Abstract
Background
We investigated the effect of propofol on activities and tumor-killing ability of natural killer (NK) cells in patients with colon cancer.
Material/Methods
Twenty colon cancer patients and 20 healthy subjects were included. Peripheral blood (5 ml) was collected from all patients and healthy subjects. NK cells in peripheral blood were separated by negative screening using immunomagnetic beads. Flow cytometry was used to determine expression of activated receptors, inhibitory receptors, killing effector molecules, and proliferation-associated markers on NK cell surfaces. After in vitro treatment with propofol for 24 h, expression of activated receptors, inhibitory receptors, killing effector molecules, and proliferation-associated markers on NK cell surfaces was examined again. In addition, the tumor-killing effect of NK cells was studied by co-culture with K562 cells or colon cancer SW620 cells at a ratio of 1: 1.
Results
The number of NK cells in peripheral blood from colon cancer patients was increased compared with healthy subjects, but activities and proliferation ability of the NK cells were decreased. The tumor-killing effect of NK cells isolated from colon cancer patients was decreased. Of note, propofol promoted activation of NK cells from colon cancer patients. In addition, propofol increased expression of tumor-killing effector molecules by NK cells and the proliferation ability of NK cells. Propofol also enhanced the killing effect of NK cells on colon cancer cells.
Conclusions
The present study demonstrates that propofol promotes the activity and tumor-killing ability of NK cells in peripheral blood of patients with colon cancer.
MeSH Keywords: Natural Killer T-Cells, Peripheral Blood Stem Cell Transplantation, Propofol
Background
Colon cancer is a clinically common malignant tumor of the digestive tract, which is caused by malignant lesions of intestinal mucosal epithelia [1]. In the USA, about 160 000 new cases of colon cancer occur and 57 000 patients die of colon cancer each year [2]. Moreover, the incidence of colon cancer worldwide is also the second highest among all cancers, and the disease has become a serious threat to human health [3]. Like other tumors, the pathogenesis and mechanism of colon cancer are not clear at present. Recurrence and metastasis are the main reasons for poor clinical treatment effects in colon cancer patients [4]. Studies show that the recurrence and metastasis of colon cancer is a complex multigene, multistage process involving a variety of factors [5,6]. Immune escape is one of the key reasons for the recurrence and metastasis of colon cancer [7]. In fact, immune cells are distributed in many types of human tissues, and highly metastatic colon cancer cells must escape the killing of immune cells in order to metastasize [8]. However, it is not clear yet how colon cancer cells escape being killed by immune cells.
Natural killer (NK) cells, the main effector cells of the innate immune system, are the first natural defense lines in preventing infection by viruses and bacteria, as well as the occurrence of tumors [9, 10]. Animal experiments show that defective NK cells can significantly induce the occurrence of tumors in mice [11]. In clinical practice, adoptive immunotherapy with NK cells was first applied to melanoma and blood cancer, and has achieved good clinical effects [12]. With the maturing of CAR-T technology, CAR-NK has been greatly developed and has shown good prospects in the treatment of cancers [13]. NK cells account for about 10–15% of peripheral blood lymphocytes, and the activity NK cells is regulated by inhibitory and activated receptors on cell surfaces. After activation, NK cells play a role in tumor-killing via the FAS-FASL pathway and granzyme-perforin pathway [14,15]. It is reported that the proportion of NK cells in peripheral blood and tissues from various tumor patients is increased, and the degree of NK cell infiltration in the tissues is positively correlated with prognosis [16]. Therefore, studies on the regulation of the killing function of NK cells are of great value in treatment of colon cancer.
Propofol (2,6-diisopropylphenol) is a glutamic acid antagonist and a calcium channel antagonist at the NMDA receptor level, with GABAergic and antioxidant activities. It is widely used in anesthesia induction and maintenance in the intensive care unit, and it is the most commonly used intravenous anesthetic agent for tumor resection under general anesthesia [17,18]. Cellular studies have shown that propofol directly inhibits the proliferation, invasion, and migration of a variety of tumor cell lines. For example, propofol reduces the expression of matrix metalloprotein (MMP)-2 through miR-451 and inhibits the proliferation, invasion, and metastasis of gastric cancer cells [19]. Propofol can inhibit the activity of the HOTAIR-mediated mTOR signaling pathway and thus promote the apoptosis of cervical cancer cells [20]. In addition, Wang et al. discovered that propofol inhibits invasion and metastasis of pancreatic cancer cells by up-regulation of miR-133a expression [21]. Propofol can also inhibit the proliferation, invasion, and migration of non-small-cell lung cancer A549 and HCC827 cells [22]. However, the effect of propofol on NK cell phenotype in peripheral blood from patients with colon cancer is not clear. In the present study, we investigated the effect of propofol on the function of NK cells in killing colon cancer cells at the cellular level, and tried to provide an experimental basis for the application of NK cells in tumor immunotherapy.
Material and Methods
Patients
A total of 20 colon cancer patients who received treatment at our hospital between January 2016 and December 2017 were included. In addition, 20 healthy subjects were included as controls. Peripheral blood (5 ml) was collected from all patients and healthy subjects. All procedures were approved by the Ethics Committee of Taishan Medical University. Written informed consents were obtained from all patients or their families.
NK cell sorting
To isolate peripheral blood mononuclear lymphocytes, 5 ml of whole blood was mixed with 5 ml of phosphate-buffered saline (PBS) in 15-ml centrifuge tubes. Then, the diluted blood was gently added onto the surface of 10 ml of Ficoll solution, followed by centrifugation at 2000 g and 4°C for 20 min. The middle mist-like layer was lymphocytes, which was transferred to a new 15-ml tube. The separated lymphocytes were mixed with PBS to reach 10 ml, and centrifuged at 2000 g and 4°C for 20 min before removing the supernatant. Then, the lymphocytes were washed again with PBS before NK cell sorting using an NK cell-negative separation kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s manual. Briefly, isolated peripheral blood mononuclear lymphocytes were resuspended in 0.5 ml X BD IMag buffer, and mixed with 200 μl biotin. After incubation at room temperature in the dark for 30 min, the labelled cells were transferred to a new tube which was kept on a magnet stirrer for 7 min. The supernatant was transferred to a new tube. This procedure was repeated 3 times, and NK cells without beads and biotin were obtained.
Treatment of NK cells with propofol
Separated and purified NK cells were mixed with RPMI-1640 medium containing 100 U interleukin (IL)-2, 100 U IL-12, or 100 U IL-15, and cultured at 37°C and 5% CO2 for 48 h. The cells were divided into a propofol group and a negative control (NC) group. NK cells in the propofol group was mixed with propofol dissolved in DMSO (final concentration, 25 μmol/ml), and cells in the NC group were mixed with an equal volume of DMSO without propofol. After incubation at 37°C and 5% CO2 for 24 h, the cells were used for subsequent experiments.
Detection of NK cell surface receptors and effector molecules by flow cytometry
A total of 1×105 cells were suspended in 100 μl RPMI-1640 medium, and fluorescence-labelled antibodies were added, including activated receptors p30, p44, p46, and G2D, inhibitory receptors 158b and G2A, and proliferative activity marker Ki67. After incubation at room temperature in the dark for 30 min, the cells were washed with PBS twice and centrifuged at 800 g for 5 min to collect NK cells. Finally, 200 μl PBS was added to resuspend the labelled cells, which were subsequently used for flow cytometry.
Detection of cell cycle of NK cells by flow cytometry
NK cells (1×105) were washed with PBS twice, and subjected to flow cytometry using the Cell Cycle Assay Kit (BD Biosciences, Franklin Lakes, NJ, USA) for the detection of cell cycles. Briefly, the cells were incubated with 200 μl liquid A at room temperature for 10 min, and then mixed with 150 μl liquid B before incubation at room temperature for 10 min. Then, 120 μl liquid C was added before incubation at room temperature in the dark for 10 min. Finally, the cells were used for flow cytometry and the results were analyzed using ModFit software (Verity Software House, Topsham, ME, USA).
Determination of tumor-killing effect of NK cells by flow cytometry
NK cells (1×105) from normal subjects (control group) or patients (experimental group) were mixed with K562 cells or colon cancer SW620 cells at a ratio of 1: 1 and cultured at 37°C and 5% CO2 overnight. Then, the density of the cells in all groups was adjusted to 1×105/100 μl and subjected to flow cytometry using the ANXN V FITC APOPTOSIS DTEC KIT I (BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer’s manual for the detection of apoptosis. Cells with ANNEXIN V-positive values were early apoptotic cells, those with PI-positive values were necrotic cells, and those with double-positive values were late apoptotic cells.
Statistical analysis
The results were analyzed using SPSS 18.0 statistical software (IBM, Armonk, NY, USA). The data are expressed as means ± standard deviations. Data were tested for normality. Multigroup measurement data were analyzed using one-way ANOVA. In case of homogeneity of variance, least significant difference test and Student-Newman-Keuls analysis were used; in case of heterogeneity of variance, Tamhane’s T2 or Dunnett’s T3 method was used. Comparison between 2 groups was carried out using the t test. P<0.05 indicated statistically significant differences.
Availability of data
Our data from the present study are available on request from the corresponding author.
Results
The number of NK cells in peripheral blood from colon cancer patients was increased, but the activities and proliferation ability of the NK cells were decreased
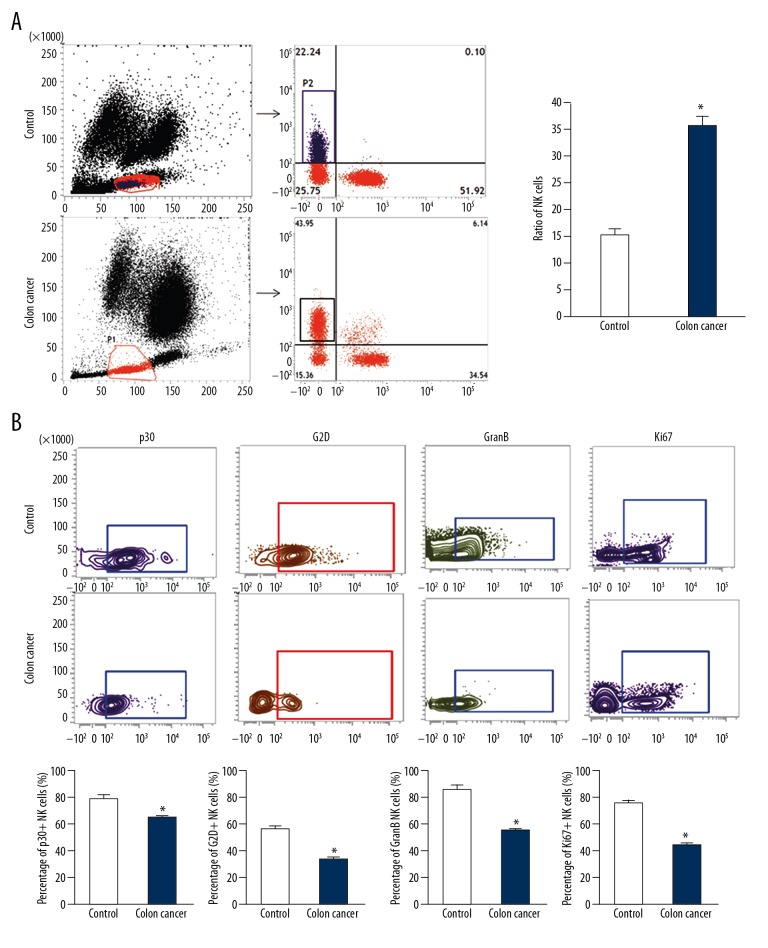
To examine NK cell number and activities, cell sorting and flow cytometry were used. The NK cell ratio in peripheral blood of colon cancer patients was significantly higher than that in healthy subjects (P<0.05) (Figure 1A). Flow cytometry showed that the ratio of NK cells with positive expression of activated receptors p30 and G2D on cell surfaces in colon cancer patients was significantly lower than that in healthy subjects (P<0.05), while the ratio of NK cells with positive expression of tumor-killing effector molecule GranB in colon cancer patients was significantly lower than that in healthy subjects (P<0.05) (Figure 1B). Moreover, the percentage of NK cells with positive expression of proliferation marker Ki67 on cell surfaces in colon cancer patients was significantly reduced compared with that in healthy subjects (P<0.05) (Figure 1B). The results suggest that the number of NK cells in peripheral blood from colon cancer patients is increased but the activities and proliferation ability of the NK cells are decreased.
Figure 1.
Ratio of NK cells in peripheral blood of colon cancer patients and the expression of markers. (A) The ratio of CD3-CD56+NK cells in peripheral blood from colon cancer patients determined by flow cytometry. * P<0.05 compared with control. (B) Percentage of NK cells with positive expression of p30, G2D, GranB, and Ki67. NK cell markers were detected by flow cytometry. * P<0.05 compared with control.
Tumor-killing effect of NK cells isolated from colon cancer patients is decreased
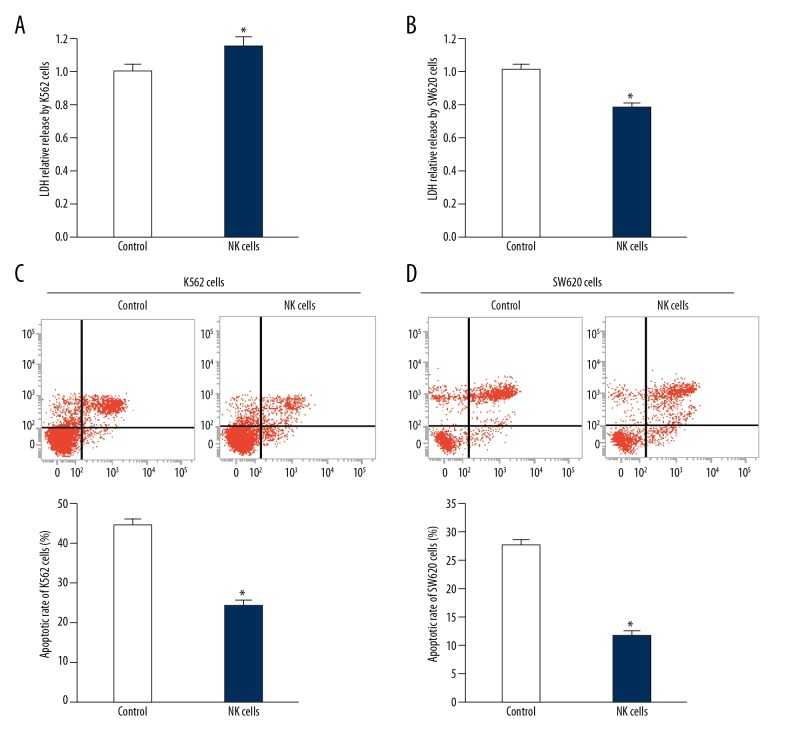
To determine the tumor-killing effect of NK cells separated from colon cancer patients, the NK cells were co-cultured with K562 cells or SW620 cells and flow cytometry was performed. The data showed that LDH level in culture medium of mixed K562 cells and NK cells was significantly lower than that of the control group (P<0.05), and the LDH level in culture medium of mixed SW620 cells and NK cells was also significantly lower than that of the control group (P<0.05) (Figure 2A, 2B). Moreover, the apoptosis of K562 cells or SW620 cells co-cultured with NK cells were decreased compared with the apoptosis of K562 cells or SW620 cells alone (P<0.05) (Figure 2C, 2D). These results indicate that the tumor-killing effect of NK cells isolated from colon cancer patients is decreased.
Figure 2.
Tumor cell-killing activity of NK cells from peripheral blood from colon cancer patients. (A, B) Relative LDH release in supernatant of (A) K562 cells and (B) SW620 cells before and after co-culture with NK cells from colon cancer patients. * P<0.05 compared with control. (C, D) Apoptotic rate of (C) K562 cells and (D) SW620 cells before and after co-culture with NK cells from colon cancer patients. * P<0.05 compared with control.
Propofol promotes the activation of NK cells from colon cancer patients
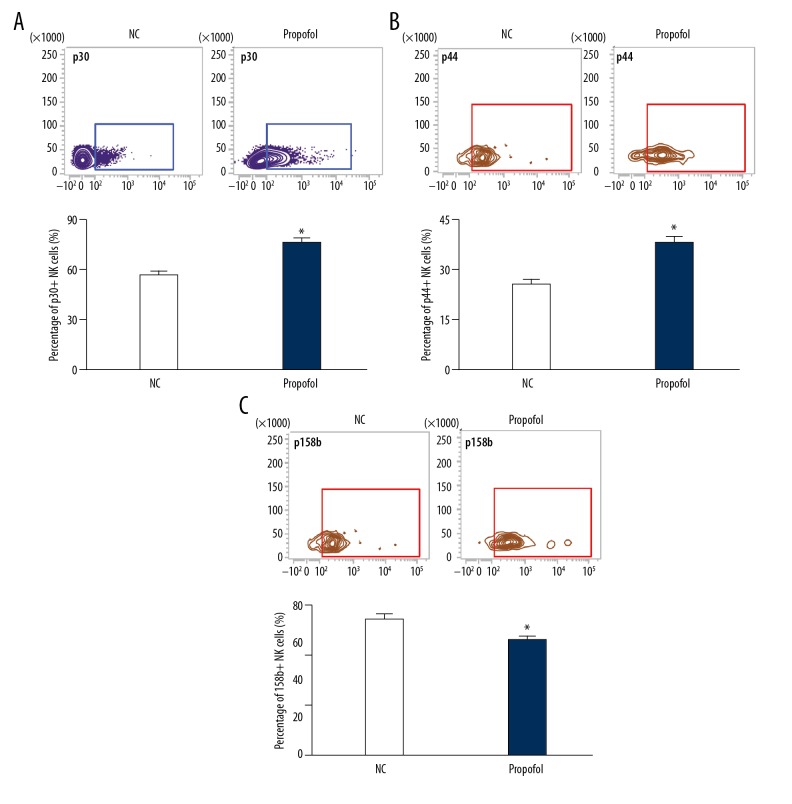
To study the effect of propofol on the receptors on the surface of NK cells, we treated NK cells from colon cancer patients with propofol (25 μmol/ml) for 24 h. The data showed that the percentages of NK cells with positive expression of activated receptors p30 and p44 were significantly enhanced after treatment with propofol (P<0.05) (Figure 3A, 3B). In addition, the percentage of NK cells with positive expression of inhibitory receptors 158b was significantly decreased after treatment with propofol (P<0.05) (Figure 3C), suggesting that propofol promotes activation of NK cells from colon cancer patients.
Figure 3.
Effect of propofol on the expression of NK cell surface receptors. The percentages of NK cells with positive expression of (A) p30, (B) p44, and (C) 158b. NK cells in the propofol group were treated with 25 μmol/ml propofol for 24 h. NK cells cultured in DMSO were used as negative control (NC). Flow cytometry was used to detect the expression of the receptors. * P<0.05 compared with NC.
Propofol increases the expression of tumor-killing effector molecules by NK cells and the proliferation ability of NK cells
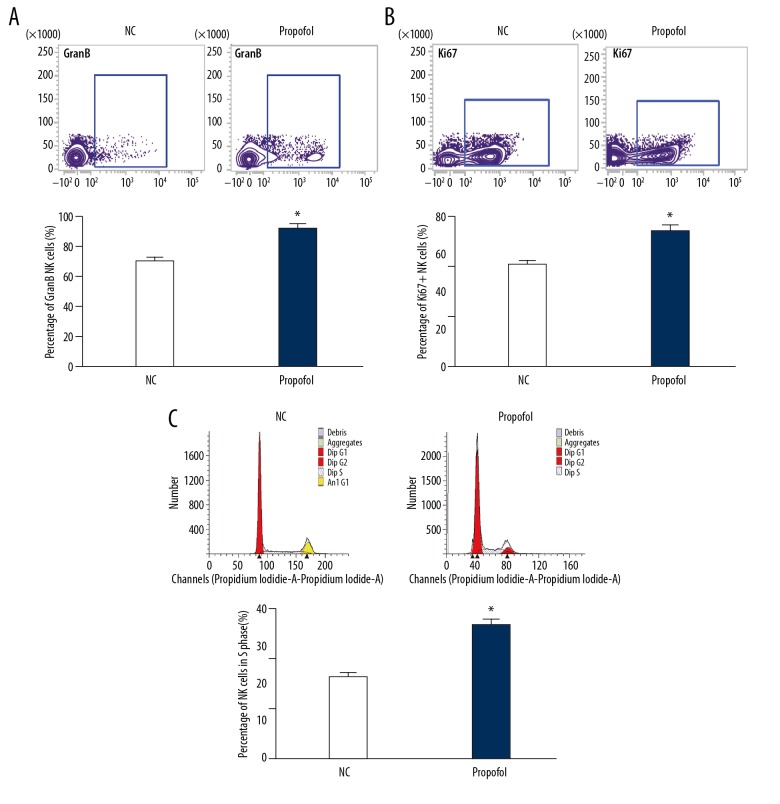
To test how propofol affects the expression of tumor-killing effector molecules by NK cells, flow cytometry was carried out. The data showed that propofol treatment significantly increased the percentage of NK cells with positive expression of GranB or Ki67 compared with the NC group (P<0.05) (Figure 4A, 4B). In addition, treatment with propofol enhanced the percentage of NK cells in S phase compared with the NC group (P<0.05) (Figure 4C). The result indicates that propofol increases the expression of tumor-killing effector molecules by NK cells and the proliferation ability of NK cells.
Figure 4.
Effect of propofol on the expression of tumor-killing effector molecules and proliferation of NK cells. (A, B) The percentages of NK cells with positive expression of (A) GranB and (B) Ki67. Flow cytometry was used to detect the expression of GranB and Ki67. (C) Percentage of NK cells in S phase determined by flow cytometry. NK cells in the propofol group were treated with 25 μmol/ml propofol for 24 h. NK cells cultured in DMSO were used as negative control (NC). * P<0.05 compared with NC.
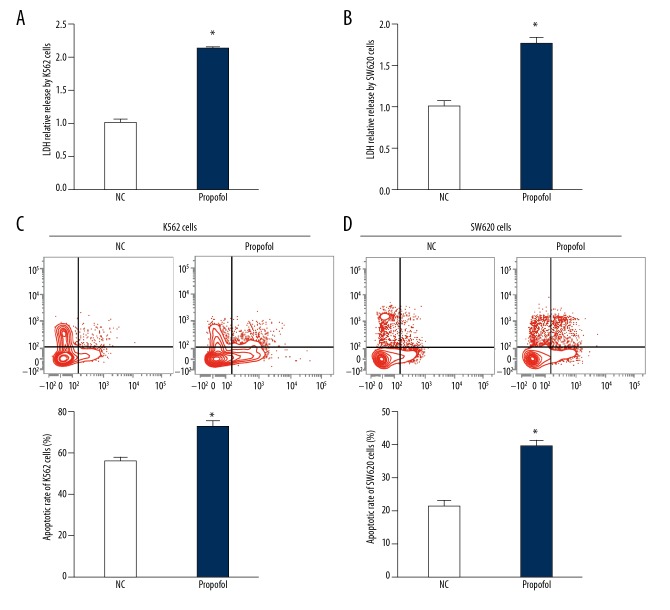
Propofol enhances the killing effect of NK cells on colon cancer cells
To investigate whether propofol could enhance the tumor-killing effect of NK cells, we cultured K562 cells or SW620 cells with NK cells pretreated with propofol (25 μmol/ml). The data showed that the release of LDH in supernatant from K562 cells and SW620 cells in the propofol group was significantly higher than in their respective negative control groups (P<0.05) (Figure 5A, 5B). Flow cytometry showed that the apoptosis of K562 cells and SW620 cells was significantly enhanced after co-culture with NK cells pretreated with propofol (P<0.05) (Figure 5C, 5D). The results suggest that propofol enhances the killing effect of NK cells on colon cancer cells.
Figure 5.
Effect of propofol on the tumor cell-killing activity of NK cells from peripheral blood from colon cancer patients. (A, B) Relative LDH release in supernatant of (A) K562 cells and (B) SW620 cells before and after co-culture with NK cells from colon cancer patients, which were pretreated with 25 μmol/ml propofol for 24 h. (C, D) Apoptotic rate of (C) K562 cells and (D) SW620 cells before and after co-culture with NK cells from colon cancer patients, which were pretreated with 25 μmol/ml propofol for 24 h. K562 cells or SW620 cells co-cultured with NK cells that were pretreated with DMSO for 24 h were used as negative control (NC). * P<0.05 compared with NC.
Discussion
The postoperative period is an important “empty window” for immune recovery of patients with colon cancer, and residual tumor cells can easily metastasize at this stage [23]. Innate immunity is the first line of defense against tumor and infection. NK cells are a main type of cells in natural immunity and they play important roles in surveillance of the occurrence and metastasis of tumors [24]. Propofol is a commonly used drug for colon cancer surgery, and is reported to be closely related to tumor cell metastasis and immune system function [25]. At present, it is not clear whether propofol can promote the postoperative immune surveillance of patients. In the present study, we confirmed in vitro that propofol can promote the proliferation, activity and cytotoxicity of NK cells in patients with colon cancer, suggesting that propofol can improve postoperative natural immunity of patients and inhibit the metastasis of the tumor.
Immunotherapy has long been an important topic in cancer research, including adoptive immunotherapy and cytokine-induced killer therapy [26,27]. With the deepening of understanding of immune cells, the number of immunotherapy methods for cancers is growing. For example, the application of DC tumor vaccine in colon cancer has been proven to be safe and effective [28]. PD1 and PDL1 McAb can improve the prognosis of patients with multiple tumors [29]. CAR-T technology has a good effect in the treatment of blood cancer, but the effect of CAR-T technology in solid tumors still needs to be improved because of the complex microenvironment and the absence of specific targets of solid tumors [30]. In colon cancer, CAR-T cells targeting CEA have shown certain effects in controlling colon cancer [31]. Compared with T cells, NK cells do not need costimulatory signals and have no MHC restriction. Therefore, CAR-NK is considered to be a potential immunotherapy technology and it has shown a strong target-killing ability in renal cell carcinoma, non-small cell lung cancer, and breast cancer [32,33]. However, little research has been performed on colon cancer.
NK cells are among the most important natural immune cells, participating in the resistance of invasion by pathogenic microorganisms, the inhibition of tumor formation, and the regulation of immune functions [34]. Animal studies have shown that the risk of cancer in mice is significantly increased after knocking out NK cells, suggesting that NK cells play an important role in tumor immune surveillance [35]. Clinical studies showed that the number of NK cells is increased but their activity is decreased in peripheral blood of tumor patients. In the tumor tissue microenvironment, NK cells show significant activation, but their activity is almost lost [36]. Our study shows that the number of NK cells in peripheral blood of colon cancer patients is significantly higher than that of normal healthy subjects. However, the expression of activated receptors p30 and G2D on the surface of NK cells from patients with colon cancer was decreased and the expression of inhibitory receptor G2A was increased. Moreover, expression of killing effector molecule GraB is decreased. The results of in vitro cytotoxicity testing show that the killing effect of NK cells on K562 and colon cancer SW620 cells is significantly reduced. These results suggest that the number of NK cells in the peripheral blood of colon cancer patients is increased, but the killing activity of NK cells is reduced. Consistent with the above results, Peng et al. showed that NK cell activity in peripheral blood and tumor tissues from patients with gastric cancer is down-regulated, and tumor-related macrophages are involved in this regulation process [37].
Propofol, a commonly used intravenous anesthetic agent, is widely used in colon cancer surgery. Propofol is usually completely metabolized 24 h after surgery and some patients with weak immune system need more than 24 h [38]. In addition to its anesthetic effect, propofol also has regulatory effects on inflammation and ischemia-reperfusion injury [39]. It is also reported that propofol directly regulates the proliferation, metastasis, and apoptosis of tumor cells in vitro [40]. Colon cancer patients are extremely weak after surgery, and the recovery of immune surveillance has a very important role in inhibiting the metastasis of residual tumor cells. It was shown that propofol promotes the activity of NK cells in patients with breast cancer, suggesting that propofol may be associated with the function and activity of NK cells [41]. In the present study, we discovered that treatment with propofol for 24 h enhanced the activity of NK cells from colon cancer patients, which is characterized by increased levels of activated receptors p30 and p44, and decreased expression of inhibitory receptor 158b. In addition, flow cytometry shows that propofol also promotes the expression of the killing effector molecule GranB. After the activation of NK cells, perforin must be released before GranB can kill target cells. The up-regulation of GranB indicates that the killing activity of NK cells is enhanced. Furthermore, we also discovered that the proliferation activity of NK cells from colon cancer patients is increased after propofol treatment, suggesting that propofol can promote the proliferation of NK cells in the body after surgery. Our results of cell-killing testing confirm that treatment with propofol enhances the in vitro killing effect of NK cells from colon cancer patients on K562 or SW620 cells. These results suggest that the activity of NK cells in the peripheral blood of colon cancer patients is reduced, and propofol can promote the activity and killing activity of NK cells in vitro. Studies show that NK cell surface receptors, killing effector molecules, and proliferation are regulated at multiple levels, such as miRNA, lncRNA, or Jak-Stat signaling pathway. We hypothesize that propofol may be involved in the regulation of NK cells at these levels, but the specific mechanisms remain to be further studied [42,43].
Conclusions
In conclusion, the present study demonstrates that propofol promotes the activity and tumor-killing ability of NK cells in peripheral blood of patients with colon cancer in vitro. In addition, propofol has a potential promoting effect on the postoperative recovery of immune surveillance in patients with colon cancer.
Acknowledgements
We are grateful for support from the Department of Anesthesiology, Taishan Medical University.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Innos K, Reima H, Baburin A, et al. Subsite- and stage-specific colorectal cancer trends in Estonia prior to implementation of screening. Cancer Epidemiol. 2018;52:112–19. doi: 10.1016/j.canep.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Yi H, Wang K, Jin JF, et al. Elevated adenylyl cyclase 9 expression is a potential prognostic biomarker for patients with colon cancer. Med Sci Monit. 2018;24:19–25. doi: 10.12659/MSM.906002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negoi I, Hostiuc S, Negoi RI, Beuran M. Laparoscopic vs open complete mesocolic excision with central vascular ligation for colon cancer: A systematic review and meta-analysis. World J Gastrointest Oncol. 2017;9:475–91. doi: 10.4251/wjgo.v9.i12.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy VG, Bonam SR, Reddy TS, et al. 4beta-amidotriazole linked podophyllotoxin congeners: DNA topoisomerase-IIalpha inhibition and potential anticancer agents for prostate cancer. Eur J Med Chem. 2018;144:595–611. doi: 10.1016/j.ejmech.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 5.Hadjiliadis D, Khoruts A, Zauber AG, et al. Cystic fibrosis colorectal cancer screening consensus recommendations. Gastroenterology. 2018;154:736–45. doi: 10.1053/j.gastro.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di J, Zhuang M, Yang H, et al. Clinical significance of circulating immune cells in left- and right-sided colon cancer. Peer J. 2017;5:e4153. doi: 10.7717/peerj.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havrlentova L, Faistova H, Mazur M, et al. Comparative analysis of human omental milky spots between the patients with colon cancer and the control group. Bratis Lek Listy. 2017;118:580–84. doi: 10.4149/BLL_2017_111. [DOI] [PubMed] [Google Scholar]
- 8.Kistner L, Doll D, Holtorf A, et al. Interferon-inducible CXC-chemokines are crucial immune modulators and survival predictors in colorectal cancer. Oncotarget. 2017;8:89998–90012. doi: 10.18632/oncotarget.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turin I, Delfanti S, Ferulli F, et al. In vitro killing of colorectal carcinoma cells by autologous activated NK cells is boosted by anti-epidermal growth factor receptor-induced ADCC regardless of RAS mutation status. J Immunother. 2018;41:190–200. doi: 10.1097/CJI.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Qin X, Zhu X, et al. Oral cancer-derived exosomal NAP1 enhances cytotoxicity of natural killer cells via the IRF-3 pathway. Oral Oncol. 2018;76:34–41. doi: 10.1016/j.oraloncology.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Counotte J, Drexhage HA, Wijkhuijs JM, et al. Th17/T regulator cell balance and NK cell numbers in relation to psychosis liability and social stress reactivity. Brain Behav Immun. 2018;69:408–17. doi: 10.1016/j.bbi.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Zhang M, Liu RT, et al. Statins reduce the expressions of Tim-3 on NK cells and NKT cells in atherosclerosis. Eur J Pharmacol. 2018;821:49–56. doi: 10.1016/j.ejphar.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Hu B, Liu Y, et al. IL-12/IL-18-preactivated donor NK cells enhance GVL effects and mitigate GvHD after allogeneic hematopoietic stem cell transplantation. Eur J Immunol. 2018;48:670–82. doi: 10.1002/eji.201747177. [DOI] [PubMed] [Google Scholar]
- 14.Hua S, Vigano S, Tse S, et al. Pegylated IFN-alpha-induced NK cell activation is associated with HIV-1 DNA decline in ART-treated HIV-1/HCV co-infected patients. Clin Infect Dis. 2018;66(12):1910–17. doi: 10.1093/cid/cix1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muta T, Yoshihiro T, Jinnouchi F, et al. Expansion of NKG2C-expressing natural killer cells after umbilical cord blood transplantation in a patient with peripheral T-cell lymphoma with cytotoxic molecules. Intern Med. 2018;57:861–66. doi: 10.2169/internalmedicine.9437-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrow AD, Edeling MA, Trifonov V, et al. Natural killer cells control tumor growth by sensing a growth factor. Cell. 2018;172:534–48.e19. doi: 10.1016/j.cell.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu XQ, Lin CS, Guo PP, et al. [Effects of propofol on myelin basic protein expression in oligodendrocytes of SD rats at different developmental stages]. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(12):1615–19. doi: 10.3969/j.issn.1673-4254.2017.12.09. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian HT, Duan XH, Yang YF, et al. Effects of propofol or sevoflurane anesthesia on the perioperative inflammatory response, pulmonary function and cognitive function in patients receiving lung cancer resection. Eur Rev Med Pharmacol Sci. 2017;21:5515–22. doi: 10.26355/eurrev_201712_13943. [DOI] [PubMed] [Google Scholar]
- 19.Peng Z, Zhang Y. Propofol inhibits proliferation and accelerates apoptosis of human gastric cancer cells by regulation of microRNA-451 and MMP-2 expression. Genet Mol Res. 2016;15(2) doi: 10.4238/gmr.15027078. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D, Zhou XH, Zhang J, et al. Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem Biophys Res Commun. 2015;468:561–67. doi: 10.1016/j.bbrc.2015.10.129. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZT, Gong HY, Zheng F, et al. Propofol suppresses proliferation and invasion of pancreatic cancer cells by upregulating microRNA-133a expression. Genet Mol Res. 2015;14:7529–37. doi: 10.4238/2015.July.3.28. [DOI] [PubMed] [Google Scholar]
- 22.Ye HJ, Bai JJ, Guo PP, et al. [Propofol suppresses invasion of human lung cancer A549 cells by down-regulating aquaporin-3 and matrix metalloproteinase-9]. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(9):1286–90. [in Chinese] [PubMed] [Google Scholar]
- 23.Luber V, Wagner J, Lock JF, et al. [The use of tumor therapeutics in the perioperative period]. Chirurg. 2018;89:108–15. doi: 10.1007/s00104-017-0528-7. [in German] [DOI] [PubMed] [Google Scholar]
- 24.Sharma R, Das A. IL-2 mediates NK cell proliferation but not hyperactivity. Immunol Res. 2018;66:151–57. doi: 10.1007/s12026-017-8982-3. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Gao W, Zhou H, et al. Propofol induces apoptosis of breast cancer cells by downregulation of miR-24 signal pathway. Cancer Biomark. 2018;21:513–19. doi: 10.3233/CBM-170234. [DOI] [PubMed] [Google Scholar]
- 26.Meng Y, Sun J, Hu T, et al. Rapid expansion in the WAVE bioreactor of clinical scale cells for tumor immunotherapy. Hum Vaccin Immunother. 2018 doi: 10.1080/21645515.2018.1480241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Liu J, Yang X, et al. Bacillus Calmette-Guérin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. Onco Targets Ther. 2018;11:2891–99. doi: 10.2147/OTT.S165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong W, Wei R, Shen H, et al. Combination of DC vaccine and conventional chemotherapeutics. Anticancer Agents Med Chem. 2016;16(5):558–67. doi: 10.2174/1871520615666150907094139. [DOI] [PubMed] [Google Scholar]
- 29.Dai B, Qi N, Li J, et al. Temozolomide combined with PD-1 antibody therapy for mouse orthotopic glioma model. Biochem Biophys Res Commun. 2018;501(4):871–76. doi: 10.1016/j.bbrc.2018.05.064. [DOI] [PubMed] [Google Scholar]
- 30.Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–80. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzinger A, Abken H. CAR T cells targeting solid tumors: Carcinoembryonic antigen (CEA) proves to be a safe target. Cancer Immunol Immunother. 2017;66(11):1505–7. doi: 10.1007/s00262-017-2045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Tian K, Xu J, et al. Synergistic effects of Cabozantinib and EGFR-specific CAR-NK-92 cells in renal cell carcinoma. J Immunol Res. 2017;2017 doi: 10.1155/2017/6915912. 6915912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinz KG, Yakaboski E, Jares A, et al. Targeting T-cell malignancies using anti-CD4 CAR NK-92 cells. Oncotarget. 2017;8(68):112783–96. doi: 10.18632/oncotarget.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milo I, Blecher-Gonen R, Barnett-Itzhaki Z, et al. The bone marrow is patrolled by NK cells that are primed and expand in response to systemic viral activation. Eur J Immunol. 2018;48(7):1137–52. doi: 10.1002/eji.201747378. [DOI] [PubMed] [Google Scholar]
- 35.Seo H, Kim BS, Bae EA, et al. IL21 therapy combined with PD-1 and Tim-3 blockade provides enhanced NK cell antitumor activity against MHC Class I-deficient tumors. Cancer Immunol Res. 2018;6(6):685–95. doi: 10.1158/2326-6066.CIR-17-0708. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari de Andrade L, Tay RE, Pan D, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359:1537–42. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng LS, Zhang JY, Teng YS, et al. Tumor-associated monocytes/macrophages impair NK-Cell function via TGFbeta1 in human gastric cancer. Cancer Immunol Res. 2017;5:248–56. doi: 10.1158/2326-6066.CIR-16-0152. [DOI] [PubMed] [Google Scholar]
- 38.Sobbeler FJ, Carrera I, Pasloske K, et al. Effects of isoflurane, sevoflurane, propofol and alfaxalone on brain metabolism in dogs assessed by proton magnetic resonance spectroscopy ((1)H MRS) BMC Vet Res. 2018;14:69. doi: 10.1186/s12917-018-1396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira-Paula GH, Lacchini R, Pinheiro LC, et al. Endothelial nitric oxide synthase polymorphisms affect the changes in blood pressure and nitric oxide bioavailability induced by propofol. Nitric Oxide. 2018;75:77–84. doi: 10.1016/j.niox.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Zhang S, Zhang A, Yan C. Propofol prevents the progression of malignant pheochromocytoma in vitro and in vivo. DNA Cell Biol. 2018;37:308–15. doi: 10.1089/dna.2017.3972. [DOI] [PubMed] [Google Scholar]
- 41.Lim JA, Oh CS, Yoon TG, et al. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: An in vitro analysis. BMC Cancer. 2018;18:159. doi: 10.1186/s12885-018-4064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge J, Huang Z, Liu H, et al. Lower expression of microRNA-155 contributes to dysfunction of natural killer cells in patients with chronic hepatitis B. Front Immunol. 2017;8:1173. doi: 10.3389/fimmu.2017.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu Q, Sun Y, Tao Y, et al. Involvement of the JAK-STAT pathway in collagen regulation of decidual NK cells. Am J Reprod Immunol. 2017;78(6) doi: 10.1111/aji.12769. [DOI] [PubMed] [Google Scholar]