Abstract
Nodulocystic acne is a severe form of acne which can result in significant damage to the skin with great impact on quality of life. Oral isotretinoin considered to be the best treatment for such cases. Although it has a high rate of success and its efficacy is well established in the treatment of nodulocystic acne, it may occasionally fail to control the disease. We report a case of a patient who presented to our skin clinic with severe facial nodulocystic acne in which treatment with isotretinoin failed to achieve disease control and caused worsening of his baseline condition. Therefore, oral dapsone was administered as an alternative treatment, and we reached a complete remission of acne lesions within six months. Oral dapsone could be an adequate and safe drug in severe acne, and it might also be a promising and hopeful alternative treatment for nodulocystic acne when isotretinoin fails.
Keywords: Acne Vulgaris, Acne Conglobata, Isotretinoin, Dapsone
Introduction
Acne vulgaris is a common chronic inflammatory skin disorder of the pilosebaceous unit with multifactorial pathogenesis, variable morphology and a great psychological impact especially in severe forms including nodulocystic acne (acne conglobata). Treatment depends on the type and severity of acne lesions. The available main therapeutic classes are topical and systemic antimicrobials, topical and systemic retinoids, and systemic hormonal therapy. Systemic isotretinoin is the best option when other treatments fail. Even though it is a very effective potent drug, it has been implicated in numerous reports that describe a wide range of systemic side effects. Moreover, it could result in worsening of acne lesions, up to an extremely serious form of acne known as acne fulminant, characterized by systemic symptoms, including fever, weight loss, myalgia, and arthralgia. However, when the available treatments fail to control the disease, oral dapsone could be an effective and useful alternative therapy for nodulocystic acne. Here, we review a case of a patient with severe nodulocystic acne not responding to isotretinoin who was successfully treated with oral dapsone.
Case Report
A 21-year-old Omani male referred from a private clinic to our dermatology outpatient clinic with a clinical picture of nodulocystic acne affecting his face. He had been on isotretinoin for two months, but his acne lesions became worse and more extensive. His past medical history was unremarkable, and he had no comorbidities.
Examination revealed a young male with normal vital signs and extensive papules, nodules, and cystic lesions on his face [Figure1].
Figure 1.
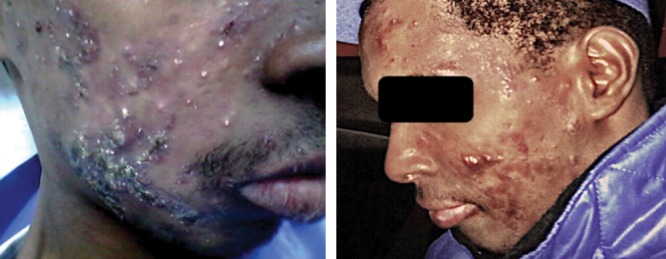
Extensive facial papules, nodules, and cystic lesions.
Biochemical profiles at presentation were within normal range. Culture and sensitivity test using a pus swab sample showed no bacterial growth. The patient completed two months of isotretinoin 40 mg/day (0.6 mg/kg) at a private clinic, with no significant improvement.
Once he was referred to us, the dose of isotretinoin was increased to 60 mg/day (1 mg/kg), and he took a short course of prednisolone for two weeks. The condition showed mild improvement of lesions at the beginning and a flare after cessation of prednisolone. Isotretinoin was then given alone for two weeks, but unfortunately, the patient stopped the treatment as he could not tolerate some of the side effects (severe myalgia, conjunctival congestion, and severe dryness of eyes).
During the next follow-up, a low dose (10 mg daily) of isotretinoin was started along with a gradually tapering course of prednisolone over three months. Initially, the patient showed great improvement; however, his lesions got progressively worse with tapering of prednisolone.
Because of unresponsiveness to isotretinoin and long-term use of prednisolone, these treatments were stopped, and the patient was started on systemic antibiotics and topical treatments which yielded the same results.
Finally, oral dapsone was introduced along with topical benzoyl peroxide (BPO) 2.5%. Laboratory tests including complete blood count and glucose-6-phosphate dehydrogenase (G6PD) were performed before starting and during treatment with dapsone and were within normal range.
Dapsone was given for a total period of six months, initially 100 mg daily for four months then 50 mg daily for another two months. After six months, clear improvement of lesions was noticed, and good control of condition was achieved [Figure 2].
Figure 2.
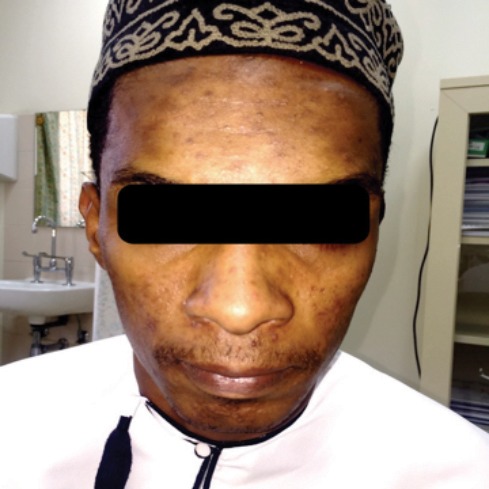
Resolution of nodulocystic acne after six months of treatment with oral dapsone.
Follow-up after one year showed no recurrence, except for few occasional acne lesions which were controlled with BPO 2.5% gel [Figure 3].
Figure 3.
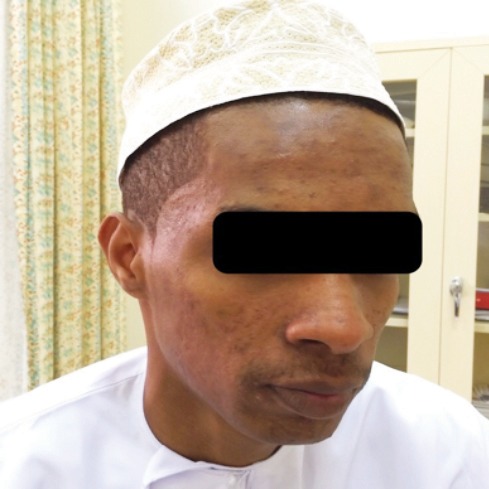
At one-year follow-up, no recurrence of nodulocystic acne was observed.
Discussion
Acne is the most common chronic skin disorder among adolescents affecting about 85% of teenagers.1 It has both financial and psychosocial impact; the latter increases significantly with the severity of the disease.2 Treating severe forms of acne including nodulocystic acne is challenging and always requires systemic treatment.3 Different therapeutic options are available including oral antibiotics, hormonal antiandrogens for female patients, oral isotretinoin, and other combination treatments.4
Systemic isotretinoin is an extremely useful drug for the treatment of not only severe refractory acne but also moderate acne that fails to respond to conventional therapy or that produces physical scarring or significant psychological distress.5,6 It is considered the only drug which changes the course of the disease permanently in severe forms as well as the only drug that targets all four pathogenesis of acne.1,3 It reduces sebaceous gland size, sebum secretion, comedone formation, and follicular colonization of Propionibacterium acnes.6 However, the reported serious systemic side effects from isotretinoin usage such as liver damage, depression, behavioral change, suicidality, and teratogenicity have created an obstacle to its use as it has been recommended by many clinicians to restrict such treatment for those with severe recalcitrant acne.1,6
Dapsone is of primary importance in the treatment of leprosy. It has also been used in a wide variety of skin disorders; for instance, dermatitis herpetiformis, neutrophilic dermatosis, and autoimmune bullous dermatoses.7
The mechanism of action of dapsone is most likely related to its duality to act as a chemotherapeutic agent as well as anti-inflammatory. The antibacterial action is usually bacteriostatic and seems to mimic sulfonamides (competitive inhibition of the enzyme necessary for the synthesis of folic acid). This antimicrobial activity against P. acnes is usually low (comparable with anti-inflammatory effects).7 The precise anti-inflammatory mechanism remains unclear. Recent investigation shows that dapsone alone (and through its metabolites) has similarities to nonsteroidal anti-inflammatory drugs.8 Dapsone also appears to inhibit chemoattractant signaling, which prevents neutrophil recruitment at the site of inflammation.9 There is also evidence that dapsone inhibits the myeloperoxidase enzyme system in neutrophils, which produce reactive oxygen species that not only kill micro-organisms but also cause tissue damage and inflammation.10
Topical dapsone 5% recently received Food and Drug Administration approval for the treatment of acne vulgaris. It showed modest to moderate efficacy in clinical trials, primarily in the reduction of inflammatory lesions.11 By contrast, there are only a few case reports on using oral dapsone as a treatment for severe acne, most likely due to its well-known systemic side effects including hemolytic anemia and methemoglobinemia, which can be prevented by checking G6PD levels before dapsone administration along with close monitoring of the patient by performing regular routine laboratory tests and clinical examinations over time. These reports demonstrated that oral dapsone might be a useful and effective alternative therapy in severe acne when other treatments are unsuccessful.12,13
Comparison between oral dapsone and isotretinoin was carried out in a randomized, prospective trial in 1988 when isotretinoin was a newly introduced therapy.14 This study proved that isotretinoin remains superior to oral dapsone in the treatment of nodulocystic acne, as oral dapsone produce no significant effect either on sebum excretion rate or skin surface microflora compared with isotretinoin, along with the striking feature of isotretinoin which is the prolonged remission period following completion of therapy.14
Based on our report and existing literature, it is worthy to highlight that oral dapsone could be an effective alternative to isotretinoin in severe acne refractory to isotretinoin and other systemic drugs provided that close monitoring of laboratory blood tests are performed regularly, because of the potential systemic side effects.
Conclusion
Nodulocystic acne is a severe form of acne affecting face, chest, and back. Systemic isotretinoin is a well-established treatment for severe acne, and it is superior to any other therapy. Nevertheless, we may consider using oral dapsone as an alternative therapy for severe acne when isotretinoin and other topical and systemic treatments fail to achieve disease resolution.
Disclosure
The authors declared no conflicts of interest.
References
- 1.Knutsen-Larson S, Dawson AL, Dunnick CA, Dellavalle RP. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin 2012. Jan;30(1):99-106, viii-ix. viii-ix. 10.1016/j.det.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 2.AL-Shidhani A Al-Rashdi S, Al-Habsi H, Rizvi S. Impact of acne on quality of life of students at Sultan Qaboos University. OMJ 2015;1(30):42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zouboulis CC, Bettoli V. Management of severe acne. Br J Dermatol 2015. Jul;172(Suppl 1):27-36. 10.1111/bjd.13639 [DOI] [PubMed] [Google Scholar]
- 4.Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016. May;74(5):945-73.e33. 10.1016/j.jaad.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 5.Titus S, Hodge J. Diagnosis and treatment of acne. Am Fam Physician 2012. Oct;86(8):734-740. [PubMed] [Google Scholar]
- 6.Rigopoulos D, Larios G, Katsambas AD. The role of isotretinoin in acne therapy: why not as first-line therapy? facts and controversies. Clin Dermatol 2010. Jan-Feb;28(1):24-30. 10.1016/j.clindermatol.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 7.Wozel VE. Innovative use of dapsone. Dermatol Clin 2010. Jul;28(3):599-610. 10.1016/j.det.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 8.Wozel G, ed. Dapson—Pharmakologie, Wirkme- chanismus und klinischer Einsatz. Stuttgart (Germany): Georg Thieme Verlag; 1996. [Google Scholar]
- 9.Debol SM, Herron MJ, Nelson RD. Anti-inflammatory action of dapsone: inhibition of neutrophil adherence is associated with inhibition of chemoattractant-induced signal transduction. J Leukoc Biol 1997. Dec;62(6):827-836. 10.1002/jlb.62.6.827 [DOI] [PubMed] [Google Scholar]
- 10.Wozel G, Barth J. Current aspects of modes of action of dapsone. Int J Dermatol 1988. Oct;27(8):547-552. 10.1111/j.1365-4362.1988.tb02401.x [DOI] [PubMed] [Google Scholar]
- 11.Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol 2009;10(4):221-227. 10.2165/00128071-200910040-00002 [DOI] [PubMed] [Google Scholar]
- 12.Didona D, Paolino G, Donati P, Muscardin LM. Resolution of nodulocystic acne with oral dapsone. Dermatol Ther 2017;30(1):1-2. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi M, Fujii N, Fujimoto N, Tanaka T. Usefulness of dapsone for the treatment of Asian severe acne. J Dermatol 2013. Jun;40(6):502-504. 10.1111/1346-8138.12142 [DOI] [PubMed] [Google Scholar]
- 14.Prendiville JS, Logan RA, Russell-Jones R. A comparison of dapsone with 13-cis retinoic acid in the treatment of nodular cystic acne. Clin Exp Dermatol 1988. Mar;13(2):67-71. 10.1111/j.1365-2230.1988.tb00659.x [DOI] [PubMed] [Google Scholar]


