Abstract
Background
We explored the possible relationship between Modic changes (MCs) and sagittal parameters of the cervical spine.
Material/Methods
We enrolled 150 patients with cervical MC on the magnetic resonance imaging (MRI) scans in the MC (+) group and divided them into 3 sub-groups with 50 patients each: the MC1 sub-group, the MC2 sub-group, and the MC3 sub-group. Another 150 healthy subjects receiving routine health examinations were also enrolled in the study as the MC (−) group. The sagittal parameters in the cervical spine were measured and compared and multiple logistic regression analysis was performed to analyze the risk factor for the occurrence of MC.
Results
Four cervical sagittal parameters were measured and compared between all the enrolled groups, including neck tilt (NT), T1 slope (T1s), thoracic inlet angle (TIA), and Cobb C2–C7. The results confirmed that the parameter of Cobb C2–C7 was much smaller in the MC(+) group when compared with that in the MC(−) group (P<0.05), while no significant differences were detected between the MC(+) and MC(−) groups for the parameters of NT, T1 T1s, and TIA (P>0.05). Multiple logistic regression analysis showed that Cobb C2–C7 (less than 8.5°) could be regarded as the risk factor for the occurrence of MC, and the receiver operating characteristic (ROC) curve showed that moderate diagnostic significance was obtained with an area under curve (AUC) of 0.82.
Conclusions
The present study demonstrated that Cobb C2–C7 (less than 8.5°) is a potential risk factor for the development of MC.
MeSH Keywords: Logistic Models; Magnetic Resonance Imaging; Osteoarthritis, Spine
Background
More attention has been paid to Modic change (MC) in recent years, which is a common and rapidly degenerative change observed in magnetic resonance imaging (MRI) in the degenerative spine summarized by Modic et al. in 1988 [1]. MC is usually observed as an abnormal bone signal under the vertebral endplate and can be divided into 3 types: Modic type 1 change (MC1) refers to edema and granulation tissue at the endplate area with low T1 and high T2 signal, MC2 refers to adipose tissue at the endplate area with high T1 and T2 signal, and MC3 refers to sclerosing bone at the endplate area with low T1 and T2 signal [2,3]. MC of the cervical spine is usually observed; as the cervical spine is the most mobile region of the spine and withstands the axial load of the head, overloading of the endplate may accelerate spine degeneration. The cervical MC degeneration may result in cervical morbidities, such as neck pain, motion disorder, and neurologic deficits, and thus warrants increased research attention [4,5].
The shape of the spine can be reflected by sagittal balance, which can allows human to maintain standing position with little muscle effort [6]. Sagittal balance status has been demonstrated to be an independent predictor of many aspects of the spine, such as clinical status and outcomes in subjects with adult scoliosis, in patients undergoing surgery for adult deformity, and degenerative disc disease and degenerative spondylolisthesis [7–10]. The relationship between the occurrence of MC and sagittal balance is important because malalignment of the spine in the sagittal plane has been proven to accelerate segment degeneration and can also lead to MC. To the best of our knowledge, there have been no previously published studies evaluating the correlation between MC and sagittal alignment in the cervical spine; therefore, we performed the present study.
Material and Methods
Ethical considerations
The study protocol was approved by the Ethics Committee of Yixing People’s Hospital, Jiangsu University. Informed consent was obtained from all enrolled patients before the study began.
Subjects were selected and divided into 2 groups
From January 2010 to January 2018, 150 patients with cervical MC on MRI scans were enrolled in the MC (+) group and divided 3 sub-groups of 50 patients each: the MC1 sub-group, the MC2 sub-group, and the MC3 sub-group. Another 150 healthy subjects receiving routine health examinations were also enrolled in the study as the MC (−) group. Inclusion criteria were: neck pain for more than 6 months; consecutive patients; only MC and no other structural findings; age range 30–60 years old; and seeking treatment at our institution. Patients with trauma, infectious spondylitis, rheumatoid arthritis, prior cervical fractures or dislocations, spinal tumors, or cervical spine surgery history were excluded from this study.
Radiographic method
All images of MC were taken using an MRI Scanner (Siemens, AG, Germany), while the sagittal parameter (SP) of the cervical vertebra were measured by spiral computed tomography (CT) (Siemens, AG, Germany). SP were measured by 2 senior orthopedic surgeons using Centricity Enterprise Web V3.0 software, consisting of neck tilt (NT), T1 slope (T1s), thoracic inlet angle (TIA), and cervical lordosis (CL: Cobb C2–C7) (Figure 1) [8]. The basic characteristics were also evaluated, including patient age, sex, BMI, and MC segment.
Figure 1.
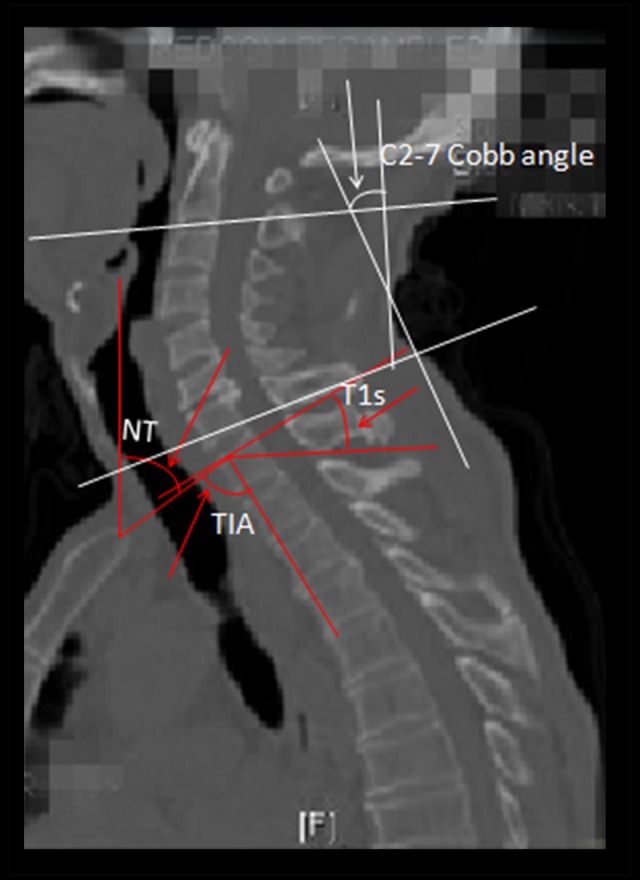
Sagittal parameters in the cervical spine. The C2–C7 cobb angle was measured by formal Cobb methods that checked the angle between the horizontal line of the C2 lower endplate and the horizontal line of the C7 lower endplate. T1 slope (T1s) is the angle formed by a line along the superior endplate of T1 and horizontal reference line; the thoracic inlet angle (TIA) denotes an angle formed by a line from the center of the T1 upper endplate (T1UEP) vertical to the T1UEP and a line connecting the center of the T1UEP and the upper end of the sternum. The 2 lines come from the upper sternum and form the neck tilt angle: one is the vertical line and the other is the line to the center of the T1UEP.
Statistical analysis
The values of all the parameters are presented as the mean±standard deviation (SD). We used the t test or χ2 test to assess continuous and categorical data to compare the MC group and control groups, while analysis of variance was carried out to compare the differences between the different MC groups. Multivariate logistic analysis was performed to identify factors independently associated with the incidence of MC. The receiver operating characteristic (ROC) curve was constructed. The area under the curve (AUC) is the concordant index, which was used to identify the parameters that best predicted MC. The cutpoint value was determined by calculating the maximum value of sensitivity adding specificity and it determines the normal value of a certain index to distinguish normal subjects from abnormal subjects. Tests were two-tailed and p<0.05 was considered significant. SPSS (version 21.0; SPSS, Chicago, IL) was used for statistical analyses.
Results
Results of the main characteristics
A total of 300 subjects were finally enrolled in the study, including 150 cases with MC (MC1, 50; MC2, 50; and MC3, 50) and 150 healthy subjects. No significant (p>0.05) differences were observed between the MC(+) and the MC(−) groups for the main characteristics: (PAge of the patients 0.58, PSex 0.73, PBMI 0.36, PMC segment0.22) (MC group vs. control; MC1 group vs. MC2 group vs. MC3 group) (Table 1).
Table 1.
Characteristics of enrolled subjects.
| Characteristics | Control group (n=150) | MC groups (n=50/n=50/n=50) | χ2 or t | P | ||||
|---|---|---|---|---|---|---|---|---|
| MC1 | MC2 | MC3 | F or χ2 | P | ||||
| Age of the patients (years) | ||||||||
| Sex | 48.3±10.3 | 47.2±7.6 | 48.1±6.4 | 46.0±7.7 | 0.62 | 0.36 | 0.55 | 0.58 |
| Male | 71 | 23 | 25 | 26 | 0.83 | 0.12 | 0.73 | |
| Female | 79 | 27 | 25 | 24 | 0.37 | 0.10 | 0.22 | 0.36 |
| BMI | 22.1±6.3 | 23.2±5.5 | 22.0±6.6 | 22.7±7.4 | 0.26 | 0.67 | 4.34 | 0.22 |
| MC segment | ||||||||
| C3–C4 | 26 | 9 | 12 | 7 | ||||
| C4–C5 | 45 | 15 | 14 | 16 | ||||
| C5–C6 | 52 | 16 | 10 | 12 | ||||
| C6–C7 | 27 | 10 | 14 | 15 | 4.01 | |||
BMI – body mass index.
Results of radiograph measurements in sagittal plane
We measured 4 cervical sagittal parameters in all the enrolled groups, including NT, T1 T1s, TIA, and Cobb C2–C7. The results confirmed that the parameter of Cobb C2–C7 was much smaller in the MC(+) group when compared with that in the MC(−) group (P<0.05), while no significant differences were detected between the MC(+) and MC(−) groups for the parameters of NT, T1 T1s, or TIA (P>0.05) (Table 2).
Table 2.
Results of parameters in sagittal plane of cervical vertebra.
| Characteristics | Control group (n=150) | MC groups (n=50/n=50/n=50) | t | P | ||||
|---|---|---|---|---|---|---|---|---|
| MC1 | MC2 | MC3 | F | P | ||||
| NT (°) | 46.7±9.8 | 45.0±6.5 | 44.6±8.2 | 45.3±7.4 | 0.11 | 0.82 | 0.96 | 0.13 |
| T1s (°) | 24.3±2.6 | 22.1±3.4 | 24.1±6.6 | 23.2±4.1 | 0.33 | 0.70 | 0.71 | 0.19 |
| Cobb C2–C7 | 11.2±0.9 | 7.9±3.0 | 7.4±0.6 | 7.3±1.2 | 0.60 | 0.31 | 6.01 | 0.00 |
| TIA | 70.1±12.6 | 67.6±7.3 | 68.2±5.9 | 68.9±15.3 | 2.01 | 0.10 | 1.197 | 0.06 |
NT – neck tilt; T1s – T1 slope; TIA – thoracic inlet angle.
Results of multivariate logistic regression
Multiple logistic regression analysis was carried out for the significant parameters, showing that Cobb C2–C7 (less than 8.5°) is as an independent significant risk factor of the incidence of MC (Table 3). The ROC showed that moderate diagnostic value was obtained for the significant variable with the area under the curve (AUC) of 0.82 (Figure 2).
Table 3.
Multivariate logistic regression analysis of risk factors.
| Risk Factors | B | S.E. | Wald | df | p | Exp(B) | 95% CI |
|---|---|---|---|---|---|---|---|
| Cobb C2–C7 | −0.20 | 0.07 | 6.59 | 1 | 0.01 | 0.32 | 0.12~0.44 |
Figure 2.
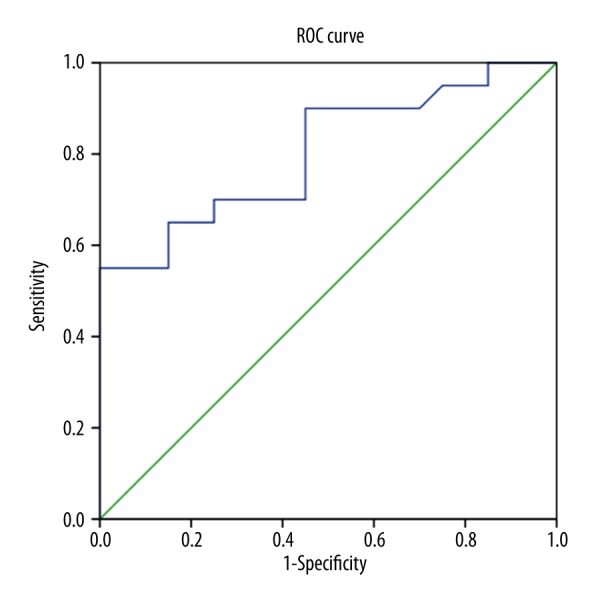
0ROC curve of the Cobb C2–C7. The area under the curve (AUC) is 0.82. ROC: receiver operating characteristic.
Discussion
Although the MC was first described in the lumbar spine, it is also observed in the cervical spine. The occurrence of MC has been confirmed to be related to the presence of cervical spondylosis and Modic typing also applies to cervical spondylotic myelopathy (CSM) [11,12]. The occurrence of MC can be changeable and MC1 and MC2 are interconvertible over time and can eventually convert to MC3, and about 20% of the MC lesion are mixed-type (MC1/2 or MC2/3) [13–16]. Many risk factors have been proven to relate to the occurrence of MC, systemic factors (aging, smoking, genetics, male sex), disc/endplate damage (disc herniation, endplate defects) and hyperloading (obesity, spinal deformities, high occupational load) [17–21]. Despite an abundance of imaging data from MC studies, fewer studies report details on the relationship between occurrence of MC and sagittal balance in the cervical spine. Malalignment of the cervical spine in the sagittal plane has been proven to accelerate segment degeneration, which results in the occurrence of cervical MC.
A previous study conducted a retrospective review of 100 subjects, discussing the relationship between the cervical sagittal parameters, and the results concluded that T1 slope is a potential risk factor for the development of MC due to impaired sagittal balance, especially in the C5–C6 cervical segment [22]. Due to the limited sample size of the enrolled subjects of the previous study, we performed the present study with a total number of 300 subjects to explore the correlation between the occurrence of MC and sagittal alignment in the cervical spine. Four cervical sagittal parameters were measured and compared between all the enrolled groups: NT, T1 T1s, TIA, and Cobb C2–C7. The results showed that Cobb C2–C7 was much smaller in the MC(+) group when compared with that in the MC(−) group (P<0.05), while no significant differences were observed between the groups for the other parameters (NT, T1 T1s, and TIA) (P>0.05). The lower Cobb C2–C7 (less than 8.5°) can also be regarded as a risk factor for predicting the occurrence of MC, and moderate diagnostic significance was obtained for the parameter (AUC, 0.82). Cobb C2–C7 could be a good reflection of the cervical curvature, which plays important roles in maintaining the physiological curve of the spine and the balance of the human body, while its biological function is to increase the ability of the cervical spine to resist longitudinal compression load, as well as to buffer concussions. The curvature of the cervical spine is an important factor affecting the balance of the sagittal plane; a smaller curvature of the cervical vertebra corresponds to shifting of the center of head gravity, resulting in the redistribution of stress. The shear force between the intervertebral disc of the vertebral body gradually reduces and the pressure loading on the sagittal position increases gradually. The endplate is particularly sensitive to the axial load, while excessive axial load can lead to bending deformation of cartilage endplate, osseous endplate, and subplate trabecular bone, resulting in MC of the cervical spine. Karchevsky et al. [23] confirmed that structural destruction of the vertebral endplate and cancellous bone can be caused by the compression force, and larger loads may lead to more microfractures or other forms of damage. A high signal was found on the T1-weighted image (T1WI) of MRI when the curvature of the spine becomes straight, and this is believed to be the result of the transformation of red bone marrow into yellow bone marrow [24,25].
Conclusions
The present study demonstrated that Cobb C2–C7 (less than 8.5°) is a potential risk factor for the development of MC.
Footnotes
Source of support: Departmental sources
References
- 1.Modic MT, Masaryk TJ, Ross JS, et al. Imaging of degenerative disk disease. Radiology. 1988;168(1):177–86. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 2.Song J, Wang HL, Ma XS, et al. The value of radiographic indexes in the diagnosis of discogenic low back pain: A retrospective analysis of imaging results. Oncotarget. 2017;8(36):60558–67. doi: 10.18632/oncotarget.18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laustsen AF, Bech-Azeddine R. Do Modic changes have an impact on clinical outcome in lumbar spine surgery? A systematic literature review. Eur Spine J. 2016;25(11):3735–45. doi: 10.1007/s00586-016-4609-y. [DOI] [PubMed] [Google Scholar]
- 4.Mann E, Peterson CK, Hodler J. Degenerative marrow (Modic) changes on cervical spine magnetic resonance imaging scans: Prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine (Phila Pa 1976) 2011;36(14):1081–85. doi: 10.1097/BRS.0b013e3181ef6a1e. [DOI] [PubMed] [Google Scholar]
- 5.Dudli S, Fields AJ, Samartzis D, et al. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–34. doi: 10.1007/s00586-016-4459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamartina C, Berjano P, Petruzzi M, et al. Criteria to restore the sagittal balance in deformity and degenerative spondylolisthesis. Eur Spine J. 2012;21(Suppl 1):S27–31. doi: 10.1007/s00586-012-2236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glassman SD, Berven S, Bridwell K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 2005;30(6):682–88. doi: 10.1097/01.brs.0000155425.04536.f7. [DOI] [PubMed] [Google Scholar]
- 8.Videbaek TS, Bunger CE, Henriksen M, et al. Sagittal spinal balance after lumbar spinal fusion: The impact of anterior column support results from a randomized clinical trial with an eight- to thirteen-year radiographic follow-up. Spine (Phila Pa 1976) 2011;36(3):183–91. doi: 10.1097/BRS.0b013e3181cc8fce. [DOI] [PubMed] [Google Scholar]
- 9.Kim MK, Lee SH, Kim ES, et al. The impact of sagittal balance on clinical results after posterior interbody fusion for patients with degenerative spondylolisthesis: A pilot study. BMC Musculoskelet Disord. 2011;12:69. doi: 10.1186/1471-2474-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JS, Shaffrey CI, Glassman SD, et al. Risk-benefit assessment of surgery for adult scoliosis: An analysis based on patient age. Spine (Phila Pa 1976) 2011;36(10):817–24. doi: 10.1097/BRS.0b013e3181e21783. [DOI] [PubMed] [Google Scholar]
- 11.Mann E, Peterson CK, Hodler J. Degenerative marrow (Modic) changes on cervical spine magnetic resonance imaging scans: Prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine (Phila Pa 1976) 2011;36(14):1081–108. doi: 10.1097/BRS.0b013e3181ef6a1e. [DOI] [PubMed] [Google Scholar]
- 12.Zhou H, Fan J, Sun P, et al. Correlation analysis between Modic change of cervical vertebrae and intramedullary high signal intensity. Clin Spine Surg. 2017;30(9):E1298–305. doi: 10.1097/BSD.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Videman T, Battie MC. Modic changes: Prevalence, distribution patterns, and association with age in white men. Spine J. 2012;12(5):411–16. doi: 10.1016/j.spinee.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaapa E, Luoma K, Pitkaniemi J, et al. Correlation of size and type of Modic types 1 and 2 lesions with clinical symptoms: A descriptive study in a subgroup of patients with chronic low back pain on the basis of a university hospital patient sample. Spine (Phila Pa 1976) 2012;37(2):134–39. doi: 10.1097/BRS.0b013e3182188a90. [DOI] [PubMed] [Google Scholar]
- 15.Jensen TS, Bendix T, Sorensen JS, et al. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet Disord. 2009;10:81. doi: 10.1186/1471-2474-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuisma M, Karppinen J, Haapea M, et al. Modic changes in vertebral endplates: A comparison of MR imaging and multislice CT. Skeletal Radiol. 2009;38(2):141–47. doi: 10.1007/s00256-008-0590-9. [DOI] [PubMed] [Google Scholar]
- 17.Kuisma M, Karppinen J, Niinimaki J, et al. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine (Phila Pa 1976) 2006;31(15):1714–18. doi: 10.1097/01.brs.0000224167.18483.14. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer P, Korsholm L, Bendix T, et al. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15(9):1312–19. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen TS, Kjaer P, Korsholm L, et al. Predictors of new vertebral endplate signal (Modic) changes in the general population. Eur Spine J. 2010;19(1):129–35. doi: 10.1007/s00586-009-1184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert HB, Briggs AM, Kent P, et al. The prevalence of MRI-defined spinal pathoanatomies and their association with Modic changes in individuals seeking care for low back pain. Eur Spine J. 2011;20(8):1355–62. doi: 10.1007/s00586-011-1794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maatta JH, Kraatari M, Wolber L, et al. Vertebral endplate change as a feature of intervertebral disc degeneration: A heritability study. Eur Spine J. 2014;23(9):1856–62. doi: 10.1007/s00586-014-3333-8. [DOI] [PubMed] [Google Scholar]
- 22.Ma Z, Liu P, Liu J, et al. Kinematic analysis of the relationship between Modic changes and sagittal balance parameters in the cervical spine. Medicine (Baltimore) 2017;96(33):e7699. doi: 10.1097/MD.0000000000007699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karchevsky M, Schweitzer ME, Carrino JA, et al. Reactive endplate marrow changes: A systematic morphologic and epidemiologic evaluation. Skeletal Radiol. 2005;34(3):125–29. doi: 10.1007/s00256-004-0886-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang LL, Du J, Fu XN, et al. [Correlation between thigh muscle magnetic resonance imaging findings and clinical features of congenital muscular dystrophies: A preliminary study]. Zhonghua Er Ke Za Zhi. 2016;54(10):756–60. doi: 10.3760/cma.j.issn.0578-1310.2016.10.009. [in Chinese[ [DOI] [PubMed] [Google Scholar]
- 25.Xia Q, Sun JM. MRI delineation of the morphometric characteristics of type I split cord malformations: A retrospective analysis of 29 cases. Acta Orthop Traumatol Turc. 2016;50(1):49–56. doi: 10.3944/AOTT.2016.14.0381. [DOI] [PubMed] [Google Scholar]


