquinazoline Derivatives Containing Chalcone Moiety. Molecules 2015, 20, 11861–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (252).Narayanapillai SC; Leitzman P; O’Sullivan MG; Xing C Flavokawains a and B in kava, not dihydromethysticin, potentiate acetaminophen-induced hepatotoxicity in C57BL/6 mice. Chem. Res. Toxicol 2014, 27, 1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Niu L; Ding L; Lu C; Zuo F; Yao K; Xu S; Li W; Yang D; Xu X Flavokawain A inhibits Cytochrome P450 in in vitro metabolic and inhibitory investigations. J. Ethnopharmacol 2016, 191, 350–359. [DOI] [PubMed] [Google Scholar]
- (254).Zhou B; Yu X; Zhuang C; Villalta P; Lin Y; Lu J; Xing C Unambiguous Identification of beta-Tubulin as the Direct Cellular Target Responsible for the Cytotoxicity of Chalcone by Photoaffinity Labeling. ChemMedChem 2016, 11, 1436–1445. [DOI] [PubMed] [Google Scholar]
- (255).Pahl HL Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [DOI] [PubMed] [Google Scholar]
- (256).Baldwin AS Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunol. Rev 2012, 246, 327–345. [DOI] [PubMed] [Google Scholar]
- (257).Gamble C; McIntosh K; Scott R; Ho KH; Plevin R; Paul A Inhibitory kappa B Kinases as targets for pharmacological regulation. Br. J. Pharmacol 2012, 165, 802–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Pandey MK; Sandur SK; Sung B; Sethi G; Kunnumakkara AB; Aggarwal BB Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaB-regulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue. J. Biol. Chem 2007, 282, 17340–17350. [DOI] [PubMed] [Google Scholar]
- (259).Kim JY; Park SJ; Yun KJ; Cho YW; Park HJ; Lee KT Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. Eur. J. Pharmacol 2008, 584, 175–184. [DOI] [PubMed] [Google Scholar]
- (260).Kumar S; Sharma A; Madan B; Singhal V; Ghosh B Isoliquiritigenin inhibits IkappaB kinase activity and ROS generation to block TNF-alpha induced expression of cell adhesion molecules on human endothelial cells. Biochem. Pharmacol 2007, 73, 1602–1612. [DOI] [PubMed] [Google Scholar]
- (261).Folmer F; Blasius R; Morceau F; Tabudravu J; Dicato M; Jaspars M; Diederich M Inhibition of TNFalpha-induced activation of nuclear factor kappaB by kava (Piper methysticum) derivatives. Biochem. Pharmacol 2006, 71, 1206–1218. [DOI] [PubMed] [Google Scholar]
- (262).Lee YH; Jeon SH; Kim SH; Kim C; Lee SJ; Koh D; Lim Y; Ha K; Shin SY A new synthetic chalcone derivative, 2hydroxy-3′,5,5′-trimethoxychalcone (DK-139), suppresses the Tolllike receptor 4-mediated inflammatory response through inhibition of the Akt/NF-kappaB pathway in BV2 microglial cells. Exp. Mol. Med 2012, 44, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (263).He W; Wang Q; Srinivasan B; Xu J; Padilla MT; Li Z; Wang X; Liu Y; Gou X; Shen HM; et al. A JNK-mediated autophagy pathway that triggers c-IAP degradation and necroptosis for anticancer chemotherapy. Oncogene 2014, 33, 3004–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).Xu J; Xu X; Shi S; Wang Q; Saxton B; He W; Gou X; Jang JH; Nyunoya T; Wang X; et al. Autophagy-Mediated Degradation of IAPs and c-FLIP Potentiates Apoptosis Induced by Combination of TRAIL and Chal-24. J. Cell. Biochem 2016, 117, 1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Shi S; Wang Q; Xu J; Jang JH; Padilla MT; Nyunoya T; Xing C; Zhang L; Lin Y Synergistic anticancer effect of cisplatin and Chal-24 combination through IAP and c-FLIPL degradation, Ripoptosome formation and autophagy-mediated apoptosis. Oncotarget 2015, 6, 1640–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (266).Zhang Y; Wu J; Ying S; Chen G; Wu B; Xu T; Liu Z; Liu X; Huang L; Shan X; et al. Discovery of new MD2 inhibitor from chalcone derivatives with anti-inflammatory effects in LPSinduced acute lung injury. Sci. Rep 2016, 6, 25130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Liu Y; Li Y; Yu S; Zhao G Recent advances in the development of thioredoxin reductase inhibitors as anticancer agents. Curr. Drug Targets 2012, 13, 1432–1444. [DOI] [PubMed] [Google Scholar]
- (268).Mahmood DF; Abderrazak A; El Hadri K; Simmet T; Rouis M The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox Signaling 2013, 19, 1266–1303. [DOI] [PubMed] [Google Scholar]
- (269).Zhang B; Duan D; Ge C; Yao J; Liu Y; Li X; Fang J Synthesis of xanthohumol analogues and discovery of potent thioredoxin reductase inhibitor as potential anticancer agent. J. Med. Chem 2015, 58, 1795–1805. [DOI] [PubMed] [Google Scholar]
- (270).Kwak MK; Kensler TW Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol 2010, 244, 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Narasimhan M; Rajasekaran NS Exercise, Nrf2 and Antioxidant Signaling in Cardiac Aging. Front. Physiol 2016, 7, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Forman HJ Redox signaling: An evolution from free radicals to aging. Free Radical Biol. Med 2016, 97, 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Koenitzer JR; Freeman BA Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann. N. Y. Acad. Sci 2010, 1203, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Van-Assche T; Huygelen V; Crabtree MJ; Antoniades C Gene therapy targeting inflammation in atherosclerosis. Curr. Pharm. Des 2011, 17, 4210–4223. [DOI] [PubMed] [Google Scholar]
- (275).Howden R Nrf2 and cardiovascular defense. Oxid. Med. Cell. Longevity 2013, 2013, 104308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Sandberg M; Patil J; D’Angelo B; Weber SG; Mallard C NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Johnson DA; Johnson JA Nrf2–a therapeutic target for the treatment of neurodegenerative diseases. Free Radical Biol. Med 2015, 88, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Bhakkiyalakshmi E; Sireesh D; Rajaguru P; Paulmurugan R; Ramkumar KM The emerging role of redox-sensitive Nrf2Keap1 pathway in diabetes. Pharmacol. Res 2015, 91, 104–114. [DOI] [PubMed] [Google Scholar]
- (279).Tekiner-Gulbas B; Westwell AD; Suzen S Oxidative stress in carcinogenesis: new synthetic compounds with dual effects upon free radicals and cancer. Curr. Med. Chem 2013, 20, 4451–4459. [DOI] [PubMed] [Google Scholar]
- (280).Itoh K; Wakabayashi N; Katoh Y; Ishii T; Igarashi K; Engel JD; Yamamoto M Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 1999, 13, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (281).Zhuang C; Miao Z; Sheng C; Zhang W Updated research and applications of small molecule inhibitors of Keap1-Nrf2 proteinprotein interaction: a review. Curr. Med. Chem 2014, 21, 1861–1870. [DOI] [PubMed] [Google Scholar]
- (282).Magesh S; Chen Y; Hu L Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev 2012, 32, 687–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Kumar V; Kumar S; Hassan M; Wu H; Thimmulappa RK; Kumar A; Sharma SK; Parmar VS; Biswal S; Malhotra SV Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J. Med. Chem 2011, 54, 4147–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Lounsbury N; Mateo G; Jones B; Papaiahgari S; Thimmulappa RK; Teijaro C; Gordon J; Korzekwa K; Ye M; Allaway G; et al. Heterocyclic chalcone activators of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) with improved in vivo efficacy. Bioorg. Med. Chem 2015, 23, 5352–5359. [DOI] [PubMed] [Google Scholar]
- (285).Cabrera M; Mastandrea I; Otero G; Cerecetto H; Gonzalez M In vivo phase II-enzymes inducers,as potential chemopreventive agents, based on the chalcone and furoxan skeletons. Bioorg. Med. Chem 2016, 24, 1665–1674. [DOI] [PubMed] [Google Scholar]
- (286).Lee SH; Seo GS; Kim JY; Jin XY; Kim HD; Sohn DH Heme oxygenase 1 mediates anti-inflammatory effects of 2′,4′,6′-tris(methoxymethoxy) chalcone. Eur. J. Pharmacol 2006, 532, 178–186. [DOI] [PubMed] [Google Scholar]
- (287).Kim SS; Lim J; Bang Y; Gal J; Lee SU; Cho YC; Yoon G; Kang BY; Cheon SH; Choi HJ Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem 2012, 23, 1314–1323. [DOI] [PubMed] [Google Scholar]
- (288).Yao J; Zhang B; Ge C; Peng S; Fang J Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J. Agric. Food Chem 2015, 63, 1521–1531. [DOI] [PubMed] [Google Scholar]
- (289).Brodziak-Jarosz L; Fujikawa Y; Pastor-Flores D; Kasikci S; Jirasek P; Pitzl S; Owen RW; Klika KD; Gerhauser C; Amslinger S; et al. A click chemistry approach identifies target proteins of xanthohumol. Mol. Nutr. Food Res 2016, 60, 737–748. [DOI] [PubMed] [Google Scholar]
- (290).Lawrence NJ; McGown AT; Ducki S; Hadfield JA The interaction of chalcones with tubulin. Anti-Cancer Drug Des 2000, 15, 135–141. [PubMed] [Google Scholar]
- (291).Dumontet C; Jordan MA Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat. Rev. Drug Discovery 2010, 9, 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Jordan MA; Wilson L Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [DOI] [PubMed] [Google Scholar]
- (293).Yang Z; Wu W; Wang J; Liu L; Li L; Yang J; Wang G; Cao D; Zhang R; Tang M; et al. Synthesis and biological evaluation of novel millepachine derivatives as a new class of tubulin polymerization inhibitors. J. Med. Chem 2014, 57, 7977–7989. [DOI] [PubMed] [Google Scholar]
- (294).Wang G; Peng F; Cao D; Yang Z; Han X; Liu J; Wu W; He L; Ma L; Chen J; et al. Design, synthesis and biological evaluation of millepachine derivatives as a new class of tubulin polymerization inhibitors. Bioorg. Med. Chem 2013, 21, 6844–6854. [DOI] [PubMed] [Google Scholar]
- (295).Wang G; Li C; He L; Lei K; Wang F; Pu Y; Yang Z; Cao D; Ma L; Chen J; et al. Design, synthesis and biological evaluation of a series of pyrano chalcone derivatives containing indole moiety as novel anti-tubulin agents. Bioorg. Med. Chem 2014, 22, 2060–2079. [DOI] [PubMed] [Google Scholar]
- (296).Zhu C; Zuo Y; Wang R; Liang B; Yue X; Wen G; Shang N; Huang L; Chen Y; Du J; et al. Discovery of potent cytotoxic ortho-aryl chalcones as new scaffold targeting tubulin and mitosis with affinity-based fluorescence. J. Med. Chem 2014, 57, 6364–6382. [DOI] [PubMed] [Google Scholar]
- (297).Shen KH; Chang JK; Hsu YL; Kuo PL Chalcone arrests cell cycle progression and induces apoptosis through induction of mitochondrial pathway and inhibition of nuclear factor kappa B signalling in human bladder cancer cells. Basic Clin. Pharmacol. Toxicol 2007, 101, 254–261. [DOI] [PubMed] [Google Scholar]
- (298).Martel-Frachet V; Keramidas M; Nurisso A; DeBonis S; Rome C; Coll JL; Boumendjel A; Skoufias DA; Ronot X IPP51, a chalcone acting as a microtubule inhibitor with in vivo antitumor activity against bladder carcinoma. Oncotarget 2015, 6, 14669–14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (299).Canela MD; Noppen S; Bueno O; Prota AE; Bargsten K; Saez-Calvo G; Jimeno ML; Benkheil M; Ribatti D; Velazquez S; et al. Antivascular and antitumor properties of the tubulin-binding chalcone TUB091. Oncotarget 2016, DOI: 10.18632/oncotarget.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (300).Hojjat-Farsangi M Small-molecule inhibitors of the receptor tyrosine kinases: promising tools for targeted cancer therapies. Int. J. Mol. Sci 2014, 15, 13768–13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (301).Chatzopoulou M; Pegklidou K; Papastavrou N; Demopoulos VJ Development of aldose reductase inhibitors for the treatment of inflammatory disorders. Expert Opin. Drug Discovery 2013, 8, 1365–1380. [DOI] [PubMed] [Google Scholar]
- (302).Maccari R; Ottana R Targeting aldose reductase for the treatment of diabetes complications and inflammatory diseases: new insights and future directions. J. Med. Chem 2015, 58, 2047–2067. [DOI] [PubMed] [Google Scholar]
- (303).Aida K; Tawata M; Shindo H; Onaya T; Sasaki H; Nishimura H; Chin M; Mitsuhashi H The existence of aldose reductase inhibitors in some kampo medicines (Oriental herb prescriptions). Planta Med 1989, 55, 22–26. [DOI] [PubMed] [Google Scholar]
- (304).Aida K; Tawata M; Shindo H; Onaya T; Sasaki H; Yamaguchi T; Chin M; Mitsuhashi H Isoliquiritigenin: a new aldose reductase inhibitor from glycyrrhizae radix. Planta Med 1990, 56, 254–258. [DOI] [PubMed] [Google Scholar]
- (305).Lim SS; Jung SH; Ji J; Shin KH; Keum SR Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. J. Pharm. Pharmacol 2001, 53, 653–668. [DOI] [PubMed] [Google Scholar]
- (306).Iwata S; Nagata N; Omae A; Yamaguchi S; Okada Y; Shibata S; Okuyama T Inhibitory effect of chalcone derivatives on recombinant human aldose reductase. Biol. Pharm. Bull 1999, 22, 323–325. [DOI] [PubMed] [Google Scholar]
- (307).Claria J; Romano M Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways. Impact on inflammation and cancer. Curr. Pharm. Des 2005, 11, 3431–3447. [DOI] [PubMed] [Google Scholar]
- (308).Goossens L; Pommery N; Henichart JP COX-2/5-LOX dual acting anti-inflammatory drugs in cancer chemotherapy. Curr. Top. Med. Chem 2007, 7, 283–296. [DOI] [PubMed] [Google Scholar]
- (309).Herencia F; Ferrandiz ML; Ubeda A; Dominguez JN; Charris JE; Lobo GM; Alcaraz MJ Synthesis and antiinflammatory activity of chalcone derivatives. Bioorg. Med. Chem. Lett 1998, 8, 1169–1174. [DOI] [PubMed] [Google Scholar]
- (310).Ozdemir A; Altintop MD; Turan-Zitouni G; Ciftci GA; Ertorun I; Alatas O; Kaplancikli ZA Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents. Eur. J. Med. Chem 2015, 89, 304–309. [DOI] [PubMed] [Google Scholar]
- (311).El-Sabbagh OI; Mostafa S; Abdel-Aziz HA; Ibrahim HS; Elaasser MM Synthesis and biological evaluation of some Narylpyrazoles and pyrazolo[3,4-d]pyridazines as anti-inflammatory agents. Arch. Pharm (Weinheim, Ger.) 2013, 346, 688–698. [DOI] [PubMed] [Google Scholar]
- (312).Pinz S; Unser S; Brueggemann S; Besl E; Al-Rifai N; Petkes H; Amslinger S; Rascle A The synthetic alpha-bromo2′,3,4,4′-tetramethoxychalcone (alpha-Br-TMC) inhibits the JAK/ STAT signaling pathway. PLoS One 2014, 9, e90275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (313).Wang LH; Li HH; Li M; Wang S; Jiang XR; Li Y; Ping GF; Cao Q; Liu X; Fang WH; et al. SL4, a chalcone-based compound, induces apoptosis in human cancer cells by activation of the ROS/MAPK signalling pathway. Cell Proliferation 2015, 48, 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (314).Hara H; Ikeda R; Ninomiya M; Kamiya T; Koketsu M; Adachi T Newly synthesized ‘hidabeni’ chalcone derivatives potently suppress LPS-induced NO production via inhibition of STAT1, but not NF-kappaB, JNK, and p38, pathways in microglia. Biol. Pharm. Bull 2014, 37, 1042–1049. [DOI] [PubMed] [Google Scholar]
- (315).Viegas-Junior C; Danuello A; da Silva Bolzani V; Barreiro EJ; Fraga CAM Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem 2007, 14, 1829–1852. [DOI] [PubMed] [Google Scholar]
- (316).Zhan P; Liu XY Designed Multiple Ligands: An Emerging Anti-HIV Drug Discovery Paradigm. Curr. Pharm. Des 2009, 15, 1893–1917. [DOI] [PubMed] [Google Scholar]
- (317).Zhuang CL; Miao ZY; Wu YL; Guo ZZ; Li J; Yao JZ; Xing CG; Sheng CQ; Zhang WN Double-Edged Swords as Cancer Therapeutics: Novel, Orally Active, Small Molecules Simultaneously Inhibit p53-MDM2 Interaction and the NF-kappa B Pathway. J. Med. Chem 2014, 57, 567–577. [DOI] [PubMed] [Google Scholar]
- (318).Qiao Z; Wang Q; Zhang F; Wang Z; Bowling T; Nare B; Jacobs RT; Zhang J; Ding D; Liu Y; et al. Chalconebenzoxaborole hybrid molecules as potent antitrypanosomal agents. J. Med. Chem 2012, 55, 3553–3557. [DOI] [PubMed] [Google Scholar]
- (319).DiCesare N; Lakowicz JR Chalcone-analogue fluorescent prfobes for saccharides signaling using the boronic acid group. Tetrahedron Lett 2002, 43, 2615–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (320).Kumar SK; Hager E; Pettit C; Gurulingappa H; Davidson NE; Khan SR Design, synthesis, and evaluation of novel boronic-chalcone derivatives as antitumor agents. J. Med. Chem 2003, 46, 2813–2815. [DOI] [PubMed] [Google Scholar]
- (321).Sasayama T; Tanaka K; Mizukawa K; Kawamura A; Kondoh T; Hosoda K; Kohmura E Trans-4-lodo,4′-boranylchalcone induces antitumor activity against malignant glioma cell lines in vitro and in vivo. J. Neuro-Oncol 2007, 85, 123–132. [DOI] [PubMed] [Google Scholar]
- (322).Achanta G; Modzelewska A; Feng L; Khan SR; Huang P A boronic-chalcone derivative exhibits potent anticancer activity through inhibition of the proteasome. Mol. Pharmacol 2006, 70, 426–433. [DOI] [PubMed] [Google Scholar]
- (323).Perez-Cruz F; Vazquez-Rodriguez S; Matos MJ; HerreraMorales A; Villamena FA; Das A; Gopalakrishnan B; Olea-Azar C; Santana L; Uriarte E Synthesis and electrochemical and biological studies of novel coumarin-chalcone hybrid compounds. J. Med. Chem 2013, 56, 6136–6145. [DOI] [PubMed] [Google Scholar]
- (324).Vazquez-Rodriguez S; Lama Lopez R; Matos MJ; Armesto-Quintas G; Serra S; Uriarte E; Santana L; Borges F; Munoz Crego A; Santos Y Design, synthesis and antibacterial study of new potent and selective coumarin-chalcone derivatives for the treatment of tenacibaculosis. Bioorg. Med. Chem 2015, 23, 7045–7052. [DOI] [PubMed] [Google Scholar]
- (325).Jamier V; Marut W; Valente S; Chereau C; Chouzenoux S; Nicco C; Lemarechal H; Weill B; Kirsch G; Jacob C; et al. Chalcone-Coumarin derivatives as potential anti-cancer drugs: an in vitro and in vivo investigation. Anti-Cancer Agents Med. Chem 2014, 14, 963–974. [DOI] [PubMed] [Google Scholar]
- (326).Sashidhara KV; Kumar A; Kumar M; Sarkar J; Sinha S Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett 2010, 20, 7205–7211. [DOI] [PubMed] [Google Scholar]
- (327).Bombardelli E; Valenti P PCT Int. Appl. Patent 2001017984A1, 2001; Chem. Abstr 2001, 134, 222628.
- (328).Borges F; Roleira F; Milhazes N; Santana L; Uriarte E Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr. Med. Chem 2005, 12, 887–916. [DOI] [PubMed] [Google Scholar]
- (329).Brunet E; Alonso MT; Juanes O; Velasco O; RodriguezUbis JC Novel polyaminocarboxylate chelates derived from 3aroylcoumarins. Tetrahedron 2001, 57, 3105–3116. [Google Scholar]
- (330).Mazzone G; Galano A; Alvarez-Idaboy JR; Russo N Coumarin-Chalcone Hybrids as Peroxyl Radical Scavengers: Kinetics and Mechanisms. J. Chem. Inf. Model 2016, 56, 662–670. [DOI] [PubMed] [Google Scholar]
- (331).Sashidhara KV; Kumar M; Modukuri RK; Sonkar R; Bhatia G; Khanna AK; Rai S; Shukla R Synthesis and antiinflammatory activity of novel biscoumarin-chalcone hybrids. Bioorg. Med. Chem. Lett 2011, 21, 4480–4484. [DOI] [PubMed] [Google Scholar]
- (332).Al-Sehemi AG; Pannipara M; Kalam A; Asiri AM A Combined Experimental and Computational Investigation on Spectroscopic and Photophysical Properties of a Coumarinyl Chalcone. J. Fluoresc 2016, 26, 1357–1365. [DOI] [PubMed] [Google Scholar]
- (333).Eftekhari-Sis B; Zirak M; Akbari A Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev 2013, 113, 2958–3043. [DOI] [PubMed] [Google Scholar]
- (334).Van Order RB; Lindwall HG Indole. Chem. Rev 1942, 30, 69–96. [Google Scholar]
- (335).Cui M; Ono M; Kimura H; Liu BL; Saji H Synthesis and biological evaluation of indole-chalcone derivatives as beta-amyloid imaging probe. Bioorg. Med. Chem. Lett 2011, 21, 980–982. [DOI] [PubMed] [Google Scholar]
- (336).Sashidhara KV; Dodda RP; Sonkar R; Palnati GR; Bhatia G Design and synthesis of novel indole-chalcone fibrates as lipid lowering agents. Eur. J. Med. Chem 2014, 81, 499–509. [DOI] [PubMed] [Google Scholar]
- (337).Robinson MW; Overmeyer JH; Young AM; Erhardt PW; Maltese WA Synthesis and evaluation of indole-based chalcones as inducers of methuosis, a novel type of nonapoptotic cell death. J. Med. Chem 2012, 55, 1940–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (338).Loch-Neckel G; Bicca MA; Leal PC; Mascarello A; Siqueira JM; Calixto JB In vitro and in vivo anti-glioma activity of a chalcone-quinoxaline hybrid. Eur. J. Med. Chem 2015, 90, 93–100. [DOI] [PubMed] [Google Scholar]
- (339).Mielcke TR; Mascarello A; Filippi-Chiela E; Zanin RF; Lenz G; Leal PC; Chirardia LD; Yunes RA; Nunes RJ; Battastini AM; et al. Activity of novel quinoxaline-derived chalcones on in vitro glioma cell proliferation. Eur. J. Med. Chem 2012, 48, 255–264. [DOI] [PubMed] [Google Scholar]
- (340).Shankaraiah N; Siraj KP; Nekkanti S; Srinivasulu V; Sharma P; Senwar KR; Sathish M; Vishnuvardhan MV; Ramakrishna S; Jadala C; et al. DNA-binding affinity and anticancer activity of beta-carboline-chalcone conjugates as potential DNA intercalators: Molecular modelling and synthesis. Bioorg. Chem 2015, 59, 130–139. [DOI] [PubMed] [Google Scholar]
- (341).Kamal A; Balakrishna M; Nayak VL; Shaik TB; Faazil S; Nimbarte VD Design and synthesis of imidazo[2,1-b]thiazolechalcone conjugates: microtubule-destabilizing agents. ChemMedChem 2014, 9, 2766–2780. [DOI] [PubMed] [Google Scholar]
- (342).Kamal A; Dastagiri D; Ramaiah MJ; Reddy JS; Bharathi EV; Srinivas C; Pushpavalli SN; Pal D; Pal-Bhadra M Synthesis of imidazothiazole-chalcone derivatives as anticancer and apoptosis inducing agents. ChemMedChem 2010, 5, 1937–1947. [DOI] [PubMed] [Google Scholar]
- (343).Kolundzija B; Markovic V; Stanojkovic T; Joksovic L; Matic I; Todorovic N; Nikolic M; Joksovic MD Novel anthraquinone based chalcone analogues containing an imine fragment: synthesis, cytotoxicity and anti-angiogenic activity. Bioorg. Med. Chem. Lett 2014, 24, 65–71. [DOI] [PubMed] [Google Scholar]
- (344).Romagnoli R; Baraldi PG; Carrion MD; Cruz-Lopez O; Cara CL; Balzarini J; Hamel E; Canella A; Fabbri E; Gambari R; et al. Hybrid alpha-bromoacryloylamido chalcones. Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett 2009, 19, 2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (345).Fu DJ; Zhang SY; Liu YC; Zhang L; Liu JJ; Song J; Zhao RH; Li F; Sun HH; Liu HM; et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate-chalcone derivates. Bioorg. Med. Chem. Lett 2016, 26, 3918–3922. [DOI] [PubMed] [Google Scholar]
- (346).Yang Y; Hahne H; Kuster B; Verhelst SH A simple and effective cleavable linker for chemical proteomics applications. Mol. Cell. Proteomics 2013, 12, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (347).Dong X; Du L; Pan Z; Liu T; Yang B; Hu Y Synthesis and biological evaluation of novel hybrid chalcone derivatives as vasorelaxant agents. Eur. J. Med. Chem 2010, 45, 3986–3992. [DOI] [PubMed] [Google Scholar]
- (348).Kamal A; Shankaraiah N; Prabhakar S; Reddy Ch. R.; Markandeya N; Reddy KL; Devaiah V Solid-phase synthesis of new pyrrolobenzodiazepine-chalcone conjugates: DNA-binding affinity and anticancer activity. Bioorg. Med. Chem. Lett 2008, 18, 2434–2439. [DOI] [PubMed] [Google Scholar]
- (349).Kamal A; Prabhakar S; Janaki Ramaiah M; Venkat Reddy P; Ratna Reddy C; Mallareddy A; Shankaraiah N; Lakshmi Narayan Reddy T; Pushpavalli SN; Pal-Bhadra M Synthesis and anticancer activity of chalcone-pyrrolobenzodiazepine conjugates linked via 1,2,3-triazole ring side-armed with alkane spacers. Eur. J. Med. Chem 2011, 46, 3820–3831. [DOI] [PubMed] [Google Scholar]
- (350).Gaur R; Pathania AS; Malik FA; Bhakuni RS; Verma RK Synthesis of a series of novel dihydroartemisinin monomers and dimers containing chalcone as a linker and their anticancer activity. Eur. J. Med. Chem 2016, 122, 232–246. [DOI] [PubMed] [Google Scholar]
- (351).Kamal A; Mallareddy A; Suresh P; Shaik TB; Lakshma Nayak V; Kishor C; Shetti RV; Sankara Rao N; Tamboli JR; Ramakrishna S; et al. Synthesis of chalcone-amidobenzothiazole conjugates as antimitotic and apoptotic inducing agents. Bioorg. Med. Chem 2012, 20, 3480–3492. [DOI] [PubMed] [Google Scholar]
- (352).Schobert R; Biersack B; Dietrich A; Knauer S; Zoldakova M; Fruehauf A; Mueller T Pt(II) complexes of a combretastatin A-4 analogous chalcone: effects of conjugation on cytotoxicity, tumor specificity, and long-term tumor growth suppression. J. Med. Chem 2009, 52, 241–246. [DOI] [PubMed] [Google Scholar]
- (353).Wendel KA; Workowski KA Trichomoniasis: challenges to appropriate management. Clin. Infect. Dis 2007, 44 (Suppl. 3), S123–S129. [DOI] [PubMed] [Google Scholar]
- (354).Anthwal A; Rajesh UC; Rawat MS; Kushwaha B; Maikhuri JP; Sharma VL; Gupta G; Rawat DS Novel metronidazole-chalcone conjugates with potential to counter drug resistance in Trichomonas vaginalis. Eur. J. Med. Chem 2014, 79, 89–94. [DOI] [PubMed] [Google Scholar]
- (355).Kolb HC; Finn MG; Sharpless KB Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- (356).Pingaew R; Saekee A; Mandi P; Nantasenamat C; Prachayasittikul S; Ruchirawat S; Prachayasittikul V Synthesis, biological evaluation and molecular docking of novel chalconecoumarin hybrids as anticancer and antimalarial agents. Eur. J. Med. Chem 2014, 85, 65–76. [DOI] [PubMed] [Google Scholar]
- (357).Guantai EM; Ncokazi K; Egan TJ; Gut J; Rosenthal PJ; Smith PJ; Chibale K Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds. Bioorg. Med. Chem 2010, 18, 8243–8256. [DOI] [PubMed] [Google Scholar]
- (358).Zhao L; Mao L; Hong G; Yang X; Liu T Design, synthesis and anticancer activity of matrine-1H-1,2,3-triazole-chalcone conjugates. Bioorg. Med. Chem. Lett 2015, 25, 2540–2544. [DOI] [PubMed] [Google Scholar]
- (359).O’Boyle NM; Carr M; Greene LM; Bergin O; Nathwani SM; McCabe T; Lloyd DG; Zisterer DM; Meegan MJ Synthesis and evaluation of azetidinone analogues of combretastatin A-4 as tubulin targeting agents. J. Med. Chem 2010, 53, 8569–8584. [DOI] [PubMed] [Google Scholar]
- (360).Singh P; Raj R; Kumar V; Mahajan MP; Bedi PM; Kaur T; Saxena AK 1,2,3-Triazole tethered beta-lactam-chalcone bifunctional hybrids: synthesis and anticancer evaluation. Eur. J. Med. Chem 2012, 47, 594–600. [DOI] [PubMed] [Google Scholar]
- (361).Sashidhara KV; Avula SR; Doharey PK; Singh LR; Balaramnavar VM; Gupta J; Misra-Bhattacharya S; Rathaur S; Saxena AK; Saxena JK Designing, synthesis of selective and highaffinity chalcone-benzothiazole hybrids as Brugia malayi thymidylate kinase inhibitors: In vitro validation and docking studies. Eur. J. Med. Chem 2015, 103, 418–428. [DOI] [PubMed] [Google Scholar]
- (362).Sashidhara KV; Avula SR; Palnati GR; Singh SV; Srivastava K; Puri SK; Saxena JK Synthesis and in vitro evaluation of new chloroquine-chalcone hybrids against chloroquineresistant strain of Plasmodium falciparum. Bioorg. Med. Chem. Lett 2012, 22, 5455–5459. [DOI] [PubMed] [Google Scholar]
- (363).Guantai EM; Ncokazi K; Egan TJ; Gut J; Rosenthal PJ; Bhampidipati R; Kopinathan A; Smith PJ; Chibale K Enoneand chalcone-chloroquinoline hybrid analogues: in silico guided design, synthesis, antiplasmodial activity, in vitro metabolism, and mechanistic studies. J. Med. Chem 2011, 54, 3637–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Pettit GR; Cragg GM; Herald DL; Schmidt JM; Lohavanijaya P Antineoplastic agents. Part 84. Isolation and structure of combretastatin. Can. J. Chem 1982, 60, 1374–1376. [Google Scholar]
- (365).Kamal A; Ramakrishna G; Raju P; Viswanath A; Ramaiah MJ; Balakishan G; Pal-Bhadra M Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorg. Med. Chem. Lett 2010, 20, 4865–4869. [DOI] [PubMed] [Google Scholar]
- (366).Sobolev VS; Horn BW; Potter TL; Deyrup ST; Gloer JB Production of stilbenoids and phenolic acids by the peanut plant at early stages of growth. J. Agric. Food Chem 2006, 54, 3505–3511. [DOI] [PubMed] [Google Scholar]
- (367).Sharma N; Mohanakrishnan D; Shard A; Sharma A; Saima; Sinha, A. K.; Sahal, D. Stilbene-chalcone hybrids: design, synthesis, and evaluation as a new class of antimalarial scaffolds that trigger cell death through stage specific apoptosis. J. Med. Chem 2012, 55, 297–311. [DOI] [PubMed] [Google Scholar]
- (368).Markovic V; Debeljak N; Stanojkovic T; Kolundzija B; Sladic D; Vujcic M; Janovic B; Tanic N; Perovic M; Tesic V; et al. Anthraquinone-chalcone hybrids: synthesis, preliminary antiproliferative evaluation and DNA-interaction studies. Eur. J. Med. Chem 2015, 89, 401–410. [DOI] [PubMed] [Google Scholar]
- (369).Wani ZA; Guru SK; Rao AV; Sharma S; Mahajan G; Behl A; Kumar A; Sharma PR; Kamal A; Bhushan S; et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem. Toxicol 2016, 87, 1–11. [DOI] [PubMed] [Google Scholar]
- (370).Meng CQ; Ni L; Worsencroft KJ; Ye Z; Weingarten MD; Simpson JE; Skudlarek JW; Marino EM; Suen KL; Kunsch C; et al. Carboxylated, heteroaryl-substituted chalcones as inhibitors of vascular cell adhesion molecule-1 expression for use in chronic inflammatory diseases. J. Med. Chem 2007, 50, 1304–1315. [DOI] [PubMed] [Google Scholar]
- (371).Bahekar SP; Hande SV; Agrawal NR; Chandak HS; Bhoj PS; Goswami K; Reddy MV Sulfonamide chalcones: Synthesis and in vitro exploration for therapeutic potential against Brugia malayi. Eur. J. Med. Chem 2016, 124, 262–269. [DOI] [PubMed] [Google Scholar]
- (372).Tuncel S; Fournier-dit-Chabert J; Albrieux F; Ahsen V; Ducki S; Dumoulin F Towards dual photodynamic and antiangiogenic agents: design and synthesis of a phthalocyanine-chalcone conjugate. Org. Biomol. Chem 2012, 10, 1154–1157. [DOI] [PubMed] [Google Scholar]
- (373).Tuncel S; Trivella A; Atilla D; Bennis K; Savoie H; Albrieux F; Delort L; Billard H; Dubois V; Ahsen V; et al. Assessing the dual activity of a chalcone-phthalocyanine conjugate: design, synthesis, and antivascular and photodynamic properties. Mol. Pharmaceutics 2013, 10, 3706–3716. [DOI] [PubMed] [Google Scholar]
- (374).Ziegler S; Pries V; Hedberg C; Waldmann H Target identification for small bioactive molecules: finding the needle in the haystack. Angew. Chem., Int. Ed 2013, 52, 2744–2792. [DOI] [PubMed] [Google Scholar]
- (375).Ducki S; Mackenzie G; Lawrence NJ; Snyder JP Quantitative structure-activity relationship (5D-QSAR) study of combretastatin-like analogues as inhibitors of tubulin assembly. J. Med. Chem 2005, 48, 457–465. [DOI] [PubMed] [Google Scholar]
- (376).Ducki S; Rennison D; Woo M; Kendall A; Chabert JF; McGown AT; Lawrence NJ Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: synthesis and biological evaluation of antivascular activity. Bioorg. Med. Chem 2009, 17, 7698–7710. [DOI] [PubMed] [Google Scholar]
- (377).Edwards ML; Stemerick DM; Sunkara PS Chalcones: a new class of antimitotic agents. J. Med. Chem 1990, 33, 1948–1954. [DOI] [PubMed] [Google Scholar]
- (378).Peyrot V; Leynadier D; Sarrazin M; Briand C; Menendez M; Laynez J; Andreu JM Mechanism of binding of the new antimitotic drug MDL 27048 to the colchicine site of tubulin: equilibrium studies. Biochemistry 1992, 31, 11125–11132. [DOI] [PubMed] [Google Scholar]
- (379).Kim DY; Kim KH; Kim ND; Lee KY; Han CK; Yoon JH; Moon SK; Lee SS; Seong BL Design and biological evaluation of novel tubulin inhibitors as antimitotic agents using a pharmacophore binding model with tubulin. J. Med. Chem 2006, 49, 5664–5670. [DOI] [PubMed] [Google Scholar]
- (380).Zhang H; Liu JJ; Sun J; Yang XH; Zhao TT; Lu X; Gong HB; Zhu HL Design, synthesis and biological evaluation of novel chalcone derivatives as antitubulin agents. Bioorg. Med. Chem 2012, 20, 3212–3218. [DOI] [PubMed] [Google Scholar]
- (381).Ruan BF; Lu X; Tang JF; Wei Y; Wang XL; Zhang YB; Wang LS; Zhu HL Synthesis, biological evaluation, and molecular docking studies of resveratrol derivatives possessing chalcone moiety as potential antitubulin agents. Bioorg. Med. Chem 2011, 19, 2688–2695. [DOI] [PubMed] [Google Scholar]
- (382).Kamal A; Kumar GB; Vishnuvardhan MV; Shaik AB; Reddy VS; Mahesh R; Sayeeda IB; Kapure JS Synthesis of phenstatin/isocombretastatin-chalcone conjugates as potent tubulin polymerization inhibitors and mitochondrial apoptotic inducers. Org. Biomol. Chem 2015, 13, 3963–3981. [DOI] [PubMed] [Google Scholar]
- (383).Konieczny MT; Bulakowska A; Pirska D; Konieczny W; Skladanowski A; Sabisz M; Wojciechowski M; Lemke K; Pieczykolan A; Strozek W Structural factors affecting affinity of cytotoxic oxathiole-fused chalcones toward tubulin. Eur. J. Med. Chem 2015, 89, 733–742. [DOI] [PubMed] [Google Scholar]
- (384).Konieczny MT; Bulakowska A; Polak J; Pirska D; Konieczny W; Gryn P; Skladanowski A; Sabisz M; Lemke K; Pieczykolan A; et al. Structural factors affecting cytotoxic activity of (E)-1-(Benzo[d][1,3]oxathiol-6-yl)-3-phenylprop-2-en-1-one derivatives. Chem. Biol. Drug Des 2014, 84, 86–91. [DOI] [PubMed] [Google Scholar]
- (385).Zhang HJ; Qian Y; Zhu DD; Yang XG; Zhu HL Synthesis, molecular modeling and biological evaluation of chalcone thiosemicarbazide derivatives as novel anticancer agents. Eur. J. Med. Chem 2011, 46, 4702–4708. [DOI] [PubMed] [Google Scholar]
- (386).Shin SY; Yoon H; Ahn S; Kim DW; Kim SH; Koh D; Lee YH; Lim Y Chromenylchalcones showing cytotoxicity on human colon cancer cell lines and in silico docking with aurora kinases. Bioorg. Med. Chem 2013, 21, 4250–4258. [DOI] [PubMed] [Google Scholar]
- (387).Kommidi DR; Pagadala R; Rana S; Singh P; Shintre SA; Koorbanally NA; Jonnalagadda SB; Moodley B Novel carbapenem chalcone derivatives: synthesis, cytotoxicity and molecular docking studies. Org. Biomol. Chem 2015, 13, 4344–4350. [DOI] [PubMed] [Google Scholar]
- (388).Sashidhara KV; Modukuri RK; Jadiya P; Dodda RP; Kumar M; Sridhar B; Kumar V; Haque R; Siddiqi MI; Nazir A Benzofuran-chalcone hybrids as potential multifunctional agents against Alzheimer’s disease: synthesis and in vivo studies with transgenic Caenorhabditis elegans. ChemMedChem 2014, 9, 2671–2684. [DOI] [PubMed] [Google Scholar]
- (389).Terstappen GC; Schlupen C; Raggiaschi R; Gaviraghi G Target deconvolution strategies in drug discovery. Nat. Rev. Drug Discovery 2007, 6, 891–903. [DOI] [PubMed] [Google Scholar]
- (390).Sato S; Murata A; Shirakawa T; Uesugi M Biochemical target isolation for novices: affinity-based strategies. Chem. Biol 2010, 17, 616–623. [DOI] [PubMed] [Google Scholar]
- (391).Lomenick B; Olsen RW; Huang J Identification of direct protein targets of small molecules. ACS Chem. Biol 2011, 6, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (392).Cong F; Cheung AK; Huang SM Chemical geneticsbased target identification in drug discovery. Annu. Rev. Pharmacol. Toxicol 2012, 52, 57–78. [DOI] [PubMed] [Google Scholar]
- (393).Sieber SA; Niessen S; Hoover HS; Cravatt BF Proteomic profiling of metalloprotease activities with cocktails of active-site probes. Nat. Chem. Biol 2006, 2, 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Fonovic M; Bogyo M Activity based probes for proteases: applications to biomarker discovery, molecular imaging and drug screening. Curr. Pharm. Des 2007, 13, 253–261. [DOI] [PubMed] [Google Scholar]
- (395).Kanoh N Photo-cross-linked small-molecule affinity matrix as a tool for target identification of bioactive small molecules. Nat. Prod. Rep 2016, 33, 709–718. [DOI] [PubMed] [Google Scholar]
- (396).Morimoto K; van der Hoorn RA The Increasing Impact of Activity-Based Protein Profiling in Plant Science. Plant Cell Physiol 2016, 57, 446–461. [DOI] [PubMed] [Google Scholar]
- (397).Krysiak J; Breinbauer R Activity-based protein profiling for natural product target discovery. Top. Curr. Chem 2011, 324, 43–84. [DOI] [PubMed] [Google Scholar]
- (398).Evans MJ; Cravatt BF Mechanism-based profiling of enzyme families. Chem. Rev 2006, 106, 3279–3301. [DOI] [PubMed] [Google Scholar]
- (399).Niphakis MJ; Cravatt BF Enzyme inhibitor discovery by activity-based protein profiling. Annu. Rev. Biochem 2014, 83, 341–377. [DOI] [PubMed] [Google Scholar]
- (400).Williams SJ; Hekmat O; Withers SG Synthesis and testing of mechanism-based protein-profiling probes for retaining endo-glycosidases. ChemBioChem 2006, 7, 116–124. [DOI] [PubMed] [Google Scholar]
- (401).Vocadlo DJ; Bertozzi CR A strategy for functional proteomic analysis of glycosidase activity from cell lysates. Angew. Chem., Int. Ed 2004, 43, 5338–5342. [DOI] [PubMed] [Google Scholar]
- (402).Stubbs KA; Scaffidi A; Debowski AW; Mark BL; Stick RV; Vocadlo DJ Synthesis and use of mechanism-based proteinprofiling probes for retaining beta-D-glucosaminidases facilitate identification of Pseudomonas aeruginosa NagZ. J. Am. Chem. Soc 2008, 130, 327–335. [DOI] [PubMed] [Google Scholar]
- (403).Chauvigne-Hines LM; Anderson LN; Weaver HM; Brown JN; Koech PK; Nicora CD; Hofstad BA; Smith RD; Wilkins MJ; Callister SJ; et al. Suite of activity-based probes for cellulose-degrading enzymes. J. Am. Chem. Soc 2012, 134, 20521–20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Kato D; Boatright KM; Berger AB; Nazif T; Blum G; Ryan C; Chehade KA; Salvesen GS; Bogyo M Activity-based probes that target diverse cysteine protease families. Nat. Chem. Biol 2005, 1, 33–38. [DOI] [PubMed] [Google Scholar]
- (405).Greenbaum DC; Arnold WD; Lu F; Hayrapetian L; Baruch A; Krumrine J; Toba S; Chehade K; Bromme D; Kuntz ID; et al. Small molecule affinity fingerprinting. A tool for enzyme family subclassification, target identification, and inhibitor design. Chem. Biol 2002, 9, 1085–1094. [DOI] [PubMed] [Google Scholar]
- (406).Patricelli MP; Szardenings AK; Liyanage M; Nomanbhoy TK; Wu M; Weissig H; Aban A; Chun D; Tanner S; Kozarich JW Functional interrogation of the kinome using nucleotide acyl phosphates. Biochemistry 2007, 46, 350–358. [DOI] [PubMed] [Google Scholar]
- (407).Tsai CS; Yen HY; Lin MI; Tsai TI; Wang SY; Huang WI; Hsu TL; Cheng YS; Fang JM; Wong CH Cellpermeable probe for identification and imaging of sialidases. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 2466–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (408).Liu Y; Patricelli MP; Cravatt BF Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. U. S. A 1999, 96, 14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (409).Patricelli MP; Giang DK; Stamp LM; Burbaum JJ Direct visualization of serine hydrolase activities in complex proteomes using fluorescent active site-directed probes. Proteomics 2001, 1, 1067–1071. [DOI] [PubMed] [Google Scholar]
- (410).Blair JA; Rauh D; Kung C; Yun CH; Fan QW; Rode H; Zhang C; Eck MJ; Weiss WA; Shokat KM Structure-guided development of affinity probes for tyrosine kinases using chemical genetics. Nat. Chem. Biol 2007, 3, 229–238. [DOI] [PubMed] [Google Scholar]
- (411).Heal WP; Dang TH; Tate EW Activity-based probes: discovering new biology and new drug targets. Chem. Soc. Rev 2011, 40, 246–257. [DOI] [PubMed] [Google Scholar]
- (412).Cisar JS; Cravatt BF Fully functionalized small-molecule probes for integrated phenotypic screening and target identification. J. Am. Chem. Soc 2012, 134, 10385–10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (413).George Cisar EA; Nguyen N; Rosen H A GTP affinity probe for proteomics highlights flexibility in purine nucleotide selectivity. J. Am. Chem. Soc 2013, 135, 4676–4679. [DOI] [PubMed] [Google Scholar]
- (414).Hulce JJ; Cognetta AB; Niphakis MJ; Tully SE; Cravatt BF Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat. Methods 2013, 10, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Li Z; Hao P; Li L; Tan CY; Cheng X; Chen GY; Sze SK; Shen HM; Yao SQ Design and synthesis of minimalist terminal alkyne-containing diazirine photo-crosslinkers and their incorporation into kinase inhibitors for cell- and tissue-based proteome profiling. Angew. Chem., Int. Ed 2013, 52, 8551–8556. [DOI] [PubMed] [Google Scholar]
- (416).Shi H; Zhang CJ; Chen GY; Yao SQ Cell-based proteome profiling of potential dasatinib targets by use of affinitybased probes. J. Am. Chem. Soc 2012, 134, 3001–3014. [DOI] [PubMed] [Google Scholar]
- (417).Ranjitkar P; Perera BG; Swaney DL; Hari SB; Larson ET; Krishnamurty R; Merritt EA; Villen J; Maly DJ Affinitybased probes based on type II kinase inhibitors. J. Am. Chem. Soc 2012, 134, 19017–19025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (418).Salisbury CM; Cravatt BF Optimization of activity-based probes for proteomic profiling of histone deacetylase complexes. J. Am. Chem. Soc 2008, 130, 2184–2194. [DOI] [PubMed] [Google Scholar]
- (419).Speers AE; Adam GC; Cravatt BF Activity-based protein profiling in vivo using a copper(i)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc 2003, 125, 4686–4687. [DOI] [PubMed] [Google Scholar]
- (420).Adibekian A; Martin BR; Chang JW; Hsu KL; Tsuboi K; Bachovchin DA; Speers AE; Brown SJ; Spicer T; Fernandez-Vega V; et al. Confirming target engagement for reversible inhibitors in vivo by kinetically tuned activity-based probes. J. Am. Chem. Soc 2012, 134, 10345–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (421).Chang JW; Cognetta AB 3rd; Niphakis MJ; Cravatt BF Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition. ACS Chem. Biol 2013, 8, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (422).Ahn K; Johnson DS; Mileni M; Beidler D; Long JZ; McKinney MK; Weerapana E; Sadagopan N; Liimatta M; Smith SE; et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem. Biol 2009, 16, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Battenberg OA; Yang Y; Verhelst SH; Sieber SA Target profiling of 4-hydroxyderricin in S. aureus reveals seryl-tRNA synthetase binding and inhibition by covalent modification. Mol. BioSyst 2013, 9, 343–351. [DOI] [PubMed] [Google Scholar]
- (424).Akihisa T; Tokuda H; Ukiya M; Iizuka M; Schneider S; Ogasawara K; Mukainaka T; Iwatsuki K; Suzuki T; Nishino H Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett 2003, 201, 133–137. [DOI] [PubMed] [Google Scholar]
- (425).Zhang X; Luo L; Ma Z A deuterium-labelling mass spectrometry-tandem diode-array detector screening method for rapid discovery of naturally occurring electrophiles. Anal. Bioanal. Chem 2011, 400, 3463–3471. [DOI] [PubMed] [Google Scholar]
- (426).Luo L; Wang R; Wang X; Ma Z; Li N Compounds from Angelica keiskei with NQO1 induction, DPPH scavenging and α-glucosidase inhibitory activities. Food Chem 2012, 131, 992–998. [Google Scholar]
- (427).Mandelli D; Chiacchio KC; Kozlov YN; Shul’pin GB Hydroperoxidation of alkanes with hydrogen peroxide catalyzed by aluminium nitrate in acetonitrile. Tetrahedron Lett 2008, 49, 6693–6697. [Google Scholar]
- (428).Gerhauser C Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [DOI] [PubMed] [Google Scholar]
- (429).Albini A; Dell’Eva R; Vene R; Ferrari N; Buhler DR; Noonan DM; Fassina G Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB and Akt as targets. FASEB J 2006, 20, 527–529. [DOI] [PubMed] [Google Scholar]
- (430).Magalhaes PJ; Carvalho DO; Cruz JM; Guido LF; Barros AA Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat. Prod. Commun 2009, 4, 591–610. [PubMed] [Google Scholar]
- (431).Jacobs AT; Marnett LJ Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc. Chem. Res 2010, 43, 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (432).Geurink PP; Prely LM; van der Marel GA; Bischoff R; Overkleeft HS Photoaffinity labeling in activity-based protein profiling. Top. Curr. Chem 2011, 324, 85–113. [DOI] [PubMed] [Google Scholar]
- (433).Lapinsky DJ Tandem photoaffinity labeling-bioorthogonal conjugation in medicinal chemistry. Bioorg. Med. Chem 2012, 20, 6237–6247. [DOI] [PubMed] [Google Scholar]
- (434).Smith E; Collins I Photoaffinity labeling in target- and binding-site identification. Future Med. Chem 2015, 7, 159–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (435).Webb Y; Zhou X; Ngo L; Cornish V; Stahl J; ErdjumentBromage H; Tempst P; Rifkind RA; Marks PA; Breslow R; et al. Photoaffinity labeling and mass spectrometry identify ribosomal protein S3 as a potential target for hybrid polar cytodifferentiation agents. J. Biol. Chem 1999, 274, 14280–14287. [DOI] [PubMed] [Google Scholar]
- (436).Hein JE; Fokin VV Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev 2010, 39, 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (437).Ong SE; Blagoev B; Kratchmarova I; Kristensen DB; Steen H; Pandey A; Mann M Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 2002, 1, 376–386. [DOI] [PubMed] [Google Scholar]
- (438).Ong SE; Schenone M; Margolin AA; Li X; Do K; Doud MK; Mani DR; Kuai L; Wang X; Wood JL; et al. Identifying the proteins to which small-molecule probes and drugs bind in cells. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 4617–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (439).Ong SE; Kratchmarova I; Mann M Properties of 13Csubstituted arginine in stable isotope labeling by amino acids in cell culture (SILAC). J. Proteome Res 2003, 2, 173–181. [DOI] [PubMed] [Google Scholar]
- (440).Bezerra DP; Militao GC; de Castro FO; Pessoa C; de Moraes MO; Silveira ER; Lima MA; Elmiro FJ; Costa-Lotufo LV Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol. In Vitro 2007, 21, 1–8. [DOI] [PubMed] [Google Scholar]
- (441).Adams DJ; Dai M; Pellegrino G; Wagner BK; Stern AM; Shamji AF; Schreiber SL Synthesis, cellular evaluation, and mechanism of action of piperlongumine analogs. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 15115–15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (442).Wu Y; Min X; Zhuang C; Li J; Yu Z; Dong G; Yao J; Wang S; Liu Y; Wu S; et al. Design, synthesis and biological activity of piperlongumine derivatives as selective anticancer agents. Eur. J. Med. Chem 2014, 82, 545–551. [DOI] [PubMed] [Google Scholar]
- (443).Zou P; Xia Y; Ji J; Chen W; Zhang J; Chen X; Rajamanickam V; Chen G; Wang Z; Chen L; et al. Piperlongumine as a direct TrxR1 inhibitor with suppressive activity against gastric cancer. Cancer Lett 2016, 375, 114–126. [DOI] [PubMed] [Google Scholar]
- (444).Duarte CD; Barreiro EJ; Fraga CA Privileged structures: a useful concept for the rational design of new lead drug candidates. Mini-Rev. Med. Chem 2007, 7, 1108–1119. [DOI] [PubMed] [Google Scholar]
- (445).Welsch ME; Snyder SA; Stockwell BR Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol 2010, 14, 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (446).DeSimone RW; Currie KS; Mitchell SA; Darrow JW; Pippin DA Privileged structures: applications in drug discovery. Comb. Chem. High Throughput Screening 2004, 7, 473–494. [DOI] [PubMed] [Google Scholar]
- (447).Berthet M; Cheviet T; Dujardin G; Parrot I; Martinez J Isoxazolidine: A Privileged Scaffold for Organic and Medicinal Chemistry. Chem. Rev 2016, 116, 15235–15283. [DOI] [PubMed] [Google Scholar]
- (448).Silva CF; Pinto DC; Silva AM Chromones: A Promising Ring System for New Anti-inflammatory Drugs. ChemMedChem 2016, 11, 2252–2260. [DOI] [PubMed] [Google Scholar]
- (449).Song Y; Chen W; Kang D; Zhang Q; Zhan P; Liu X ″Old friends in new guise″: exploiting privileged structures for scaffold re-evolution/refining. Comb. Chem. High Throughput Screening 2014, 17, 536–553. [DOI] [PubMed] [Google Scholar]
- (450).Giacomini E; Rupiani S; Guidotti L; Recanatini M; Roberti M The Use of Stilbene Scaffold in Medicinal Chemistry and Multi- Target Drug Design. Curr. Med. Chem 2016, 23, 2439–2489. [DOI] [PubMed] [Google Scholar]
- (451).Song Y; Zhan P; Liu X Heterocycle-thioacetic acid motif: a privileged molecular scaffold with potent, broad-ranging pharmacological activities. Curr. Pharm. Des 2013, 19, 7141–7154. [DOI] [PubMed] [Google Scholar]
- (452).Song Y; Zhan P; Zhang Q; Liu X Privileged scaffolds or promiscuous binders: a glance of pyrrolo[2,1-f][1,2,4]triazines and related bridgehead nitrogen heterocycles in medicinal chemistry. Curr. Pharm. Des 2013, 19, 1528–1548. [PubMed] [Google Scholar]
- (453).Li Z; Zhan P; Liu X 1,3,4-oxadiazole: a privileged structure in antiviral agents. Mini-Rev. Med. Chem 2011, 11, 1130–1142. [DOI] [PubMed] [Google Scholar]
- (454).Petrov O; Ivanova Y; Gerova M SOCl2/EtOH: Catalytic system for synthesis of chalcones. Catal. Commun 2008, 9, 315–316. [Google Scholar]
- (455).Yamada YMA; Uozumi Y Development of a convoluted polymeric nanopalladium catalyst: α-alkylation of ketones and ringopening alkylation of cyclic 1,3-diketones with primary alcohols. Tetrahedron 2007, 63, 8492–8498. [Google Scholar]
- (456).Yoshizawa K; Shioiri T Convenient stereoselective synthesis of (Z)-chalcone derivatives from 1,3-diaryl-2-propynyl silyl ethers. Tetrahedron Lett 2006, 47, 4943–4945. [Google Scholar]