Abstract
Although the form‐function relation of muscles and tendons has been studied extensively, little in vivo data exist on the musculotendon properties of the gastrocnemius complex in dogs. Using a combination of ultrasound and 3D motion tracking, musculotendon parameters were obtained in vivo from the lateral gastrocnemius muscle and the gastrocnemius tendon in nine healthy Labrador Retrievers. These parameters include musculotendon length and excursion potential, tendon slack length, muscle belly length, muscle fibre length, pennation angle and architectural index. This study also examined the variation of muscle and tendon length contributions to musculotendon length, as well as the relation between musculotendon excursion potential and muscle fibre length or tendon length. To facilitate comparison between dog breeds, the femur length as a potential scaling parameter was examined. In the Labrador gastrocnemius musculotendon complex, the tendon contributes 41% (± 9%) of musculotendon length. In longer musculotendon complexes, the contribution of the muscle belly increases while the tendon contribution decreases. Longer muscle belly and musculotendon complexes were, however, associated with shorter muscle fibres. No significant relations were found between musculotendon excursion potential and muscle fibre length or tendon slack length, and femur length did not prove to be a reliable scale factor for the length‐related musculotendon parameters examined in this study. Longer musculotendon complexes exhibit relatively longer muscle bellies, which are in turn associated with shorter muscle fibre lengths. This trade‐off between gastrocnemius muscle belly length and muscle fibre length might have the advantage that muscle volume stays constant regardless of the length of the limbs.
Keywords: dog, excursion, gastrocnemius, muscle, tendon
Introduction
The canine gastrocnemius muscle, being a superficial muscle with a long tendon, plays an important role in gait. The muscle consists of a lateral and medial head, originating respectively from the lateral and medial supracondylar tuberosities of the femur, and merging into a single gastrocnemius tendon in the proximal crus, which inserts on the proximal dorsal surface of the calcaneus (Gardell, 1980; Dyce, 2010). The canine common calcaneal tendon is actually composed of the gastrocnemius tendon, superficial digital flexor tendon and the common tendon of the biceps femoris, gracilis and semitendinosus muscles. The gastrocnemius musculotendon complex is not only responsible for extension of the tarsal joint but is also thought to contribute to the stifle flexion.
As in most canine anatomy studies, the study of the gastrocnemius muscle has been almost entirely descriptive in nature, describing muscle origin, insertion, function and innervation (Gardell, 1980; Dyce, 2010). Because of this, the relative importance of the muscle vs. the tendinous unit is less clear. This in contrast to human musculoskeletal research where key parameters describing muscle and tendon function and force production have been parametrized for most lower limb muscles. Despite the large body of work done in human musculotendon research, few of these parameters and associated methods have found their way into the field of canine musculoskeletal research. The acquisition of a similar parameter set describing muscle and tendon function in dogs has a great potential significantly to advance our understanding of the role of different muscle groups and, more specifically, their muscle–tendon interaction and consequent effect on force production, in canine locomotion. To avoid confusion with terminology referring to studies on muscle and tendon function during locomotion and muscle function, this study will refer to the parameters measured as musculotendon properties. These musculotendon properties include the tendon slack length, the muscle fibre and muscle belly length, the pennation angle and the musculotendon excursion potential.
The first musculotendon property is the tendon slack length, defined as the tendon length beyond which further stretching induces the development of passive elastic force. The tendon slack length is important in ensuring the most effective moment production over the functional range of motion (ROM) of the joint by optimally aligning the muscle's effective operating range and maximal force production with the optimal moment arm. In a relaxed muscle (i.e. not activated and without external forces stretching it), the muscle fibre length at tendon slack length approximates the optimal fibre length, as no passive forces are present that stretch the muscle fibres beyond optimal fibre length. Therefore, the tendon slack length and the optimal muscle fibre length are strongly related (Garner & Pandy, 2003). Currently, tendon slack length data are sparse in literature, both in human and animals (Garner & Pandy, 2003).
Two other important musculotendon properties are the muscle fibre length and the muscle belly length. Muscle fibre length is directly related to the maximal contraction velocity of a muscle (Arnold et al. 2013), enabling longer muscle fibres to generate faster muscle contractions. The relation between muscle belly length and the muscle fibre length is reflected in the architectural index (AI) and depends on the pennation angle (α) of the muscle fibres. This angle at which the muscle fibres insert into the aponeurosis, determines how much of the muscle belly excursion is translated into muscle fibre excursion. This relation is functionally important because muscle fibres can produce less active force when the muscle fibre length is longer or shorter than optimal muscle fibre length (Zajac, 1989). The optimal muscle fibre length is defined as the length of the muscle fibres at which muscles can perform maximal isometric force (Zajac, 1989) and is crucial in defining the active and passive force‐length properties of the muscle. A muscle needs to achieve maximal muscle force production at specific joint angles. To enable this, musculotendon length has to be fine‐tuned to optimally match optimal fibre length to the appropriate joint angle, by balancing the contribution of muscle (fibre) length and tendon length with the musculotendon length. Therefore, it is important to study the relative contribution of the muscle (fibre) length and tendon length to the total muscle tendon length. Furthermore, because of the pinnate architecture of the gastrocnemius muscle, changes in muscle belly length do not necessarily correspond to changes in muscle fibre lengths. To account for this, the relation between muscle fibre length, muscle belly and overall musculotendon length needs to be investigated.
Finally, the musculotendon excursion potential, i.e. the difference between the maximal and minimal possible physiological musculotendon length (Brand et al. 1981), also characterizes muscle function, as it determines the physiological operating range or ROM of the musculotendon complex. Reduced ROM indicates restricted mobility of the joint (Ismail et al. 2015) and can reduce gait economy (Godges et al. 1989) as well as increase the likelihood of injury (Bradley & Portas, 2007). Muscle fibre length plays a crucial role in determining muscle excursion potential, as a longer muscle fibre should yield a larger musculotendon excursion potential for a similar tendon excursion. Furthermore, differences in muscle and tendon contributions to musculotendon lengthening will likely also affect the excursion potential of the musculotendon complex. Because the tendon is relatively stiffer than the muscle (Cook & McDonagh, 1996), and a longer muscle belly can be expected to yield longer muscle fibres, a larger muscle length contribution can theoretically be expected to yield a larger musculotendon excursion potential. Therefore, to identify the function of the muscle tendon complex, the contribution of the tendon length and fibre length to the musculotendon length and consequent musculotendon excursion potential needs to be characterized.
The general aim of this study is to determine the muscle and tendon properties of the gastrocnemius muscle, and to determine associations of these properties with the functional behaviour of the canine musculotendon units. We will first determine the in vivo tendon slack length, muscle (fibre) length and musculotendon excursion potential, and subsequently examine the relation between these parameters. The most important parameters examined in this study, namely the maximal and minimal musculotendon unit lengths, and the musculotendon excursion, need to be studied in living dogs. Therefore, the current study is the first to examine these relations in vivo in dogs.
Finally, because of the enormous variety in limb sizes and proportions among dog breeds, the ability of anthropometrical data (more specifically, femur length) as potential scaling factors for these muscle and tendon length‐related parameters was evaluated. This should allow cross‐breed usage of the reported muscle function parameters.
Materials and methods
Study animals and ethics statement
Measurements were taken from nine Labradors between 8 and 10 months of age. Approval from the local ethics (Faculty of Veterinary Medicine, Ghent University, Belgium) committee was granted (EC2013_53, 7/5/2013).
The protocol of this study was performed at the same time as a routine clinical CT and radiograph evaluation of the subjects, for which anaesthesia is necessary. This study therefore only prolonged the anaesthesia during the measurements taken for this study. These radiographs included the ventro‐dorsal radiographs of the hips and both femurs (Fig. 2) used in this study.
Anaesthesia protocol. After placement of a 22‐gauge catheter in a cephalic vein, premedication was achieved with 5 μg kg−1 dexmedetomidine or 10 μg kg−1 medetomidine i.v., followed 15 min later by induction of anaesthesia with 2 mg kg−1 propofol i.v. given until anaesthesia was achieved. Following endotracheal intubation with an appropriately sized tube, anaesthesia was maintained with inhalation of isoflurane vaporized in oxygen and delivered by a rebreathing circle system. Lactated Ringer's solution was infused at a rate of 10 mL kg−1 h−1 throughout anaesthesia.
Positioning of the dogs for all other measurements was achieved with a custom‐build joint positioning device (Fig. 1) to maintain the dog in a state of ventral recumbency with the hind limbs in a fixed position. The hair coat was clipped at the level of the gastrocnemius muscle tendon and the skin surface was disinfected with alcohol solution, followed by a thin layer of ultrasound coupling gel to increase skin conductivity.
Figure 1.
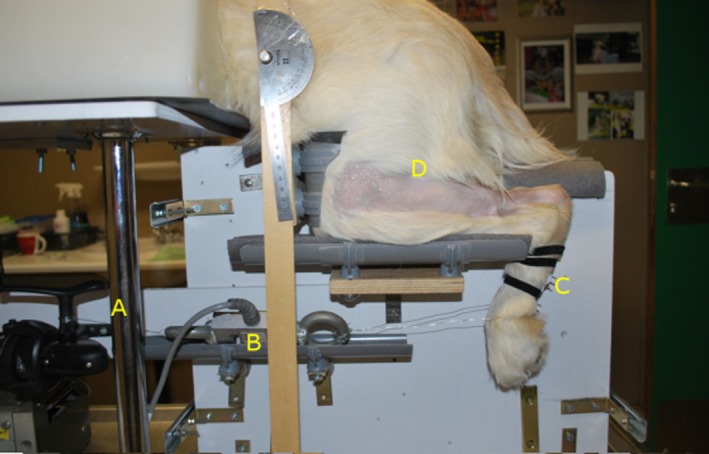
Joint‐positioning device. (A) Spool to wind up and keep the cable at a steady length, (B) load cell, (C) foot plate, (D) clipped area covering gastrocnemius muscle and common calcaneal tendon area.
Terminology
Musculotendon length was defined as the length of the musculotendon complex with the tarsus in a relaxed position, i.e. without any externally imposed tarsus (dorsi‐) flexion]. Maximal musculotendon length was defined as the length of the musculotendon complex during maximal imposed tarsus (dorsi‐) flexion with a fixed stifle angle (range 65–90°). Tendon length was determined at tendon slack length, and was defined as the gastrocnemius tendon length at the maximal tarsus (dorsi‐) flexion angle at which gastrocnemius muscle fibres remain slack. Muscle fibre and muscle belly lengths were also measured at tendon slack length.
As an important note, even though the gastrocnemius tendon is the major contributor to the canine common calcaneal tendon, this study only examined the gastrocnemius tendon.
Protocol
First, the dogs were placed in a custom‐built joint positioning device (Fig. 1) specifically designed to support the hind limbs at a stifle angle of approximately 73° (range 65–90°), measured with a goniometer. In this setup, the between‐subject difference in the stifle angle caused concurrent changes in the tarsus angle, resulting in comparable musculotendon lengths. A small plastic plate was attached to the plantar aspect of the metatarsus to prevent foot deformation during the muscle activity and to protect the foot against potential harm from the pulling force of the steel wire. The foot plate was connected to a spool with a steel wire to increase tarsus (dorsi‐) flexion by winding up the steel cable. A calibrated load cell (Tedea‐Huntleigh – 614‐300M‐D2; VPG, Malvern, PA, USA), coupled with an amplifier (KWS 3073, full bridge; HBM, Darmstadt, Germany) attached in series with the steel wire, allowed the examiner to maintain similar tensile forces during the maximal tarsus flexion trials.
Musculotendon lengths were obtained through a combination of ultrasonography using a linear transducer with a range of 12–5 MHz (Philips CX50 ultrasound system), and a ‘flock of bird’ (FOB) electromagnetic six degrees‐of‐freedom tracking device (Model MiniBird; Ascension Techology Corp., Burlington, VT, USA). The FOB receiver was placed close to the joint positioning device, and the FOB probe was attached to the ultrasound probe. In combination with a data acquisition card (NI USB‐6008; National Instruments, Austin, TX, USA), this setup allowed the ultrasound probe movements to be measured accurately in three dimensions.
The ultrasound probe (with the FOB probe attached to it) was positioned lateral to the origin of the lateral gastrocnemius muscle head, with the muscle insertion site on the far left of the image. The ultrasound film data and the FOB probe position data were simultaneously acquired during a distal movement of the ultrasound probe along the skin surface, and the recording was stopped with the insertion site of the tendon at the far left of the image. Tendon length was measured with the probe starting position at the end of the muscle (the aponeurosis) and the insertion site of the tendon as the probe end position.
Three repetitions were performed respectively to acquire musculotendon length, maximal musculotendon length and tendon slack length. First, the musculotendon length was obtained by measuring the musculotendon length with the tarsus in a relaxed state (i.e. the natural length of the musculotendon complex with the spool unwound and gravity as the only external force acting upon the foot). Following this, the steel cable was tensioned to bring the tarsus into the most flexed position that could be manually induced. The musculotendon length measured in this state was defined as the maximal musculotendon length. For the last measurement set, the muscle was relaxed again by unwinding the spool. Using the ultrasound probe to visualize the muscle fibres, the tarsus was flexed (by winding up the spool) to the position just before visible tension occurred in the gastrocnemius muscle fibres. The tendon length measured at this position was defined as the tendon slack length. Finally, while maintaining the tarsus angle, the ultrasound probe was positioned along the long axis of the muscle fibres, and images of the lateral head of the gastrocnemius muscle were taken to measure pennation angles and fibre lengths.
Post‐processing
Analysis of the ultrasound images was performed with imagej software (ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/).
To determine the musculotendon and tendon slack lengths, the three‐dimensional co‐ordinates from the FOB data were filtered with a fourth‐order low pass Butterworth filtre with a cut‐off frequency of 5 Hz. The Euclidean distance between the start and the end points of the motion was calculated as musculotendon length for that measurement. For each measurement, images from the ultrasound film were analysed to account for an offset between the edge of the first and last images of the ultrasound probe and the origin and insertion sites of the musculotendon complex. The musculotendon length for each subject was calculated as the average of the three measurements. A ratio of musculotendon excursion potential to musculotendon length was calculated to provide a value for relative excursion potential. A ratio of tendon slack length to musculotendon length was calculated to provide a measure for relative tendon contribution to musculotendon length.
Femur lengths were measured using osirix imaging software, by calculating the distance between the trochlear fossa and the intercondylar depression (Fig. 2) on the radiographs.
Figure 2.
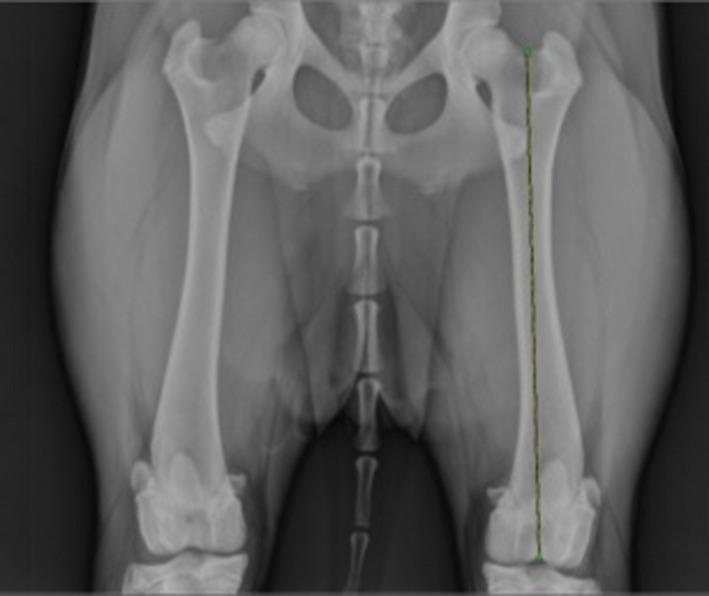
Radiograph of the femur. Ventro‐dorsal radiograph of the hips and both femurs, showing left femur length as the distance between the trochlear fossa and the intercondylar depression.
Muscle fibre lengths were measured as the distance between the abaxial superficial aponeurosis and the central aponeurosis, as acquired from the ultrasound images of the gastrocnemius lateral muscle head, and were calculated as the average fibre length from ten randomly selected muscle fibres on different parts of the muscle (Fig. 3). Muscle fibre pennation angles were measured as the angle between the fibre length line and that of the internal aponeurosis, and were calculated as the average of 10 randomly selected pennation angles (Fig. 3). Musculotendon excursion potential was calculated as the difference between the maximal and minimal musculotendon lengths. Muscle belly length was calculated as tendon slack length subtracted from the musculotendon length. AI for the (lateral) gastrocnemius muscle was calculated as muscle fibre length divided by the muscle belly length.
Figure 3.
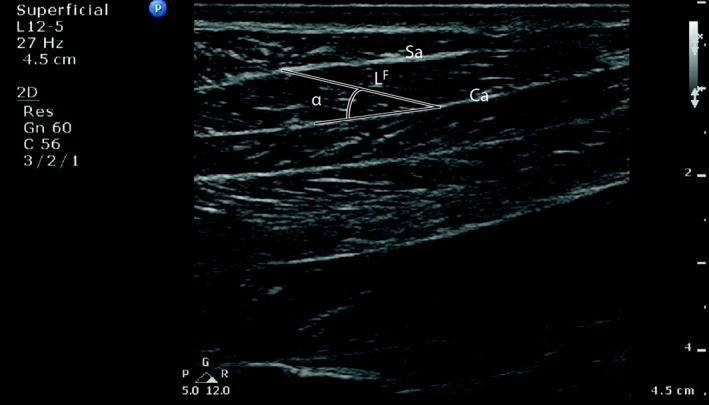
Sagittal ultrasound image used for measurements. Length‐related ultrasound image of the lateral gastrocnemius muscle used for measurement of: fibre length (LF) as the distance between the abaxial superficial aponeurosis (Sa) and the central aponeurosis (Ca), pennation as the angle between the fibre length line and that of the internal aponeurosis. Actual measurements were taken as the average of 10 measurements per subject. Left in the image is proximal, top is lateral.
Muscle thicknesses were measured as the distance between the upper and lower superficial aponeurosis of the muscle, perpendicular to the direction of the central aponeurosis. Muscle thickness was calculated as the average of 10 measurements.
Statistical analysis
The repeatability for each of the measurement techniques involved in this study was calculated as:
where n represents the number of measurements, and n = 3 for the calculation of the musculotendon length, maximal musculotendon length and the tendon slack length repeatability, and n = 10 for the fibre length, pennation angle and muscle thickness repeatability.
To examine whether an increase in musculotendon length changes the ratio of muscle and tendon contribution to the musculotendon length, a linear regression analysis was performed between muscle belly length and musculotendon length, as well as between tendon slack length and musculotendon length. To account for the possibility that changes in muscle belly length do correspond to similar changes in muscle fibre lengths, a linear regression analysis was performed between muscle fibre length and muscle belly, as well as between muscle fibre length and musculotendon length.
To examine the impact of muscle fibre length and tendon length on musculotendon excursion potential, a linear regression analysis was performed between the relative musculotendon excursion to musculotendon length ratio and the relative tendon slack to musculotendon length ratio, as well as between the relative musculotendon excursion to musculotendon length ratio and the relative muscle to fibre to musculotendon length. ‘Relative parameters’ indicate that values (i.e. musculotendon excursion, tendon slack length, muscle fibre length) were divided by the musculotendon length of that dog.
To examine the relation between the length‐related proportions of the limb and those of the gastrocnemius muscle and tendon, Pearson's product‐moment correlation tests and regression analyses were performed between the femur length (proposed scaling factor) and the musculotendon length, the maximal musculotendon length, the tendon slack length and the muscle excursion potential.
Results
Between‐measurement repeatability was 0.49 cm (0.12–1.27) for the musculotendon length (2.9% of average musculotendon length), 0.44 cm (0.14–0.98) for the maximal musculotendon length (2.5% of average maximal musculotendon length), 0.23 cm (0.11–0.39) for the fibre length (12.7% of average fibre length), 2.44° (2.02–3.61) for the pennation angle (17.7% of average pennation angle), 0.33 cm (0.16–0.52) for the tendon slack length (4.5% of average tendon slack length), and 0.016 cm (0.003–0.036) for the muscle thickness (1.5% of average muscle thickness).
The following values are the averages for the Labrador gastrocnemius muscle and tendon parameters. Musculotendon length was 16.10 cm (± 2.49), maximal musculotendon length was 17.25 cm (± 2.69), average musculotendon excursion potential was 1.15 cm (± 0.26) and the average ratio of excursion to musculotendon length was 7.45% (± 2.48). Muscle belly length was 9.51 cm (± 2.58), muscle fibre length was 1.86 cm (± 0.40), pennation angle was 13.26° (± 1.73) and AI was 0.21 (± 0.09). Tendon slack length was 6.59 cm (± 1.50) with relative tendon slack length being 41% (± 9%). The average femur length of the dog group was 19.81 cm (± 0.73). Table 1 lists the individual values per subject. The average muscle thickness of the dog group was 1.07 cm (± 0.05).
Table 1.
Musculotendon and femur lengths per subject
| S | M (kg) | L MT (cm) | T M (mm) | L MT max (cm) | L T s (cm) | L MT exc (cm) | L Fe (cm) | L F (cm) | αF (°) | L M (cm) | AI | L T s/L MT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 19.09 | 0.925 | 19.94 | 8.84 | 0.85 | 19.49 | 1.15 | 16.48 | 10.24 | 0.11 | 0.46 |
| 2 | 30 | 21.34 | 0.951 | 22.53 | 6.69 | 1.19 | 21.00 | 1.53 | 12.32 | 14.65 | 0.10 | 0.31 |
| 3 | 33.5 | 15.47 | 1.261 | 16.65 | 4.15 | 1.18 | 19.62 | 1.80 | 14.33 | 11.32 | 0.16 | 0.27 |
| 4 | 22 | 17.86 | 0.997 | 18.84 | 6.45 | 0.98 | 19.45 | 1.68 | 11.68 | 11.41 | 0.15 | 0.36 |
| 5 | 25 | 13.24 | 1.097 | 14.83 | 4.99 | 1.60 | 19.23 | 2.08 | 12.44 | 8.24 | 0.25 | 0.38 |
| 6 | 22.5 | 14.48 | 1.084 | 15.86 | 7.41 | 1.38 | 19.71 | 2.23 | 12.86 | 7.07 | 0.32 | 0.51 |
| 7 | 21 | 14.91 | 0.995 | 16.26 | 7.74 | 1.36 | 19.21 | 1.82 | 13.69 | 7.16 | 0.25 | 0.52 |
| 8 | 28 | 15.42 | 1.372 | 16.25 | 7.60 | 0.83 | 19.46 | 1.91 | 14.70 | 7.82 | 0.24 | 0.49 |
| 9 | 25 | 13.08 | 0.949 | 14.09 | 5.44 | 1.02 | 21.14 | 2.54 | 10.84 | 7.64 | 0.33 | 0.42 |
αF, muscle fibre pennation angle; L F, muscle fibre length; L Fe, femur length; L M, muscle belly length; L MT, musculotendon length; L MT exc, musculotendon excursion potential; L MT max, maximal musculotendon length; L T s, tendon slack length; M, body mass; S, subject number; T M, muscle thickness. Architectural index (AI) and tendon (slack) length to musculotendon length ratio L T s/L MT min.
Regression analysis yielded a significant positive correlation between muscle belly length and musculotendon length (R 2 = 0.72, P = 0.004) (Fig. 4) but not between tendon slack length and musculotendon length (R 2 = 0.16, P = 0.29) (Fig. 4). Regression analysis yielded a significant negative correlation between muscle fibre length and musculotendon length (R 2 = 0.68, P = 0.007) (Fig. 5), and a slight negative trend between muscle fibre length and muscle belly length (R 2 = 0.38, P = 0.08) (Fig. 6).
Figure 4.
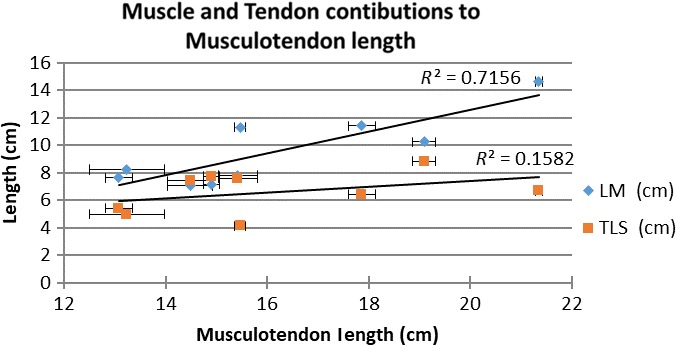
Scatterplot of muscle and tendon contributions to musculotendon length. LM, muscle belly length; R 2, R‐square of the trendline; TSL, tendon slack length. Error bars denote the standard error.
Figure 5.
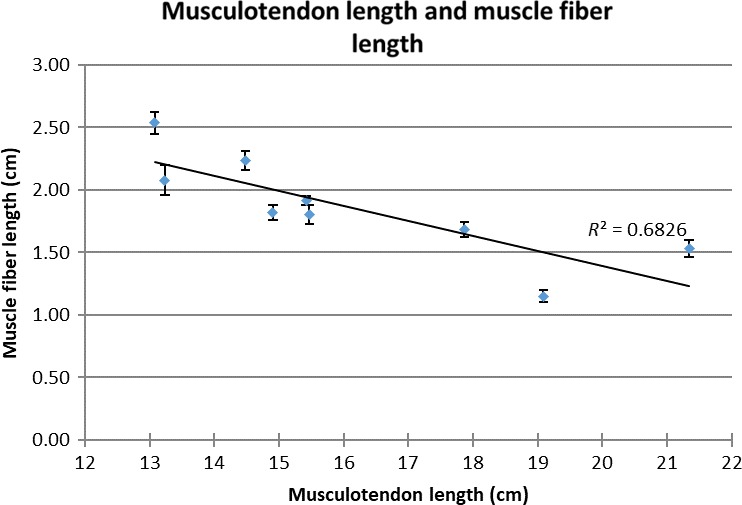
Scatterplot of muscle fibre length and musculotendon length. R 2, R‐square of the trendline. E, error bars denote the standard error.
Figure 6.
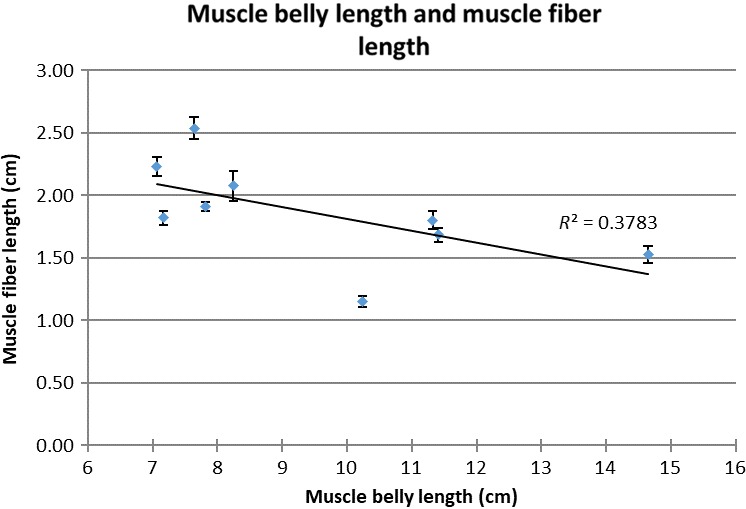
Scatterplot of muscle fibre length and muscle belly length. R 2, R‐square of the trendline. E, error bars denote the standard error.
Regression analysis yielded no significant correlation between relative musculotendon excursion and the relative tendon slack (P = 0.82) or between the relative musculotendon excursion to musculotendon length ratio and the relative muscle fibre to musculotendon length (P = 0.19).
Pearson's product‐moment correlation tests and regression analyses yielded no significant association between the femur length and the musculotendon length‐related parameters (R 2 ≤ 0.05, P ≥ 0.57), and no significant association between the musculotendon excursion potential and tendon slack length (R 2 = 0.19, P = 0.21) or association between muscle fibre and tendon slack length (R 2 = 0.15, P = 0.27).
Discussion
This study describes the in vivo musculotendon properties of the gastrocnemius muscle and tendon in Labrador dogs, employing a dedicated ultrasound‐based methodology adopted from human biomechanics research.
This study found that, in Labradors, the gastrocnemius tendon contributes 41% (± 9%) of the musculotendon complex. Currently, relatively little research has focused on canine hind limb muscle and tendon parameters, and the only potential data available for comparison are data on muscle fibre lengths and muscle belly lengths data for a 23‐kg mixed‐breed dog (Shahar & Milgram, 2001). However, musculotendon properties of the gastrocnemius muscle and tendon are available for Greyhounds (Williams et al. 2008) and may yield interesting insights into the functional muscle and tendon adaptations of a dog breed specifically bred for sprinting performance. Comparing the Labrador gastrocnemius tendon contribution of 41% to the 52% contribution in Greyhounds (Williams et al. 2008), it is clear that the Greyhound breed has indeed undergone significant specializations towards elastic storage and distal limb mass reduction (Pasi & Carrier, 2003; Williams et al. 2008). The (lateral) gastrocnemius pennation angle in Labradors is also markedly lower than in Greyhounds. However, Labrador muscle fibres length is relatively comparable to Greyhound muscle fibre length, and both are markedly longer than the reported mixed‐breed dog muscle fibre length (Shahar & Milgram, 2001). In contrast, Labradors have a slightly higher AI than Greyhounds and the mixed‐breed dog, which is consistent with the combination of similar fibre lengths but longer muscle bellies in Labradors. Because the differences in muscle properties between breeds can have profound functional implications, this argues against extrapolating data from a specific breed to dogs in general. In summary, this study found that Labradors have a larger contribution of muscle belly length to musculotendon length than Greyhounds, supporting the theory that Greyhounds reduce distal limb mass and increase elastic storage to increase running efficiency.
The significant correlation of muscle belly length with musculotendon length (Fig. 3), and the non‐significant correlation between tendon slack length and musculotendon length (Fig. 4), indicate a functional variation in musculotendon composition within the Labrador population. It appears that Labradors with a longer musculotendon complex also have a higher contribution of muscle belly length, while tendon slack length remains relatively constant. Remarkably, longer musculotendon complexes, and therefore longer muscle belly lengths, also correspond to shorter muscle fibre lengths (Figs 5, 6 ), and this without significant changes in muscle fibre pennation angle. This is possible due to the pennate organization of the gastrocnemius muscle, which allows muscle belly length to increase by adding more muscle fibres in parallel. This implies that longer muscle bellies also correspond to an increase in muscle physical cross‐sectional area (PCSA) and that the combination of longer muscle bellies and shorter muscle fibres represents an attempt to maintain a similar muscle volume in longer musculotendon complexes without increasing muscle excursion potential. Maintaining a similar volume for muscles located distally in the limb may be important in preventing important increases in the moment of inertia of the limb (Steudel & Beattie, 1993). However, because no data on muscle volume are available from this study, no definite conclusions on this matter can be reached.
One of the assumptions when performing muscle scaling is that longer muscle bellies correlate with longer muscle fibres. Surprisingly, this study found that in the Labrador gastrocnemius muscle, shorter muscle fibre length corresponded with longer muscle belly length. This has important implications for muscle function, as it suggests an ‘uncoupling’ of muscle belly length and muscle fibre length for pennate muscles. Specifically, muscles in which the muscle fibres do not run along the entire length of the muscle may not follow the same scaling rules as muscle fibres in fusiform muscles with a high AI, for which fibre length is directly related to muscle belly length.
Further evidence of this ‘uncoupling’ of muscle fibre length and muscle belly length can be found in the lack of significant correlation between relative tendon length and musculotendon excursion potential. This is contrary to the hypothesis that increases in relative tendon length would decrease the musculotendon excursion potential, and indicates that changes in relative tendon length or muscle fibre length do not reliably predict changes in the musculotendon excursion potential. A possible explanation for this is that changes in muscle fibre length could offset the changes in relative tendon contribution to musculotendon length, thereby nullifying any changes in effective excursion potential.
This study did not find femur length to be a reliable scaling factor for any of the musculotendon parameters investigated. This is surprising, as indicators of limb length could be expected to be a primary component in determining the length‐related aspects of the musculotendon complex and could therefore be expected to be useful scaling factors. While it is possible that the low variability in femur lengths within the study is responsible for the lack of correlation between femur lengths and the length‐related aspects of the musculotendon complex, studies on fibre lengths also indicate that measures of limb length are poor predictors of fibre length in the canine hind limb (Dries et al. 2015, 2016). Finding scaling factors may prove to be one of the biggest hurdles in canine musculotendon research due to the massive musculoskeletal variation between dog breeds (Dries et al. 2016). Further research focusing on musculotendon properties of a large variety of dog breeds may yield further insights into this matter.
Experimental setup
Most muscle tendon research in humans requires voluntary controlled motions and contractions that are not applicable to canine research. Therefore, this study employed state‐of‐the‐art methods from human musculotendon research that did not require controlled muscle contractions (Fry et al. 2003, 2004; Barber et al. 2009). Repeatability values for musculotendon and tendon lengths indicate that, at least for muscles with origin and insertion sites close to the skin surface, a combination of ultrasound measurements and 3D motion tracking yields reliable measurements for estimating in vivo muscle and tendon lengths.
However, as a result of adaptation of these techniques for use in dogs, several limitations apply to the current experimental setup:
First, during the experiment an increase in tarsus angle was noted after the stretching of the musculotendon complex during the trial measuring maximal musculotendon length compared with the trial measuring the tendon slack length. Because the calculation of muscle belly length uses data from the tendon slack length trial, an overestimation of muscle belly length may have occurred. However, this does not affect the other parameters examined.
Secondly, measuring musculotendon length with the tarsus in a relaxed position may have overestimated the length. The shortest physiological muscle belly length likely occurs when the muscle is fully shortened (activated) and it was not possible to measure this with the current study setup. In human research, the shortest musculotendon length could be achieved through voluntary contractions. While functional electrical stimulation of the muscle could have been used to produce muscle contraction, the measurement duration of the musculotendon length was too long to maintain a constant stimulation, and the stimulation process would have interfered with ultrasound/3D motion tracking measurements. Assuming the muscle fibres are at an optimal fibre length in their relaxed state, and that the maximal amount of active muscle contraction will shorten the muscle fibres to about two‐thirds of optimal fibre length (Brand et al. 1981), this yields a maximal overestimation of about 0.6 cm, or 2.5%.
Thirdly, the dogs employed in this study were relatively young. Although Labradors at this age are nearly adult and very close to adult size, there is a possibility that their relative youth influenced minimal and maximal musculotendon lengths. Previous research has shown that Labradors reach around 87 and 96% of their adult body mass by respectively 8 and 10 months of age (Hawthorne et al. 2004).
Fourthly, repeatability for the measurements of fibre length and pennation angle was quite poor. This may be due to a high variability in the fibre lengths and pennation angles within the canine gastrocnemius muscle (Fig. 3), either inherent in the muscle itself or because of muscle deformation during measurement. For the purposes of this study, however, muscle fibre lengths and pennation angles were always based on an average of 10 measurements, mitigating – at least partially – the effect of limited repeatability.
Finally, although the use of ultrasound to measure musculotendon parameters is already well established in human research (Lan et al. 2009; Noorkoiv et al. 2010), this study is the first to employ anaesthesia and a joint‐positioning device for ultrasound measurements in dogs. Although this may raise some questions about the reliability of these measurements, these new measurement techniques provide practical measurement solutions for non‐invasive research in animals and may give additional insight into understanding functional musculotendon anatomy. The determination of maximal musculotendon length through an imposed (dorsi‐) flexion of the canine tarsus using maximal manual force and therefore the applied force is unknown, but it provides a realistic estimate of the maximal physiological extension of the musculotendon complex, while eliminating the risk of muscle or tendon rupture. Determination of tendon slack length through ultrasound visualization of the muscle fibres during passive limb movement allows for a practical non‐invasive in vivo measurement. Indeed, as the muscle component is placed in series with the tendon, any force applied to the tendon is bound to cause a stretching of the muscle fibres.
Conclusion
In the Labrador gastrocnemius musculotendon complex, the tendon contributes 41% of the total length. Labradors with longer musculotendon complexes also have longer muscle belly lengths and shorter muscle fibres, while tendon slack length remains relatively constant. This also results in an increase in relative muscle contribution to musculotendon length in larger musculotendon complexes. This suggests that Labradors with longer musculotendon have more muscle fibres in parallel, increasing the PCSA and preventing increasing gastrocnemius muscle volume. This study found no evidence to support a relation between musculotendon excursion length and tendon slack length or muscle fibre length. Furthermore, femur length did not prove to be a reliable scaling factor for length‐related musculotendon parameters in dogs.
Conflict of interests
No competing interests declared.
Author contributions
Conceptualization: B. Dries, B. Vanwanseele, I. Jonkers, J. Vander Sloten, H. van Bree, I. Gielen; Methodology: B. Dries, B. Vanwanseele; Formal analysis: B. Dries; Investigation: B. Dries; Resources: B. Vanwanseele, I. Jonkers, W. Dingemanse, J. Vander Sloten, A. Villamonte‐Chevalier, E. Vander Vekens, I. Polis, H. van Bree, I. Gielen; Writing – original draft preparation: B. Dries; Writing – review and editing: B. Vanwanseele, I. Jonkers, I. Gielen; Visualization: B. Dries; Supervision: H. van Bree, I. Gielen; Project administration: K. Vanderperren.
References
- Arnold EM, Hamner SR, Seth A, et al. (2013) How muscle fiber lengths and velocities affect muscle force generation as humans walk and run at different speeds. J Exp Biol 216, 2150–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber L, Barrett R, Lichtwark G (2009) Validation of a freehand 3D ultrasound system for morphological measures of the medial gastrocnemius muscle. J Biomech 42, 1313–1319. [DOI] [PubMed] [Google Scholar]
- Bradley PS, Portas MD (2007) The relationship between preseason range of motion and muscle strain injury in elite soccer players. J Strength Cond Res 21, 1155. [DOI] [PubMed] [Google Scholar]
- Brand PW, Beach RB, Thompson DE (1981) Relative tension and potential excursion of muscles in the forearm and hand. J Hand Surg 6, 209–219. [DOI] [PubMed] [Google Scholar]
- Cook CS, McDonagh MJ (1996) Measurement of muscle and tendon stiffness in man. Eur J Appl Physiol 72, 380–382. [DOI] [PubMed] [Google Scholar]
- Dries B, Jonkers I, Van den Broeck W, et al. (2015) Scaling muscle parameters in a canine musculoskeletal model. 25th Congress of the International Society of Biomechanics. ISB congress, 12–16 July 2015, Glasgow, UK. Poster presentation. 1802 pp.
- Dries B, Jonkers I, Dingemanse W, et al. (2016) Musculoskeletal modelling in dogs: challenges and future perspectives. Vet Comp Orthop Traumatol 29, 181–187. [DOI] [PubMed] [Google Scholar]
- Dyce KM, Sack WO, Wensing CJG (2010). Textbook of Veterinary Anatomy. Saunders/Elsevier: St. Louis. [Google Scholar]
- Fry NR, Childs CR, Eve LC, et al. (2003) Accurate measurement of muscle belly length in the motion analysis laboratory: potential for the assessment of contracture. Gait Posture 17, 119–124. [DOI] [PubMed] [Google Scholar]
- Fry NR, Gough M, Shortland AP (2004) Three‐dimensional realisation of muscle morphology and architecture using ultrasound. Gait Posture 20, 177–182. [DOI] [PubMed] [Google Scholar]
- Gardell C (1980) Miller's Anatomy of the Dog. Second edition. Can Vet J 21, 118. [Google Scholar]
- Garner BA, Pandy MG (2003) Estimation of musculotendon properties in the human upper limb. Ann Biomed Eng 31, 207–220. [DOI] [PubMed] [Google Scholar]
- Godges JJ, Macrae H, Longdon C, et al. (1989) The effects of two stretching procedures on hip range of motion and gait economy. J Orthop Sports Phys Ther 10, 350–357. [DOI] [PubMed] [Google Scholar]
- Hawthorne AJ Booles D, Nugent PA, et al. (2004) Body‐weight changes during growth in puppies of different breeds. J Nutr 134(8 Suppl), 2027S–2030S. [DOI] [PubMed] [Google Scholar]
- Ismail A, et al. (2015) Comparative kinematic gait analysis in young and old Beagle dogs. J Vet Sci 18, 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Jin LH, Zhu KY, et al. (2009) Musculo‐tendon parameters estimation by ultrasonography for modeling of human motor system. IFMBE Proceedings 23, 1761–1765. [Google Scholar]
- Noorkoiv M, Stavnsbo A, Aagaard P, et al. (2010) In vivo assessment of muscle fascicle length by extended field‐of‐view ultrasonography. J Appl Physiol 109, 1974–1979. [DOI] [PubMed] [Google Scholar]
- Pasi BM, Carrier DR (2003) Functional trade‐offs in the limb muscles of dogs selected for running vs. fighting. J Evol Biol 16, 324–332. [DOI] [PubMed] [Google Scholar]
- Shahar R, Milgram J (2001) Morphometric and anatomic study of the hind limb of a dog. Am J Vet Res 62, 928–933. [DOI] [PubMed] [Google Scholar]
- Steudel K, Beattie J (1993) Scaling of cursoriality in mammals. J Morphol 217, 55–63. [DOI] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Rhodes L, et al. (2008) Functional anatomy and muscle moment arms of the pelvic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris). J Anat 213, 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17, 359–411. [PubMed] [Google Scholar]


