Abstract
Trastuzumab, an epidermal growth factor receptor 2 (HER2) targeting humanized monoclonal antibody, has been approved for the treatment HER2-positive breast cancer and HER2-positve metastatic gastric cancer. However, cardiotoxicity associated with its clinical application poses challenges for clinicians and patients, mechanisms of which are still evolving. This review will summarize the current mechanistic understanding of trastuzumab-mediated cardiotoxicity, discuss the novel role of DNA topoisomerase IIB as a shared target for enhanced cardiotoxicity induced by trastuzumab and anthracyclines-based combination regimens, and speculate the potential impact of trastuzumab intervention in immune checkpoint inhibitors-based therapies.
Keywords: trastuzumab, cardiotoxicity, HER2, TOP2B
Statement of Significance
Mechanisms of trastuzumab-mediated cardiotoxicity are still evolving. This review highlights novel discovery of DNA Topoisomerase II as a shared target for anthracycline and trastuzumab-induced cardiotoxicity and speculates on cardiac risks associated with trastuzumab and immune checkpoint-based therapies.
TRASTUZUMAB AND MECHANISMS OF ACTION
Trastuzumab, a humanized monoclonal antibody directed against epidermal growth factor receptor 2 (HER2), has been approved to treat multiple oncologic conditions, including HER2-positive breast cancers in adjuvant, neoadjuvant, and metastatic settings and metastatic gastric cancer [1,2,3]. HER2 is overexpressed in approximately 20–25% of breast cancers, which is characterized by aggressive growth and poor prognosis. While it has been postulated that there is no recognized ligand binding to HER2 extracellular domain, understanding of HER2 structural intricacies and HER2 biology has spurred development of multiple HER2 targeting agents, often with complex and previously unrecognized mechanisms of action. Trastuzumab binds to domain IV of extracellular domain of HER2 and triggers its tumor-suppressive actions through multiple mechanisms, including activation of antibody-dependent cell-mediated cytotoxicity, inhibition of HER2 extracellular domain cleavage, disruption of HER2 receptor homodimerization and heterodimerization, abrogation of oncogenic cellular signaling, and downregulation of angiogenesis and DNA repair pathways [1,4,5]. Knowledge of the anti-tumor mechanisms of trastuzumab is constantly developing, due to better understanding of HER2-associated signaling pathways, and many aspects of mechanisms of action still need to be clarified. Accumulating data suggest that unlike tyrosine kinase inhibitors or cetuximab, a monoclonal antibody directed against epidermal growth factor receptor (EGFR), trastuzumab increases tyrosine kinase activity of HER2 and induces phosphorylation of HER2 and EGFR at tyrosine 1248 (Y1248) and tyrosine 845 (Y845), respectively, in HER2-positive breast cancer cells [6,7,8]. The enhanced HER2 phosphorylation at Y1248 increases the interaction of HER2 with non-receptor Csk-homologous kinase that negatively regulates the activity of HER2 signaling, leading to growth inhibition of breast cancer cells [6]. Clinical data suggest that the status of HER2 phosphorylation at Y1248 correlates with the response to trastuzumab treatment in neoadjuvant settings, consistent with findings obtained from the cellular model [6].
Clinical benefits of trastuzumab in conjunction with chemotherapy are well documented; however, therapeutic application of trastuzumab-based regimens is limited by occurrence of significant adverse cardiac events. While clinical data supporting the in vivo relevance of different mechanisms of trastuzumab action and side effects are still lacking, expanded use of trastuzumab in clinical practice has spurred interest in research models that aid understanding of trastuzumab-associated clinical phenomena, including cardiotoxicity. In this review, we attempted to summarize some of the highlights and challenges that will be at the frontiers of trastuzumab research.
CARDIOTOXIC EVENTS REPORTED IN CLINICAL TRIALS
Early phase I and phase II clinical trials in which trastuzumab was used alone or in combination with cisplatin appear to be well tolerated and did not report any significant adverse cardiac events in the patients [9,10]. The pivotal, randomized multicenter phase III trial by Slamon and colleagues showed that combining trastuzumab with anthracyclines caused cardiac dysfunctions and heart failures in up to 27% of HER2-positive metastatic breast cancer patients compared with less than 7% in anthracyclines only group [11]. Retrospective evaluation of incidence of cardiac dysfunctions caused by trastuzumab in adjuvant setting concluded that approximately one in four women might develop left ventricular systolic dysfunctions after trastuzumab administration [12]. Likewise, another large population-based, observational study of 12 500 women diagnosed with invasive breast cancer reported that incidences of cardiotoxicity and cardiomyopathy were higher in trastuzumab-treated group compared to anthracycline alone, thus complementing the findings from clinical trial data on trastuzumab safety [13]. Concomitant administration of anthracyclines and trastuzumab in most clinical trials reported higher percentage of cardiac toxicity [14]. Unexpected high incidences of cardiac dysfunctions in these studies changed the design of subsequent clinical trials in which concurrent treatment of trastuzumab and anthracyclines was substituted with sequential therapy. Large clinical trial Breast Cancer International Research Group 006 investigating trastuzumab efficacy in adjuvant setting reported that 2% incidence of congestive heart failure occurred in chemotherapy and trastuzumab sequential therapy group in comparison to 0.7% in chemotherapy only group [15]. Similarly, final analysis from HERceptin Adjuvant trial also reported much lower rate of cardiotoxicity with 7.25% incidence of secondary cardiac endpoint (class I or II toxicity as defined by New York Heart Association) in 2-year trastuzumab group and 4.4% in 1-year trastuzumab [16]. To minimize the cardiotoxicity, these large adjuvant clinical trials implemented few guidelines that included sequential administration of trastuzumab after chemotherapy, careful monitoring of left ventricle ejection fraction (LVEF) during trastuzumab treatment, excluding the patients with pre-existing cardiovascular illness and discontinuation of trastuzumab when patients develop cardiac symptoms [17]. To assess whether pharmacological interventions can reduce trastuzumab-mediated cardiotoxicity, two clinical studies, The Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research and Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy, used angiotensin enzyme inhibitor, β-blocker, and angiotensin-receptor blocker in adjuvant trastuzumab settings; however, both studies reported no significant benefit to patients when these cardioprotective agents were added to trastuzumab-based regimen [17]. Despite enforcement of stringent patient enrollment criteria, trastuzumab-based regimens are still regarded as significant cardiotoxicity risk, although the numbers of cardiotoxic events from recent clinical studies are less than reported in earlier trials.
MOLECULAR MECHANISMS OF CARDIOTOXICITY
Despite considerable efforts to uncover molecular aspects of trastuzumab-induced cardiotoxicity, its mechanisms remain incompletely understood. Trastuzumab-induced cardiac dysfunctions were regarded as less severe and largely reversible because primary cardiomyocyte did not show ultrastructure changes that were associated with anthracycline-induced cardiotoxicity, and primary myocyte injury did not occur in patients that were treated with trastuzumab [18]. Concerns have been raised about immediate and long-term cardiovascular risks associated with trastuzumab therapy and the need for longer cardiac follow-up for patients was emphasized [19, 20]. In vivo investigation from our lab showed that trastuzumab caused ultrastructural alternations in mice heart tissues as detected by electron microscopy. This suggested that trastuzumab might produce lasting effect on myocyte structure, thus questioning the reversibility concept of trastuzumab-induced cardiotoxicity [21]. Additionally, trastuzumab treatment also significantly changed expression profiles of genes necessary for cardiac and mitochondrial functions and DNA repair [21]. Interestingly, trastuzumab-treated mice had significantly elevated serum levels of cardiac myosin light chain, which could be evaluated as potential biomarker for trastuzumab-induced cardiotoxicity in humans [21].
Integral role of HER2 signaling in cardiac development is well established [22, 23]. Conditional mutant mice carrying cardiac-specific deletion of HER2 showed multiple independent parameters of dilated cardiomyopathy, thus emphasizing the pivotal role of HER2 in embryonic and postnatal cardiogenesis [24]. Recent investigation from our lab confirmed the direct link between impaired HER2 signaling and trastuzumab-induced cardiotoxicity and showed that trastuzumab dysregulates HER2 signaling and suppresses autophagy in cardiomyocytes to trigger accumulation of toxic reactive oxygen species (ROS) in human cardiomyocytes [25]. Trastuzumab disrupts HER signaling by inducing phosphorylation of HER1 and HER2 at 845 and 1248 sites, respectively, and activates autophagy-inhibitory Erk/mTOR/Ulk 1 signaling cascade, thereby compromising cardiomyocyte’s ability to recycle toxic cellular substrates causing cardiotoxicity [25]. On the contrary, pertuzumab, another anti-HER2 monoclonal antibody with distinct epitope, did not affect HER2 signaling cascade (as evaluated by HER1 and HER2 phosphorylation), at least in cardiomyocyte in vitro model, which is consistent with results from clinical studies reporting that addition of pertuzumab to trastuzumab-based regimens did not have additive or synergistic effect on cardiotoxicity [26]. Not unexpectedly, Akt activity, which was downregulated by trastuzumab in HER2-positive breast cancer cells [6], remained unchanged in cardiomyocytes treated with trastuzumab, suggesting that HER2 downstream signaling is differentially regulated by trastuzumab dependent on status of HER2 expression levels in cells. Differential regulation of HER2 signaling pathways following trastuzumab binding in human cardiomyocytes and tumor cells remains underexplored and could hold potential for understanding problems such as trastuzumab-associated cardiotoxicity.
TOP2B AS A SHARED TARGET FOR THE ENCHANTED CARDIOTOXICITY INDUCED BY THE COMBINATION OF TRASTUZUMAB AND DOXORUBICIN TREATMENT
Although combination of trastuzumab and anthracycline-based chemotherapy, such as doxorubicin, is used as a standard of care in the treatment for patients at different stages of breast cancers, understanding of the mechanisms of cardiotoxicity associated with this combination treatment remains elusive. Despite their efficacy in cancer treatment, both doxorubicin and trastuzumab have been found to induce severe cardiotoxicity, either alone or in combination. DNA topoisomerase IIB (TOP2B) was previously identified as a major cellular target for doxorubicin-induced cardiotoxicity [27]. Doxorubicin binds to TOP2B protein and DNA resulting in double strand DNA breaks, activation of DNA damage response pathway, and induction of programmed cell death (PD) in cardiomyocytes causing cardiotoxicity. It is tempting to speculate that in the absence of trastuzumab, anthracycline-induced damage via binding to TOP2B might be repaired by yet unidentified mechanism that is likely associated with normal HER2 function in cardiomyocytes [18]; in the presence of trastuzumab, an anti-HER2 binding agent, the HER2 function would be compromised and the more likely outcome would be significant damage in cardiomyocytes [18, 28]. Although plausible, little experimental evidence is available to support this “repair-interfering model” [18] for trastuzumab/anthracycline combination therapy associated cytotoxicity.
In our recent publication, we attempted to address the issue of cardiotoxicity associated with doxorubicin/trastuzumab combination therapy [29]. Using a high-throughput DNA microarray method to screen for genes affected by trastuzumab in the primary human cardiomyocytes, we found that trastuzumab downregulated gene expression of TOP2B [29]. Using primary cardiomyocytes as a cellular model, we identified that trastuzumab either alone or in combination with doxorubicin strongly downregulated TOP2B, a major intracellular target in doxorubicin-induced cardiotoxicity [27, 29]. Furthermore, treatment of cardiomyocytes with combination of trastuzumab and doxorubicin either sequentially or concurrently significantly enhances downregulation of the protein levels of TOP2B, increases apoptosis and cell growth inhibition, and promotes production of reactive oxidative and nitrative species in human cardiomyocytes as compared to either trastuzumab or doxorubicin treatment (Fig. 1) [26].
Figure 1.
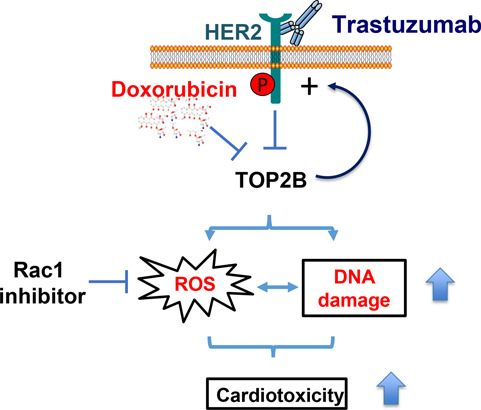
TOP2B: a shared target for the cardiotoxicity induced by doxorubicin and trastuzumab.
Both of doxorubicin and trastuzumab inhibit TOP2B in human cardiomyocytes. Their combined cardiotoxic effect at the cellular level is associated with increased ROS production and DNA damage enhancement. While doxorubicin inhibits TOP2 activity, it also enhances the levels of HER2 expression in human cardiomyocytes, which may render the cardiomyocytes to become addicted to HER2 signaling for survival under stressed conditions, leading to cardiomyocytes becoming more sensitive to trastuzumab treatment after doxorubicin exposure. Our proposed model explains the elevated cardiotoxicity induced by trastuzumab and doxorubicin combination therapy. In addition, Rac1, a small GTPase inhibitor, prevents trastuzumab from inducing DNA damage and suppresses increased ROS production induced by either trastuzumab alone or combination of doxorubicin and trastuzumab, suggesting that Rac1 inhibitor may be used to antagonize cardiotoxicity induced by trastuzumab or trastuzumab and doxorubicin combination therapy.
In our recent study, we also revealed that the treatment of human cardiomyocytes with doxorubicin increased HER2 protein level and activated downstream HER2 signaling pathway, which might render the cardiomyocytes to become addicted to HER2 signaling for survival under stressed conditions. Because doxorubicin treatment enhanced HER2 expression, we believed this could be the reason that cardiomyocytes become more sensitive to trastuzumab-mediated HER2-targeted treatment and subsequently more stressful under dual exposures. The finding of doxorubicin-induced upregulation of HER2 expression in human cardiomyocytes supports the “repair-interfering model”, in that trastuzumab may prevent recovery of cardiotoxicity induced by doxorubicin. Taken together, our study suggests a novel molecular model for the significantly increased cardiotoxicity induced by combination of trastuzumab and doxorubicin.
POTENTIAL CARDIOTOXICITY FROM IMMUNE CHECKPOINT INHIBITORS AND TRASTUZUMAB
Immune checkpoint proteins, cytotoxic T lymphocyte associated 4 and PD-1 that are frequently expressed in T cells, can interact with their ligands CD80/CD86 and programmed cell death ligand 1 (PD-L1). Their interaction can effectively block anti-tumor T cell responses and assist tumor cells in escaping T cell–mediated killing [30]. Several monoclonal antibodies targeted against immune checkpoints gained Food and Drug Administration (FDA) approval in recent years including anti-CTLA-4 antibodies (ipilimumab and tremelimumab), anti-PD-1 antibodies (nivolumab and pembrolizumab), and anti-PD-L1 antibodies (atezolizumab, avelumab, and durvalumab) [30]. Although, immune checkpoint inhibitors have revolutionized the clinical management of advanced staged malignancies with dismal prognosis [31], activation of immune system by these therapeutic monoclonal antibodies also causes wide spectrum of adverse events, including myocarditis, myocardial fibrosis, cardiomyopathy, and heart failure; mechanisms of which are not yet identified. Recently, Johnson et al. reported two death cases of lethal myocarditis accompanied with myositis in melanoma patients who received nivolumab and ipilimumab [32]. Both patients did not have history of any major cardiac risk factor except hypertension, but they developed severe cardiac symptoms within two weeks of administration of first dose [32]. Authors performed next generation sequencing of complementarity-determining region 3 of T cell receptors from tumor as well as heart and skeletal muscles biopsies, and observed that T cell receptor sequences were shared among cardiac muscle, skeletal muscle, and tumor tissues, which raises the possibility that T cells could be responding to common antigen [32]. Authors further analyzed large corporate safety database of Bristol-Meyers Squibb and concluded that patients treated with combination of nivolumab and ipilimumab are more vulnerable to adverse cardiac events compared to nivolumab alone (0.27% vs. 0.06%; P < 0.001), but condition remains rare in both regimen affecting only less than 1% of the patients [28,29]. Currently, many clinical trials are ongoing for the treatment of breast and gynecologic malignancies using immune-checkpoint antibodies alone and in combination with trastuzumab and anthracycline-based regimens [33]. Initial safety data from Phase IB of PANACEA trial evaluating efficacy of trastuzumab and pembrolizumab combination in PD-L1 positive patients were presented at 2017 San Antonio Breast Cancer Symposium, which did not observe any cardiac events in patients; however, 19% of the patients in this study developed immune-related adverse event [34]. Further data from these and other trials on trastuzumab clinical studies are awaited.
Since the population of cancer patients treated with anti-HER2 targeted therapy and/or immune checkpoint inhibitors is growing rapidly, the potential risk of cardiotoxicity in these patients is increasing. Thus, it is urgent to understand how these drugs induce cardiotoxicity and whether combination therapies could further increase the cardiac risk for cancer patients. It is also crucial to determine specific major histocompatibility phonotype, T cell characteristics, and other immune features that might predispose immune-related adverse cardiac events in patients exposed to immune checkpoint inhibitors and trastuzumab combination. Development of time-sensitive and specific bioassays and other innovative non-invasive methods that could detect early onset of cardiotoxicity is urgently needed to support post-marketing surveillance program for these drugs and improve the quality of life for cancer patients.
FUNDING
This work was supported by FDA Office of Women's Health Research Science Program award to Wen Jin Wu.
ACKNOWLEDGMENTS
The authors thank Drs. Ashwinkumar Bhirdeand and Nozomi Sakakibara for critical internal review of this article. This project was supported in part by an appointment to the ORISE Research Participation Program at the Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and FDA. Dr. Jiangsong Jiang is an ORISE research fellow supported by Food and Drug Administration Office of Women's Health.
Disclaimer: This article reflects the views of the authors and should not be construed to represent FDA's views or policies.
References
- 1. Hudis, CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007; 357: 39–51. [DOI] [PubMed] [Google Scholar]
- 2. Gianni, L, Eiermann, W, Semiglazov, Vet al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014; 15: 640–7. [DOI] [PubMed] [Google Scholar]
- 3. Bang, YJ, Van Cutsem, E, Feyereislova, Aet al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–97. [DOI] [PubMed] [Google Scholar]
- 4. Spector, NL, Blackwell, KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2009; 27: 5838–47. [DOI] [PubMed] [Google Scholar]
- 5. Dokmanovic, M, Wu, WJ. Trastuzumab-induced HER2 phosphorylation: exploring the mechanisms and implications. Receptors Clin Investig 2014; 1: e340. [Google Scholar]
- 6. Dokmanovic, M, Wu, Y, Shen, Yet al. Trastuzumab-induced recruitment of Csk-homologous kinase (CHK) to ErbB2 receptor is associated with ErbB2-Y1248 phosphorylation and ErbB2 degradation to mediate cell growth inhibition. Cancer Biol Ther 2014; 8: 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohan, N, Wu, WJ. Trastuzumab is not a tyrosine kinase inhibitor. Nat Rev Cardiol 2015; 12: 669. [DOI] [PubMed] [Google Scholar]
- 8. Gijsen, M, King, P, Perera, Tet al. HER2 phosphorylation is maintained by a PKB negative feedback loop in response to anti-HER2 herceptin in breast cancer. PLoS Biol 2010; 8: e1000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baselga, J, Tripathy, D, Mendelsohn, Jet al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol 1999; 26: 78–83. [PubMed] [Google Scholar]
- 10. Baselga, J. Phase I and II clinical trials of trastuzumab. Ann Oncol 2001; 12: S49–55. [DOI] [PubMed] [Google Scholar]
- 11. Slamon, DJ, Leyland-Jones, B, Shak, Set al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92. [DOI] [PubMed] [Google Scholar]
- 12. Wadhwa, D, Fallah-Rad, N, Grenier, Det al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res Treat 2009; 117: 357–64. [DOI] [PubMed] [Google Scholar]
- 13. Bowles, EJ, Wellman, R, Feigelson, HSet al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012; 104: 1293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nemeth, BT, Varga, ZV, Wu, WJet al. Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br J Pharmacol 2017; 174: 3727–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slamon, D, Eiermann, W, Robert, Net al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011; 365: 1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cameron, D, Piccart-Gebhart, MJ, Gelber, RDet al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017; 389: 1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dang, CT, Yu, AF, Jones, LWet al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol 2016; 34: 1030–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ewer, MS, Ewer, SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 2015; 12: 547–58. [DOI] [PubMed] [Google Scholar]
- 19. Telli, ML, Hunt, SA, Carlson, RWet al. Trastuzumab related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 2007; 25: 3525–33. [DOI] [PubMed] [Google Scholar]
- 20. Riccio, G, Coppola, C, Piscopo, Get al. Trastuzumab and target-therapy side effects: is still valid to differentiate anthracycline Type I from Type II cardiomyopathies? Hum Vaccin Immunother 2016; 12: 1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ElZarrad, MK, Mukhopadhyay, P, Mohan, Net al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS One 2013; 8: e79543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Negro, A, Brar, BK, Lee, KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res 2004; 59: 1–12. [DOI] [PubMed] [Google Scholar]
- 23. Dokmanovic, M, King, KE, Mohan, Net al. Cardiotoxicity of ErbB2-targeted therapies and its impact on drug development, a spotlight on trastuzumab. Expert Opin Drug Metab Toxicol 2017; 13: 755–66. [DOI] [PubMed] [Google Scholar]
- 24. Crone, SA, Zhao, YY, Fan, Let al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 2002; 8: 459–65. [DOI] [PubMed] [Google Scholar]
- 25. Mohan, N, Shen, Y, Endo, Yet al. Trastuzumab, but not pertuzumab. dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol Cancer Ther2016; 15: 321–31. [DOI] [PubMed] [Google Scholar]
- 26. Yu, AF, Singh, JC, Wang, Ret al. Cardiac safety of dual anti-HER2 therapy in the neoadjuvant setting for treatment of HER2-positive breast cancer. Oncologist 2017; 22: 642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang, S, Liu, X, Bawa-Khalfe, Tet al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012; 18: 1639–42. [DOI] [PubMed] [Google Scholar]
- 28. Vejpongsa, P, Yeh, ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014; 64: 938–45. [DOI] [PubMed] [Google Scholar]
- 29. Jiang, J, Mohan, N, Endo, Yet al. Type IIB DNA topoisomerase is downregulated by trastuzumab and doxorubicin to synergize cardiotoxicity. Oncotarget 2017; 9: 6095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abril-Rodriguez, G, Ribas, A. SnapShot: immune checkpoint inhibitors. Cancer Cell 2017; 31: 848–8. [DOI] [PubMed] [Google Scholar]
- 31. Varricchi, G, Galdiero, MR, Marone, Get al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017; 2: e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson, DB, Balko, JM, Compton, MLet al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016; 375: 1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hartkopf, AD, Taran, FA, Wallwiener, Met al. PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care (Basel) 2016; 11: 385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loi, S, Giobbe-Hurder, A, Gombos, Aet al. Phase Ib/II study evaluating safety and efficacy of pembrolizumab and trastuzumab in patients with trastuzu-mab-resistant HER2-positive metastatic breast cancer: results from the -PANACEA (IBCSG 45-13//KEYNOTE-014) study. In: 2017 San Antonio Breast Cancer Symposium, 2017Abstract GS2-06.


