Clinical presentations of hemophagocytic lymphohistiocytosis (HLH) and septic shock share many similarities, including multiple organ dysfunction and overall clinical and biological symptoms. However, these life-threatening conditions require specific and opposing treatments. Currently, no single biomarker is available to differentiate septic shock from HLH at patient admission [1, 2]. HLH is classified as a primary (genetically inherited) or a secondary, i.e., induced by various inflammatory conditions (viral infections, autoimmune processes, lymphoid malignancies, or drug allergies), immune disorder. We report here the case of a young woman with febrile shock which proved to be a HLH caused by drug-induced hypersensitivity syndrome (DIHS). As septic shock was initially suspected, the patient benefited from broad immunological screening during the first week of evolution [3]. Strikingly, this revealed massively increased expression of monocyte human leukocyte antigen-DR (mHLA-DR) at 137,021 ABC (antibody bound per cell), even though expected values in septic shock are usually drastically decreased [3]. In addition, a positive response to increasing doses of corticosteroids was observed over time (Fig. 1). More precisely, while the patient’s mHLA-DR expression was measured at 137,021 ABC at admission, it decreased to 38,961 ABC after the introduction of corticosteroids (day 3). Following the reactivation of inflammatory processes (day 5), mHLA-DR rose again (66,829 ABC). Finally, mHLA-DR returned to a normal range after increasing corticosteroid doses (20,499 ABC, day 8). All clinical features are provided in Additional file 1.
Fig. 1.
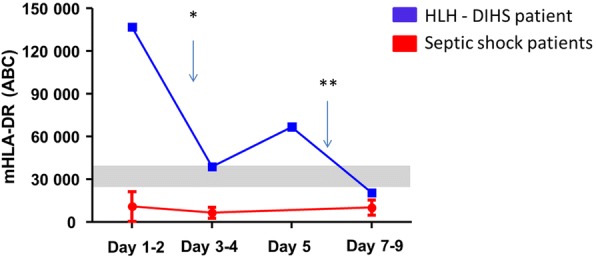
Time course of mHLA-DR in a HLH patient. Blue squares depict mHLA-DR values in the HLH patient. Red circles represent pediatric septic shock values [3]. Gray range represents interquartile range values obtained previously in healthy children [3]. *Corticosteroids introduced, receiving 2 mg/kg/day; **corticosteroid adjustment to 4 mg/kg/day
In the present patient, the extremely increased inaugural mHLA-DR value (i.e., 137,021 ABC) helped to unequivocally exclude a diagnosis of septic shock. Indeed, in our experience (more than 600 septic shock patients monitored over several years), the vast majority of mHLA-DR values measured within the first 3 days after septic shock are reported to be < 30,000 ABC and mostly found below 10,000 ABC (normal values ranged from 15,000 to 40,000 ABC). This agrees with pathophysiology since HLH is secondary to overproduction of interferon-γ (IFN-γ), a cytokine known to be a strong inducer of mHLA-DR expression, whereas sepsis induces downregulation of mHLA-DR expression.
In conclusion, mHLA-DR may discriminate septic shock from HLH at admission despite both situations with multiple organ dysfunction sharing very common clinical and biological features (e.g., sCD25, elevated ferritin levels) [4, 5]. This result obviously needs further assessment in various types of HLH. Upon confirmation, as these two deadly conditions (i.e., septic shock and HLH) would require opposing treatments, mHLA-DR may be of crucial help for clinicians regarding patients’ care and management.
Additional file
Additional online information. (DOCX 27 kb)
Acknowledgments
Funding
This work was supported by Hospices Civils de Lyon and University Claude Bernard Lyon 1.
Availability of data and materials
According to the French National Data Protection Commission, we are not authorized to provide the individual clinical data.
Abbreviations
- ABC
Antibody bound per cell
- DIHS
Drug-induced hypersensitivity syndrome
- HLH
Hemophagocytic lymphohistiocytosis
- IFN-γ
Interferon-γ
- mHLA-DR
Monocyte human leukocyte antigen-DR
Authors’ contributions
EJ and GM conceptualized and designed the PedIRIS study and reviewed and revised each draft of the manuscript. SR, AP, and AB participated in patient treatment and reviewed and revised the manuscript. SR, MG, FV, and JH performed biological analysis and reviewed and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This case report belongs to the PedIRIS study. This observational prospective clinical study was approved by our Institutional Review Board (Comité de Protection des Personnes, Lyon Sud-Est II, number 2014–010-2, in accordance with Article L1121–1 of the French Public Health Code). According to legislation in place at the time of the study, this study required only the non-opposition of the study participants (written informed consent was not required). An information leaflet was systematically distributed to holders of parental authority. Participants and/or holders of parental authority could withdraw consent at any time. The study was registered: Pediatric Immune Response to Infectious Shock (PedIRIS), NCT02848144.
Consent for publication
Written informed consent for publication of their clinical details was obtained from the parent of the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Solenn Remy, Email: solene.remy@chu-lyon.fr.
Morgane Gossez, Email: morgane.gossez@chu-lyon.fr.
Alexandre Belot, Email: alexandre.belot@chu-lyon.fr.
Jack Hayman, Email: jack.hayman@student.manchester.ac.uk.
Aurelie Portefaix, Email: aurelie.portefaix@chu-lyon.fr.
Fabienne Venet, Email: fabienne.venet@chu-lyon.fr.
Etienne Javouhey, Email: etienne.javouhey@chu-lyon.fr.
Guillaume Monneret, Phone: +33 4 72 11 97 58, Email: guillaume.monneret@chu-lyon.fr.
References
- 1.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10(3):387–392. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]
- 2.Machowicz R, Janka G. Similar but not the same: differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol. 2017;114:1–12. doi: 10.1016/j.critrevonc.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Remy S, Kolev-Descamps K. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: a pilot study. Ann Intensive Care. 2018;8(1):36. doi: 10.1186/s13613-018-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosario C, Zandman-Goddard G. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Møller HJ, Moestrup S. Macrophage serum markers in pneumococcal bacteremia: prediction of survival by soluble CD163. Crit Care Med. 2006;34(10):2561–2566. doi: 10.1097/01.CCM.0000239120.32490.AB. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional online information. (DOCX 27 kb)
Data Availability Statement
According to the French National Data Protection Commission, we are not authorized to provide the individual clinical data.


