Abstract
Introduction
Freeze-thaw instability may contribute to preanalytical variation in blood-based biomarker studies. We investigated the effects of up to four freeze-thaw cycles on single molecule array immunoassays of serum neurofilament light chain and plasma total tau, amyloid β 1–40 (Aß40), and Aβ 1–42 (Aβ42).
Methods
Individuals who had peripheral venepuncture during investigation of suspected neurodegenerative disease were recruited. After standardized preprocessing, 200 μL of plasma and serum aliquots were stored at −80°C within 60 minutes. Aliquots underwent one to four freeze-thaw cycles.
Results
There was no significant difference across four freeze-thaw cycles for serum neurofilament light chain (n = 12), plasma total tau (n = 11), or plasma Aβ42 (n = 12). For plasma Aβ40 (n = 14), there were significant median reductions by ratios of .96 and .92 at the third and fourth cycles, respectively.
Discussion
Up to four freeze-thaw cycles do not influence single molecule array blood biomarkers of neurofilament light chain, total tau, or Aβ42, with at most minor reductions in Aβ40.
Keywords: Freeze-thaw, Blood, Biomarker, Simoa
1. Introduction
Preanalytical factors may be an important source of variation among studies investigating putative blood-based biomarkers of Alzheimer's disease, in which replication across studies has posed a significant challenge. Guidelines for standardizing these preanalytic variables have stated that it is desirable to minimize the number of freeze-thaw cycles to which samples are exposed [1]. The evidence for this comes predominantly from studies showing that more than three freeze-thaw cycles reduce plasma amyloid β 1–40 and 1–42 (Aβ40 and Aβ42) concentrations by as much as 20% [2] and also reduce cerebrospinal fluid (CSF) Aβ42 concentration [3] as quantified on immunoassay platforms. There is some conflicting evidence on the stability in CSF of total tau, which in one report remained stable over up to six freeze-thaw cycles [3], but in another study, both CSF total tau and phospho-tau-181 concentrations as measured by enzyme-linked immunosorbent assay reduced over three freeze-thaw cycles [4]. Conversely, CSF neurofilament light chain (NFL) remains stable over up to four freeze-thaw cycles [5]. However, there has been no systematic examination of the stability of these biomarkers in blood. More recently, the Single molecule array (Simoa) HD-1 analyzer, a highly sensitive digital immunoassay platform, has been developed, which is capable of measuring these and other biomarkers with sensitivity in the femtomolar range [6]. In this study, we examined the effects of up to four freeze-thaw cycles on serum NFL and plasma total tau, Aβ40, and Aβ42.
2. Methods
2.1. Participants
We recruited individuals seen at the specialist cognitive disorders clinics at the National Hospital for Neurology and Neurosurgery, who were having diagnostic lumbar puncture with paired venous blood testing for investigation of a suspected neurodegenerative condition. At the time of clinical sampling, all individuals donated an additional blood sample for research, and basic demographic data were recorded. All participants gave written informed consent, or for those who were deemed lacking in capacity to do so by the assessing clinician, proxy consent was obtained from a consultee. The study was conducted in accordance with local clinical research regulations and was approved by the local ethics committee (reference: 12/0344).
2.2. Sample handling
The plasma was collected in 8-mL ethylenediaminetetraacetic acid tubes and serum in 6.5-mL serum separator tube gel separator tubes by peripheral venepuncture with a tourniquet, using either a 21G or 23G butterfly needle and BD Vacutainer® collecting system. Participants were not instructed to fast, but all blood samples for each participant were taken between 9 AM and 1 PM on a single venepuncture. Plasma and serum were centrifuged at 1800 × g for 5 minutes at room temperature. Plasma was centrifuged within a median of 20 minutes after sampling, whereas serum was allowed to clot for a median of 15 minutes before centrifugation. The supernatant was pipetted into 200-μL aliquots into identical polypropylene tubes, for freezing at −80°C, within 60 minutes of sampling, in keeping with current guidelines [1].
2.3. Freeze-thaw cycles
Each additional freeze-thaw cycle after the first consisted of 1 hour of direct thaw to room temperature, followed by 1 hour of direct replacement into −80° C. After the final thaw, samples were transferred to 1.5-mL polypropylene centrifuge tubes and centrifuged as per the kit-manufacturer's recommendation at 13,000 × g for 10 minutes, and the supernatant of each sample was pipetted onto the plate for analysis. For each biomarker, all aliquots from the same participant were assayed using the same batch of reagents in the same run.
2.4. Blood fraction choice
To examine the effects of freeze-thaw cycle number, we chose to measure NFL in serum as we and others have shown that NFL may be measured reliably in serum to show differences between disease groups among neurodegenerative diseases [7], [8] and that there is a linear relationship between plasma and serum NFL within individuals, with serum returning higher values when paired samples are analyzed on the same run (Supplementary Fig. S1A). Similarly, we chose to measure total tau, Aβ40, and Aβ42 in plasma as it returns higher values than serum for these biomarkers (Supplementary Fig. S1B–D).
2.5. Analysis
Commercially available Simoa total tau, NFL, Aβ40, and Aβ42 kits (Quanterix) were used according to the manufacturer's instructions. The principles of these assays are similar; a detailed account of the basis of Simoa technology has been provided by Wilson and colleagues [6].
All measurements were conducted on the same automated HD-1 analyzer (Quanterix). For serum NFL, plasma Aβ40, and plasma Aβ42, all samples were assayed in duplicate. For plasma tau, all samples were assayed in triplicate because of kit availability. The mean of the replicates for each sample was calculated and represented graphically. Results were included in the analysis if the coefficient of variation across replicates was <15%. The number of individuals included was therefore as follows: serum NFL, 12; plasma total tau, 11; plasma Aβ40, 14; and plasma Aβ42, 12.
2.6. Statistics
A Friedman analysis of variance (ANOVA) test (nonparametric repeated measures) was used to assess differences within individuals across four freeze-thaw cycles (SPSS Statistics, version 24). Where statistically significant differences were found at the P = .05 level, the median ratio of the concentration at a specific freeze-thaw cycle to the concentration at the first cycle was calculated across individuals.
3. Results
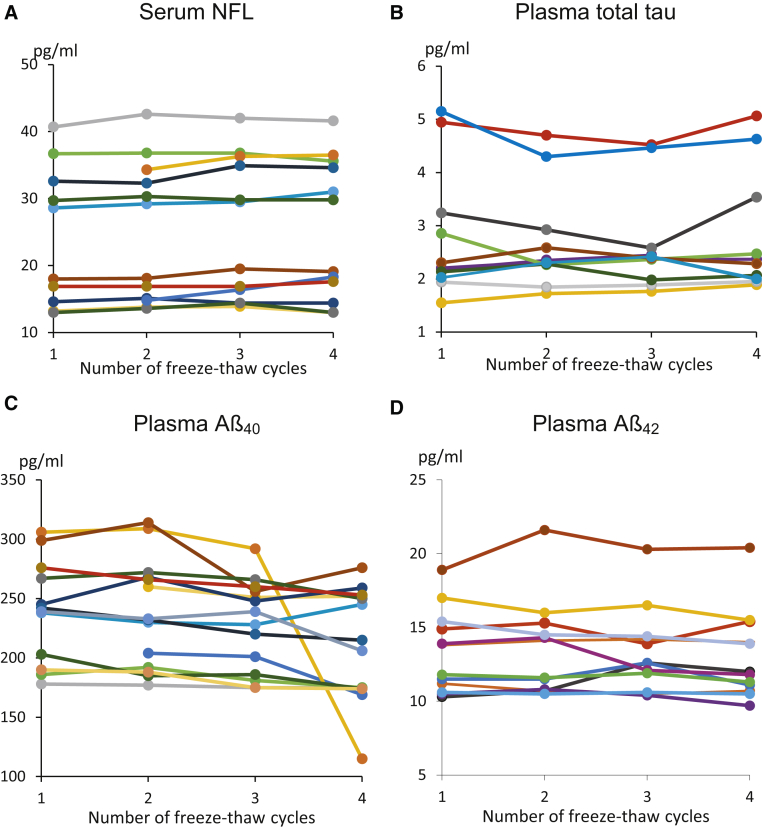
Fig. 1 shows the measured concentration of each analyte against the number of freeze-thaw cycles.
Fig. 1.
Concentration of the biomarker versus number of freeze-thaw cycles for (A) serum NFL, n = 12; (B) plasma total tau, n = 11; (C) plasma Aβ40, n = 14; and (D) Plasma Aβ42, n = 12. Each colored line represents an individual. Abbreviations: Aβ, amyloid β; NFL, neurofilament light chain.
The Friedman ANOVA test showed a two-tailed significance of >.05 for a change in the means for serum NFL, plasma total tau, and plasma Aβ42 over four cycles.
For plasma Aβ40, the median concentration ratio between the third and first cycles was .96 (interquartile range, .92–.99; Friedman ANOVA P = .015). The median concentration ratio between the fourth and first cycles was .92 (interquartile range, .90–.96; Friedman ANOVA P = .001), and this survived the exclusion of the one clear outlier individual.
4. Discussion
We show that for up to four freeze-thaw cycles, each consisting of at least 1 hour of thawing to room temperature, in aliquot volumes of 200 μL, there is no consistent trend for change in the concentrations of serum NFL, plasma total tau, or plasma Aβ42 as measured using the Simoa digital immunoassay platform. We found some evidence for a small reduction in plasma Aβ40 after the third and further after the fourth cycle. This latter finding is in keeping with the experience of Lachno and colleagues, who used a Luminex bead-based immunoassay platform and a 1-hour thaw per cycle to demonstrate a reduction in plasma Aβ 1–40 from the fourth cycle in five individuals [2]. Here, we show, in a larger sample size, a statistically significant, albeit very small, reduction by a median ratio of 0.96 at the third cycle and 0.92 at the fourth cycle relative to the first. The latter reduction survived removal of an outlier individual for whom there was a ratio of 0.38 between the concentrations at fourth and first cycles but no obvious technical difference identified in sample treatment. It is not clear whether the results from this outlier were in any way related to the freeze-thaw effect, but in keeping with the group results, we suggest that samples in which plasma Aβ40 is to be measured should be restricted to a maximum of two freeze-thaw cycles.
The conditions chosen in this study are consistent with the aliquot volumes and treatment recommended by current consensus guidelines [1] and mimic the likely conditions in large-cohort studies on blood-based biomarkers of neurodegenerative disease, in which minimizing sample volumes is desirable and cross-validation between laboratories will be essential. The ability to subaliquot samples will allow for sharing of samples across centers while reducing concerns about the effects of small numbers of short-duration freeze-thaw cycles on measured analyte concentrations.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using PubMed. There are several publications on preanalytical factors including the effects of freeze-thaw cycles on core cerebrospinal fluid (CSF), biomarkers of Alzheimer's disease (AD), and CSF neurofilament light chain (NFL), but there has been no systematic examination of the effects of multiple freeze-thaw cycles of these biomarkers in blood despite the existence of a growing literature on the use of these blood biomarkers of AD in large cohorts.
-
2.
Interpretation: This work demonstrates that up to four freeze-thaw cycles on aliquot volumes of 200 μL do not affect measured concentrations of serum NFL or plasma total tau and Aβ42. Beyond two freeze-thaw cycles, plasma Aβ40 may drop significantly in a few samples.
-
3.
Future directions: Sample sharing across research centers may be facilitated by the ability to freeze and thaw small aliquots up to the limits described in this article.
Acknowledgments
The authors are grateful to all the study participants; to Dr Ross Paterson who helped with the study cohort and to collect the samples; to Jamie Toombs, Martha Foiani, and Elena Veleva who processed the samples before initial storage; and to Dr Jennifer Nicholas for statistical advice.
This work was funded as part of a Leonard Wolfson Experimental Neurology Clinical Fellowship awarded to A.K.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.06.001.
Supplementary data
References
- 1.O'Bryant S.E., Gupta V., Henriksen K., Edwards M., Jeromin A., Lista S. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer's disease research. Alzheimers Dement. 2015;11:549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lachno D.R., Emerson J.K., Vanderstichele H., Gonzales C., Martenyi F., Konrad R.J. Validation of a multiplex assay for simultaneous quantification of amyloid-beta peptide species in human plasma with utility for measurements in studies of Alzheimer's disease therapeutics. J Alzheimers Dis. 2012;32:905–918. doi: 10.3233/JAD-2012-121075. [DOI] [PubMed] [Google Scholar]
- 3.Schoonenboom N.S., Mulder C., Vanderstichele H., Van Elk E.J., Kok A., Van Kamp G.J. Effects of processing and storage conditions on amyloid beta (1-42) and tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin Chem. 2005;51:189–195. doi: 10.1373/clinchem.2004.039735. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen A.H., Bahl J.M., Danborg P.B., Lindstrom V., Larsen S.O., Grubb A. Pre-analytical factors influencing the stability of cerebrospinal fluid proteins. J Neurosci Methods. 2013;215:234–240. doi: 10.1016/j.jneumeth.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Koel-Simmelink M.J., Vennegoor A., Killestein J., Blankenstein M.A., Norgren N., Korth C. The impact of pre-analytical variables on the stability of neurofilament proteins in CSF, determined by a novel validated SinglePlex Luminex assay and ELISA. J Immunol Methods. 2014;402:43–49. doi: 10.1016/j.jim.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Wilson D.H., Rissin D.M., Kan C.W., Fournier D.R., Piech T., Campbell T.G. The simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. 2016;21:533–547. doi: 10.1177/2211068215589580. [DOI] [PubMed] [Google Scholar]
- 7.Rohrer J.D., Woollacott I.O., Dick K.M., Brotherhood E., Gordon E., Fellows A. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87:1329–1336. doi: 10.1212/WNL.0000000000003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weston P.S.J., Poole T., Ryan N.S., Nair A., Liang Y., Macpherson K. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology. 2017;89:2167–2175. doi: 10.1212/WNL.0000000000004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.