Abstract
Medical informatics is defined largely by its host disciplines in clinical and biological medicine, and to project the agenda for informatics into the next decade, the health community must envision the broad context of biomedical research. This paper is a sketch of this vision, taking into account pressures from changes in the U.S. health care system, the need for more objective information on which to base health care decisions, and the accelerating progress and clinical impact of genomics research. The lessons of modern genomics research demonstrate the power of computing and communication tools to facilitate rapid progress through the adoption of open community standards for information exchange and collaboration. While aspects of this vision are speculative, it seems clear that the core agenda for informatics must be the development of interoperating systems that can facilitate the secure gathering, interchange, and analysis of high-quality information and can gain leverage from worldwide collaboration in advancing and applying new medical knowledge.
Medical informatics may be thought of as the discipline at the intersection of computer science and medicine—both clinical medicine and basic biomedical science. As such, informatics has a technology-based agenda of its own, but the needs and opportunities of its host medical disciplines drive the most important priorities for its agenda (and distinguish it from computer science per se). As we look forward to the challenges for medical informatics in the next decade, we are led to anticipate the clinical and basic science settings in which informatics will be developed and applied. This paper is an attempt to project future directions for clinical and genomic research for the coming decade and to focus in particular on their likely interactions—with each other and with informatics. The discussion is divided into two main sections, one focusing on prospects for clinical research and one on molecular biology and genomics research. The impact of these on informatics research and development is interwoven throughout and summarized in a concluding section.
An analysis such as this is unavoidably speculative—ten years is a long time in any modern technologic or scientific discipline. This vision is intended to promote discussion about the long-term role and agenda for informatics. Despite this speculative nature, it is difficult to imagine any scenario in which standards-based information management and interchange technologies, pioneered by the Internet, will not play increasingly important roles. Thus, in many ways we can project that the informatics agenda for 2008 will derive from and be elaborated on the basis of what we already know today. Given the successes of the 1990s, the biggest differences will be in the broader standardization and deeper integration and dissemination of information technologies throughout bio-medicine. We must expect that most research and collaboration will be mediated through more and more powerful digital information resources and communication and computation tools. This will facilitate a synthesis between clinical medicine and genomic science, fueled by rapid advances in the understanding of the biomolecular basis of life and disease processes.
Clinical Research
The Need for Clinical Research, and Its Agenda
Despite the profound changes in the U.S. health care system in the 1990s, caused by the move toward managed care to contain the costs of health care delivery, we can expect the importance of biomedical research to be re-emphasized. Paradoxically, an initial and direct result of managed care has been cutbacks in funding for medical research, especially in academic medical centers.1,2,3,4 However, these cutbacks come at a time when such research is one of the only ways to establish a rational basis for managed care—determining which interventions work and which do not and providing clear and rational practice guidelines for the cost-effective prevention and treatment of disease. We can expect that short-term efforts by managed care administrators simply to increase physician loads or to cut back on care will prove simplistic and will not result in sustainable cost cutting.
As we enter the 21st century, the U.S. population continues to grow and age. This means that the incidence of cardiovascular disease, cancer, pulmonary diseases, and chronic diseases such as diabetes, hypertension, skeletal and joint problems, obesity, autoimmune diseases, digestive disorders, depression, and stress-related problems will increase along with the cost of health care and medications. As one example, the cost of cardiovascular disease treatment reached a record $274 billion in 1996.5 In that year 570,000 coronary artery bypass grafting operations were performed, compared with 501,000 in 1995 (up 14%), at an average cost of $45,000 per operation. In 1996, 420,000 angioplasties were performed, compared with 404,000 the year before (up 4%), at an average cost of $20,000 per procedure. In 1996, 734,000 people died from cardiovascular disease and 160,000 died from strokes.
At the same time, one third of the pharmaceutical industry's growth has come from products less than 2 years old. New drugs are appearing with ever-increasing frequency because of the new technology of molecular biology and genomics. In the short term these technologies are allowing the rapid screening of drug candidates for bioactivity, and in the longer term they will allow the targeted design of drugs for particular diseases and even individual treatment. But new drugs are expensive—some new cholesterol management drugs cost $1,200 per year—and drugs are often prescribed without clear evidence of the need for them or of their effectiveness.
Finally, a significant proportion of the causes of heart disease and its predisposing risk factors continue to arise from unhealthy habits (e.g., lack of exercise, poor diet, smoking, and obesity) in conjunction with unfavorable physical, economic, and psychosocial environments. Similar circumstances predispose patients to other diseases and complicate their treatment, but attempts to educate people about these risks and modify their behavior to reduce them have been, to date, only marginally successful, if at all.6,7,8
These three trends will drive up the costs of health care relentlessly unless there is vigorous investment in clinical and health services research. Broadly, we can expect the research program for the next decade to include:
Increasing numbers of clinical-trial studies to support the rationalization of care decisions through evidence-based medicine.
Collaborative work with basic science to facilitate the discovery, testing, and integration of rapidly advancing etiologic, diagnostic, and treatment innovations from molecular biology and genomics research.
Efforts to better educate and involve patients in disease prevention and management, including emphasis or such habits as exercise, diet, alcohol use, smoking, substance abuse, obesity, immunizations, exposure to environmental chemicals, and awareness of accident risks.
Support for the global scope of public health and epidemiologic studies, and work toward improving education, immunization, basic hygiene measures, and the quality of food and water sources, which are required to understand the mechanisms of diseases and control their spread.
Efforts to keep physicians and other health care professionals current in their understanding of new technologies, diagnostic methods, treatment interventions, patient education, and prevention methods.
Re-engineering of the delivery and business side of health care for optimal efficiency, taking advantage of paramedical personnel, primary-care providers, secondary-care specialists, and tertiary-care settings in appropriate ways.
Definition of effective measures, and the routine assessment and reporting of outcomes, in health care settings.
Effective Standards for Information Collection and Exchange
Biomedical research is, of course, information intensive, and renewed research goals will lead to an increased demand for accurate and timely information that can be aggregated for analysis and decision support. This need for information will lead in turn to a renewed commitment to the goals and infrastructure of research and to the need for ubiquitous and interoperating electronic medical record (EMR) systems. In the current era, clinical-trial (and other) studies are very expensive, partly because each study is largely handcrafted with data collected by manual procedures. Often the data are recorded and transformed multiple times by hand instead of being accessed electonically from EMR sources. Few data structures and descriptions facilitate automatic interpretation of relations among data collected at multiple places for studying issues over broad populations. Only through substantial investment of human effort, such as that being done by the Cochrane Group,9 can clinical studies be combined and synthesized to support best-evidence clinical guidelines.
In addition, as noted by Sim in her 1997 doctoral thesis10:
Millions of dollars are spent annually on the conduct of randomized clinical trials, a type of experiment widely regarded as yielding the most valuable evidence for improving our understanding of medicine. Yet the results of many large and important clinical trials are published only as text-based articles in the clinical literature, articles that both practitioners and clinical researchers have difficulty finding, interpreting, and applying to clinical care. The result is an inefficient transfer of evidence from the research world to the clinic and a waste of precious resources. It is, however, not only the deficiencies of randomized-trial reports that contribute to this evidence-transfer problem; our difficulties with using randomized-trial evidence stem from problems that involve the entire life cycle of trials—from their design, registration, standardization, and publication to the synthesis of their results....
In an optimistic view, we can expect that the work of Sim and others, such as the Cochrane Group, will lead to the definition of community-based data standards and software tools to facilitate the design, execution, and reporting of clinical trials. In addition to the publication of trial results in articles, such studies would be communicated in standardized, structured, electronic databases. Most often the data for these trials would be derived directly from the EMRs used in the care of patients. This would happen through data filtering and normalization, anonymization against direct or inferred identification,11,12 and use of reporting scripts that can minimize human intervention to the judgmental efforts of quality control.
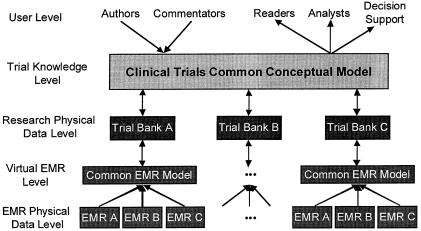
Informatics work has already produced systems that, given a codified EMR environment, can help with physician decision support to guide eligibility and treatment decisions for protocol-based care13,14 and help ensure more accurate and complete information. ▶ provides a conceptual diagram of this flow, which is adapted and extended from Sim's work.10,15 Legacy systems that continue in operation will need to be accommodated, of course, but technologies for virtual medical record definitions, such as the W3-EMRS system,16 can help bridge these differences to provide a normalized view of even older EMR data. Research “data warehouses” will become available to support these efforts, organized optimally for longitudinal searches rather than the care-oriented transactions of actual patient EMRs. In this view, databases of clinical-trial results will become interoperable through the adoption of common definitions and ontologies of clinical-trial concepts and nomenclature and the development of declarative methods to describe trial goals, procedures, study populations, and results. This will allow the sharing and critiquing of results, the extension of prior studies rather than their repetition, easier systematic reviews and meta-analyses, broad collaborations in carrying out intercomparable studies to achieve more definitive results, and more effective dissemination of results in the context of the EMR to affect decisions at the point of care.
Figure 1.
The flow of information from structured electronic medical records to research and clinical-trial data banks.
Basic informatics and computer science tools that can help with this transformation of medical record keeping and clinical-trial support have already been developed. However, much informatics research needs to be done before we understand how to develop and deploy an infrastructure that will scale and integrate smoothly into practice.
Interestingly, while efforts to put EMRs into routine use and to structure clinical knowledge have moved very slowly during the 1980s and 1990s, similar efforts to standardize nomenclature and information structures in molecular biology have met with extraordinary success during the same period. From the earliest work to develop computer-based tools for the analysis of sequence information, such as MOLGEN and BIONET in the 1970s and 1980s,17 shared and highly interoperable databases have evolved in the bioscience communities and have facilitated rapid progress in research and practice.
Related tools and information disciplines can offer similar leverage to clinical medicine. If everyone cooperated in this effort, it might be shown that minimizing the wasted effort in collecting and using clinical information would be a major step toward improving the quality of that information, containing the costs of delivering health care, and developing and evaluating new interventions.
If this standardization could be achieved, it would profoundly affect the relationships among patients, providers, administrators, payers, researchers, and supervisory groups. From a research point of view, it would be relatively easy to carry out studies that build on and complement previous work. New information tools would make it possible to assemble large-enough patient populations, even if they were not geographically local, and to engage community physicians in research projects, because the costs of information collection and reporting would have been reduced substantially by interfaces between standardized EMRs. Academic medical centers would still be heavily involved in the most advanced clinical research, primarily in the design and supervision of trials, the synthesis of diverse results, and the interface between clinical care and basic science. The evaluation of new diagnostic techniques, the design and evaluation of new drugs and genetic interventions, and the ongoing study of the underlying biomolecular basis of life and disease all require a clinical sophistication not often found outside academic centers.
In many ways, this transition to broad standards will be very difficult and costly. Many of the legacy EMR systems adopted during the early- to mid-1990s will have to be replaced. People working in health care will have to be trained to adopt the reporting standards required for the interoperability of diverse information systems. It is unlikely that this process could be completed by 2008, because the needed record systems will not yet have fully penetrated rural health care practices in the United States. Pressures will increase for better information systems worldwide to meet public health needs and humanitarian goals to raise the quality of health care. Also, with the rapid growth of new information about diagnostic and treatment alternatives, and guidelines for their use, individual physicians, nurses, and other providers will face an increasing problem of lifetime learning. Digital information publishing and Web-based delivery technologies, customized for particular areas of practice, will help health care personnel stay current.
Molecular Biology and Genomics Research
Agenda for Genomics Research
In the classical Mendelian view, organisms contain cells with many characteristic units, or genes, which define a wide variety of observable traits for the organism and which are inherited and expressed according to the laws of genetic dominance. The core success of modern molecular biology and genetics has been accelerating progress to unravel the biomolecular basis of these processes. The goals of the human genome project are to identify the 100,000 or so different genes in humans (as expressed in DNA sequences) and to understand their modes of expression (through RNA translation processes) and their functions in proteins. Another goal is to understand how the cell can enable, control, and manage the machinery involved in embryogenesis, development, growth, reproduction, metabolism, aging, and response to environmental factors.18
Researchers in the field of genomics study genes, their interactions, their mutations, and the relationships they reveal between normal function and disease. Through the understanding of “normal” functions of various parts of an organism and their variants, many malfunctions or diseases may be understood at the biomolecular level. Genomics also provides tools for diagnosing disease, both inherited and infectious, through the study of the genomes of pathogenic agents. A key result of even this early work is that genomics is already having a profound effect on modern medicine. Some diseases are caused overtly by genetic disorders—e.g., single-gene diseases such as cystic fibrosis, familial hypercholesterolemia, hereditary hemochromatosis, and sickle cell anemia, and chromosomal disorders such as Down syndrome, Klinefelter syndrome, and Turner syndrome. Many more diseases have important genetic components, including diabetes mellitus, hypertension, and cancer. These effects are not rare. Single-gene defects occur in at least one of every 100 persons; chromosomal disorders occur in at least one of every 150 live-born children; and multifactorial diseases with a genetic component occur in at least one of every 10 persons. Our genetic makeup defines our inborn functional processes and capacities and the effects of many of our interactions with our environment, such as dietary intake, exposure to infectious agents, exposure to physical factors, and lifestyle. Mutation of genes due to random processes or the influence of outside agents can change the balance we achieve with our environments and hence move us from “normal” function to impaired function. Understanding these functions and processes allows us not only to understand at the deepest level the identity and sources of many diseases but also to design effective ways of intervening to control or eliminate them.
Genomics Research: A Model for Informatics as a Tool
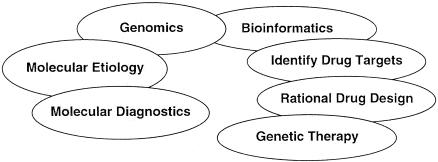
One of the central goals of bioinformatics is the use of computing and communication technologies to facilitate genomic studies. Using the conventional dichotomy between “diagnosis” and “therapy” (▶), we can view genomics and bioinformatics as attempts to understand the molecular etiology of disease, leading to better diagnostic tools. Equally important, genomics and bioinformatics seek to rationalize the development of drug and genetic interventions to cure or ameliorate disease.
Figure 2.
The clinical impact of genomics and bioinformatics on diagnosis and therapy.
We have already mentioned that since the 1980s and 1990s, basic science researchers have recognized that tremendous leverage could be obtained by standardizing the structure and content of genomic databases so that sequences could be studied through relations to other sequences whose function was already known. These include DNA sequence databases (e.g., GenBank19), protein sequence and structure databases (e.g., PIR20), and structure-to-phenotype databases (e.g., Online Mendelian Inheritance in Man [OMIM]21) as well as many specialized voluntary databases, such as Roberts' restriction enzyme database.22 Appropriate standards for describing genomic sequences and function have been adopted and have come to be enforced by professional societies and journal publishers. An author has to submit not only the text of an article describing research results but also standardized descriptions of the sequences, structures, and genetic functions related to the results. The National Center for Biotechnology Information (NCBI) at the National Library of Medicine was founded in 1991 as a global repository for databases of genomic information23, ▶ shows the home page of its Web site.
Figure 3.
The home page for the Web site of the National Center for Biotechnology Information at the National Library of Medicine (available at http://www.ncbi.nlm.nih.gov/).
The NCBI Web pages cross-link bibliographic citations of the medical literature, full-text publications, information in the diverse genomic databases, and a growing body of information about the biomolecular basis of life and disease. Through modern communication and computing technology, NCBI and related resources in Europe and Japan have come to serve as facilitators of broad collaborations in genomic research, including the large-scale genome mapping projects for humans, bacteria, yeasts, and many other organisms. For example, the RiboWeb project uses tools relevant to the study of such biomolecular structures as the 30S ribosome (the site of activity for most common antibiotics) to facilitate collaborative studies, over distance, of the structure and function of this ribosome, as a basis for the development of more effective drugs.24 Without the informatics base to record and interrelate genomics results, and the computational tools to compare and analyze structures, progress would have been much slower, if possible at all.
Intersection of Genomics Research and Clinical Research
There are many modern examples of gene mutations that have been correlated with disease. For example, most cases of cystic fibrosis (CF) are the result of a three-base-pair deletion in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This deletion removes a phenylalanine residue from a protein crucial to chloride transport in pulmonary and gastrointestinal systems. The CFTR gene has been isolated on human chromosome 7 (where the “obese” gene also resides). The NCBI Web page for chromosome 7 has pointers to other Web pages, such as that of the CF Research Foundation, with information on mutations giving rise to CF. The pointer to the OMIM database shows historical descriptions and clinical synopses as well as pointers to all the molecular databases related to CF. The Web provides a natural medium for navigating the genomic information distributed among the numerous research sites around the world that are collaborating in their research on the genomic basis of various diseases.
Genomics can provide highly effective diagnostic probes to detect inherited traits, often before any symptoms are manifest. Genomics provides novel methods for diagnosing disease at all levels—at the levels of DNA, RNA, and bioactive proteins. By studying the mechanism, specificity, and regulation of genomic processes, we can make diagnoses at the most fundamental level, on the basis of evidence that is at the same time more sensitive and more specific—much less ambiguous than classical symptoms, which can often arise from several different diseases.
Genomic methods can also be mechanized using technologies similar to those used in microcircuit manufacture. Thousands of diseases can be screened simultaneously using such microarrays. We can expect that the new analytic instruments that will result from this research will deeply affect the informatics environments of clinical laboratories. For example, one such microarray, developed by Affymetrix, can be used to diagnose CF. Each spot on the microarray contains a short sequence of DNA that is hybridized to a specific sequence on the gene, encoding the normal or mutant form of the CF gene. Microarrays of DNA sequences can be made on silicon chips, on glass slides, or on a number of other supporting media. The DNA from a patient is broken into short pieces and combined chemically with a fluorescent compound. The patient's DNA binds to DNA on the microarray, giving a characteristic pattern of fluorescent spots. Such diagnoses can be performed in a few hours, with milligram amounts of tissues.
Affymetrix now routinely makes diagnostic chips, which can test for 64,000 inherited sequences simultaneously. Similar arrays are used for studying the expression of genes rather than their mutations—for example, to screen RNA from promyelocytes in patients with acute promyelocytic leukemia to help decide on and guide treatment with retinoic acid.
Still other examples of progress include the studies of renal cell carcinoma using two-dimensional electrophoresis, of treatment-resistant tuberculosis, and of protease inhibitor resistance developed with treatment in HIV infections. In the last case, Shafer et al.25 have been able to identify five structural mutations in HIV-1 protease that make available protease inhibitors ineffective against the rapidly mutating HIV virus in heavily treated patients. Successful cloning experiments of the late 1990s might hold promise for the availability of customized tissues needed to repair disease damage or injuries without rejection problems.
Tools such as these can be expected to bring unparalleled power to clinical diagnosis in the form of DNA probes for infectious disease, inherited disease, and genetic damage; analysis of gene expression; and analysis of protein expression.26,27 Equally important will be new therapeutic tools in the form of recombinant gene products, novel drug targets, rational drug design, and gene therapy. This work will require forging stronger ties between basic and clinical scientists to provide populations for studies of genomic function and to evaluate new diagnostic and therapeutic interventions.
Despite all the projected benefits of genomic research, it is unlikely that genomics will reduce the cost of health care in the short term. In fact, many of the “high-tech” diagnostic and treatment tools developed as a result of genomic studies will be very expensive initially and may raise the cost of health care. On the other hand, many of the genetic techniques for molecular diagnosis can be readily automated with hardware and informatics techniques, and even though they may increase the capital costs, they may eventually reduce the cost per patient. However, significant challenges remain to find ways to deliver the benefits of these technologies to large populations, in the United States and worldwide.
Implications for Informatics
The visions we have projected for clinical research and genomics research, and especially their intersection, are speculative in many ways. Still, in any scenario one might imagine, the computer-based collection, management, and analysis of information will only increase in importance over time. The lessons from early examples of locally idiosyncratic clinical information, decision support, and research database systems make it clear that standards for encoding and processing information must be the cornerstone of facilitating research and thereby the cost-effective delivery of care. The lessons of the modern genomics research effort are far-ranging—both in the impact this effort will have in providing a more scientific basis for clinical practice and interventions and in the way its use of computing and communication tools has facilitated rapid progress through the adoption of open community standards for information exchange and collaboration. These lessons in turn derive from lessons of the Internet era: Build your systems with an open and layered architecture for interoperability, scalability, and extensibility. Informatics is central to these goals.
These goals will also require better integration of genomics and biomedical informatics into curricula for clinical researchers and providers. The building of standards is a domain-specific task; that is, it requires the synthesis of informatics and computer science methodologies with the needs, concepts, terminologies, and structures of the practice of medicine. Interdisciplinary education, including a strong foundation in informatics, will be essential. Also, while genomics will bring great advances to basic science and clinical medicine, it will also bring perplexing ethical, social, and economic issues. Genetic information, such as that provided by analyses of the BrCA1 or HER2/neu genes, may signal an increased likelihood of serious disease (breast cancer in these cases), but then what should be done about this information in the absence of overt disease? Does it make sense even to test for such genetic factors before there is any evidence of disease? Genomics and bioinformatics methods often yield diagnostic results that are more quantitative than those based on other observable symptoms. Nevertheless, molecular diagnostics also have well-defined uncertainties, as embodied in the probabilistic rules of inheritance and penetrance of inherited traits. For these tools to provide sound and useful information on which patients can base informed decisions, it is critical that physicians be trained in methods of medical decision analysis to help interpret the molecular results. The discipline of informatics-based genetic counseling will have to become a key part of a physician's training.
Finally, as we understand in greater detail the mechanisms that enable, control, and manage the processes involved in embryogenesis, development, growth, reproduction, metabolism, aging, and responding to environmental factors, we can expect renewed opportunities to develop powerful functional models. We are already making good progress in modeling biomolecular dynamics, and we should expect complementary methods to scale to more detailed models of cells, populations of cells, organs, organ systems, and organisms. Such functional models, based on a sound knowledge of the consequences of the biomolecular processes of life and disease, will likely require hierarchic approximations to control complexity but can be expected to help broadly with teaching, basic research, clinical research and practice, and drug design.
As we begin to realize this vision of the growing interrelationships between clinical research, genomics research, and informatics research, and their interdependence, we must take a long view. Even though we are often led to expect explosive short-term results from investments in research—e.g. through press coverage of new drugs, genomic research breakthroughs, and informatics applications like the World Wide Web—we must realize that these have followed decades of investment in difficult basic research. Informatics has had success in providing methodologies to facilitate clinical and genomics research, but progress in informatics itself is just as difficult and must have sustained support for research.
References
- 1.Gaus CR, Fraser I. Shifting paradigms and the role of research. Health Aff. 1996;15(2): 234-42. [DOI] [PubMed] [Google Scholar]
- 2.Mechanic RE, Dobson A. The impact of managed care on clinical research: a preliminary investigation. Health Aff. 1996;15(3): 72-89. [DOI] [PubMed] [Google Scholar]
- 3.Burnett DA. Evolving market will change clinical research. Health Aff. 1996;15(3): 90-2. [DOI] [PubMed] [Google Scholar]
- 4.Cutler CM. Research needs for managed care. Health Aff. 1996;15(3): 93-4. [DOI] [PubMed] [Google Scholar]
- 5.NCHS home page. National Center for Health Statistics. Web site. Available at: http://www.cdc.gov/nchswww/. Accessed July 13, 1998.
- 6.Fortmann SP, Flora JA, Winkleby MA, Schooler C, Taylor CB, Farquhar JW. Community intervention trials: reflections on the Stanford Five-City Project experience. Am J Epidemiol. 1995;142(6): 576-86. [DOI] [PubMed] [Google Scholar]
- 7.Carleton RA, Lasater TM, Assaf AR, Feldman HA, McKinlay S. The Pawtucket Heart Health Program: community changes in cardiovascular risk factors and projected disease risk. Am J Public Health. 1995;85(6): 777-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luepker RV, Rastam L, Hannan PJ, Murray DM, Gray C, Baker WL, et al. Community education for cardiovascular disease prevention: morbidity and mortality results from the Minnesota Heart Health Program. Am J Epidemol. 1996;144(4): 351-62. [DOI] [PubMed] [Google Scholar]
- 9.The Cochrane Library home page. Cochrane Library Web site. Available at: http://www.Cochrane.co.uk/. Accessed July 13, 1998.
- 10.Sim I. Sharable Databases of Randomized Clinical Trials: In Support of Computer-assisted Evidence-based Medicine [PhD thesis]. Stanford, Ca.: Stanford University, 1997. (Medical Information Sciences Report SMI-97-0701.
- 11.Sweeney L. Replacing personally identifying information in medical records: the Scrub system. Proc AMIA Annu Fall Symp. 1996: 333-7. [PMC free article] [PubMed]
- 12.Sweeney L. Guaranteeing anonymity when sharing medical data: the Datafly system. Proc AMIA Annu Fall Symp. 1997: 51-5. [PMC free article] [PubMed]
- 13.Hickam DH, Shortliffe EH, Bischoff MB, Scott AC, Jacobs CD. The treatment advice of a computer-based cancer chemotherapy protocol advisor. Ann Intern Med. 1985;103(6, Pt 1): 928-36. [DOI] [PubMed] [Google Scholar]
- 14.Musen MA, Carlson RW, Fagan LM, Deresinski SC, Shortliffe EH. T-HELPER: automated support for community-based clinical research. Proc 16th Annu Symp Comput Appl Med Care. 1992: 719-23. [PMC free article] [PubMed]
- 15.Sim I, Rennels G. Standardized Reporting of Clinical Trials into Electronic Trial Banks: In Support of Computer-assisted Evidence-based Medicine. Stanford, Ca.: Stanford University, 1996. Medical Information Sciences Report SMI-96-0630.
- 16.Kohane IS, Greenspun P, Fackler J, Cimino C, Szolovits P. Building national electronic medical record systems via the World Wide Web. J Am Med Inform Assoc. 1996;3(3): 191-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DH, Brutlag D, Friedland P, Kedes LH. BIONET: national computer resource for molecular biology. Nucleic Acids Res. 1986;14(1): 17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leder P, Clayton DA, Rubenstein E (eds). Introduction to Molecular Medicine. New York: Scientific American, 1994.
- 19.Kelly MJ. Computers: the best friends a human genome ever had. Genome. 1989;31(2): 1027-33. [DOI] [PubMed] [Google Scholar]
- 20.Barker WC, George DG, Mewes HW, Tsugita A. The PIR-International Protein Sequence Database. Nucleic Acids Res. 1992;20(Suppl): 2023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKusick VA, Amberger JS. The morbid anatomy of the human genome: chromosomal locations of mutations causing disease. J Med Genet. 1993;30(1): 1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gingeras TR, JP MI, Roberts RJ. A computer assisted method for the determination of restriction enzyme recognition sites. Nucleic Acids Res. 1978;5(11): 4105-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The NCBI home page. National Center for Biotechnology Information Web site. Available at: http://www.ncbi.nlm.nih.gov/. Accessed July 13, 1998.
- 24.Altman RB. The RiboWeb Project [Stanford University Web Site]. Available at: http://smi-web.stanford.edu/projects/helix/riboweb.html. Accessed July 13, 1998.
- 25.Shafer RW, Winters MA, Palmer S, Merigan TC. Multiple concurrent reverse transcriptase and protease mutations and multidrug resistance of HIV-1 isolates from heavily treated patients. Ann Intern Med. 1998;128(11): 906-11. [DOI] [PubMed] [Google Scholar]
- 26.American Association for the Advancement of Science. Genome Issue. Science. 1997;278(5338): 541-768. [Google Scholar]
- 27.American Association for the Advancement of Science. Frontiers in Cancer Research. Science. 1997;278(5340): 981-1192. [Google Scholar]