Abstract
Increase of blood brain barrier (BBB) permeability after acute ischemia stroke is a predictor to intracerebral hemorrhage transformation (HT) for tissue plasminogen activator (tPA) thrombolysis and post-endovascular treatment. Previous studies showed that 2-h ischemia induced damage of BBB integrity and matrix metalloproteinase-2 (MMP-2) made major contribution to this disruption. A recent study showed that blocking β2-adrenergic receptor (β2-AR) alleviated ischemia-induced BBB injury by reducing hypoxia-inducible factor-1 alpha (HIF-1α) level. In this study, we sought to investigate the interaction of HIF-1α with MMP-2 and vascular endothelial growth factor (VEGF) in BBB injury after acute ischemia stroke. Rat suture middle cerebral artery occlusion (MCAO) model was used to mimic ischemia condition. Our results showed that ischemia produced BBB damage and MMP-2/9 upregulation was colocalized with Rhodamine-dextran leakage. Pretreatment with YC-1, a HIF-1α inhibitor, alleviated 2-h ischemia-induced BBB injury significantly accompanied by decrease of MMP-2 upregulation. In addition, YC-1 also prevented VEGF-induced BBB damage. Of note, VEGF was shown to be colocalized with neurons but not astrocytes. Taken together, BBB damage was reduced by inhibition of interaction of HIF-1α with MMP-2 and VEGF during acute cerebral ischemia. These findings provide mechanisms underlying BBB damage after acute ischemia stroke and may help reduce thrombolysis- and post-endovascular treatment-related cerebral hemorrhage.
Keywords: blood brain barrier, HIF-1α, ischemia stroke, matrix metalloproteinase, vascular endothelial growth factor
Introduction
Damage of blood brain barrier (BBB) integrity after acute ischemia stroke is a promising target (Jin et al., 2014; Liu et al., 2016) for clinical intervention to reduce hemorrhage transformation (HT) in patients with intravenous tissue plasminogen activator (tPA; Leigh et al., 2014) or post-endovascular treatment (Leigh et al., 2016). Most of studies focus on reperfusion-induced BBB damage because most of the damaging consequences of BBB injury (hemorrhage and edema) won’t be presented until the blood flow to the ischemic brain is restored (Simard et al., 2007; Hafez et al., 2014; Shi et al., 2016). However, BBB damage at the ischemia stage, particularly at the early stage of the 4.5 h thrombolytic time window (Hacke et al., 2008), remains a much less-well studied topic.
Recent studies showed that 2-h ischemia caused damage of BBB integrity in non-infarcted ventromedial striatum (Jin et al., 2012; Wang et al., 2016; Sun et al., 2017) and 2-h ischemia-induced matrix metalloproteinase-2 (MMP-2) induction lead to the disruption of the BBB integrity (Jin et al., 2012; Liu et al., 2012; Wang et al., 2016). In addition, blocking β2-adrenergic receptor (β2-AR) alleviated ischemia-induced BBB damage by reducing hypoxia-inducible factor-1 alpha (HIF-1α) level (Sun et al., 2017). However, it is not clear about the interaction of HIF-1α with MMP-2 in BBB damage during acute ischemia.
HIF-1α and its downstream vascular endothelial growth factor (VEGF) have been shown to play important role in the damage of BBB integrity after ischemia and reperfusion (Chen et al., 2009, 2010). VEGF has been shown to disrupt integrity of BBB by altering tight junction proteins under ischemic and inflammatory conditions (Yeh et al., 2007; Argaw et al., 2009; Engelhardt et al., 2014). However, the role of VEGF in BBB damage after acute ischemia as well as its cellular distribution is not known.
Therefore, in this study, using suture middle cerebral artery occlusion (MCAO) model to mimic ischemia stroke, we aimed to investigate the relationship of HIF-1α with MMP-2 and VEGF in BBB damage during acute cerebral ischemia. Our hypothesis is that acute ischemia stroke induced upregulation of HIF-1α and HIF-1α plays a critical role in BBB damage through interacting with its downstream MMP-2 and VEGF.
Materials and Methods
Animal Model of Focal Cerebral Ischemia
The Sprague-Dawley rats, which were ordered from SLAC Company (Shanghai, China), were housed 2–3 per cage under constant temperature (23 ± 1°C) and light-controlled vivarium (12-h light/12-h dark cycle). Food and water were available ad libitum. The animal procedures were approved by the University Committee on Animal Care of Soochow University and performed according to the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to reduce the number of animals and to minimize animal suffering. Rats with body weight from 270 g to 290 g were subjected to MCAO for 2 h using the suture model, as has been described previously (Liu et al., 2017). Briefly, rats were anesthetized with isoflurane (4% for induction, 1.75% for maintenance) during surgical procedures. Body temperature was maintained at 37.5 ± 0.5°C using a heating pad. The external carotid artery (ECA) and internal carotid artery (ICA) were exposed. A 4-0 silicone-coated monofilament nylon suture was inserted into the ICA via a cut on the ECA. Reperfusion was produced by gently withdrawing the suture out of the ECA. Successful surgery was further confirmed by tissue staining with 2,3,5-triphenyltetrazolium chloride (TTC).
Rhodamine-Conjugated Dextran and Evan’s Blue Leakage Detection
Immediately after 2-h ischemia, Rhodamine-conjugated dextran (2,000 kDa, Invitrogen, Carlsbad, CA, USA) or Evan’s blue dye (EB; Sigma, St. Louis, MO, USA, 2% wt/vol in PBS, 3 mL/kg) was administered via the tail vein. All rats were reperfused for 10 min to allow sufficient circulation of Rhodamine-conjugated dextran or EB to the ischemic hemisphere (I), but minimize the effect of reperfusion on integrity of BBB. After reperfusion, the rat was transcardially perfused with ice-cold PBS followed by quickly taking the brain out (Liu et al., 2017). The EB maximally absorbs light of 620 nm and emits red fluorescence of 680 nm (Saria and Lundberg, 1983).
Rhodamine-conjugated dextran leakage was used to observe the co-localization of BBB injury. EB leakage was applied to check the co-localization of BBB injury and MMP activity or VEGF expression. Twenty-micrometer-thick cryosection was cut from the 8-mm-thick brain region as described (Jin et al., 2012) and mounted for fluorescence microscopy observation. BBB injury was visualized as leakage of Rhodamine-conjugated dextran or EB, which appeared as red fluorescence on brain sections. Brain sections were subjected to immunostain analysis for VEGF expression.
YC-1 Administration
Rat was treated with 3-(5’-Hydroxymethyl-2’-furyl)-1-benzyl indazole (YC-1), the HIF-1α inhibitor (Cayman Chemical Company, Ann Arbor, MI, USA dissolved in a solution of 1% dimethyl sulfoxide, DMSO) which is known to downregulate HIF-1α at the post-translational level. In addition, YC-1 inactivates the COOH-terminal transactivation domain (CAD) of HIF-1α, and stimulates factor inhibiting HIF (FIH) binding, further suppressing HIF-1α (Dewitz et al., 2017). YC-1 was administered at 2 mg/kg body weight through femoral vein at 24 h and 30 min prior to the onset of ischemia. We chose the dose based on previous publication (Sun et al., 2017).
Immunostaining
Immediately after 2-h ischemia, rat was perfused with ice-cold PBS followed by 4% PFA. Analysis of VEGF was carried out by immunostaining using the 20-μm-thick cryosection as described (Wang et al., 2017). In brief, tissue was pre-incubated for 1 h at room temperature in PBS containing 0.1% Triton X-100, 1% BSA, and 5% goat serum to block non-specific binding sites. Then anti-VEGF antibody (1:200, Abcam), anti-NeuN (1:200, Millipore), anti-GFAP (1:2,000, Millipore) primary antibody were applied to the sections and incubated at 4°C overnight. Cy3 conjugated secondary antibody (anti-mouse, 1:800) or 488-conjugated secondary antibody (anti-rabbit, 1:800) was incubated with the brain cryosection for 2 h at room temperature. The staining was visualized under LSM 700 confocal laser-scanning microscope (Zeiss), and images were taken from the ischemic and the mirrored non-ischemic region.
Gel and in situ Gelatin Zymography
In situ Zymography
In situ zymography was used to check the gelatinolytic activity of MMP-2/9 in brain tissue by using Kit (EnzCheck Collagenase Kit, Invitrogen) following the manufacturer’s instructions as described (Shu et al., 2015). Rat brain was sliced into 20-μm-thick cryosections and cryosections were incubated in a reaction buffer which contained 30 μg/ml of FITC-labeled DQ-gelatin in a humidity chamber for 1 h at 37°C. The sections were rinsed and mounted for fluorescent microscopic observation. The disruption of BBB integrity was reflected by Rhodamine dextran or EB extravasation (red), and the gelatin-FITC is cleaved by gelatinases, yielding peptides whose fluorescence is representative of net proteolytic activity (green).
Gel Gelatin Zymography
Tissues from ischemic (I) and non-ischemic (NI) hemisphere were homogenized in matrix metalloproteinase lysis buffer, and levels of MMP-2/9 in the homogenates were detected by gel gelatin zymography as described previously (Sun et al., 2017). A mixture of human MMP-2/9 (Invitrogen) was used as a positive control (gelatinase standards).
Statistical Analysis
The data are showed as mean ± SEM. Statistical analysis was done using one way or two-way ANOVA (SPSS software, version 17.0). A value of P < 0.05 was considered statistically significant.
Results
Effect of 2-h Ischemia on BBB Damage and MMP-2/9 Activity
Rhodamine-dextran leakage which showed BBB damage was clearly seen in the ischemic area (Figure 1). In situ zymography was done to check the spatial distribution of gelatinase activation and its colocalization with 2-h ischemia-induced BBB injury. After incubating the brain slice with FITC-labeled DQ gelatin, compared to the non-ischemic (NI) hemisphere, there was a significant increase of gelatinolytic activity (green fluorescence) in the ischemic (I) hemisphere where dextran leakage was detected (Figure 1A) and quantitative data confirmed the ischemia-induced MMP-2/9 activity upregulation (Figure 1B). Remarkably, more gelatinolytic activities were found with the leaked ischemic microvessels (Figure 1A).
Figure 1.
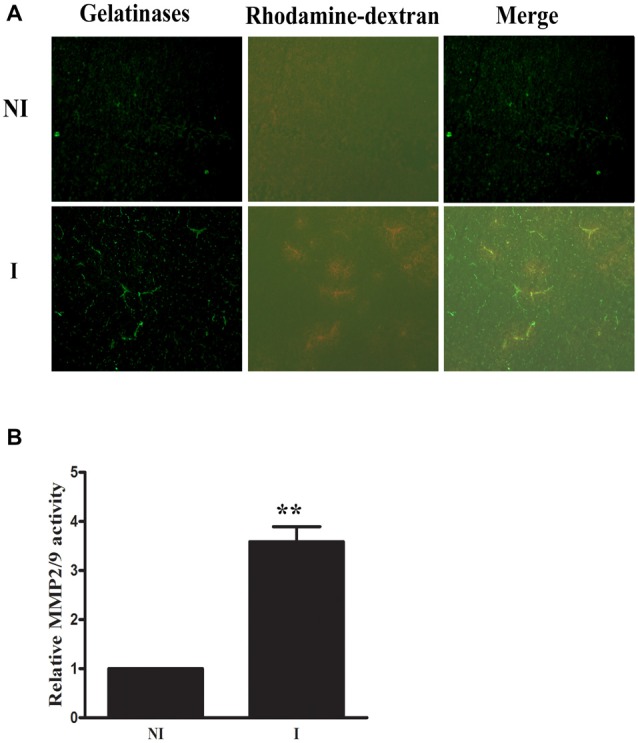
Effect of 2-h ischemia on blood brain barrier (BBB) damage and matrix metalloproteinase-2 (MMP-2)/9 activity. (A) After in situ zymography procedure, ischemic region (I) with dextran leakage (bottom left panel) and its corresponding tissue (upper left panel) in the non-ischemic hemisphere (NI) were chosen for microscopic observation. Fluorescent micrographs showed increased gelatinolytic activity of MMP-2/9 along ischemic microvessels (bright green fluorescence), where dextran leakage concurrently occurred. Spotted distribution of increased gelatinolytic activity was also seen in the leaky area. No dextran leakage and weak gelatinolytic activity were seen in the corresponding region of the NI hemisphere. (B) Quantitative data of MMP-2/9 activity (n = 3). **P < 0.01 compared to MMP2/9 activity in NI hemisphere.
MMP-2 Induction After 2-h Ischemia
The levels of MMP-2/9 were analyzed with gel zymography after 2-h of ischemia (Figure 2). There is no significant difference between the levels of MMP-2 in the non-ischemic (NI) hemisphere and similar results were observed for MMP-9 (Figure 2A). The level of MMP-2 was significantly increased after 2-h ischemia (Figure 2B), while the level of MMP-9 was much lower and there is no significant increase (Figure 2C).
Figure 2.
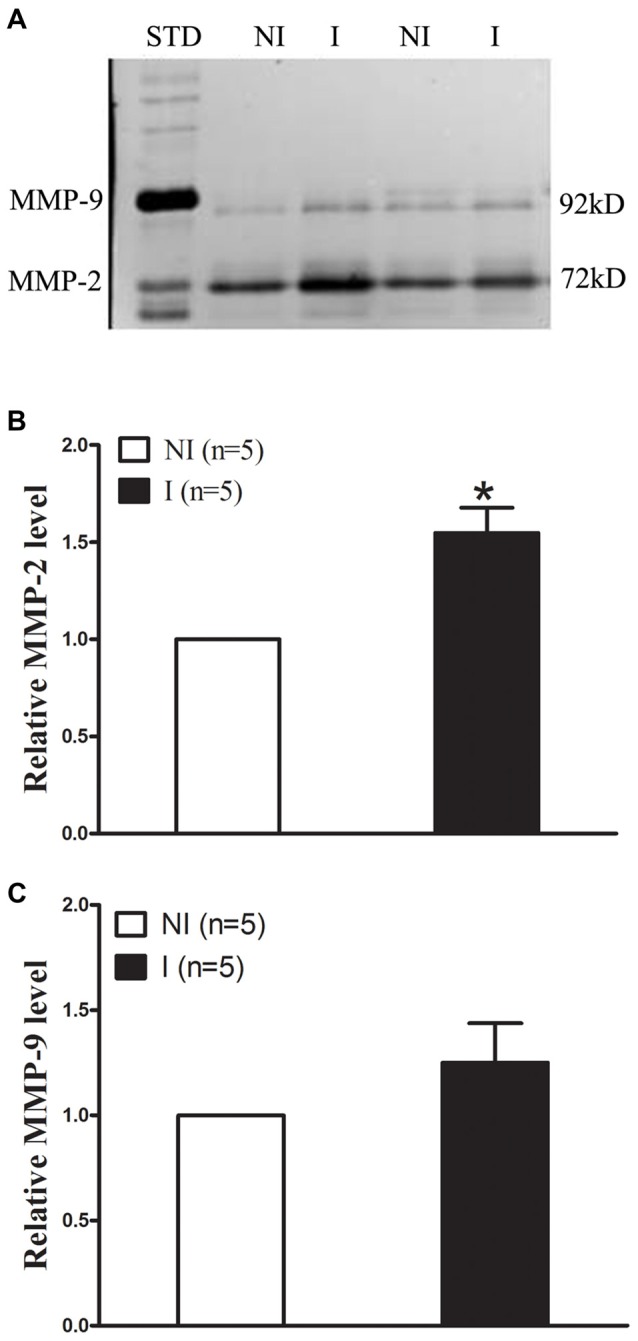
Effect of 2-h ischemia on MMP-2/9 induction. MMP-2/9 was induced in ischemic brain tissue after 2-h ischemia. (A) Representative gelatin zymogram showed MMP-2/9 levels in the non-ischemic (NI) and ischemic (I) hemispheric tissue. MMP-2 bands were much stronger than MMP-9 bands on zymogram gels. STD is a mixture of standard MMP-2/9. The relative band intensity of MMP-2 (B) and MMP-9 (C) was quantified. A significant increase was observed for MMP-2 in the ischemic tissue after 2-h middle cerebral artery occlusion (MCAO; *P < 0.05 vs. NI, n = 5), while there was no significant increase for MMP-9 (P > 0.05 vs. NI, n = 5). Data were expressed as mean ± SEM.
HIF-1α Inhibitor YC-1 Alleviated 2-h Ischemia-Induced BBB Disruption as Well as MMP-2 Upregulation
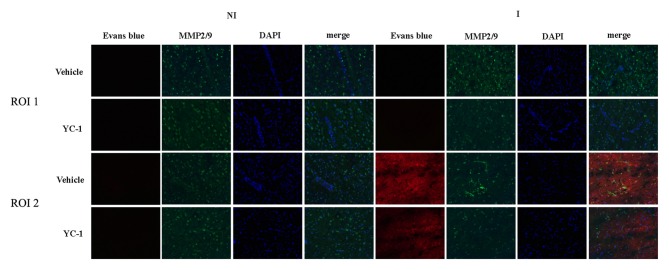
EB leakage was often used to evaluate the integrity of BBB (Wang et al., 2017). After 2-h ischemia, there was an obvious EB leakage in the ipsilateral hemisphere of rat brain (Figure 3). HIF-1α is a key mediator of the adaptive cellular response to hypoxia condition. To determine the interaction HIF-1α and MMP-2 in BBB damage after 2-h ischemia, treatment with HIF-1α inhibitor YC-1 dramatically reduced the EB leakage, indicating that HIF-1α inhibition with YC-1 protected BBB against 2-h ischemia-induced damage. Using in situ zymography, our results showed that MMP-2 was significantly increased in region of interest 2 (ROI 2) and a colocalization was observed for MMP-2 and EB leakage. YC-1 administration significantly decreased BBB damage as well as MMP-2 activity (Figure 3).
Figure 3.
Effect of 3-(5’-Hydroxymethyl-2’-furyl)-1-benzyl indazole (YC-1) treatment on 2-h ischemia-induced BBB damage and MMP-2 induction. Rats received YC-1 at 24 h and 30 min before the onset of ischemia. Evan’s blue (EB) leakage (red) was seen in the ischemic region of interest 2 (ROI 2) after 2-h ischemia and in situ zymmography showed increased MMP-2 activity in the brain area where EB leakage occurred. Pretreatment with YC-1 significantly prevented MMP-2 induction as well as EB leakage. n = 3/group.
Effect of HIF-1α Inhibition on Colocalization of VEGF With EB Leakage
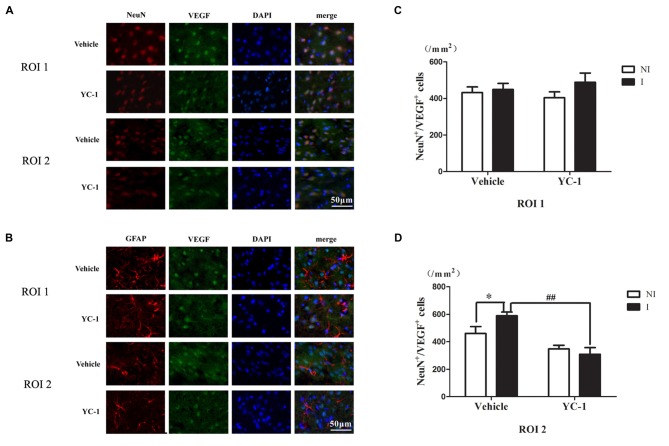
VEGF has been implicated in BBB permeability increase (Schoch et al., 2002; Yan et al., 2011). To check the interaction of HIF-1α and VEGF in 2-h ischemia-induced BBB injury, immunofluoresence was performed to determine the spatial distribution of VEGF and its co-localization with BBB damage. After 2-h ischemia, VEGF (green fluorescence) was seen in the ischemic hemisphere (ROI 2) where EB leakage was observed (red), there is a co-localization of VEGF and EB leakage (Figure 4). YC-1 pretreatment significantly decreased the VEGF as well as decreased the BBB leakage (Figure 4).
Figure 4.
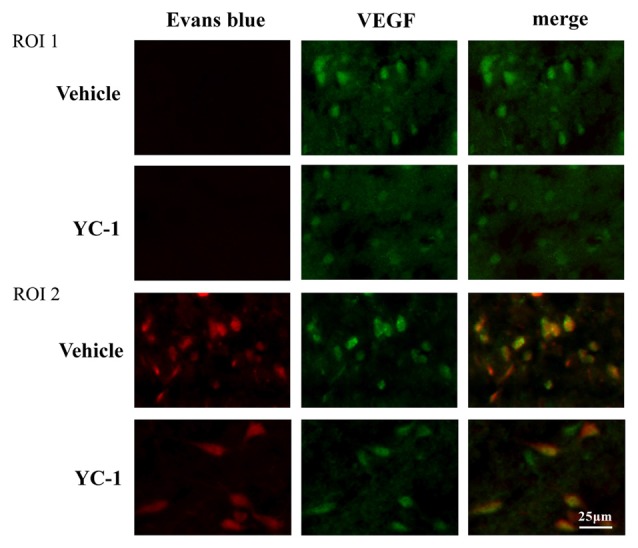
Effect of hypoxia-inducible factor-1 alpha (HIF-1α) inhibition on colocalizaion of vascular endothelial growth factor (VEGF) with EB leakage. Representative fluorescent micrographs of EB leakage (red) and VEGF expression in non-ischemic (NI) and ischemic site (I). Two-hour MCAO induced significant EB leakage (BBB damage) in ROI 2 of ischemic site and inhibition of HIF-1α with YC-1 significantly decreased EB leakage. n = 4.
Colocalization of VEGF With Neurons and Astrocytes
A cell type-specific reaction for HIF-1α within endothelial cells, astrocytes and neurons after 2-h ischemia stroke has been defined (Sun et al., 2017). In addition, it has been reported that there were differential changes of glutathione levels in astrocytes and neurons in ischemic hemisphere (Bragin et al., 2010). However, the cellular distribution of VEGF after acute ischemia stroke was not known. Double-immunostaining was applied to check the cellular distribution of VEGF and the nuclear localization was confirmed by colocalization with DAPI staining. As shown in Figure 5, VEGF was mainly localized in neurons (Figure 5A), but not in astrocytes (Figure 5B) in the ROI 2 after 2-h ischemia. YC-1 significantly downregulated the proportion of VEGF-positive neurons (Figures 5C,D). Therefore, it was neuron that continuously produced VEGF after 2-h ischemia.
Figure 5.
Effect of YC-1 on 2-h ischemia-induced VEGF expression in neurons and astrocytes. The cellular distribution of VEGF was analyzed by immunostaining with NeuN (marker of neurons) or GFAP (marker of astrocytes) after 2-h MCAO. Double immunostain of VEGF (green) and NeuN (red) showed a good colocalization of VEGF and neurons and YC-1 treatment significantly decreased the proportion of VEGF-positive neurons (A). Double immunostain of VEGF (green) and GFAP (red) showed no co-localization of VEGF and astrocytes (B). After quantification, two-way ANNOVA showed an increased proportion of VEGF positive neurons and YC-1 significantly decreased this upregulation (C,D). n = 3/group. Scale bar = 50 μm. n = 3/group. *P < 0.05 compared to the NeuN+/VEGF+ cells in the NI hemisphere. ##P < 0.01 compared to NeuN+/VEGF+ cells in I hemisphere of Vehicle group.
Discussion
The integrity of BBB after acute ischemia stroke is important for determining the efficacy of thrombolysis with tPA (Leigh et al., 2014) or post-endovascular treatment (Leigh et al., 2016). This has motivated us to study early ischemic BBB injury, with an attempt to improve acute stroke management (the critical time window for rescuing ischemic neurons) in the long run and give some clue for future tPA thrombolysis. In our current study, we showed that: (1) ischemia damaged BBB integrity and MMP-2/9 upregulation was colocalized with Rhodamine-dextran leakage. (2) Pretreatment with YC-1, a HIF-1α inhibitor, significantly alleviated 2-h ischemia-induced BBB damage accompanied by inhibition of MMP-2 upregulation. In addition, inhibition of HIF-1α prevented VEGF-induced BBB damage. (3) VEGF was shown to be colocalized with neurons but not astrocytes. Taken together, BBB damage was reduced by inhibition of interaction of HIF-1α with MMP-2 and VEGF during acute cerebral ischemia.
Interaction of HIF-1α with MMP-2 and VEGF has been shown to play a critical role in barrier integrity. For example, pharmacological inhibition of HIF-1α by YC-1 markedly suppressed the expression of HIF-1α, VEGF and MMP-2, mitigated the severity of BBB disruption and attenuated isoflurane-induced cognitive deficits in the Morris water maze task in aged rat model of postoperative cognitive dysfunction (Cao et al., 2018).
MMP-2/9 has been shown to play important role in BBB damage at the stage of reperfusion (Liu and Rosenberg, 2005; Yang et al., 2007; Jin et al., 2013) and at acute ischemia stage (Jin et al., 2012; Wang et al., 2016). Previous study showed that 2-h MCAO induced BBB damage through upregulating HIF-1α which appeared to be mostly induced in the non-infarcted ventral striatum and preoptic area (Sun et al., 2017). Consistent with previous study showing that HIF-1α is implicated in the control of MMP (Jalal et al., 2015), our current study provide evidence that MMP-2 activity was significantly increased in ventral striatum and preoptic area, interaction of HIF-1α with MMP-2 play important role in BBB damage during acute ischemia and inhibition of HIF-1α could reduce BBB damage through regulating MMP-2 activity. Thus, HIF-1α may be a promising target for reducing BBB damage after acute ischemia stroke.
In our current study, we showed that VEGF played critical role in BBB damage during acute ischemia which was consist with previous studies showing that VEGF is involved in BBB permeability increase after ischemia stroke (Schoch et al., 2002; Argaw et al., 2009; Yan et al., 2011). In addition, HIF-1α-VEGF-mediated tight junction dysfunction has been shown in choriocarcinoma cells (Zhang et al., 2017) and HIF-1α-VEGF signaling has been shown to play an important role in BBB protection against ischemia-reperfusion-induced injury (Yeh et al., 2007; Yan et al., 2011). For example, inhibition of HIF-1α and VEGF reduced HT in the ischemic brain which was induced by acute hyperglycemia (Chen et al., 2010; Zhang et al., 2016) and inhibition of HIF-1α-VEGF signaling reduced BBB damage in rat neonatal stroke model (Mu et al., 2003).
Of note, hypoxic Müller cells-secreted VEGF increased MMP-2 activity in endothelial cells (Rodrigues et al., 2013). In addition, early VEGF inhibition attenuated BBB disruption in ischemic rat brains by regulating the expression of MMPs (Zhang et al., 2017) and VEGF-MMP pathway played important role in progesterone’s protective effect on delayed tPA treatment-induced hemorrhagic transformation (Won et al., 2014). Therefore, interaction of HIF-1α with MMP-2 and VEGF play an important role in BBB damage.
Our result that VEGF was colocalized with neurons but not astrocytes is consistent with previous study showing that HIF-1α was co-localized with neurons but not astrocytes or endothelial cells (Sun et al., 2017). Astrocytes have the ability to be resistant to hypoxic condition and severe oxygen deprivation was needed to activate HIF-1α signaling pathway and regulate survival and proliferation in astrocytes (Schmid-Brunclik et al., 2008). Two-h ischemia may not be serious enough for astrocytes to produce VEGF. However, with duration of ischemia was extended, astrocytes played more important role in BBB damage, for example, Li et al. (2014) showed that 3-h ischemia facilitated neurons to activate astrocytes to damage barrier of endothelial cells via increasing expression of VEGF.
Nitric oxide (NO)-mediated many conditions can lead to disruption of BBB integrity, eventually leading to vasogenic edema and secondary brain damage (Gu et al., 2012). One of the major actions of NO is activation of soluble guanylate cyclase (sGC) resulting in generation of cGMP (Lamothe et al., 2004). YC-1, a dual functioning chemical, functions as both an inhibitor of HIF-1α and a direct activator of sGC (Huh et al., 2011). For example, YC-1 has been shown to protect white matter axons from NO toxicity and metabolic stress (Garthwaite et al., 2002). The effect of YC-1 on ischemia-induced BBB damage may be through modulation of NO. However, it has been previously shown that 2-h ischemia induced occludin degradation, but not claudin-5 degradation, although 2-h OGD increased NO, only 4-h OGD induced NO-dependent claudin-5 degradation (Liu et al., 2016). In our current study, 2-h ischemia induced BBB damage and this disruption is independent of NO (Gu et al., 2012), excluding the possibility that YC-1 affect the BBB integrity through modulating NO.
In summary, the findings may give new insights to prevent the BBB from ischemic damage and to extend the time window of tPA treatment or endovascular treatment and alleviate HT.
Author Contributions
The work was performed and accomplished by all authors. YS, JG, ZL, CX, SQ, XZ, BZ, YS and QG contributed to the execution of the entire project and the statistical analyses. YS, JG, XJ and YW wrote the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- BBB
blood brain barrier
- β2-AR
β2-adrenergic receptor
- EB
Evan’s blue dye
- ECA
external carotid artery
- GSH
glutathione
- HIF-1α
hypoxia-inducible factor-1 alpha
- HT
hemorrhagic transformation
- I
ischemic hemisphere
- ICA
internal carotid artery
- MCAO
middle cerebral artery occlusion
- MMP
matrix metalloproteinase
- NI
non-ischemic
- NO
nitric oxide
- ROI
region of interest
- sGC
soluble guanylate cyclase
- tPA
tissue plasminogen activator
- TTC
2,3,5-triphenyltetrazolium chloride
- VEGF
vascular endothelial growth factor.
Footnotes
Funding. This work was supported by grants from Health and Family Planning Commission of Zhejiang Province (grant no. 2017KY653) and Science Technology Department of Zhejiang Province (grant no. 2018C37093). This work was also supported by Natural Science Foundation of Jiangsu Province of China (BK20151225), by Suzhou Science and Technology Plan (SYS201518), by Jiangsu Provincial College of Natural Science research project (17KJB180012). This work was also partly supported by Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
References
- Argaw A. T., Gurfein B. T., Zhang Y., Zameer A., John G. R. (2009). VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. U S A 106, 1977–1982. 10.1073/pnas.0808698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin D. E., Zhou B., Ramamoorthy P., Müller W. S., Connor J. A., Shi H. (2010). Differential changes of glutathione levels in astrocytes and neurons in ischemic brains by two-photon imaging. J. Cereb. Blood Flow Metab. 30, 734–738. 10.1038/jcbfm.2010.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li Z., Li H., Ni C., Li L., Yang N., et al. (2018). Hypoxia-inducible factor-1α is involved in isoflurane-induced blood-brain barrier disruption in aged rats model of POCD. Behav. Brain Res. 339, 39–46. 10.1016/j.bbr.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Chen C., Hu Q., Yan J., Yang X., Shi X., Lei J., et al. (2009). Early inhibition of HIF-1α with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol. Dis. 33, 509–517. 10.1016/j.nbd.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Chen C., Ostrowski R. P., Zhou C., Tang J., Zhang J. H. (2010). Suppression of hypoxia-inducible factor-1α and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J. Neurosci. Res. 88, 2046–2055. 10.1002/jnr.22361 [DOI] [PubMed] [Google Scholar]
- Dewitz C., McEachern E., Shin S., Akong K., Nagle D. G., Broide D. H., et al. (2017). Hypoxia-inducible factor-1α inhibition modulates airway hyperresponsiveness and nitric oxide levels in a BALB/c mouse model of asthma. Clin. Immunol. 176, 94–99. 10.1016/j.clim.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Engelhardt S., Al-Ahmad A. J., Gassmann M., Ogunshola O. O. (2014). Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J. Cell. Physiol. 229, 1096–1105. 10.1002/jcp.24544 [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Goodwin D. A., Neale S., Riddall D., Garthwaite J. (2002). Soluble guanylyl cyclase activator YC-1 protects white matter axons from nitric oxide toxicity and metabolic stress, probably through Na+ channel inhibition. Mol. Pharmacol. 61, 97–104. 10.1124/mol.61.1.97 [DOI] [PubMed] [Google Scholar]
- Gu Y., Zheng G., Xu M., Li Y., Chen X., Zhu W., et al. (2012). Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 120, 147–156. 10.1111/j.1471-4159.2011.07542.x [DOI] [PubMed] [Google Scholar]
- Hacke W., Kaste M., Bluhmki E., Brozman M., Dávalos A., Guidetti D., et al. (2008). Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N. Engl. J. Med. 359, 1317–1329. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- Hafez S., Coucha M., Bruno A., Fagan S. C., Ergul A. (2014). Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl. Stroke Res. 5, 442–453. 10.1007/s12975-014-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J. W., Kim S. Y., Lee J. H., Lee Y. S. (2011). YC-1 attenuates hypoxia-induced pulmonary arterial hypertension in mice. Pulm. Pharmacol. Ther. 24, 638–646. 10.1016/j.pupt.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Jalal F. Y., Yang Y., Thompson J. F., Roitbak T., Rosenberg G. A. (2015). Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J. Cereb. Blood Flow Metab. 35, 1145–1153. 10.1038/jcbfm.2015.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Liu J., Liu W. (2014). Early ischemic blood brain barrier damage: a potential indicator for hemorrhagic transformation following tissue plasminogen activator (tPA) thrombolysis? Curr. Neurovasc. Res. 11, 254–262. 10.2174/1567202611666140530145643 [DOI] [PubMed] [Google Scholar]
- Jin X., Liu J., Liu K. J., Rosenberg G. A., Yang Y., Liu W. (2013). Normobaric hyperoxia combined with minocycline provides greater neuroprotection than either alone in transient focal cerebral ischemia. Exp. Neurol. 240, 9–16. 10.1016/j.expneurol.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Liu J., Yang Y., Liu K. J., Liu W. (2012). Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3 h after ischemia onset. Neurobiol. Dis. 48, 309–316. 10.1016/j.nbd.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Lamothe M., Chang F. J., Balashova N., Shirokov R., Beuve A. (2004). Functional characterization of nitric oxide and YC-1 activation of soluble guanylyl cyclase: structural implication for the YC-1 binding site? Biochemistry 43, 3039–3048. 10.1021/bi0360051 [DOI] [PubMed] [Google Scholar]
- Leigh R., Christensen S., Campbell B. C., Marks M. P., Albers G. W., Lansberg M. G. (2016). Pretreatment blood-brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology 87, 263–269. 10.1212/WNL.0000000000002862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R., Jen S. S., Hillis A. E., Krakauer J. W., Barker P. B. (2014). Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke 45, 2030–2035. 10.1161/STROKEAHA.114.005249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. N., Pan R., Qin X. J., Yang W. L., Qi Z., Liu W., et al. (2014). Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J. Neurochem. 129, 120–129. 10.1111/jnc.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Jin X., Liu K. J., Liu W. (2012). Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. 32, 3044–3057. 10.1523/JNEUROSCI.6409-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu W. C., Sun Y., Shen X., Wang X., Shu H., et al. (2017). Normobaric hyperoxia extends neuro- and vaso-protection of N-acetylcysteine in transient focal ischemia. Mol. Neurobiol. 54, 3418–3427. 10.1007/s12035-016-9932-0 [DOI] [PubMed] [Google Scholar]
- Liu K. J., Rosenberg G. A. (2005). Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic. Biol. Med. 39, 71–80. 10.1016/j.freeradbiomed.2005.03.033 [DOI] [PubMed] [Google Scholar]
- Liu J., Weaver J., Jin X., Zhang Y., Xu J., Liu K. J., et al. (2016). Nitric oxide interacts with caveolin-1 to facilitate autophagy-lysosome-mediated claudin-5 degradation in oxygen-glucose deprivation-treated endothelial cells. Mol. Neurobiol. 53, 5935–5947. 10.1007/s12035-015-9504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D., Jiang X., Sheldon R. A., Fox C. K., Hamrick S. E., Vexler Z. S., et al. (2003). Regulation of hypoxia-inducible factor 1α and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol. Dis. 14, 524–534. 10.1016/j.nbd.2003.08.020 [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Xin X., Jee K., Babapoor-Farrokhran S., Kashiwabuchi F., Ma T., et al. (2013). VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 62, 3863–3873. 10.2337/db13-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria A., Lundberg J. M. (1983). Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J. Neurosci. Methods 8, 41–49. 10.1016/0165-0270(83)90050-x [DOI] [PubMed] [Google Scholar]
- Schmid-Brunclik N., Burgi-Taboada C., Antoniou X., Gassmann M., Ogunshola O. O. (2008). Astrocyte responses to injury: VEGF simultaneously modulates cell death and proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R864–R873. 10.1152/ajpregu.00536.2007 [DOI] [PubMed] [Google Scholar]
- Schoch H. J., Fischer S., Marti H. H. (2002). Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 125, 2549–2557. 10.1093/brain/awf257 [DOI] [PubMed] [Google Scholar]
- Shi Y., Leak R. K., Keep R. F., Chen J. (2016). Translational stroke research on blood-brain barrier damage: challenges, perspectives, and goals. Transl. Stroke Res. 7, 89–92. 10.1007/s12975-016-0447-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H., Zheng G. Q., Wang X., Sun Y., Liu Y., Weaver J. M., et al. (2015). Activation of matrix metalloproteinase in dorsal hippocampus drives improvement in spatial working memory after intra-VTA nicotine infusion in rats. J. Neurochem. 135, 357–367. 10.1111/jnc.13283 [DOI] [PubMed] [Google Scholar]
- Simard J. M., Kent T. A., Chen M., Tarasov K. V., Gerzanich V. (2007). Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 6, 258–268. 10.1016/s1474-4422(07)70055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Chen X., Zhang X., Shen X., Wang M., Wang X., et al. (2017). β2-adrenergic receptor-mediated HIF-1α upregulation mediates blood brain barrier damage in acute cerebral ischemia. Front. Mol. Neurosci. 10:257. 10.3389/fnmol.2017.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu Y., Sun Y., Liu W., Jin X. (2016). Blood brain barrier breakdown was found in non-infarcted area after 2-h MCAO. J. Neurol. Sci. 363, 63–68. 10.1016/j.jns.2016.02.035 [DOI] [PubMed] [Google Scholar]
- Wang X., Xue G. X., Liu W. C., Shu H., Wang M., Sun Y., et al. (2017). Melatonin alleviates lipopolysaccharide-compromised integrity of blood-brain barrier through activating AMP-activated protein kinase in old mice. Aging Cell 16, 414–421. 10.1111/acel.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won S., Lee J. H., Wali B., Stein D. G., Sayeed I. (2014). Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway. J. Cereb. Blood Flow Metab. 34, 72–80. 10.1038/jcbfm.2013.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Zhou B., Taheri S., Shi H. (2011). Differential effects of HIF-1 inhibition by YC-1 on the overall outcome and blood-brain barrier damage in a rat model of ischemic stroke. PLoS One 6:e27798. 10.1371/journal.pone.0027798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Estrada E. Y., Thompson J. F., Liu W., Rosenberg G. A. (2007). Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 27, 697–709. 10.1038/sj.jcbfm.9600375 [DOI] [PubMed] [Google Scholar]
- Yeh W. L., Lu D. Y., Lin C. J., Liou H. C., Fu W. M. (2007). Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1α accumulation and VEGF expression. Mol. Pharmacol. 72, 440–449. 10.1124/mol.107.036418 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yan J., Shi H. (2016). Role of hypoxia inducible factor 1 in hyperglycemia-exacerbated blood-brain barrier disruption in ischemic stroke. Neurobiol. Dis. 95, 82–92. 10.1016/j.nbd.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. T., Zhang P., Gao Y., Li C. L., Wang H. J., Chen L. C., et al. (2017). Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol. Med. Rep. 15, 57–64. 10.3892/mmr.2016.5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhao H. J., Xia X. R., Diao F. Y., Ma X., Wang J., et al. (2017). Hypoxia-induced and HIF1α-VEGF-mediated tight junction dysfunction in choriocarcinoma cells: implications for preeclampsia. Clin. Chim. Acta [Epub ahead of print]. 10.1016/j.cca.2017.12.010 [DOI] [PubMed] [Google Scholar]