Abstract
Recently, alterations in fascial gliding‐like movement have been invoked as critical in the etiology of myofascial pain. Various methods have been attempted for the relief of this major and debilitating clinical problem. Paramount have been attempts to restore correct gliding between fascial layers and the movement over bone, joint, and muscular structures. One of the key elements that underlies such fascial movement is hyaluronan. However, until now, the precise content of hyaluronan within fasciae has been unknown. This study quantifies for the first time the hyaluronan content of human fascial samples obtained from a variety of anatomic sites. Here, we demonstrate that the average amount varies according to anatomic site, and according to the different kinds of sliding properties of the particular fascia. For example, the fascia lata has 35 μg of hyaluronan per gram of tissue, similar to that of the rectus sheath (29 μg g−1). However, the types of fascia adherent to muscle contain far less hyaluronan: 6 μg g−1 in the fascia overlying the trapezius and deltoid muscles. In the fascia that surrounds joints, the hyaluronan increases to 90 μg g−1, such as in the retinacula of the ankle, where greater degrees of movement occur. Surprisingly, no significant differences were detected at any site as a function of age or sex (P‐value > 0.05, t‐test) with the sole exception of the plantar fascia. This work can provide a better understanding of the role of hyaluronan in fascia. It will facilitate a better comprehension of the modulation of the hyaluronan‐rich layer that occurs in relation to the various conditions that affect fascia, and the diverse factors that underlie the attendant pathologies.
Keywords: densification, extracellular matrix, fascia, fibrosis, gliding, hyaluronan, myofascial pain
Introduction
As has been demonstrated recently (Klein et al. 1999; Stecco et al. 2011; Cowman et al. 2015a,2015b), fascia presents a lubricating layer of hyaluronan (HA), allowing sliding between fascial sublayers and between fasciae and adjacent muscles, bones, and joints. HA is the major glycosaminoglycan (GAG) of the extracellular matrix (ECM). It is composed of d‐glucuronic acid and N‐acetyl‐d‐glucosamine, linked via alternating β‐(1→4) and β‐ (1→3) glycosidic bonds. Polymers of HA can range in size from a few kDa up to 8 MDa. The molecular size strongly influences the biological, physiological, and pathological functions of the molecule (Cowman et al. 2015a,2015b). The HA content in in vivo tissues is usually constant and is the result of the simultaneous action of the synthetases, HAS1, HAS2, HAS3 (Itano & Kimata, 1996; DeAngelis, 1999), the hyaluronidases (HYAL) in its various isoforms (Stern & Jedrzejas, 2006; Csoka & Stern, 2013), and other degrading molecules, such as reactive oxygen species (Triggs‐Raine & Natowicz, 2015).
HA plays a key role in tissue hydration, lubrication of sliding surfaces, activation of specific signal pathways by way of membrane receptors that affect cell motility, inflammation, and cancer progression (Stern et al. 2006). It has important lubricating functions between fascia and underlying muscle, bones, and joints (Temple‐Wong et al. 2016) and within fascial layers (Laurent et al. 1991; McCombe et al. 2001).
In clinical practice, it is observed that a reduced gliding of thoracolumbar fascia occurs in patients with chronic low back pain (Langevin et al. 2011), and in the neck fascia of patients affected by nonspecific neck pain (Stecco et al. 2013, 2014a). More precisely, Langevin et al. (2011) have found significant correlations between the shear strain capability of the loose connective layers and trunk flexion range of motion (ROM) and trunk extension ROM. Stecco et al. (2014a) documents fascial alteration occurring in patients with chronic neck pain in the loose connective tissue between fascial sublayers. This suggests a key role for HA in the genesis of myofascial pain. Restoring the natural sliding mechanism of fasciae is also a major task for massage therapy (Chaitow, 2014; Luomala et al. 2014) and other manual technologies. However, until now, no estimates of HA content within fasciae have been available, or of the concentrations of HA that occur in relation to various pathologies or as a function of age, sex, and anatomic site.
In the last few years, many studies have focused on the quantification of HA content in different human tissues, with the aim of examining the role of this GAG in normal physiology. It was demonstrated that the amount of HA is approximately 35 μg g−1 in human muscle tissue (Piehl‐Aulin et al. 1991), 2–3 mg mL−1 in synovial fluid, 0.5–2.5 mg g−1 in articular cartilage, 0.4–0.5 mg g−1 in skin, with some variations depending on sex and age (Cowman et al. 2015a,2015b). Our aim here is for the first time to determine the HA content in healthy human fascia. The outcomes will serve as a fundamental starting point for further investigation of pathological conditions, in particular in relation to myofascial pain. The latter continues to be a vexing clinical problem that has up to now evaded effective treatment modalities. It is a major cause of the epidemic of painkiller medications and the related addiction currently so pervasive internationally.
Methods
Sample collection
This study was approved by the Institutional Ethical Review Board (approval no. 3722/AO/16). The institute's ethical regulations on research conducted on human tissues were followed, and written informed consent was obtained from each donor.
Samples of fascia 1 × 1 cm were collected from 15 volunteer patients, six males and nine females with an average age of 58 ± 20 years. All were undergoing elective surgical procedures at the Orthopedic Clinic of Padova University. We excluded patients with cancer, patients who have previously undergone surgery in the same region, and patients with rheumatological or infectious diseases. We selected four different types of fascia, each sample from a different patient, based on their different gliding capacities with respect to the underlying muscles (Stecco et al. 2009; Stecco, 2015): six samples of aponeurotic fasciae, which are thick, fibrous, and freely gliding with respect to the underlying muscles; three samples of retinacula, which are fibrous reinforcements of the aponeurotic fasciae around the joints; three samples of epimysial fasciae, which are thin and completely adherent to the underlying muscles; and three samples of plantar fascia. The latter is the thickest and most fibrous aponeurotic fascia of the body. Details of samples are given in Table 1. The samples were transported to the laboratory in phosphate‐buffered saline (PBS) within a few hours of collection and were either frozen or used fresh.
Table 1.
Details of sample collection and mean of μg HA contained in 1 g of human tissue
| Subject | Sex | Age | Collection area | Fascial type | Mean HA (μg g−1) |
|---|---|---|---|---|---|
| 1 | M | 76 | Fascia lata | Aponeurotic fascia | 43.2 ± 8.5* |
| 2 | M | 64 | Fascia lata | Aponeurotic fascia | 38.5 ± 1.0 |
| 3 | M | 50 | Fascia lata | Aponeurotic fascia | 27.1 ± 0.1* |
| 4 | F | 81 | Fascia lata | Aponeurotic fascia | 32.7 ± 1.9** |
| 5 | F | 73 | Fascia lata | Aponeurotic fascia | 35.5 ± 0.9 |
| 6 | F | 42 | Rectus sheath | Aponeurotic fascia | 29.1 ± 0.2 |
| 7 | F | 98 | Plantar fascia | Aponeurotic fascia | 44.8 ± 0.2 |
| 8 | M | 49 | Plantar fascia | Aponeurotic fascia | 131.0 ± 3.0 |
| 9 | M | 32 | Plantar fascia | Aponeurotic fascia | 5.8 ± 0.2 |
| 10 | M | 64 | Trapezius | Epimysial fascia | 6.0 ± 0.2 |
| 11 | F | 27 | Deltoid fascia | Epimysial fascia | 6.1 ± 0.2 |
| 12 | F | 27 | Deltoid fascia | Epimysial fascia | 6.9 ± 0.2 |
| 13 | F | 43 | Ankle extensor | Retinaculum | 94.5 ± 1.0 |
| 14 | F | 75 | Ankle peroneal | Retinaculum | 80.5 ± 6.5 |
| 15 | F | 64 | Ankle peroneal | Retinaculum | 96.3 ± 0.7 |
Values of each subject are the mean of two extractions, with the only exceptions *in three and **four extractions.
Isolation and quantification of hyaluronan
The fascial samples were weighed and were typically 200 ± 10 mg They were then cut into small fragments using a surgical scalpel (Fig. 1A). As a sample standard, we selected 100 ± 10 mg of human adult skin from four different subjects (two females and two males, average age 75 ± 6 years) to validate the extraction method and for the accuracy of the kit, as there are no reference data regarding fascia in the literature.
Figure 1.
Macroscopic image of a human fascial sample before (A) and after (B) the digestion by Proteinase K.
To measure the HA content, we used the Purple‐Jelley HA assay (Biocolor Ltd.), a colorimetric method with a detection limit of 0.2 μg. All samples were processed following the datasheet of the kit. Briefly, we carried out a protein digestion in 50 mm Tris–HCl (pH 7.6) containing Proteinase K (Sigma Aldrich) overnight at 55 °C (Fig. 1B). After centrifugation at 14000 g for 10 min, the supernatants were mixed with GAG precipitation reagent. The residues obtained after centrifuge were dispersed in water and mixed with NaCl and cetylpyridinium chloride (CPC). These steps were repeated until the recovery of total GAG content. For the final HA isolation, we added 98% ethanol, centrifuged the samples, and discarded the supernatants. The tubes were dried, and the HA pellet was solubilized and hydrated in 100 μL water.
We then proceeded with the measurements of HA, transferring 20‐μL aliquots of test samples to a 96‐microwell plate, and adding 200 μL of the Purple Dye reagent. The absorbance value at 650 nm was read with a Mithras LB 940 spectrophotometer (Berthold Technologies). For each sample, the extraction was carried out twice.
Data analysis
The HA standard (200 μg mL−1) provided with the kit was used to obtain a standard curve (Fig. 2).
Figure 2.
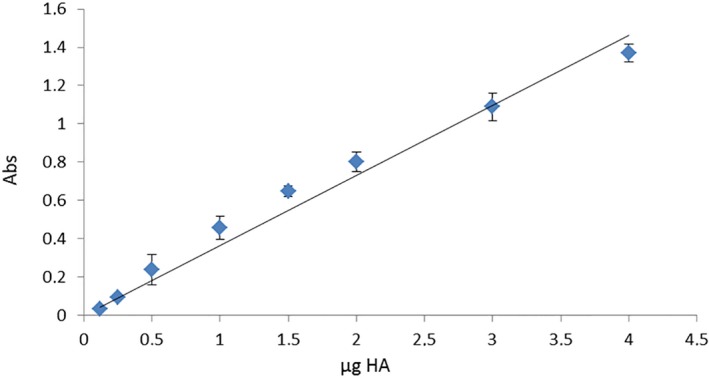
Straight line standard curve obtained by HA standard (200 μg mL−1).
With that curve, we converted the absorbance values into μg HA contained in the total volume of 100 μL. Finally, we converted the μg HA extracted from 200 mg of wet starting tissue to μg HA contained in 1 g of wet tissue.
Statistical analysis
We performed a statistical analysis (t‐test) to verify significant differences when comparing data by sex, age (greater or less than 50 years), and collection from the designated areas.
Results
Initially, the kit was validated by the quantification of HA in four samples of human adult skin used as control: the experimental mean value of HA was equal to 176.2 ± 12.3 μg g−1 of wet starting tissue (repeated twice, data not shown). No significant differences were found (P‐value > 0.05) with the literature data reporting the relative amount of HA in adult skin (160 ± 19 ng mg−1) (Tzellos et al. 2011).
HA quantification in various fasciae is reported in Table 1. The average contents are indicated in Fig. 3. The mean content of HA was equal to 35.0 ± 7.2 μg g−1 of wet tissue in the human fascia lata of the thigh, 29.1 ± 0.4 μg g−1 in the rectus sheath of the abdomen, 6.3 ± 0.5 μg g−1 in the fascia of trapezius and deltoid, and 90.4 ± 8.8 in the retinacula of ankle. In the plantar fascia, we found very divergent values. The mean HA in one old female patient (98 years) was equal to 44.8 ± 0.02 μg g−1, whereas in two younger male patients (49 and 32 years), the mean HA content was equal to 131.0 ± 3.0 and 5.8 ± 0.2 μg g−1, respectively.
Figure 3.
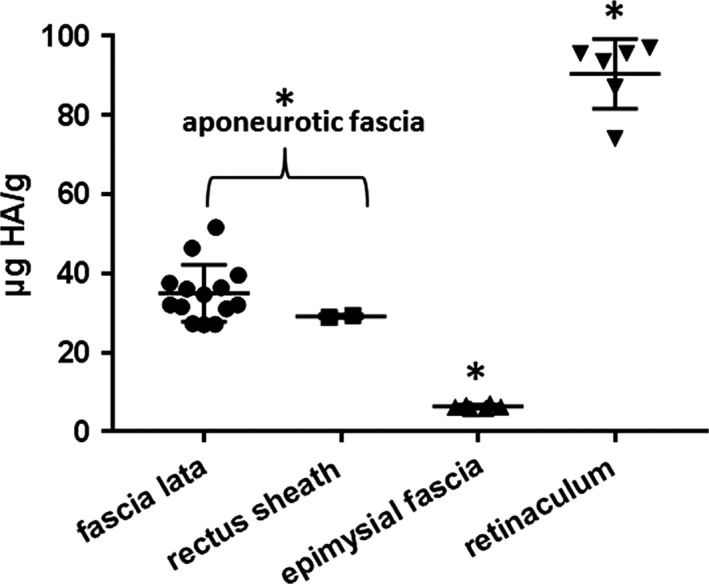
Mean HA values (μg HA g−1 of wet starting tissue) and standard deviations, derived from fascia lata and rectus sheath (aponeurotic fasciae), epimysial fasciae, and retinacula. P > 0.05, t‐test fascia lata vs. rectus sheath; *P < 0.01, t‐test comparing aponeurotic fascia, epimysial fascia, and retinaculum.
The statistical analysis demonstrated that the amount of HA extracted is significantly different according to collection site. The P‐value was < 0.01 when analyzing the differences between aponeurotic fasciae (fascia lata of the thigh and rectus sheath of the abdomen), epimysial fasciae (trapezius and deltoid), and retinacula of the ankle (Fig. 3). There were no significant differences between the same class of fascia: there was a P‐value of > 0.05 when comparing the fascia lata of the thigh and the rectus sheath of the abdomen, both of which are aponeurotic fasciae. The only three values obtained from the same anatomical site that showed significant differences (P < 0.01) were those of the plantar fascia. However, if we consider the plantar fascia together with all the aponeurotic fasciae, patient #7 had an HA content similar to all the other aponeurotic fasciae (P > 0.05), whereas patients #8 and #9 had entirely different values.
The statistical analysis demonstrated that there are no correlations that occurred as a function of sex (P > 0.05), or age (greater or less than 50 years; P > 0.05).
Discussion
In this work, we have isolated and quantified for the first time HA from human fascial samples collected from different anatomical sites in patients of both sexes and of different ages. Our data demonstrate for the first time the presence of considerable levels of HA in human fascia (45.2 μg g−1). The HA content remained almost constant in samples collected from the same fascial site. In contrast, significant differences were found depending on the collection area. The amount of HA was about 43 μg g−1 in the aponeurotic fasciae, decreased drastically (about 6 μg g−1) in epimysial fasciaem and increased in the retinacula (90.4 μg g−1). These variations corresponded perfectly with the various gliding functions of the fasciae from various sites. Indeed, the aponeurotic fasciae are free to glide over the muscles, whereas the epimysial fasciae are completely adherent to the underling muscles (Stecco et al. 2009). In addition, the retinacula are a specialization of aponeurotic fasciae that surround joints (Klein et al. 1999; Stecco et al. 2010), where movements are the most intense, and correspond with the highest levels of HA.
The results are similar to the content found in human muscle tissue (approximately 35 μg g−1) (Piehl‐Aulin et al. 1991) and rabbit muscle (30 μg g−1: Laurent & Tengblad, 1980; and 80–90 μg g−1: Armstrong & Bell, 2002). It is important to point that inside the muscle as well, HA is present only in the perimysium, endomysium, and epimysium (Stecco et al. 2011), allowing isolated activation of the various motor units. In the same manner, Rowe (1985) demonstrated in the tendon that HA is present above all in the endotenon, which allows the tertiary fascicles to slide more easily and to better manage the mechanical stress inside the tendon (Stecco et al. 2014b). Furthermore, we found no significant differences as a function of sex (P > 0.05, t‐test) in any of the collected data. These results also confirm that the amount of HA is not affected by age, unlike the skin (Tzellos et al. 2011), where it has been demonstrated that HA declines with age in the upper epidermal layers, with consequent dryness and minimal scarring (Juhlin, 1997).
Our results allow us to confirm that healthy fascia require a specific level of HA (Csoka & Stern, 2013) to permit the correct gliding and the normal functions of deep fascia. Significant changes of these physiological levels of HA may indicate an underlying pathology. Factoring out the content of the HA‐rich layers of fasciae and the changes they undergo with various pathologies will facilitate future studies in fascial sliding and correlations with pathological conditions affecting the fasciae and related tissues.
Author contributions
C.F. contributed to the writing of the work and is responsible for the hyaluronan isolation and quantification, and data analysis. A.A. and P.R. are responsible for the collection of fascia from volunteer patients and contributed to data analysis. R.S. contributed to editing the manuscript and to data analysis. V.M., A.P., and R.DC. edited the manuscript and contributed to statistical analysis. C.S. contributed to the writing and editing of the manuscript and coordinated all the work.
Acknowledgements
We thank the Orthopedic Clinic of Padua for patient data and samples collection. This work was funded by Fascial Manipulation Association. C. Fede acknowledges the support of a Fascial Manipulation Association research grant.
References
- Armstrong SE, Bell DR (2002) Measurement of high‐molecular‐weight hyaluronan in solid tissue using agarose gel electrophoresis. Anal Biochem 308, 255–264. [DOI] [PubMed] [Google Scholar]
- Chaitow L (2014) Somatic dysfunction and fascia's gliding‐potential. J Bodyw Mov Ther 18, 1–3. [DOI] [PubMed] [Google Scholar]
- Cowman MK, Lee HG, Schwertfeger KL, et al. (2015a) The content and size of hyaluronan in biological fluids and tissues. Front Immunol 6, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman MK, Schmidt TA, Raghavan P, et al. (2015b) Viscoelastic properties of hyaluronan in physiological conditions. F1000Res 4, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka AB, Stern R (2013) Hypotheses on the evolution of hyaluronan: a highly ironic acid. Glycobiology 23, 398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis PL (1999) Hyaluronan synthases: fascinating glycosyltransferases from vertebrates, bacterial pathogens, and algal viruses. Cell Mol Life Sci 56, 670–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano N, Kimata K (1996) Expression cloning and molecular characterization of HAS protein, a eukaryotic hyaluronan synthase. J Biol Chem 271, 9875–9878. [DOI] [PubMed] [Google Scholar]
- Juhlin L (1997) Hyaluronan in skin. J Intern Med 242, 61–66. [DOI] [PubMed] [Google Scholar]
- Klein DM, Katzman BM, Mesa JA, et al. (1999) Histology of the extensor retinaculum of the wrist and the ankle. J Hand Surg Am 24, 799–802. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Fox JR, Koptiuch C, et al. (2011) Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord 12, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent UB, Tengblad A (1980) Determination of hyaluronate in biological samples by a specific radioassay technique. Anal Biochem 109, 386–394. [DOI] [PubMed] [Google Scholar]
- Laurent C, Johnson‐Wells G, Hellström S, et al. (1991) Localization of hyaluronan in various muscular tissues. A morphological study in the rat. Cell Tissue Res 263, 201–205. [DOI] [PubMed] [Google Scholar]
- Luomala T, Pihlman M, Heiskanen J, et al. (2014) Case study: could ultrasound and elastography visualized densified areas inside the deep fascia? J Bodyw Mov Ther 18, 462–468. [DOI] [PubMed] [Google Scholar]
- McCombe D, Brown T, Slavin J, et al. (2001) The histochemical structure of the deep fascia and its structural response to surgery. J Hand Surg Br 26, 89–97. [DOI] [PubMed] [Google Scholar]
- Piehl‐Aulin K, Laurent C, Engstrom‐Laurent A, et al. (1991) Hyaluronan in human skeletal muscle of lower extremity: concentration, distribution, and effect of exercise. J Appl Physiol 71, 2493–2498. [DOI] [PubMed] [Google Scholar]
- Rowe RW (1985) The structure of rat tail tendon. Connect Tissue Res 14, 9–20. [DOI] [PubMed] [Google Scholar]
- Stecco C (2015) Functional Atlas of the Human Fascial System. 1st edn Edinburgh: Elsevier, pp. 51–100. [Google Scholar]
- Stecco A, Macchi V, Masiero S, et al. (2009) Pectoral and femoral fasciae: common aspects and regional specializations. Surg Radiol Anat 31, 35–42. [DOI] [PubMed] [Google Scholar]
- Stecco C, Macchi V, Porzionato A, et al. (2010) The ankle retinacula: morphological evidence of the proprioceptive role of the fascial system. Cells Tissues Organs 192, 200–210. [DOI] [PubMed] [Google Scholar]
- Stecco C, Stern R, Porzionato A, et al. (2011) Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat 33, 891–896. [DOI] [PubMed] [Google Scholar]
- Stecco A, Gesi M, Stecco C, et al. (2013) Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep 17, 352. [DOI] [PubMed] [Google Scholar]
- Stecco A, Meneghini A, Stern R, et al. (2014a) Ultrasonography in myofascial neck pain: randomized clinical trial for diagnosis and follow‐up. Surg Radiol Anat 36, 243–253. [DOI] [PubMed] [Google Scholar]
- Stecco C, Cappellari A, Macchi V, et al. (2014b) The paratendineous tissues: an anatomical study of their role in the pathogenesis of tendinopathy. Surg Radiol Anat 36, 561–572. [DOI] [PubMed] [Google Scholar]
- Stern R, Jedrzejas MJ (2006) Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev 106, 818–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Asari AA, Sugahara KN (2006) Hyaluronan fragments: an information‐rich system. Eur J Cell Biol 85, 699–715. [DOI] [PubMed] [Google Scholar]
- Temple‐Wong MM, Ren S, Quach P, et al. (2016) Hyaluronan concentration and size distribution in human knee synovial fluid: variations with age and cartilage degeneration. Arthritis Res Ther 18, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs‐Raine B, Natowicz MR (2015) Biology of hyaluronan: insights from genetic disorders of hyaluronan metabolism. World J Biol Chem 6, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzellos TG, Sinopidis X, Kyrgidis A, et al. (2011) Differential hyaluronan homeostasis and expression of proteoglycans in juvenile and adult human skin. J Dermatol Sci 61, 69–72. [DOI] [PubMed] [Google Scholar]


