Abstract
Objective:
The objective of this study was to describe the epidemiology, clinical features, outcomes, and predictors of mortality in veterans with peripheral artery disease (PAD).
Methods:
We used national data from the Veterans Health Administration from fiscal years 2009 to 2011 to identify patients with a new diagnosis of PAD. Within this cohort, we describe characteristics of the patients, use of recommended medications, and clinical outcomes during a 3-year follow-up (fiscal year 2014). We used Cox proportional hazards regression to examine predictors of mortality and adverse limb outcomes (amputation and hospitalization for critical limb ischemia [CLI]) during follow-up.
Results:
A total of 175,865 patients with a new diagnosis of PAD were included. The mean age was 69.9 years; 97.8% were male, and 67.7% were white. Nearly 77% of patients had hypertension, 46.5% had diabetes, 23% had chronic obstructive pulmonary disease, and 12.9% had renal failure. A prescription for statins was filled by 60.8%, and 34.9% received high-intensity statins within 90 days of PAD diagnosis. At 1 year, 2.6% underwent revascularization, 1.3% developed CLI, and 1.1% underwent amputation. During a median follow-up of 3.8 years, a total of 28.6% patients died (6.7% at 1 year), and 3.7% developed a limb outcome (2.0% at 1 year). Predictors of mortality included advanced age, comorbidities, and CLI or amputation at presentation. In contrast, prescription with statins was associated with lower mortality. Similar findings were present with regard to predictors of adverse limb outcomes, except that older age was associated with a lower risk of amputation or CLI.
Conclusions:
We found that veterans with PAD have a high prevalence of comorbid conditions and have a significant risk of mortality and limb loss. A substantial proportion of veterans with PAD are not prescribed recommended medications, especially statin therapy. Our data highlight important opportunities for improving care of veterans with PAD. (J Vasc Surg 2018; 68:527–35.)
Lower extremity peripheral artery disease (PAD) is common, affecting >8 million adults in the United States and >200 million people worldwide.1 Although many PAD patients may be asymptomatic, PAD often is mani-fested as intermittent claudication, atypical leg pain, or impaired mobility. 2, 3 Some patients may develop advanced disease leading to critical limb ischemia (CLI), a dreaded complication that requires emergent revascularization and may lead to amputation.4 In addition to its impact on limb-related events, PAD is also associated with an increased risk of cardiovascular events, such as acute myocardial infarction, stroke, and death.5
The prevalence of PAD risk factors, which include hypertension, diabetes mellitus, and smoking, in the veteran population exceeds national estimates. For example, nearly one in three veterans has hypertension, one in four has diabetes, and one in four is a current smoker, placing veterans at a significantly increased risk of PAD.6–8 Yet, data regarding the epidemiology of PAD in veterans and the associated clinical characteristics, risk factor management, and long-term outcomes remain limited.
To address this knowledge gap, we conducted a nationwide study of PAD among veterans in the United States. We used national Veterans Health Administration (VHA) data to identify veterans with incident PAD and to describe the associated clinical characteristics, comorbidities, use of guideline-recommended medical therapy, revascularization treatment, and long-term clinical outcomes (mortality and hospitalization for CLI or amputation).9–11 We also examined clinical predictors of long-term morality and adverse limb outcomes (ie, hospitalization for CLI and amputation).
METHODS
Data sources.
Our study was based on the national administrative VHA data, which include the VHA Patient Treatment and the Outpatient Care files. The Patient Treatment File is a national administrative database of all patients hospitalized in Veterans Affairs (VA) centers across the United States. Key data elements include de-mographics of patients, admission dates, primary diagnosis and secondary diagnosis, procedures performed during hospitalization (identified by International Classification of Diseases, Ninth Revision [ICD-9] diagnosis codes), and discharge disposition. Given that most PAD patients may be diagnosed in the ambulatory setting, we also used the Outpatient Care File, which includes all outpatient encounters at VA clinics. Data elements in the Outpatient Care File include visit date, clinic type, diagnoses, procedures performed using Current Procedural Terminology (CPT) codes, and laboratory values. Records in the Patient Treatment File and the Outpatient Care File are associated with unique patient identifiers (scrambled social security numbers) that were used to merge records for the same patient across the two data sources. For information about prescription medications filled by VA pharmacies, we used the VA National Data Extract pharmacy data. 12 Specific medications were identified within the pharmacy database using VA drug description variables located within that data set. Patients were assumed to be receiving a particular medication at baseline if they filled a prescription for that drug within 90 days of PAD diagnosis. Non-VA formulary drugs and over-the-counter medications were not recorded in our data set, including the antiplatelet aspirin. Medications not filled at VA pharmacies were not included in this data set, including medications prescribed by VA and non-VA providers filled at non-VA pharmacies. Dates of death for patients who died were obtained from the VA Vital Status File, which includes information from multiple VA and non-VA sources including VA Beneficiary Death File, VA Medicare Status File, and Social Security Administration Death Master File. Our study design and use of veteran data were reviewed and approved by the University of Iowa Institutional Review Board and the Iowa City VA Research and Development Committee, which waived the requirement of informed consent.
Study population.
The development of our study cohort is shown in the Fig. We used the national VHA data for fiscal years 2009 to 2011 to identify all adult patients with at least two outpatient or one inpatient encounter for PAD. PAD was identified using a combination of ICD-9 diagnosis codes and CPT procedure codes, as outlined in Supplementary Tables I and II (online only), based on previous methodology.13–15 We restricted this cohort to patients without a similar PAD diagnosis in the previous 12-month period to focus our study on patients with a new diagnosis of PAD, patients who were at least 45 years or old, and patients with at least one primary care encounter during the year before PAD diagnosis to ensure that only patients who are regular VHA users were included. Thus, a total of 175,865 patients were included in our final cohort. Patients were followed up until the end of fiscal year 2014 (last follow-up, September 30, 2014).
Fig.
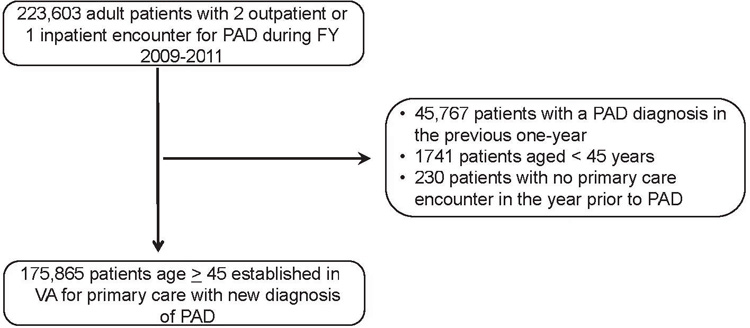
Derivation of the study cohort. FY, Fiscal year; PAD, peripheral artery disease; VA, Veterans Affairs.
As outlined in Supplementary Tables I and II (online only), the ICD-9 and CPT codes represent a range of PAD manifestations. Therefore, patients were further classified into the following categories by their first presenting PAD diagnosis: CLI or amputation, with associated PAD diagnosis; lower extremity revascularization (stenting or bypass); and stable PAD—absence of CLI, amputation, or revascularization. Patients in the stable PAD group are likely to be asymptomatic or to have stable claudication, which would correspond to Rutherford class 0, 1, or 2.16 However, we chose to not use the Rutherford classification because of lack of information on symptoms. Amputation and disarticulation codes were considered PAD equivalents only if a corresponding ICD-9 code for PAD was present to exclude patients with amputation or débridement due to other causes (eg, trauma, infection, neuropathy).
Study variables.
Demographic characteristics included age, sex, race (black, white, other), and median income, which was available at the ZIP code level. The patients’ comorbidities were determined using algorithms originally developed by Elixhauser and updated by Quan et al17; these included atherosclerosis, coronary artery disease, acute myocardial infarction, congestive heart failure, valvular heart disease, arrhythmias, cere-brovascular disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic renal disease, dialysis, chronic liver disease, cancer, and major psychiatric disorder. We also examined baseline laboratory values for lipids and hemoglobin A1c within 365 days before the initial qualifying PAD diagnosis. Lipid values included total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (LDL-C), and are expressed as milligrams per deciliter. Hemoglobin A1c, expressed as percentage of total hemoglobin, was evaluated among patients with diabetes. We also evaluated use of medications recommended by clinical practice guidelines for PAD patients. Medication use was assessed as filling a prescription within 90 days of initial diagnosis of PAD. The list of medications for each drug class was based on the VA formulary, as follows: statins—pravastatin, fluvastatin, simvastatin, atorvastatin, rosuvastatin, pitavastatin, and cerivastatin; antiplatelet agents—clopidogrel, ticagrelor, prasugrel, ticlopidine, and dipyridamole; use of aspirin was not examined because aspirin is commonly filled over the counter and therefore not recorded in the pharmacy database; medications for claudication symptoms—cilostazol, a phosphodiesterase inhibitor, and pentoxifylline, a rheologic agent; and smoking cessation (varenicline and nicotine patches or gums). Although bupropion, an antidepressant, is also commonly prescribed for smoking cessation, its use was not examined because of the difficulty in ascribing whether it was used for smoking cessation or for treatment of depression. Given that recent guidelines from the American College of Cardiology and the American Heart Association emphasize high-intensity statin therapy in patients with PAD, we also examined rates of high-intensity statin therapy in our study cohort. High-intensity statin therapy included simvastatin 80 mg, atorvastatin 40 to 80 mg, and rosuvastatin 20 to 40 mg.
Study outcomes.
Our primary outcome was all-cause mortality, which was determined using the Vital Status File and available for 100% (175,865) of our cohort. We also examined hospitalization for CLI or amputation as a secondary outcome.
Statistical analysis.
We used descriptive statistics for demographics, comorbidities, baseline values of lipid and hemoglobin A1c testing, and use of guideline recommended medical therapy. Next, we examined 1-year rates of our primary and secondary outcomes in the overall population as well as within prespecified subgroups based on age (45–64, 65–74, 75–84, and ≥85 years), race (black vs white), and presence of diabetes mellitus. Next, we used Cox proportional hazards regression modeling to determine independent predictors of mortality in our cohort. The outcome variable was time to death, starting from the date of first diagnosis of PAD. Candidate variables were demographics (age, race), comorbidities (coronary atherosclerosis, myocardial infarction, congestive heart failure, valve disease, arrhythmia, cerebrovascular disease, hypertension, COPD, diabetes mellitus, renal disease, dialysis, liver disease), initial presentation of PAD (stable PAD, CLI or amputation, and revascularization procedure), and statin use (no statin, low to moderate intensity, high intensity). To account for nonindependence of outcomes within sites (ie, clustering), we used the robust sandwich estimator of Lin and Wei18 and a working correlation matrix. We repeated these analyses for our secondary outcome of hospitalization for CLI or amputation.
All hypothesis testing was conducted with a two-sided test, and P value <.05 was used to denote statistical significance. Adjusted analyses using Cox models were not conducted for our secondary outcomes because of their low incidence during the study period.
Sensitivity analyses.
To address the issue of dual care (ie, missing information regarding clinical outcomes among veteran patients because of care that was obtained outside the VA), we repeated our analyses after restricting our cohort to patients who were eligible for Medicare, aged ≥66 years to allow for a 1-year look-back period to exclude patients with pre-existing PAD. Patients with at least one inpatient or two outpatient encounters with an ICD-9 or CPT code for PAD were identified similar to the main cohort (n = 102,708 patients). Within this cohort, we examined 1-year rates of clinical outcomes using claims in both the VA and Medicare files to determine what proportion of the outcomes were missed by the VA data and detected only using Medicare claims data.
RESULTS
The mean age was 69.9 years; 97.8% were male, and 67.7% were white. Black veterans and other races composed 13.7% and 6.4% of our study, respectively. A majority (96%) of the cohort had stable PAD (no history of CLI or amputation or revascularization at baseline). Of the remaining, 2.3% had CLI or amputation and 1.7% had revascularization without CLI or amputation as their initial PAD qualifying event (Table I). The prevalence of cardiovascular and noncardiovascular comorbidities was high in this cohort; 77.2% of patients had hypertension, 13.6% had cerebrovascular disease, and 11.8% had congestive heart failure. Likewise, diabetes mellitus (46.5%), COPD (23%), and chronic renal failure (12.9%) were also highly prevalent in this cohort (Table I).
Table I.
Characteristics of study cohort
| Demographics | N = 175,865 |
|---|---|
| Age, years | 69.9 (10.6) |
| 45–64 | 66,845 (38.0) |
| 65–74 | 45,288 (25.8) |
| 75–84 | 45,152 (25.7) |
| ≥85 | 18,580 (10.6) |
| Male sex | 171,930 (97.8) |
| Race | |
| Black | 24,043 (13.7) |
| White | 119,008 (67.7) |
| Other | 11,159 (6.4) |
| Unknown | 21,665 (12.3) |
| Qualifying diagnosis of PAD | |
| CLI or amputation | 4074 (2.3) |
| Lower extremity revascularization with no CLI or amputation |
3039 (1.7) |
| Stable PAD | 168,752 (96.0) |
| Cardiovascular comorbidities | |
| Coronary atherosclerosis | 12,142 (6.9) |
| History of AMI | 7169 (4.1) |
| CHF | 20,766 (11.8) |
| Valvular heart disease | 8443 (4.8) |
| Arrhythmias | 29,823 (17.0) |
| Cerebrovascular disease | 23,830 (13.6) |
| Hypertension | 135,727 (77.2) |
| Non-CV comorbidities | |
| Diabetes mellitus | 81,850 (46.5) |
| COPD | 40,395 (23.0) |
| Chronic renal failure | 22,628 (12.9) |
| Dialysis | 1037 (0.6) |
| Chronic liver disease | 6014 (3.4) |
| Cancer | 20,456 (11.6) |
| Major psychiatric disorder | 6667 (3.8) |
AMI, Acute myocardial infarction; CHF, congestive heart failure; CLI, critical limb ischemia; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; PAD, peripheral artery disease.
Categorical variables are presented as number (%). Continuous vari-ables are presented as mean ± standard deviation.
Use of guideline-recommended medications and laboratory values are presented in Table II. Approximately 60.8% of patients in our cohort had filled a prescription for a statin within 90 days of PAD diagnosis, and 34.9% had received high-intensity statin. Approximately 17% of patients were receiving an antiplatelet drug other than aspirin. Use of cilostazol and pentoxifylline was low at 4.1%. Smoking cessation therapy with varenicline (0.8%) and nicotine replacement therapy (6.6%) was 7.2% overall. Lipid values at baseline were available for 86% of the patients in our cohort (Table II). Median LDL-C was 87 mg/dL (interquartile range [IQR], 69–111 mg/dL), median nonhigh-density lipoprotein cholesterol was 39 mg/dL (IQR, 32–48 mg/dL), and total cholesterol was 158 mg/dL (IQR, 135–186 mg/dL). The proportion of patients with LDL-C >100 mg/dL was 35.4%. Among diabetics (n = 81,850), hemoglobin A1c values at baseline were available in 75,437 (92%) of our cohort. Median hemoglobin A1c value in diabetics was 7.1 (IQR, 6.4–8.2) at baseline.
Table II.
Medication use and laboratory values
| Medication class | No. (%) |
|---|---|
|
Nonaspirin antiplatelets |
30,120 (17.1) |
| Any statins | 106,949 (60.8) |
| High-intensity statin | 61,371 (34.9) |
| Any claudication drugs | 7168 (4.1) |
| Pentoxifylline | 2250 (1.3) |
| Cilostazol | 5054 (2.9) |
| Smoking cessation therapy | 12,723 (7.2) |
| Varenicline | 1341 (0.8) |
| Nicotine patches or gums |
11,592 (6.6) |
| Laboratory values | Median (IQR) |
|
Total cholesterol |
158 (135–186) |
| LDL cholesterol | 87.5 (69–111) |
| Non-HDL cholesterol | 39 (32.2–48) |
| Hemoglobin A1c in diabetics | 7.1 (6.4–8.2) |
HDL, High-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein.
High-intensity statin therapy was defined as simvastatin 80 mg, ator-vastatin 40 to 80 mg, or rosuvastatin 20 to 40 mg. The sample size for data regarding laboratory values was as follows: total cholesterol, 151,641 patients; LDL cholesterol, 150,896 patients; non-HDL cholesterol, 151,023 patients; and hemoglobin A1c in diabetics, 75,437 patients.
Unadjusted outcomes at 1 year are reported overall and within our predefined subgroups in Table III One-year rate of mortality was 6.7%, lower extremity revascularization was 2.6%, hospitalization for CLI was 1.3%, and amputation was 1.1%. In patients who developed CLI at 1 year, 52.8% underwent revascularization, either percutaneous or surgical, and 41.0% underwent amputation during the same hospitalization. Nearly all of the patients had codes that suggested tissue loss (eg, ulcer or gangrene; data not shown). Risk of 1-year mortality was higher in older patients, among men, and in patients with diabetes (P < .001 for all). In contrast, advanced age was associated with lower likelihood of revascularization, hospitalization for CLI, and amputation. The incidence of hospital admission for CLI was higher in diabetics compared with nondiabetics (1.5% vs 1.1%) and blacks compared with whites (2.2% vs 1.2%; P < .001 for all). Likewise, the incidence of amputation was also higher among blacks and diabetics (Table III).
Table III.
Primary and secondary outcomes at 1 year
| Outcomes at 1 year after PAD diagnosis, No. (%) |
||||
|---|---|---|---|---|
|
Mortality |
Surgical and percutaneous revascularization |
CLI |
Amputation or disarticulation | |
|
Overall population |
11,767 (6.7) |
4598 (2.6) |
2297 (1.3) |
1985 (1.1) |
| Age, years | ||||
| 45–64 | 2613 (3.9) | 2736 (4.1) | 1135 (1.7) | 1096 (1.6) |
| 65–74 | 2480 (5.5) | 1215 (2.7) | 575 (1.3) | 468 (1.0) |
| 75–84 | 3925 (8.7) | 530 (1.2) | 429 (1.0) | 340 (0.8) |
| 85+ | 2749 (14.8) | 117 (0.6) | 158 (0.9) | 81 (0.4) |
| P value | <.001 | <.001 | <.001 | <.001 |
| Race | ||||
| Black | 1435 (6.0) | 681 (2.8) | 538 (2.2) | 500 (2.1) |
| White | 8313 (7.0) | 3285 (2.8) | 1416 (1.2) | 1167 (1.0) |
| Other | 696 (6.2) | 211 (1.9) | 151 (1.4) | 157 (1.4) |
| Unknown | 1323 (6.1) | 421 (1.9) | 192 (0.9) | 161 (0.7) |
| P value | <.001 | <.001 | <.001 | <.001 |
| Diabetic | ||||
| Diabetic | 5824 (7.1) | 1707 (2.1) | 1240 (1.5) | 1561 (1.9) |
| Nondiabetic | 5943 (6.3) | 2891 (3.1) | 1057 (1.1) | 424 (0.5) |
| P value | <.001 | <.001 | <.001 | <.001 |
CLI, Critical limb ischemia; PAD, peripheral artery disease.
The median follow-up was 3.8 years, during which 28.6% of patients died. During the period of follow-up, we found a number of strong predictors of mortality in risk-adjusted analyses (Table IV). Risk of mortality was higher in older patients but lower in patients of black or other races. Likewise, mortality was higher in patients with congestive heart failure, COPD, renal disease, dialysis, and liver disease (Table IV). Initial presentation of PAD with CLI or amputation (hazard ratio, 1.58; 95% confidence interval, 1.50–1.66) and revascularization (hazard ratio, 1.36; 95% confidence interval, 1.28–1.45), which are likely to represent more severe manifestations of PAD, was also associated with higher mortality. In contrast, statins, especially high-intensity statins, were associated with a modest reduction in long-term mortality (Table IV).
Table IV.
Predictors of mortality from multivariable Cox regression models
| Hazard ratio | 95% CI | |
|---|---|---|
|
Demographics |
||
| Age, years | ||
| 65–74 | 1.45 | 1.42–1.50 |
| 75–84 | 2.48 | 2.42–2.55 |
| 85+ | 4.41 | 4.29–4.53 |
| Race | ||
| White | Reference | |
| Black | 0.85 | 0.83–0.88 |
| Other | 0.82 | 0.79–0.86 |
| Unknown | 0.93 | 0.91–0.96 |
| Comorbidities | ||
| Atherosclerosis | 0.87 | 0.82–0.91 |
| Myocardial infarction | 1.19 | 1.12–1.27 |
| CHF | 1.74 | 1.70–1.79 |
| Valve disease | 1.06 | 1.02–1.09 |
| Arrhythmia | 1.15 | 1.13–1.18 |
| Cerebrovascular disease | 1.17 | 1.14–1.20 |
| Hypertension | 0.90 | 0.88–0.92 |
| COPD | 1.42 | 1.39–1.45 |
| DM | 1.22 | 1.19–1.24 |
| Renal disease | 1.56 | 1.52–1.59 |
| Dialysis | 1.67 | 1.53–1.81 |
| Liver disease | 1.67 | 1.60–1.75 |
| Cancer | 1.33 | 1.30–1.36 |
| Psychiatric illness | 1.35 | 1.30–1.41 |
| Diagnosis of PAD | ||
| Stable PAD | Reference | |
| CLI diagnosis or amputation | 1.58 | 1.50–1.66 |
| Revascularization in absence of CLI or amputation |
1.36 |
1.28–1.45 |
| Statins | ||
| None | Reference | |
| Statin, low-moderate intensity | 0.87 | 0.85–0.88 |
| Statin, high intensity | 0.82 | 0.80–0.84 |
CHF, Congestive heart failure; CI, confidence interval; CLI, critical limb ischemia; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PAD, peripheral artery disease.
In analyses of predictors of hospitalization for CLI or amputation, we found that presence of CLI or amputation at baseline, diabetes mellitus, dialysis, history of myocardial infarction, and black race were among the strongest predictors of adverse limb-related outcomes (Table V). However, in contrast to mortality, we found that age had an inverse association with an adverse limb outcome in our cohort.
Table V.
Predictors of adverse limb outcomes from multivariable Cox regression models
| Hazard ratio | 95% CI | |
|---|---|---|
|
Demographics |
||
| Age, years | ||
| 65–74 | 0.66 | 0.62–0.70 |
| 75–84 | 0.43 | 0.40–0.46 |
| 85+ | 0.32 | 0.29–0.37 |
| Race | ||
| White | Reference | |
| Black | 1.35 | 1.27–1.44 |
| Other | 1.01 | 0.92–1.12 |
| Unknown | 0.80 | 0.73–0.87 |
| Comorbidities | ||
| Atherosclerosis | 0.76 | 0.65–0.89 |
| Myocardial infarction | 1.40 | 1.17–1.68 |
| CHF | 1.26 | 1.17–1.35 |
| Valve disease | 0.95 | 0.84–1.07 |
| Arrhythmia | 1.07 | 1.00–1.15 |
| Cerebrovascular disease | 1.14 | 1.06–1.22 |
| Hypertension | 1.06 | 0.99–1.13 |
| COPD | 0.88 | 0.82–0.93 |
| DM | 1.93 | 1.83–2.03 |
| Renal disease | 1.51 | 1.41–1.61 |
| Dialysis | 1.83 | 1.53–2.19 |
| Liver disease | 1.04 | 0.93–1.17 |
| Cancer | 0.97 | 0.89–1.05 |
| Psychiatric illness | 0.93 | 0.83–1.05 |
| Diagnosis of PAD | ||
| Stable PAD | Reference | |
| CLI diagnosis or amputation | 6.28 | 5.82–6.76 |
| Revascularization in absence of CLI or amputation |
1.30 | 1.10–1.53 |
| Statins | ||
| None | Reference | |
| Statin, low-moderate intensity | 1.26 | 1.19–1.34 |
| Statin, high intensity | 1.02 | 0.96–1.08 |
CHF, Congestive heart failure; CI, confidence interval; CLI, critical limb ischemia; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PAD, peripheral artery disease.
In sensitivity analyses, we examined the extent to which event rates for our study outcomes were influenced by use of care outside the VHA. Within the cohort of 102,708 patients aged 66 years or older that also had available Medicare claims data, we found 329 (3.6%) additional deaths, 203 (10.9%) additional revascularization events, 26 (3.1%) additional amputations, and 186 (14.7%) additional hospitalizations for CLI that were not captured using VA data alone (Supplementary Table III, online only).
DISCUSSION
In this study of patients with incident PAD within the VHA, there were a number of important findings. First, the prevalence of comorbidities, especially hypertension, diabetes, congestive heart failure, and renal failure, was high among veterans with PAD. Second, despite strong evidence to support a benefit, nearly 40% of veterans with PAD were not treated with a statin. Third, only a small proportion of PAD patients (<3%) underwent revascularization, by either surgical or percutaneous methods, at 1 year. Fourth, risk of mortality was substantial; nearly 30% of PAD patients died within 3.8 years of diagnosis. Advanced age, comorbidities, and initial diagnosis with CLI were associated with increased risk of mortality, whereas prescription with statins was associated with decreased mortality. A number of our findings are important and merit further discussion.
To our knowledge, this is the first systematic examination of the epidemiology and clinical outcomes of PAD among veterans. Compared with other non-VA studies, patients in our PAD cohort were older (mean age, 70 years), predominantly white (68%), and male (98%) patients, which reflects the general demographics of the veteran population.19–21 The high burden of cardiovascular and noncardiovascular comorbidities, such as hypertension, congestive heart failure, renal failure, and COPD, reported in our study is consistent with prior studies, except for a markedly high prevalence of diabetes mellitus in our cohort. We found that nearly one in two (46%) veterans with PAD had diabetes mellitus, which is much higher than previously reported. Whether this represents greater attention to vascular disease screening among veterans or increased disease burden within the population cannot be determined from this study. Likewise, hypertension, renal failure, and congestive heart failure were present at higher rates than previously observed for PAD populations and may support a higher disease burden within the veteran population, or it may simply reflect the shared causes in the development of these comorbid conditions.2, 25, 20, 22 A high burden of comorbidities, especially diabetes and renal failure, could lead to accelerated progression of PAD to CLI and amputation. In our study, 1.3% of our cohort developed CLI at 1 year, and this increased to 2.5% at a median follow-up of 3.8 years, which is much higher compared with a Medicare study, in which the mean annual incidence of CLI was 0.35%.20 Future studies need to determine the relative contribution of comorbidities, use of risk reduction therapy, revascularization, and specialty vascular care in explaining these differences between veteran and nonveteran populations.
Previous studies have reported suboptimal rates of medical therapy and risk factor modification in PAD patients. Using data from the National Health and Nutrition Examination Survey (NHANES) for 1999 to 2004, Pande et al found low rates of therapy with statins (30.5%) and aspirin (35.8%).23–25 Although rates of statin use in our study (60.8%) are somewhat higher, it is possible that use of these medications was overestimated in our study because a prescription fill may not equate with medication use or compliance. Moreover, only one in three veterans was prescribed high-intensity therapy. Although use of aspirin could not be ascertained, use of smoking cessation therapy was low, highlighting other opportunities to improve risk factor modification within this cohort. Given the strong recommendation of statin therapy (class I), especially high-intensity statins, these data suggest persistent gaps in quality of medical care of veterans with PAD. It is possible that an overall low rate of high-intensity statins among veterans is due to the fact that in contrast to national guidelines, the VHA guidelines do not support high-intensity statin therapy, citing insufficient evidence.26 Moreover, national guidelines recommending high-intensity statin therapy in patients with established atherosclerosis were not published until November 2013 and therefore may not have had an opportunity to influence medical practice in our study cohort, which was assembled during 2009 to 2011.
Despite guideline recommendations, use of cilostazol was also low at 2.9%, a finding that is consistent with non-VA studies.27 Factors that may underlie the low use of cilostazol include concerns for adverse effects due to a high prevalence of comorbid conditions, such as heart failure. Moreover, cilostazol is a nonformulary medication in the VHA, and therefore the inconvenience of prescribing may be an additional hurdle. Collectively, our findings highlight that numerous opportunities to improve medical therapy and risk factor modification among veterans with PAD exist. Future studies need to determine the specific factors that explain whether the low rate of medication use in this population is due to missed opportunities, compliance of the patients, reported intolerance to therapy, or prescription from a non-VA pharmacy.
We also found a low overall rate of surgical or endovascular revascularization at 1 year (<3% of patients in our cohort). An appropriate rate of revascularization in newly diagnosed PAD patients is difficult to determine as information on clinical symptoms, a key determinant of revascularization in PAD patients, was not available in our study. Moreover, revascularization decisions are also dependent on anatomic complexity, location and extent of disease, and quality of distal vessels, which were also not available in our study. Although non-VA studies suggest an exponential increase in the use of revascularization, especially endovascular stenting, in PAD patients, raising concerns for overutilization, low use of revascularization in this study may suggest underutilization.28 Future studies are necessary to understand the impact of factors such as availability of vascular services that may be associated with use of revascularization for PAD among veterans.
Within our cohort, nearly 7% of PAD patients died within 1 year of PAD diagnosis, and this number increased to 28.6% during a median follow-up of 3.8 years. As expected, older age was a significant predictor of mortality. Consistent with prior studies, risk of mortality was higher in patients who were older, patients with comorbidities (such as congestive heart failure, renal failure, and dialysis), and patients in whom the initial presentation of PAD was CLI or amputation.22, 29 However, black race was associated with lower mortality in our study. Whereas these findings are in sharp contrast to a number of non-VA studies that have consistently shown worse clinical outcomes in black patients compared with white patients for a range of cardiovascular conditions, they are not altogether surprising because similar findings have been noted in previous studies among veterans.30 For example, Jha et al31 found that black patients admitted to the VA had lower mortality compared with white patients for a range of common medical conditions. The authors surmised that absence of survival disadvantage among black patients might be due to equal opportunities for access to health care and the quality of inpatient treatment. Finally, use of statins, especially high-intensity statins, was associated with lower risk of mortality, and these findings mirrored a recent study that also found use of statins to be associated with an 18% lower hazard of adverse cardiovascular and limb-related outcomes in PAD patients.32 Improving prescription of and adherence to statins at recommended dosing and other guideline recommended medications, such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, could potentially lower the risk of mortality among veterans with PAD.
In contrast to mortality, we found that older age was associated with lower likelihood of revascularization and complications of PAD, such as CLI or amputation. Reduced mobility among older adults may be associated with lower symptom burden and therefore lower revascularization rates. Moreover, presence of comorbidities and perceived risk of complications may lead to a preference against use of invasive procedures. Finally, lower risk of CLI and amputations in older adults may suggest “survivor” bias, that is, patients who developed PAD later in life may have a less severe form of PAD compared with patients who develop it at an earlier age.
Our study findings should be interpreted in light of the following limitations. First, diagnosis of PAD in our study was based on the use of ICD-9 and CPT codes. Although we used an approach that was modeled on previous studies of PAD using administrative data, it is contingent on documentation of PAD during clinical encounters. Moreover, given that the sensitivity and specificity of identifying PAD using ICD-9 or CPT codes in the VHA have not been evaluated, it is difficult to determine to what extent patients with other vascular diseases (eg, aneurysms, mesenteric ischemia, embolism, and venous disorders without atherosclerotic lower extremity vascular disease) are included in our study.13,33 Future work should focus on using more innovative approaches to identify PAD patients, such as values of ankle-brachial index or arterial ultrasound findings extracted from electronic medical records, as they could yield greater disease specificity and classify disease severity. Second, although our study was focused on patients with newly diagnosed PAD, it is possible that some patients had pre-existing PAD and were seen outside the VHA. Third, information about clinical symptoms and their severity, key determinants of treatment intensity, was not available in this study. Fourth, medication use was defined as filling a prescription for a particular drug within 90 days, which may not equate with medication use. True compliance with therapy may be even lower. Alternatively, patients filling a prescription outside the VHA would not be captured. Fifth, it is possible that clinical outcomes, especially revascularization, amputation, and CLI, are lower in this study, as veterans may seek vascular care outside the VHA if they have commercial medical insurance (dual care). In sensitivity analyses within a sub-group of patients aged >66 years who were also eligible for Medicare, we found that the additional yield from use of Medicare data was low for mortality and amputations but exceeded 10% for revascularization (10.9%) and CLI (15%). Although these rates are not trivial, they are substantially smaller than reported for other conditions, such as receipt of colonoscopy.34 Finally, our study cohort was composed of nearly 97.8% male patients, and therefore extrapolation to women with PAD should be done with caution.
CONCLUSIONS
We found that veterans with PAD have a high prevalence of comorbid conditions and have a significant risk of mortality and adverse limb outcomes. A substantial proportion of PAD patients are not receiving therapy with statins, and use of revascularization is generally low. Our findings highlight several opportunities for improving care in veterans with PAD.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective analysis of prospectively collected data from the Veterans Health Administration database
Take Home Message: Among 175,865 veterans with peripheral artery disease with a mean age of 69.9 years, 46.5% had diabetes and 12.9% had renal failure; 28.6% died at a mean of 3.8 years. Age, comorbidities, critical limb ischemia, or amputation predicted death. Statins were prescribed to only 60.8%.
Recommendation: The authors recommend that all veterans with peripheral artery disease receive medical treatment and risk factor modifications to reduce the risk of amputation and cardiovascular death.
Acknowledgments
S.G. is supported by a career development award (K08HL122527) from the National Heart, Lung, and Blood Institute. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res 2015;116:1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res 2015;116:1527–39. [DOI] [PubMed] [Google Scholar]
- 5.Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009;120:2053–61. [DOI] [PubMed] [Google Scholar]
- 6.Brown DW. Smoking prevalence among US veterans. J Gen Intern Med 2010;25:147–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazis LE, Ren XS, Lee A, Skinner K, Rogers W, Clark J, et al. Health status in VA patients: results from the Veterans Health Study. Am J Med Qual 1999;14:28–38. [DOI] [PubMed] [Google Scholar]
- 8.Selim AJ, Berlowitz DR, Fincke G, Cong Z, Rogers W, Haffer SC, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc 2004;52:1271–6. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127: 1425–43. [DOI] [PubMed] [Google Scholar]
- 10.Moyer VA; U.S. Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;159:342–8. [DOI] [PubMed] [Google Scholar]
- 11.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;58:2020–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan-Sarrazin MS, Mazur A, Chrischilles E, Cram P. Trends in the pharmacologic management of atrial fibrillation: data from the Veterans Affairs health system. Am Heart J 2014;168:53–9.e1. [DOI] [PubMed] [Google Scholar]
- 13.Goodney PP, Travis LL, Nallamothu BK, Holman K, Suckow B, Henke PK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Car- diovasc Qual Outcomes 2012;5:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol 2012;60:2230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunis SR, Bass EB, Steinberg EP. The use of angioplasty, bypass surgery, and amputation in the management of peripheral vascular disease. N Engl J Med 1991;325: 556–62. [DOI] [PubMed] [Google Scholar]
- 16.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517–38. [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ. The robust inference for the cox proportional hazards model. J Am Stat Assoc 1989;84:1074–8. [Google Scholar]
- 19.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71:510–5. [DOI] [PubMed] [Google Scholar]
- 20.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014;60:686–95.e2. [DOI] [PubMed] [Google Scholar]
- 21.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999– 2000. Circulation 2004;110:738–43. [DOI] [PubMed] [Google Scholar]
- 22.Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Dis 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonaca MP, Creager MA. Pharmacological treatment and current management of peripheral artery disease. Circ Res 2015;116:1579–98. [DOI] [PubMed] [Google Scholar]
- 24.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subherwal S, Patel MR, Kober L, Peterson ED, Jones WS, Gislason GH, et al. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation 2012;126:1345–54. [DOI] [PubMed] [Google Scholar]
- 26.Downs JR, O’Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U. S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med 2015;163: 291–7. [DOI] [PubMed] [Google Scholar]
- 27.Neel JD, Kruse RL, Dombrovskiy VY, Vogel TR. Cilostazol and freedom from amputation after lower extremity revascularization. J Vasc Surg 2015;61:960–4. [DOI] [PubMed] [Google Scholar]
- 28.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovas- cular interventions, and major amputations. J Vasc Surg 2009;50:54–60. [DOI] [PubMed] [Google Scholar]
- 29.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in pa- tients with peripheral arterial disease. N Engl J Med 1992;326: 381–6. [DOI] [PubMed] [Google Scholar]
- 30.Brothers TE, Zhang J, Mauldin PD, Tonnessen BH, Robison JG, Vallabhaneni R, et al. Better survival for African and Hispanic/Latino Americans after infrainguinal revascularization in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg 2017;65:1062–73. [DOI] [PubMed] [Google Scholar]
- 31.Jha AK, Shlipak MG, Hosmer W, Frances CD, Browner WS. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA 2001;285: 297–303. [DOI] [PubMed] [Google Scholar]
- 32.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Goto S, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J 2014;35:2864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodney PP, Travis LL, Brooke BS, DeMartino RR, Goodman DC, Fisher ES, et al. Relationship between regional spending on vascular care and amputation rate. JAMA Surg 2014;149:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra A, Vaughan-Sarrazin M, Rosenthal GE. Elderly veterans with dual eligibility for VA and Medicare services: where do they obtain a colonoscopy? Am J Manag Care 2015;21:e264–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


