Abstract
Background
Angiogenesis plays an important role in the progression of glioblastoma, with a high degree of malignancy. Previous studies have proved that glial cell line-derived neurotrophic factor (GDNF) and fibromodulin (FMOD) are strongly expressed in human glioblastoma. The purpose of this study was to explore the roles of GDNF and FMOD in angiogenesis and the molecular mechanisms underlying these roles in human glioblastoma.
Material/Methods
The effects of GDNF on the expression and secretion of vascular endothelial growth factor (VEGF) in human glioblastoma cell line U251 and angiogenesis in human umbilical vein endothelial cells (HUVECs) were investigated. The molecular mechanism of GDNF-induced expression of FMOD was explored. The roles of FMOD in GDNF-induced expression and secretion of VEGF and angiogenesis were also examined.
Results
In the present study, we showed that GDNF promoted the expression and secretion of VEGF in U251 cells. VEGF mediated GDNF-induced angiogenesis in human glioblastoma. In addition, GDNF significantly upregulated the expression of FMOD in U251 cells. Mechanistically, the results of luciferase reporter assay and methylation-specific PCR (MSP) demonstrated that GDNF facilitated the demethylation of the FMOD promoter. More importantly, we found that FMOD acted as an important mediator in VEGF expression and angiogenesis induced by GDNF in human glioblastoma.
Conclusions
Collectively, our data show that GDNF promotes angiogenesis through demethylation of the FMOD promoter in human glioblastoma, indicating that GDNF and FMOD may be potential therapeutic targets for glioblastoma.
MeSH Keywords: Glial Cell Line-Derived Neurotrophic Factor, Glioblastoma, Human Umbilical Vein Endothelial Cells
Background
Glioblastoma is the most common and malignant tumor derived from the central nervous system in adults [1]. Despite remarkable advances in chemotherapy, radiation, surgery, and targeted therapy, the prognosis for the majority of patients with glioblastoma remains poor [2]. Accumulating evidence indicates that glioblastoma is highly vascularized, and angiogenesis is of great importance in the progression of this lethal disease [3]. In recent years, more and more researchers have paid considerable attention to angiogenesis in glioblastoma, which may be a promising therapeutic target [4,5]. Moreover, it is generally accepted that angiogenesis is regulated by a wide range of molecules in tumors [6,7]. As is well known, vascular endothelial growth factor (VEGF) serves as one of the most important factors with proangiogenic potential.
Glial cell line-derived neurotrophic factor (GDNF), a member of the transforming growth factor-β (TGF-β) superfamily, is a neurotrophic factor that is involved in regulating survival and differentiation of various neural cells [8]. Previous studies have demonstrated that GDNF is highly expressed in human glioblastoma and promotes the proliferation, migration, and invasion of glioma cells [9–11]. In addition, it has been reported that GDNF secreted from adipose-derived stem cells promotes angiogenesis in human umbilical vein endothelial cells (HUVECs) [12]. Nevertheless, little is known about the role of GDNF in angiogenesis in human glioblastoma.
Fibromodulin (FMOD), a member of the family of small leucine-rich proteoglycans, participates in regulating angiogenesis in cutaneous and optical diseases associated with angiogenesis and wound healing [13–15]. Moreover, a previous study has shown that FMOD has aberrantly high expression in human glioblastoma tissues and is implicated in promoting migration and invasion of glioma cell via regulating actin cytoskeleton remodeling [16]. However, the effect of FMOD on angiogenesis in human glioblastoma has not been investigated. Additionally, it remains undefined whether there is a regulatory interaction between GDNF and FMOD in glioblastoma cells.
In the present study, we investigated the roles of GDNF in the expression and secretion of VEGF in human glioblastoma cells and angiogenesis in HUVECs. Moreover, the molecular mechanisms of GDNF-induced FMOD expression was explored. We also assessed the roles of FMOD in GDNF-induced the expression and secretion of VEGF in glioblastoma cells and angiogenesis in HUVECs.
Material and Methods
Cell culture
U251 (human glioblastoma cell line) and HUVECs were purchased from the American Type Culture Collection. Both were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone) supplemented with 10% fetal bovine serum (FBS, Hyclone) at 37°C with 5% CO2.
Western blot analysis
U251 cells transfected with control siRNA (si-Con) or GDNF siRNA (si-GDNF) were cultured. Western blot analysis was performed as previously described [17]. The proteins were separated using SDS-PAGE and then transferred to PVDF membranes. The specific antibodies were used to detect and identify proteins of interest. Antibodies against GAPDH, GDNF, FMOD, and VEGF were obtained from Abcam (Cambridge, UK).
Quantitative real-time PCR
qRT-PCR was carried out as previously described [17]. TRIzol was purchased from Invitrogen to extract total RNA. Extraction of RNA was performed according to the manufacturer’s instructions. Primers for GAPDH, VEGF, and FMOD were obtained from Invitrogen Bioengineering Corporation (Shanghai, China).
ELISA
Human VEGF ELISA kit was obtained from Abcam and used to measure the concentration of VEGF. ELISA was performed according to the manufacturer’s instructions. The concentration of VEGF in conditioned medium was determined by detecting the absorbance at 450 nm in a microplate reader.
Transwell migration assay
HUVECs (6×104) were seeded in the upper chamber of Transwell inserts in 24-well plates (Corning, USA). Conditioned medium (CM) from U251 cells transfected with FMOD siRNA (si-FMOD) or control siRNA (si-Con) was added to the lower chamber. After culturing for 18 h, the migratory cells were counted via using a microscope.
Tube formation assay
Growth factor-reduced Matrigel (Corning, USA) was placed in 96-well plates (50 μL/well) and incubated for 30 min at 37°C. HUVECs were suspended in conditioned medium from U251 cells transfected with si-FMOD or si-Con. HUVECs (2×104/200 μL) were seeded in the Matrigel-coated wells and then cultured for 20 h at 37°C. An inverted microscope was used to assess tube formation in endothelial cells.
Methylation-specific PCR (MS-PCR)
MS-PCR was carried out to detect the methylation status of FMOD promoter in U251 cells transfected with si-Con or si-GDNF. According to the manufacturer’s instructions, MS-PCR was performed using a methylation-specific kit (Tiangen). The products were analyzed through agarose gel electrophoresis. All experiments were repeated at least 3 times.
Statistical analysis
All data are presented as mean ± standard deviation (SD). The difference between the 2 groups was analyzed using the t test. P<0.05 was considered statistically significant.
Results
GDNF promotes the expression and secretion of VEGF in human glioblastoma cells
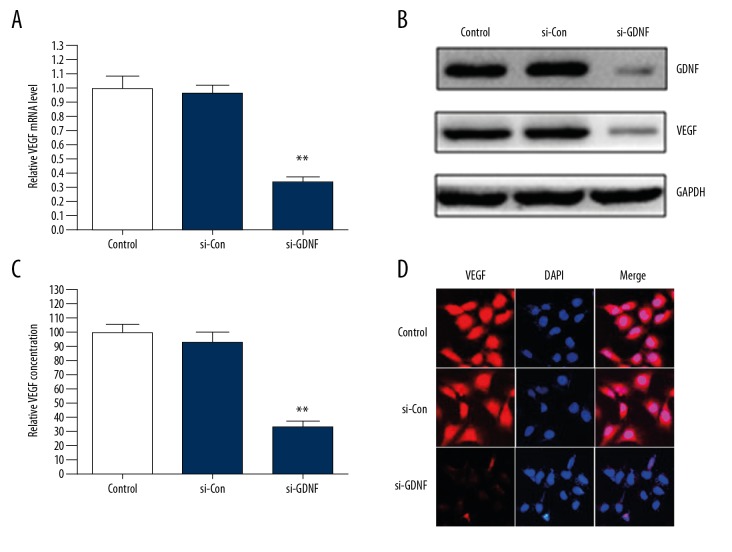
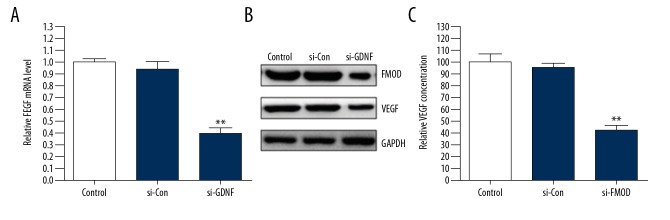
To investigate the effects of GDNF on the expression and secretion of VEGF in human glioblastoma cells, U251 cells were transfected with si-GDNF or si-Con. We found that silencing of GDNF expression significantly suppressed the protein and mRNA expression levels and secretion of VEGF compared with the si-Con group (Figure 1A–1C). In addition, the same results were further demonstrated through immunofluorescence staining (Figure 1D). These data suggest that GDNF promotes the expression and secretion of VEGF in human glioblastoma cells.
Figure 1.
GDNF induces the expression and secretion of VEGF in human glioblastoma cells. (A–C) U251 cells were transfected with GDNF siRNA (si-GDNF) or control siRNA (si-Con). The protein and mRNA expression levels and secretion of VEGF were detected through Western blot, qRT-PCR, and ELISA. ** P<0.01 vs. si-Con group. (D) The expression of VEGF in U251 cells transfected with si-GDNF or si-Con was measured by immunofluorescence.
VEGF mediates GDNF-induced angiogenesis
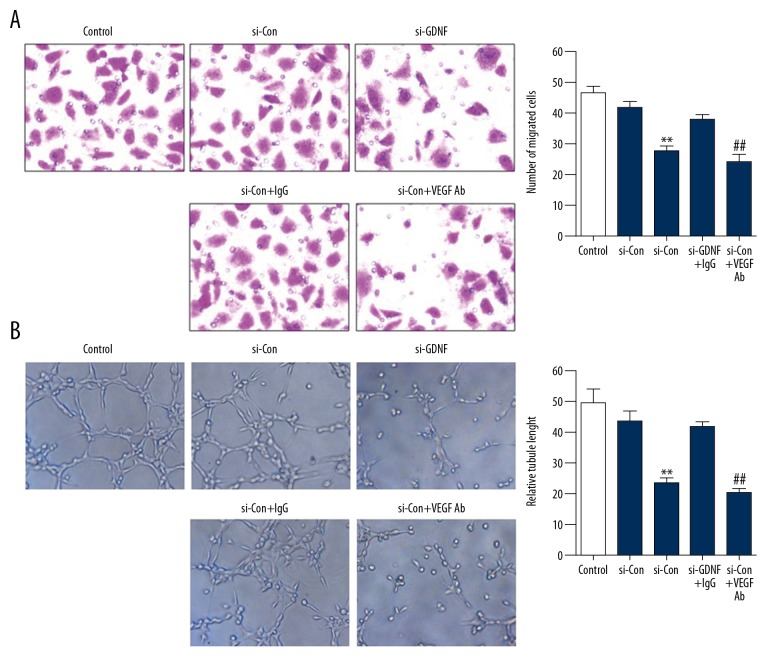
To explored whether GDNF was involved in angiogenesis in human glioblastoma, HUVECs were treated with conditioned medium (CM) from U251 cells transfected with si-Con or si-GDNF. As shown in Figure 2A and 2B, GDNF obviously promoted the migration and tube formation of HUVECs. On the contrary, ablation of GDNF inhibited these effects. Next, to identify the potential role of VEGF in GDNF-induced angiogenesis, we neutralized VEGF in the CM by specific antibody against it. The results showed that inhibiting VEGF in the CM through neutralizing antibody led to decreased migration and tube formation of HUVECs compared with the CM treated with IgG (Figure 2A, 2B). Collectively, these observations demonstrate that VEGF mediates GDNF-induced angiogenesis.
Figure 2.
VEGF is involved in GDNF-induced angiogenesis. (A, B) HUVECs were treated with conditioned medium (CM) from U251 cells transfected with si-Con or si-GDNF. Antibody against VEGF was used to neutralize it in the CM. Transwell migration assay and tube formation assay were performed. ** P<0.01 vs. si-Con group, ## P<0.01 vs. si-Con + IgG group.
GDNF promotes the expression of FMOD in glioblastoma cells
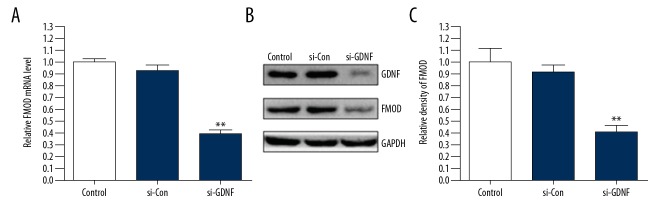
TGF-β was reported to regulate the expression of FMOD in glioblastoma cells [16]. Moreover, GDNF is a member of the TGF-β superfamily. Therefore, to demonstrate the role of GDNF in the expression of FMOD, we silenced GDNF expression in U251 cells by using siRNA. We found that silencing of GDNF expression inhibited the mRNA and protein expression of FMOD (Figure 3A–3C). These results indicate that GDNF promotes the expression of FMOD in glioblastoma cells.
Figure 3.
GDNF promotes the expression of FMOD in glioblastoma cells. (A–C) U251 cells were transfected with si-Con or si-GDNF. Western blot and qRT-PCR were used to analyze the protein and mRNA expression of FMOD. ** P<0.01 vs. si-Con group.
GDNF regulates the expression of FMOD via the demethylation of its promoter
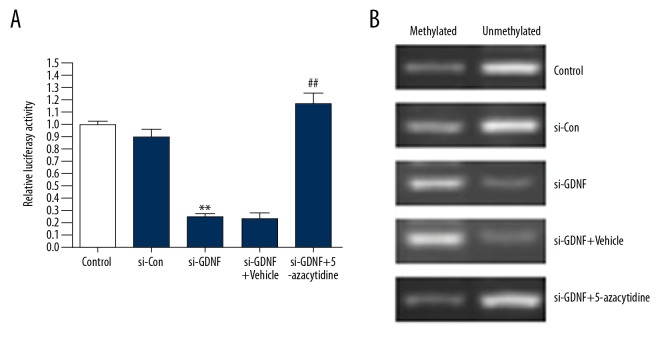
To further investigate the molecular mechanisms involved in GDNF-induced the expression of FMOD, luciferase reporter assay was performed in U251 cells. As shown in Figure 4A, ablation of GDNF resulted in a significant decrease in the FMOD promoter-dependent luciferase activity comparing with the si-Con group, whereas 5-azacytidine (a DNA methyltransferase inhibitor) treatment abolished the effect of si-GDNF in U251 cells (Figure 4A). Furthermore, the results of MS-PCR demonstrated that GDNF promoted the demethylation of the FMOD promoter (Figure 4B). Overall, these results suggest that GDNF regulates the expression of FMOD through the demethylation of its promoter.
Figure 4.
GDNF regulates the expression of FMOD via the demethylation of its promoter. (A, B) U251 cells transfected with FMOD promoter-dependent Luc construct and si-GDNF were stimulated with 5-azacytidine (1 μM) or vehicle. Luciferase reporter assay and methylation-specific PCR (MS-PCR) were performed. ** P<0.01 vs. si-Con group, ## P<0.01 vs. si-GDNF + Vehicle.
FMOD mediates GDNF-induced the expression and secretion of VEGF in glioblastoma cells
To determine whether GDNF-induced VEGF was mediated by FMOD, we silenced FMOD expression in U251 cells by siRNA. As shown in Figure 5A and 5B, the protein and mRNA expression of VEGF was induced by GDNF. Inversely, silencing of FMOD abolished the effects of GDNF (Figure 5A, 5B). Moreover, we found that the secretion of VEGF in U251 cells transfected with si-FMOD was obviously decreased compared with the si-Con group (Figure 5C). In conclusion, these data show that FMOD mediates GDNF-induced expression and secretion of VEGF in glioblastoma cells.
Figure 5.
FMOD mediates GDNF-induced the expression and secretion of VEGF in glioblastoma cells. (A–C) U251 cells were transfected with FMOD siRNA (si-FMOD) or control siRNA (si-Con). The protein and mRNA expression levels and secretion of VEGF were measured via Western blot, qRT-PCR and ELISA. ** P<0.01 vs. si-Con group.
FMOD mediates GDNF-induced angiogenesis
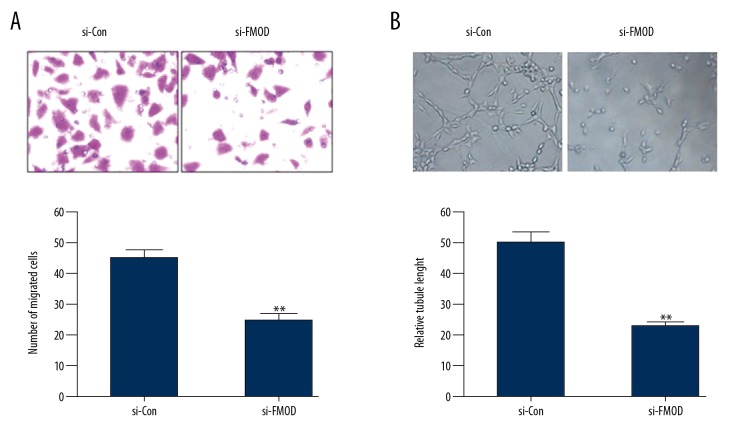
To further verify the role of FMOD in GDNF-induced angiogenesis, HUVECs were treated with conditioned medium from U251 cells transfected with si-Con or si-FMOD. We found that GDNF significantly promoted the migration and tube formation of HUVECs (Figure 2A, 2B), and silencing of FMOD eliminated the effect of GDNF (Figure 6A, 6B). Taken together, these results demonstrate that FMOD mediates GDNF-induced angiogenesis.
Figure 6.
FMOD mediates GDNF-induced angiogenesis. (A, B) HUVECs were stimulated with CM from U251 cells transfected with si-Con or si-FMOD. Transwell migration assay and tube formation assay were carried out. ** P<0.01 vs. si-Con group.
Discussion
Tumor angiogenesis is regulated by antiangiogenic and proangiogenic factors secreted from the surrounding and infiltrating host cells and tumor cells [7,18]. Sufficient angiogenesis is of great importance in growth and progression in human glioblastoma cells. There is an urgent need to better understand the molecular mechanisms of angiogenesis during progression of this deadly disease. In the present study, we demonstrated that GDNF promoted the expression and secretion of VEGF in human glioblastoma cells. VEGF mediated GDNF-induced angiogenesis in HUVECs. Moreover, we provided evidence that GDNF upregulated the expression of FMOD through the demethylation of its promoter. More importantly, we identified that FMOD mediated GDNF-induced the expression and secretion of VEGF in glioblastoma cells. In addition, we found that FMOD acted as an important mediator in GDNF-induced angiogenesis.
GDNF is one of the most potent neurotrophic factors demonstrated to be mainly involved in promoting the differentiation and survival of nerve cells [8,19,20]. Previous studies have shown that the expression of GDNF in human glioblastoma is significantly increased compared to normal brain tissues [9,21]. Additionally, it has been reported that GDNF participates in regulating the proliferation, migration, and invasion of glioblastoma cells [9,10,22,23]. It is also well known that VEGF plays an important role in neovascularization in brain tumors [24]. However, it is not clear whether GDNF promotes angiogenesis via regulating VEGF expression in human glioblastoma. Our study demonstrates that GDNF induces the expression and secretion of VEGF in human glioblastoma cells. Moreover, GDNF promotes angiogenesis by regulating VEGF expression. Our results are similar to those reported previously, in which GDNF induced VEGF-C expression in mouse neuroblastoma cells and promoted angiogenesis in hepatocellular carcinoma cells, normal skin cells, and renal cells [12,25–27].
FMOD plays an important role in angiogenesis in lung cancer and in cutaneous and optical diseases associated with angiogenesis and wound healing [14,15,28]. In addition, a previous study has shown that FMOD is highly expressed in human glioblastoma and is upregulated by TGF-β via the demethylation of its promoter in glioblastoma cells [16]. FMOD is also a member of the TGF-β superfamily. These observations prompted us to investigate whether FMOD was involved in angiogenesis and VEGF expression induced by GDNF in glioblastoma. In this study, our findings provide evidence that GDNF induces the expression of FMOD via the demethylation of its promoter in glioblastoma cells. More importantly, FMOD mediates GDNF-induced VEGF expression and angiogenesis. It is worth noting that these results are supported by previous observations [16,28].
Conclusions
We demonstrated for the first time that FMOD acts as an important mediator of VEGF expression and angiogenesis induced by GDNF in human glioblastoma. Furthermore, we provided evidence that GDNF upregulates the expression of FMOD via the demethylation of its promoter. These data indicate that GDNF and FMOD may be a promising therapeutic target for glioblastoma.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Medicine and Health Program of Zhejiang Province (2015ZDA028 and 2018RC067)
References
- 1.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17:301–12. doi: 10.1016/j.molmed.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7:1152–60. doi: 10.1016/S1474-4422(08)70260-6. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 5.Chi AS, Sorensen AG, Jain RK, Batchelor TT. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist. 2009;14:621–36. doi: 10.1634/theoncologist.2008-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 8.Lin LF, Doherty DH, Lile JD, et al. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–32. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 9.Wiesenhofer B, Stockhammer G, Kostron H, et al. Glial cell line-derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas. Acta Neuropathol. 2000;99:131–37. doi: 10.1007/pl00007416. [DOI] [PubMed] [Google Scholar]
- 10.Suter-Crazzolara C, Unsicker K. GDNF mRNA levels are induced by FGF-2 in rat C6 glioblastoma cells. Brain Res Mol Brain Res. 1996;41:175–82. doi: 10.1016/0169-328x(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 11.Song H, Moon A. Glial cell-derived neurotrophic factor (GDNF) promotes low-grade Hs683 glioma cell migration through JNK, ERK-1/2 and p38 MAPK signaling pathways. Neurosci Res. 2006;56:29–38. doi: 10.1016/j.neures.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Zhong Z, Gu H, Peng J, et al. GDNF secreted from adipose-derived stem cells stimulates VEGF-independent angiogenesis. Oncotarget. 2016;7:36829–41. doi: 10.18632/oncotarget.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinegard D, Antonsson P, Hedbom E, et al. Noncollagenous matrix constituents of cartilage. Pathol Immunopathol Res. 1988;7:27–31. doi: 10.1159/000157088. [DOI] [PubMed] [Google Scholar]
- 14.Adini I, Ghosh K, Adini A, et al. Melanocyte-secreted fibromodulin promotes an angiogenic microenvironment. J Clin Invest. 2014;124:425–36. doi: 10.1172/JCI69404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z, Jian J, Velasco O, et al. Fibromodulin enhances angiogenesis during cutaneous wound healing. Plast Reconstr Surg Glob Open. 2014;2:e275. doi: 10.1097/GOX.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondal B, Patil V, Shwetha SD, et al. Integrative functional genomic analysis identifies epigenetically regulated fibromodulin as an essential gene for glioma cell migration. Oncogene. 2017;36:71–83. doi: 10.1038/onc.2016.176. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Zhuang X, Jiang M, et al. ANGPTL4 mediates the protective role of PPARgamma activators in the pathogenesis of preeclampsia. Cell Death Dis. 2017;8:e3054. doi: 10.1038/cddis.2017.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–48. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 19.Beck KD, Valverde J, Alexi T, et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–41. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 20.Henderson CE, Phillips HS, Pollock RA, et al. GDNF: A potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–64. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 21.Yu ZQ, Zhang BL, Ren QX, et al. Changes in transcriptional factor binding capacity resulting from promoter region methylation induce aberrantly high GDNF expression in human glioma. Mol Neurobiol. 2013;48:571–80. doi: 10.1007/s12035-013-8443-5. [DOI] [PubMed] [Google Scholar]
- 22.Shabtay-Orbach A, Amit M, Binenbaum Y, et al. Paracrine regulation of glioma cells invasion by astrocytes is mediated by glial-derived neurotrophic factor. Int J Cancer. 2015;137:1012–20. doi: 10.1002/ijc.29380. [DOI] [PubMed] [Google Scholar]
- 23.Sun S, Lei Y, Li Q, et al. Neuropilin-1 is a glial cell line-derived neurotrophic factor receptor in glioblastoma. Oncotarget. 2017;8:74019–35. doi: 10.18632/oncotarget.18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 25.Piltonen M, Planken A, Leskela O, et al. Vascular endothelial growth factor C acts as a neurotrophic factor for dopamine neurons in vitro and in vivo. Neuroscience. 2011;192:550–63. doi: 10.1016/j.neuroscience.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 26.Blais M, Levesque P, Bellenfant S, Berthod F. Nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3 and glial-derived neurotrophic factor enhance angiogenesis in a tissue-engineered in vitro model. Tissue Eng Part A. 2013;19:1655–64. doi: 10.1089/ten.tea.2012.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kloth S, Suter-Crazzolara C. Modulation of renal blood vessel formation by glial cell line-derived neurotrophic factor. Microvasc Res. 2000;59:190–94. doi: 10.1006/mvre.1999.2214. [DOI] [PubMed] [Google Scholar]
- 28.Ao Z, Yu S, Qian P, et al. Tumor angiogenesis of SCLC inhibited by decreased expression of FMOD via downregulating angiogenic factors of endothelial cells. Biomed Pharmacother. 2017;87:539–47. doi: 10.1016/j.biopha.2016.12.110. [DOI] [PubMed] [Google Scholar]