Abstract
The use of various forms of tobacco is one of the most important preventable risk factors for the incidence and progression of periodontal disease. Tobacco use negatively affects treatment outcomes for both periodontal diseases and conditions, and for dental implants. Tobacco cessation programs can mitigate these adverse dental treatment outcomes, and may be the most effective component of a personalized periodontal treatment approach. In addition, heavy alcohol consumption may exacerbate the adverse effects of tobacco use. In this review, the microbiology, host/inflammatory responses, and genetic characteristics of the tobacco-using patient are presented as a framework to aid the practitioner in developing personalized treatment strategies for these patients. These personalized approaches can be used for patients who use a variety of tobacco products including cigarettes, cigars, pipes, smokeless tobacco products, e-cigarettes, and other tobacco forms, as well as patients who consume large amounts of alcohol. In addition, principles for developing personalized tobacco cessation programs using both traditional and newer motivational and pharmacological approaches are presented.
It is well established that tobacco use is among the most important, if not the most important, preventable risk factor for the incidence and progression of periodontal diseases (55, 59, 60, 73, 74, 78, 79, 96). In addition, tobacco use has negative adverse effects on the full spectrum of periodontal treatment approaches, including mechanical debridement, local and systemic antimicrobial therapy, surgery, regenerative procedures, and implants (61, 78, 79, 83, 163). The benefits of smoking cessation have been convincingly demonstrated both directly and indirectly in studies that show reduced levels of periodontal destruction, fewer missing teeth, and improved periodontal treatment outcomes in former smokers when compared to current smokers (3, 19, 22, 61, 83, 96). Not only is assessing a patient's tobacco use critical for understanding periodontal risks factors and gauging the predictability of periodontal therapy, but the dental practitioner can also help the patient achieve tremendous improvements in oral and systemic health by providing effective personalized tobacco cessation counseling and treatment.
In addition to tobacco, the dental practitioner may consider alcohol use as a possible contributing factor in periodontal diseases. Relative to tobacco use, the association between alcohol consumption and periodontal disease is less clear (6, 41, 63, 130, 154, 180) and may differ by gender or type of alcohol consumed (90, 124, 166). Recent evidence suggests that smoking combined with alcohol consumption could synergistically increase the risk of periodontal diseases (102). Currently available epidemiologic studies of alcohol consumption and periodontal disease are largely cross-sectional, thereby establishing alcohol consumption as a potential “risk indicator” for periodontal diseases. To elevate alcohol consumption as a “risk factor” for periodontal diseases requires the types of longitudinal epidemiologic studies that have been performed over the past several decades for tobacco use (7, 153, 169). Therefore, this paper focuses on the evidence, underlying mechanisms, and recommendations for a personalized dentistry approach for the tobacco-using patient that can also be applied to the patient who is also consumes a relatively large amount of alcohol. Where appropriate, the evidence for the mechanisms for a synergistic role of alcohol consumption in developing personalized approaches for treatment of periodontal diseases will be incorporated.
When encountering a tobacco-using patient in the dental office, particularly the tobacco-using patient with some form of periodontal disease that may be caused or exacerbated by tobacco use, the clinician is faced with a range of options on how to consult and treat this patient. These options require the clinician to incorporate specific circumstances and characteristics of each patient, including behavioral considerations, motivational approaches, and clinical presentation. For example, one specific clinical presentation of cigarette smokers is teeth with extrinsic tobacco staining surrounded by a pink fibrotic gingiva with loss of stippling, deeper probing depths, gingival recession, loss of clinical attachment, and less tendency for sites to bleed on probing (20, 21) (Fig. 1). In addition, diagnostic and treatment decisions should consider the microbiology, host/inflammatory responses, and genetic characteristics that are unique for each tobacco-using patient. Without a single widely accepted algorithm to account for all the specific factors for each patient, the practitioner must rely on the innate computer of human thought to assess and integrate each of these diagnostic and treatment variables into a personalized approach for each individual patient who uses tobacco in some form.
Fig. 1.
Clinical views of two heavy smokers with a moderate (top) and severe (bottom) chronic periodontitis. In both of these clinical examples there is considerable accumulation of plaque, calculus and tobacco stain. As often seen in heavy smokers, the gingiva generally appears enlarged and fibrotic, with loss of surface stippling, and with the exception of a few areas, with a less overt margin-papillary erythema when compared to similar levels of inflammatory periodontal diseases in non-smokers. There also often less of a tendency for bleeding on probing when compared to non-smokers. (Special thanks to Stacey Lee and Iman Mohammadi)
Throughout this review, we use the word "tobacco" to encompass a variety of tobacco-derived products, not limited to cigarettes, cigars, pipe tobacco, conventional smokeless tobacco (oral moist snuff and chewing tobacco), and several new or emerging products, such as tobacco waterpipe smoking (commonly called hookah). The vast majority of existing research on tobacco and periodontal diseases focuses on cigarette smoking. However, a number of studies report strong associations between periodontal diseases and pipe or cigar smoking (3, 97), smokeless tobacco use (49, 140), and waterpipe smoking (43). While it cannot be assumed that all oral health risks associated with cigarette smoking will directly translate to all tobacco products, the accumulating evidence supports a generally harmful effect on periodontal tissues across multiple classes of tobacco products. Later in this review, we present some specific considerations to help the dental practitioner take a personalized approach to treating patients who use tobacco, or are contemplating using, non-cigarette tobacco products. This includes electronic cigarettes, which frequently contain tobacco-derived nicotine and are sometimes regulated as tobacco products.
The primary objective of this review is to present an overview of the major factors the dental practitioner must consider in developing a personalized approach to the tobacco-using patient, with additional considerations for the patient who consumes larger amounts of alcohol. We focus on the underlying causes of tobacco-related periodontal diseases, options for periodontal treatment, and personalized approaches for facilitating tobacco cessation.
Microbial, host response, and genetic considerations for the tobacco-using patient
Microbial considerations
Although tobacco use is generally considered the major preventable contributing risk factor for the incidence, severity, and progression of periodontal diseases, tobacco use is one of several patient variables that must be incorporated in estimating the overall periodontal disease risk for the individual patient. Several periodontal disease risk calculators have been developed, all of which incorporate tobacco as a major risk factor, along with age, gender, medical history, dental history, clinical measures and findings, family history, microbiota, and the inflammatory response (53, 54, 107). Of particular interest to the field of personalized dentistry are the specific interactions of tobacco use, in general, and cigarette smoking, in particular, with alterations of the periodontal microbiota, the protective and destructive inflammatory responses, and genetic predisposition. Such specific interactions may underlie differences in treatment approaches for the tobacco-using patient from what would be recommended for tobacco non-users. While each of these interactions could require an individual paper to discuss in full, several key examples are presented here to illustrate how tobacco cessation and reducing the damage from tobacco use might be incorporated into developing a personalized approach to periodontal treatment.
The primary etiology of most periodontal diseases and conditions centers on the development and maturation of the plaque biofilm and the emergence of a more periodontopathic microbial profile. The potential effects of tobacco smoke on the composition of the microflora has received considerable attention over the past several decades. While some studies reported no significant differences from non-smokers in the presence and relative proportion of selected periodontal pathogens in the plaque biofilms of smokers (35, 132, 162), other studies have demonstrated significantly higher recovery rates of periodontal pathogens in smokers (85, 88, 184) and earlier establishment of periodontal pathogenic bacteria into the biofilm (98). One recent study also demonstrated a higher recovery rate of periodontal pathogens in patients who consumed alcohol in larger quantities (99).
In addition, significant shifts in biofilm ecology towards a microbiota associated with periodontal health were reported following successful smoking cessation (98). Of note, some recent studies using these newer microbial approaches have demonstrated in smokers higher levels of periodontal pathogens in shallow, clinically healthy periodontal pockets and on oral mucous membranes (64, 82, 109). In addition, significant differences in the microbiota during the initial stages of plaque formation have been found between smokers and non-smokers (98), as well as a higher recovery rate of pathogens around healthy peri-implant tissues in smokers (172). Other differences in smoking versus non-smoking periodontal patients include an increased recovery rate of superinfecting microorganisms such as Escherichia coli and Candida spp.(85), less reduction in periodontal pathogens in smokers following scaling and root planing (34, 46, 174), a diminished healing response to systemic antibiotic regimens, such as amoxicillin/metronidazole combinations (45), and a shorter rebound period to a pathogenic flora following resolution of gingival inflammation (82).
Host response considerations
One intriguing mechanism proposed for how tobacco smoke could affect the oral microbiota is via a genetic modification of Porphyromonas gingivalis after tobacco exposure, which could impair the host response to this pathogenic species (11). Thus, the role of tobacco smoking in promoting the growth of oral pathogenic bacteria may be either by directly altering the growth environment or by impairing the protective response of the host, including altering the inflammatory response, which itself would indirectly alter the biofilm environment and allow for the growth of pathogenic organisms. The interactions between the host response and oral microflora may be significantly altered both by acute exposures to high concentrations of tobacco constituents during smoking or use of a smokeless tobacco and by chronic exposures to chemicals that remain in the tissues, saliva, crevicular fluid, or bloodstream in lower concentrations after tobacco use (144).
It is now well accepted that the major driving force in periodontal breakdown, particularly in inflammatory periodontal diseases, is an imbalance been the protective/reparative and destructive/inflammatory functions of the host response (42). These imbalances are exacerbated by tobacco smoking, both during the initial host responses within the periodontal pocket and subsequently in the deeper periodontal tissues (77). The first host response events occur in the periodontal pocket between the plaque biofilm and neutrophils that have migrated out of the tissue, aided by complement and antibody (70, 143). In an effective response, these neutrophils would migrate to the plaque biofilm and engulf and kill the target bacteria. While this neutrophil activity may effectively eliminate bacteria in planktonic suspensions, it is virtually impossible for neutrophils to reach periodontopathic bacteria deep within a dense and well-organized biofilm (70, 143). As a result, these “frustrated” neutrophils release proteolytic enzymes and inflammatory mediators that can resorb or stimulate resorption of the supporting periodontal tissues. The acute and chronic exposure of the oral tissues to tobacco smoke, whether from a cigarette or cigar, has been shown to tip this balance further from the protective functions of neutrophils and antibodies in the periodontal pocket and towards greater destructive activity(70, 143).
For example, studies have demonstrated smoke-related impairment of protective host response functions, including impaired immunoglobulin response to periodontopathic and other bacteria (2, 9, 72, 80, 82, 116, 134), suppressed levels of chemokines required for a protective response (158), and a reduction in potentially protective dendritic cells in the epithelium (157). In addition, higher concentrations of products in tobacco smoke during smoking can directly impair the protective functions of neutrophils (10), including chemotaxis to target bacteria and engulfment (phagocytosis) of the bacteria (31, 95, 108). Similar impairment of neutrophils to kill bacteria has also been reported in heavy alcohol drinkers, including after adjustment for smoking (89). Tobacco smoke also stimulates the release of tissue destructive products from neutrophils, such as superoxide, hydrogen peroxide, and proteolytic enzymes (111, 145).
The proteolytic enzymes that break down periodontal tissues include a variety of matrix metalloproteinases, including several classes of collagenases, elastase, and gelatinase, which are derived from the host’s own neutrophils, mononuclear cells, epithelial cells, or from several periodontopathic bacteria species. The effects of smoking on these enzymes themselves may not be as important as the balance between the activity of these enzymes and the neutralizing activities of the intrinsic enzyme inhibitors in periodontal tissues. Several studies have demonstrated elevated levels of tissue destructive enzymes in the gingival crevicular fluid and serum of smokers with periodontitis (100, 117, 155, 156, 171), as well as higher levels of Receptor activator of nuclear factor kappa-B ligand (RANKL), a stimulator of bone resorption, and lower levels of the bone protective osteoprotegrin (117, 122). Other studies have shown no difference in levels (or even lower levels) of tissue destructive enzymes in smokers (62, 125, 173). However, impaired activity of tissue enzyme inhibitors may be even more critical in tipping the balance toward periodontal breakdown. Indeed, reduced activity of these protective enzyme inhibitors have been reported consistently in the gingival crevicular fluid and/or in the serum of smokers with chronic periodontitis (121, 128, 129). Furthermore, high concentrations of nicotine during smoking or using smokeless tobacco can impair healing through various mechanisms, such as impairment of fibroblast attachment and collagen synthesis (65, 136, 167). It appears that tobacco use contributes to periodontal tissue destruction by tipping the balance toward greater proteolytic and resorptive activity and away from anti-proteolytic activity and repair (86).
While tissue neutrophils play both a protective and destructive role in periodontal diseases, with protective functions impaired and destructive functions enhanced by tobacco products, the principal component in the host response within the periodontal tissues center on mononuclear cell populations (monocytes and lymphocytes). As with neutrophil activities, resorption and/or repair of periodontal tissues centers on a balance between destructive inflammatory cytokines and tissue resorption enzymes on one side and protective cytokines and growth factors on the other. The inflammatory/destructive profile involves increased secretion and activity of proteolytic enzymes, as well as cytokines, such as interleukin-1alpha and interleukin-1 beta tumor necrosis factor-alpha, prostaglandins, interleukin-6 and/or interleukin-8 (120). In contrast, protective/reparative cytokines and chemokines include transforming growth factor-beta, insulin-like growth factor, interleukin-4, and interferons (120). There is a large body of evidence that supports the paradigm that smoking will tip the balance toward a more destructive cytokine profile (168). As a general overview, some studies have shown that T-cell activation and the Th2 immune response, which can stimulate this destructive inflammatory cytokine profile, is elevated in smokers (36, 37). When examining the role of tobacco in enhancing or suppressing specific cytokine activities, in vitro studies have demonstrated elevated levels of secreted interleukin-1beta in isolated mononuclear blood cells when exposed to tobacco smoke (147) and elevated interleukin-1beta and interleukin-8 from periodontal ligament cells exposed to nicotine (183). In some clinical studies, the effect of tobacco smoke and other tobacco products and substances on cytokine secretion have been inconclusive (23, 25, 26, 126, 127), including no significant differences in the levels of interleukin-6 (152) and tumor necrosis factor-alpha (44). However, other studies have demonstrated that tobacco smoke enhances these destructive inflammatory profiles. For example, in a study of experimental gingivitis, smokers demonstrated an elevated level of the inflammatory cytokine, interleukin-8 but a lower level of the protective cytokine interleukin-4 (56). Other studies reported elevated interleukin-6 and interleukin-8, depressed interleukin-4 (56) and elevated tumor necrosis factor-alpha (52) in smokers. In studies of early-onset periodontitis (aggressive periodontitis), smokers exhibited elevated levels of several inflammatory cytokines, including interleukin-1beta, interleukin-6 and interleukin-8, when compared with nonsmokers (84). Elevated levels of the reparative cytokine transforming growth factor-beta also have been reported in the gingival crevicular fluid of smokers (161), which may be a response to the increased breakdown of periodontal tissues in smokers.
Genetic considerations
In the field of genetics and periodontal diseases there is a large body of evidence that both Mendelian variants and single nucleotide polymorphisms make a significant contribution to a patient’s overall risk for developing periodontal disease and to disease progression and severity. In addition, as discussed later in the paper, genetic variants may influence the response of an individual patient to pharmacological approaches to smoking cessation. In recent years, there has been considerable focus on the role of single nucleotide polymorphisms of the DNA coding for proteins critical to the host response or inflammatory destruction of periodontal tissue. Comparative studies of genetic polymorphisms and effects on periodontal diseases between smokers and non-smokers have included interleukin-1 (56, 139), tumor necrosis factor-alpha (33), interleukin-6 (33, 71), and vitamin D receptor (28), among other candidates. Smoking may enhance the risk of periodontal disease incidence and severity in patients with these genetic polymorphisms (40, 76, 104, 113, 114, 131). Heavy alcohol use may also interact with genetics to enhance periodontal disease risk, particularly for those patients with genetic variants in alcohol dehydrogenase, a key enzyme in alcohol metabolism. (118). However, a recent study did not observe interactive effects between tobacco and genetics polymorphisms for interleuken-1alpha or interleukin-1beta in the success of dental implants (176). Additionally, there was no interaction between smoking status and single nucleotide polymorphisms for complement component 5, part of the host response to periodontopathic bacteria, but the small number of smokers in this study limited statistical power to detect gene-environment interactions (27). Notably, polymorphic alleles coding for enzymes involved in the neutralization of toxic substances from tobacco have been associated with periodontal disease, suggesting elevated disease risk for tobacco-using patients with such polymorphisms (91).
Epigenetics is a third area of gene expression in which tobacco use may affect periodontal disease. Epigenetic changes are post-translational modifications of mRNA through methylation or acetylation, which may either stimulate or suppress translation to proteins. Such epigenetic changes may tip the balance away from protective effects of the host and towards a more inflammatory/destructive profile in periodontal diseases (16, 101, 106). Methylation or acetylation of post translational mRNA can be stimulated by environmental factors, such as smoking and use of other forms of tobacco, as well as the periodontal microbiota and local inflammatory environment (14). Methylation or acetylation of mRNA can then lead to an increase or decrease in the synthesis of corresponding proteins, such as destructive inflammatory cytokines, tissue resorption enzymes, and growth factors, or may lead to a more active or inactive enzyme form. At present, the specific mechanisms by which smoking may contribute to epigenetic modifications involved in periodontal disease is unclear. Nevertheless, in the context of personalized dentistry, the topic of smoking and epigenetics merits further investigation.
Implications of microbial, host response, and genetic considerations for personalized periodontal therapy
Over the past several decades there has been a considerable body of literature on periodontal diagnostic approaches and therapies that specifically target the patient's individual microbiota, genetic predisposition, and inflammatory host response (138). Since tobacco use in general and smoking in particular may exacerbate the deleterious local and systemic changes seen in periodontal disease progression in non-smoking patients, such targeted approaches may also benefit the tobacco-using patient. Broadly, therapeutic approaches fall under two categories: first, microbial testing with application of specific antimicrobials targeted to the identified microbiota; and secondly, host modulation therapies specifically designed to reduce the activities of destructive inflammatory substances and enhance the protective, regenerative effects of the host response. Currently available risk calculators for future periodontal breakdown incorporate local and systemic components including local periodontal and systemic diseases and conditions, genetic predisposition, and oral habits into their algorithms (53). These risk components can also be considered for patients who may require a personalized treatment approach and include:
Patients who use tobacco. In particular, moderate to heavy smokers who continue to smoke despite repeated attempts at smoking cessation
Patients with aggressive forms of periodontal diseases
Patients with an underlying systemic disease or condition that places the patient at higher risk such as uncontrolled diabetes
Patients who have a family history and/or genetic disposition to further breakdown
Patients whose periodontal condition continues to deteriorate despite repeated attempts at conventional periodontal therapy.
For patients who use tobacco, personalized periodontal therapy may include systemic and/or local antimicrobial therapy, host modulation therapies, and rigorous and customized smoking cessation strategies.
Systemic or local antibiotic therapies may have a beneficial effect on reducing periodontopathic bacteria in both clinically healthy and diseased sites. As previously discussed previously in this paper, the literature suggests that periodontopathic bacterial species can be isolated with a greater frequency in smokers in clinically healthy sites with shallower pockets and around dental implants without clinical evidence of inflammation. Any potential clinical benefit of reducing these bacterial populations must be weighed against the significant and well-documented possibility of contributing to development of antimicrobial resistant strains. Therefore, the current evidence to support systemic antibiotics as a personalized approach to managing periodontal diseases in patients who use tobacco is limited at best.
In contrast, with the current understanding of the role of a destructive inflammatory host response in periodontal breakdown, and the role of smoking in exacerbating this response, current and future host modulation therapies offer a promising option as personalized dentistry approaches. Perhaps the most widely used host modulators that may tip the balance toward regeneration/repair and away from destruction/inflammation are the tetracycline antibiotics, including tetracycline itself, doxycycline and minocycline. The tetracycline family of antibiotics may neutralize inflammation-inducing substances that are particularly elevated in tobacco-associated periodontal diseases. In particular, the tetracyclines have been shown to depress the activity of several matrix metalloproteinases (including collagenases), inhibit the release of interleukin-1beta from mononuclear cells, and act as a scavenger for destructive oxygen species (such as superoxide), which are released during the oxidative burst (1, 57, 177). As previously discussed in this paper, all these inflammatory substances have been reported to be elevated, either in level and/or activity with tobacco smoke exposure. Although smokers may still have a less favorable response than non-smokers to local application of this group of tetracycline antibiotics (92, 182), several clinical studies have demonstrated that either low doses of doxycycline given systemically, or higher concentrations of minocycline and doxycycline given in a local controlled delivery system, can have beneficial effects on periodontal status. For example, in a study of patients with advanced periodontitis, including a majority with a history of past or current smoking, there were marked added benefits of systemic low-dose doxycycline combined with scaling and root planing when compared with scaling and root planing alone (119). In addition, studies on the local delivery of either a doxycycline gel (146) or minocycline microspheres (123) demonstrated an improved response to non-surgical periodontal therapy in smokers. These studies indirectly support the concept that the locally applied antibiotics of the tetracycline family may not only have an antimicrobial effect, but may also exert a local host modulating effect by protecting against some of the effects of tobacco smoke on the destructive/inflammatory arm of the host response.
As of this writing, systemic delivery of low dose antibiotics in the tetracycline family or local high dose controlled delivery of these antibiotics are the only widely used approaches for host modulation therapies, particularly for their effects on the suppression of destructive host response in smokers. However, the emergence of new host modulation approaches to suppress inflammation and promote repair, which are now in at the animal model or early human trial stage, may have additive benefits in the treatment of periodontal diseases in smokers. These include current work on the local and systemic application of inflammation resolving molecules under the broad umbrella of resolvins (148). In particular, resolvins may mitigate the destructive effects of tobacco on the host response discussed in this paper, such as impairment of neutrophil migration and enhanced bone resorption (51, 175, 179). Indeed, any host modulation approach that reduces periodontal tissue destruction may be of particular benefit for tobacco users.
Nevertheless, from the large body of cross sectional and longitudinal studies comparing periodontal disease incidence and progression between non-smokers, former smokers, and current smokers, it is apparent that the single most important personalized dentistry approach to the prevention and treatment of periodontal disease in the tobacco-using patient is an effective smoking cessation strategy. Strategies for developing a personalized dentistry approach to tobacco use prevention and tobacco cessation are discussed in this next section.
Understanding tobacco dependence
To provide comprehensive, personalized cessation counseling, dental professionals must understand the physiological, psychological, behavioral, and social aspects of nicotine addiction (178). From a physiological standpoint, nicotine from tobacco products stimulates the brain to release chemicals that result in feelings of pleasure, cognitive arousal, appetite suppression, improved short-term memory, mood enhancement, decreased anxiety and stress reduction. Over time, with continued exposure to nicotine, the brain becomes tolerant and adapts by producing additional nicotine receptors and develops tolerance: requiring higher levels of nicotine to yield the same physical effects (17). Reductions in nicotine exposure lead to symptoms of nicotine withdrawal, including cravings, depression, irritability, frustration, anger anxiety, and difficulty concentrating. The severity of these withdrawal symptoms varies greatly across individuals. Levels of nicotine dependence and withdrawal are key considerations in determining the type and intensity of tobacco cessation strategies. For example, nicotine replacement therapy may be more appropriate for the dependent patient. (29, 137)
While physical aspects of nicotine addiction undoubtedly influence tobacco use, certain psychological, behavioral, and social factors also play an important role in tobacco cessation success. Patients may use tobacco products as a coping mechanism to relieve stress, depression and anxiety. For some, tobacco use may be part of a social identity. For example, smokeless tobacco use may be a bonding activity within peer groups, often associated with outdoor activities or sporting events (32, 69). For many tobacco users, environmental and social situations can pose major challenges to cessation. Indeed, individuals whose family, peers, or significant others are tobacco users are less likely to quit successfully (30). Moreover, many tobacco-using patients will develop behavioral responses to certain environmental stimuli, such as finishing a meal, driving in their car, or drinking alcohol, that generate an urge to use (178). These behavioral responses are often responsible for relapses in individuals with short-term cessation success. Understanding the socio-behavioral aspects of nicotine addiction can be just as critical as the physical aspects in helping to personalize a patient’s tobacco use and dependence treatment plan.
Incorporating a personalized approach to tobacco cessation
Dental professionals are in a unique position to help their periodontal patients be successful in tobacco cessation. Combined findings from 14 studies with over 10,500 participants demonstrated that tobacco interventions by dental professionals helped tobacco users to quit cigarettes and smokeless tobacco (47). However, it is important to keep in mind that a personalized dentistry approach is more effective than “one size fits all” when considering the broad range of individual patient attitudes, behaviors and level of dependence. The dental provider must be able to guide a patient to the awareness that their tobacco habit is the most important preventable risk factor for developing systemic and oral disease, including periodontitis and oral cancer. While a comprehensive review of tobacco cessation strategies would require its own paper, the aim of this section is to describe a general framework for treating tobacco use and dependence in a dental setting and helpful strategies for implementing a personalized approach within this framework. For additional information and resources for the dental provider and patient, see Table 1.
Table 1.
Resources for Dental Professionals and Patients
|
Dental Professionals:
|
Motivational Interviewing Resources
|
Tobacco Cessation Resources
|
|
|
| Patients: |
|
|
Online Resources
|
Smartphone applications
|
Telephone Quitlines
|
Smokeless Tobacco Cessation Resources
|
Mobile Cessation Support
|
Support Groups
|
Behavior Change and Motivational Interviewing
To effectively communicate with patients about their tobacco use, clinicians must first understand their patient’s readiness to change their behavior. The Stages of Change model (or Transtheoretical model) is a model developed by researchers that describes the process of behavior change in 5 steps: pre-contemplation, contemplation, preparation, action, and maintenance (133). When applied to tobacco cessation, the model demonstrates that individuals cycle through each of the five stages of change in both a linear and non-linear process depending on their personal circumstances (Fig. 2). It is important that clinicians understand where patients are in the stage of change process in order to provide effective tobacco cessation counseling.
Fig. 2.
Stages of Change Model Related to Tobacco Cessation
Dental practitioners must ask detailed questions and listen to each patient in order to understand their readiness and individual motivations to quit. Motivational interviewing is a method used by practitioners to stimulate a patient’s intrinsic motivation to change a behavior, such as tobacco use. There are four general principles of motivational interviewing: expressing empathy, developing discrepancy, rolling with patient resistance and supporting self-efficacy (Table 2). These principles can be used to inform patients about negative health risks and help guide them to make changes that fit their unique needs (141). It is important that dental practitioners listen and acknowledge the patients’ perspective, support their initiative to change, and provide them with relevant health and treatment information. In addition, practitioners must resist the urge to tell their patients what they should or should not do (181). This desire to persuade patients into making a behavior change tends to result in increased resistance and greater focus on the disadvantages of quitting. Rollnick at al. (2008) suggest that providers resist this “righting reflex” by listening and determining where the patient is in their own process of change (141).
Table 2.
Four Main Principles of Motivational Interviewing
Principle 1: Expressing empathy
|
Principle 2: Developing discrepancy
|
Principle 3: Roll with resistance
|
Principle 3: Support self-efficacy
|
One strategy to determine a patients’ readiness for change is to listen for change talk. Change talk is the use of certain words that suggest a willingness or openness to behavior change (Table 3). When a patient voices change talk, providers should respond with particular interest in what the patient is saying. This response can be non-verbal, such as a simple head nod, or a verbal response, asking the patient to elaborate (115). The ability to identify when a patient is ready for change is a motivational interviewing technique that is central to assisting them in taking the next step.
Table 3.
Listening for Change Talk
| Type of change talk | Examples |
|---|---|
|
| |
| Desire to change: | “I wish” “I want” I like the idea” |
|
| |
| Ability to change: | “I could probably try replacing my smoke breaks with a short walk.” |
| “I might be able to cut out dipping at practice” | |
|
| |
| Reasons to change: | “I’m sure I’d feel better if I quit” |
| “Smoking keeps me from running” | |
|
| |
| Need to change: | “I’ve must get healthier for my family” |
| “I’ve got to get my habit under control” | |
|
| |
| Commitment to change | |
| Higher level: | “I will try to cut back” |
| “I promised my kids I would quit this year” | |
| “I plan on calling a quitline today” | |
| Lower level: | |
| “I will think about what we discussed” | |
| “I really hope I can quit” | |
Examples of "change talk," which represents language and phrases that a tobacco-using patient may express prior to committing to tobacco cessation. Adapted from Rollnick, et al.(142)
The 5 A’s: Ask, Advise, Assess, Assist and Arrange
The established framework for the treatment of tobacco use and dependence is the 5 A’s approach to care (47). This approach involves asking clients about their tobacco use, advising users to quit and encouraging nonusers to remain tobacco free, assessing readiness to quit, assisting with the quitting process, and arranging follow-up. The 5 A’s framework is best designed for practices and organizations that have cessation medications, counseling, and behavioral interventions available. When there is limited time or resources, clinicians are encouraged to apply a condensed 5 A’s model, known as the “2 A’s & R” model, whereby they ask about tobacco use, advise tobacco users to quit, and refer patients who are willing to quit to a telephone quitline or other community-based tobacco cessation program. Regardless of a patient’s readiness to quit, the “5 A’s” or “2 A’s & R” model are essential interventions for every patient.
It is important that dental professionals ask about tobacco use with every patient at every appointment. With increasing popularity of non-cigarette tobacco products, dental professionals must expand their inquiry beyond cigarettes and verbally ask about all tobacco and nicotine products, recognizing that many tobacco-using patients do not consider themselves "smokers." In addition to tobacco products used, dental providers must also ask about frequency of use and their history of previous quit attempts (including methods used). If a patient does not use tobacco products, it is important to congratulate that patient and encourage continued abstinence.
If a patient reports tobacco use, the next step in the 5 A’s framework is to advise that patient to stop using tobacco in an effort to prevent associated oral and systemic health risks. While advising patients about their tobacco use, it is important that dental professionals incorporate a personalized approach that is clear and relevant to that particular patient. One potential strategy that may be effective for some tobacco-using patients is to appeal to their sense of appearance, as esthetic concerns may be a major reason a patient seeks dental care. For those patients and others, a clear demonstration of the effects of tobacco on the appearance of their teeth, gum recession, and tooth mobility may be a motivational tool. The effect of showing a patient the immediate effects of tobacco use has parallels to other smoking-related disease or conditions. For example, studies have demonstrated that cigarette smoking patients who have had a heart attack or been diagnosed with acute coronary heart disease can have fairly high levels of tobacco cessation success, exceeding those reported for healthier individuals in the general population (15). As a personalized dentistry approach, incorporating a visual demonstration of the effects of tobacco on the appearance of their teeth and soft tissues, while certainly not as dramatic as the patient’s experience of a heart attack, provides a “teachable moment” and may have a higher motivational impact on their efforts to quit long-term (135).
Once a tobacco-using patient has been advised to quit, the next step in the 5 A’s approach is to assess the patient’s readiness to quit. If a patient has expressed motivation to quit, the chances for success are far greater than if the patient is uninteresting in quitting or wishes to postpone the start of a cessation program. The patient’s response to the question, “Would you like to quit smoking in the next month?” is a branching point for the dental provider in customizing a tobacco counseling strategy.
For patients who are uninterested in quitting, dental providers should implement the “5 R’s” Relevance, Risks, Rewards, Roadblocks and Repetition (48). This approach involves: asking the patient to explore why quitting is personally relevant to them; asking the patient to identify potential risks of tobacco use and rewards or benefits of quitting; asking the patient to identify roadblocks or barriers to quitting; and repeating these motivational interventions with patients at each visit (Table 4) (48).
Table 4.
Examples of Risks, Rewards and Roadblocks to Tobacco Cessation
| Components of the 5 R’s Approach: |
Potential Personal Examples: |
|---|---|
|
| |
| Risks |
|
|
| |
| Rewards |
|
|
| |
| Roadblocks |
|
The "5 R's" Approach, adapted from Fiore, et al(47).
Some patients may not be ready to quit completely, but instead express a readiness to “cut down” on their tobacco use or switch to a different tobacco product that they perceive to be less harmful. One example would be a cigarette smoking patient that expresses an interest in switching to using cigars or pipes. Several large-scale epidemiologic studies have shown that pipe and cigar smokers have comparable levels of periodontal diseases to cigarette smokers (3, 97). In addition, when considering the overall local and systemic effects of pipe and cigar smoking, increased risks of developing cancers of the lung, mouth, and esophagus, as well as pulmonary diseases and cardiovascular disease should be discussed (12, 93). A second question patients may ask is whether cutting back on the number of cigarettes smoked per day would reduce the risk and progression of periodontal diseases. Several studies have indeed demonstrated a dose-dependent association between the number of daily cigarettes and loss of periodontal support or teeth (38). While these findings may suggest benefits from cutting back, they need to be carefully interpreted. For example, blood and urine levels of nicotine metabolites in smokers who reduced their daily numbers of cigarettes are higher than found in smokers who had continually smoked fewer cigarettes/day (67). This discrepancy may be due to the habituating effects of nicotine, which compel the smoking patient to inhale each cigarette more fully to maintain constant nicotine intake from fewer cigarettes consumed. Compared to non-smokers, even adults who smoke no more than 1 cigarette daily exhibit substantially elevated risk of lung cancer and death, suggesting that there is no risk-free level of tobacco smoke exposure (75).
Yet another alternative the patient may propose is to substitute other tobacco products, such as smokeless tobacco, for a cigarette smoking. While smokeless tobacco use may produce fewer toxins than burned cigarettes, potentially decreasing smoking-related health risks, health conditions associated with smokeless tobacco include severe loss of clinical periodontal attachment, precancerous and cancerous lesions of the oral mucosa, pancreatic cancer, and elevated nicotine in the bloodstream, which can lead to an intractable habituation and increased risk for smoking initiation, smoking relapse, or continued use of both products(4, 24, 50, 58, 105, 150). In addition, patients may consider whether cannabis use poses oral health risk. However, there appears to be a dose related association between cannabis smoking and the prevalence of periodontal diseases (151, 170). Patients may view use of other forms of tobacco, such as cigars and tobacco waterpipe (hookah), as less damaging to health than cigarette smoking (110). However, much like cigarette smoking, use of these products is associated with worse periodontal health (3, 43, 97). There is not yet strong direct evidence connecting use of electronic cigarettes to poor oral health, although these products are not harm free. The implications of the emergence of electronic cigarettes for the tobacco-using periodontal patients will be further discussed later in this review.
When patients do express an interest in quitting all tobacco products, they should be encouraged to set a quit date within the next month. During the assisting phase of the 5 A’s, dental practitioners should provide brief counseling and referral to an internal or external cessation program. These programs can be part of the dental practice in which the practitioner works, or a community-based program, such as tobacco cessation websites (e.g. www.smokefree.gov) or telephone quit lines (e.g. 1–800-QUIT-NOW). It is important that patients have social support during a quit attempt. Thus, dental professionals should encourage patients to tell their family and friends about their desire to stop using tobacco. Using the motivational interviewing strategies discussed above allows the patient to begin hearing their own motivation for change. It also creates an opportunity to use the Elicit-Provide-Elicit model to guide the patient towards a personalized cessation plan. This model is promoted by the Mayo clinic for tobacco cessation and is based on the dental practitioner’s ability to elicit the patient’s perspective, provide information about tobacco products and cessation strategies in a non-judgmental way, and elicit the patient’s thoughts and feelings about the information shared and their ideas about next steps. This approach helps dental practitioners understand the personal reasons a patient is interested in quitting and engages a patient's internal motivation.
Studies have shown that personalized behavioral counseling and pharmacological medications improve tobacco cessation efficacy, particularly when used in combination (159, 160, 164). Pharmacological aids may be especially important to personalize tobacco cessation strategies for the highly nicotine dependent patient. Dependence on nicotine can often be determined by asking about a patient’s age of initiation, frequency of tobacco use, and time of tobacco use after waking. Several assessment tools have been developed to help practitioners quantify a patients’ degree of dependence (Table 5). This degree of dependence can help predict the behavioral counseling and pharmacological treatment required and the level of difficulty a patient will experience during their quit attempt (68). Over the counter pharmacological aids are nicotine replacement therapies, which include nicotine-containing transdermal patches, chewing gum, lozenges, nasal spray, and inhalers (47). Prescription medications include varenicline (Chantix®) and buproprion (Zyban®, Wellbutrin®) and have been shown to improve smoking cessation success, especially in combination with nicotine replacement therapy (81). Contraindications and potential adverse psychological effects of prescription medications, such as buproprion should be considered before prescribing to patients.
Table 5.
Nicotine Dependence Assessment Examples
| Fagerstrom Test for Nicotine Dependence (FND) (68) |
|
| How to interpret Nicotine Dependency Score: | |
| Score of 6 or higher: Indicates high nicotine dependency and represents individuals who would be particularly likely to benefit from tapering and/or the prescription of nicotine replacement therapy (gum or patch) to decrease nicotine withdrawal symptoms as an adjunct to standard counseling. | |
| Score of 5 or less: Suggests low to moderate nicotine dependency and represents individuals who may be less likely to require tapering and/or the prescription of nicotine replacement therapy (gum or patch). Standard counseling is most appropriate. | |
|
| |
| Hooked on Nicotine Checklist (HONC) (39) |
|
When you haven’t used tobacco for a while…OR, When you tried to stop smoking…
|
|
How to interpret Nicotine Dependency Score: The HONC as an indicator of diminished autonomy.
|
|
The final step in the 5 A’s approach involves arranging follow-up with patients who have quit using tobacco products, or those who are thinking about quitting in the future. These follow-up visits are opportunities to reinforce the importance of tobacco cessation and modify cessation strategies for those who have been unsuccessful. Tobacco users who have failed in previous quit attempts should be encouraged to continue trying to quit and be reminded that successful cessation often involves multiple attempts. If a person has been successful, they should be congratulated and encouraged to continue abstinence in the future. Regardless of whether a patient has successfully quit using tobacco, clinicians must be mindful that tobacco dependence is a chronic and relapsing disorder that requires ongoing assessment and motivational interventions (47).
Recent developments affecting personalized tobacco cessation
One recent mode of tobacco use that merits more extensive discussion for consulting with patients in this era of personalized medicine and dentistry is the emergence and increasing popularity of electronic cigarettes, or “e-cigarettes.” While the types and formulations of e-cigarettes varies considerably, the basic principle is that these devices produce an inhalable, heated aerosol, commonly called a vapor, that delivers nicotine and flavoring agents but does not contain (or contains at much lower levels) the several hundred other toxic substances present in tobacco smoke. The short and long-term safety and other adverse and/or beneficial effects of e-cigarettes have been the subject of controversy both within and between countries, with some countries, such as Brazil and Norway, placing heavy restrictions or bans on their sale. Of particular note to the dental profession, one safety consideration is the possibility that poorly manufactured e-cigarette devices could explode during use, potentially leading to dental or facial injuries (94). While e-cigarettes may offer a less harmful form of nicotine delivery than combustible tobacco, e-cigarettes are not harm free. Recent studies suggest immediate cardiovascular changes in healthy volunteers after using e-cigarettes (8) and showed the release of inflammatory cytokines by isolated periodontal fibroblasts exposed to e-cigarette aerosols (165). Long-term habituation to nicotine and subsequent use of other tobacco products is a concern supported by a recent study that demonstrated children who initially used e-cigarettes were more likely to progress to using combustible tobacco (13).
How and whether e-cigarettes should be incorporated into customized approaches to tobacco cessation is a topic of much debate. For example, while in the United Kingdom, use of e-cigarettes is widely accepted by the public health community, citing the potential of e-cigarettes to reduce smoking-related disease(112) in the United States, more concern and caution has been raised, noting the rising prevalence of e-cigarette use among youth (149). To date, randomized controlled trials of e-cigarettes as a smoking cessation tool have not consistently demonstrated superior outcomes over existing cessation aids, but more trials are ongoing (66). For the dental practitioner, there is a responsibility to remain up-to-date as more research regarding the potential risks and benefits of e-cigarettes emerges. As always, the most important advice the practitioner can give to a patient in any personalized dentistry approach is to encourage evidence-based cessation strategies with the ultimate goal of reaching long-term cessation from all tobacco.
Looking toward the future of personalized tobacco cessation approaches, emerging research suggests that genetic factors may play a key role in nicotine dependence and smoking cessation outcomes. The liver enzyme cytochrome P450 (CYP) 2A6 is responsible for breaking down nicotine in the body. Genetic variation in the CYP2A6 gene determines the rate at which individuals metabolize nicotine: a phenotype reliably measured in blood, urine, or saliva as a ratio of nicotine breakdown products (nicotine metabolite ratio) (5). Variation in nicotine metabolite ratio has been associated with cigarette consumption behaviors and smokers' ability to quit successfully (18, 87, 103). Variation in tobacco cessation effectiveness according to nicotine metabolite ratio and specific pharmacologic aids supports personalized interventions based on patient phenotypes, for example, nicotine replacement therapy for slow metabolizers and varenicline for normal metabolizers (5). While there is not yet a rapid chair-side test for determining nicotine metabolite ratio, the dental practitioner may soon have the option of using genetic information or other biomarkers to tailor tobacco cessation treatment or tobacco prevention education to individual patients.
Improving periodontal outcomes for the tobacco-using patient
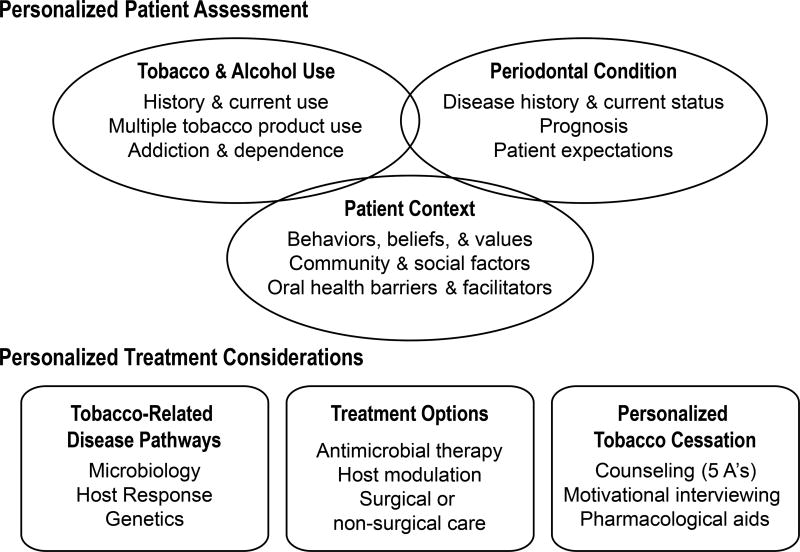
Tobacco use is quite possibly the most influential preventable risk factor for periodontal diseases and periodontal treatment outcomes. Understanding how a patient's tobacco-related behaviors interact with specific microbial, host response, and genetic determinants of periodontal health will better position the practitioner to choose appropriate therapies that best address the specific circumstances of each individual patient. While the relationship between the consumption of alcoholic beverages and at what quantities to periodontal disease progression both as a stand-alone risk factor and as a co-factor with tobacco use has yet to be determined, the dental practitioner can also inform the patient of new evidence regarding alcohol use and their periodontal condition as it becomes available. Use of tobacco and alcohol constitute one aspect of a personalized periodontal assessment, in which the practitioner aims not only to diagnosis existing disease, but to identify potentially modifiable risk factors and to center prevailing conditions within the larger context of the patient's behaviors and environment. A summary of the various biological and behavioral considerations for the evaluation, consultation, and treatment approaches for the tobacco-using patient presented in this paper is presented in Fig. 3. Equipped with a better understanding of the patient, the practitioner can align both periodontal treatment options and tobacco cessation counseling with patient-specific biological and behavioral factors (Fig. 3). In tailoring clinical approaches to each patient, the practitioner must incorporate the scientific evidence and clinical findings, as well as sound judgment and excellent patient communication in order to incorporate patient behavior and motivation into personalized approaches. The dental practitioner has a tremendous opportunity to improve patient health and wellbeing through encouraging and facilitating that all patients live tobacco-free. While patients can be expected to face significant challenges in overcoming nicotine dependence to quit tobacco use, dental practitioners can enhance the chances of cessation success with evidence-based personalized approaches.
Fig. 3.
In evaluating, counseling, and treating the tobacco-using periodontal patient, the dental care provider should consider personalized factors, such as patient values, nicotine dependence, and microbiology, to develop appropriately tailored therapies.
Acknowledgments
Thank you to Joanna Hill of the University of California San Francisco for administrative assistance in preparing the manuscript. Grant number KL2TR001870 from the U.S. National Institutes of Health National Center for Advancing Translational Sciences provided support to Dr. Chaffee. The content of the publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors report that they have no conflicts of interest related to this publication.
References
- 1.Akamatsu H, Asada M, Komura J, Asada Y, Niwa Y. Effect of doxycycline on the generation of reactive oxygen species: a possible mechanism of action of acne therapy with doxycycline. Acta Derm Venereol. 1992;72:178–179. [PubMed] [Google Scholar]
- 2.Al-Ghamdi HS, Anil S. Serum antibody levels in smoker and non-smoker saudi subjects with chronic periodontitis. J Periodontol. 2007;78:1043–1050. doi: 10.1902/jop.2007.060431. [DOI] [PubMed] [Google Scholar]
- 3.Albandar JM, Streckfus CF, Adesanya MR, Winn DM. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000;71:1874–1881. doi: 10.1902/jop.2000.71.12.1874. [DOI] [PubMed] [Google Scholar]
- 4.Alguacil J, Silverman DT. Smokeless and other noncigarette tobacco use and pancreatic cancer: a case-control study based on direct interviews. Cancer Epidemiol Biomarkers Prev. 2004;13:55–58. doi: 10.1158/1055-9965.epi-03-0033. [DOI] [PubMed] [Google Scholar]
- 5.Allenby CE, Boylan KA, Lerman C, Falcone M. Precision medicine for tobacco dependence: development and validation of the nicotine metabolite ratio. J Neuroimmune Pharmacol. 2016;11:471–483. doi: 10.1007/s11481-016-9656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaral Cda S, Luiz RR, Leao AT. The relationship between alcohol dependence and periodontal disease. J Periodontol. 2008;79:993–998. doi: 10.1902/jop.2008.070525. [DOI] [PubMed] [Google Scholar]
- 7.Amaral Cda S, Vettore MV, Leao A. The relationship of alcohol dependence and alcohol consumption with periodontitis: a systematic review. J Dent. 2009;37:643–651. doi: 10.1016/j.jdent.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, Lundback M. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis. 2016;255:179–185. doi: 10.1016/j.atherosclerosis.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 9.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 10.Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagaitkar J, Williams LR, Renaud DE, Bemakanakere MR, Martin M, Scott DA, Demuth DR. Tobacco-induced alterations to Porphyromonas gingivalis-host interactions. Environ Microbiol. 2009;11:1242–1253. doi: 10.1111/j.1462-2920.2008.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker F, Ainsworth SR, Dye JT, Crammer C, Thun MJ, Hoffmann D, Repace JL, Henningfield JE, Slade J, Pinney J, Shanks T, Burns DM, Connolly GN, Shopland DR. Health risks associated with cigar smoking. Jama. 2000;284:735–740. doi: 10.1001/jama.284.6.735. [DOI] [PubMed] [Google Scholar]
- 13.Barrington-Trimis JL, Urman R, Berhane K, Unger JB, Cruz TB, Pentz MA, Samet JM, Leventhal AM, Mcconnell R. E-cigarettes and future cigarette use. Pediatrics. 2016:138. doi: 10.1542/peds.2016-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barros SP, Offenbacher S. Modifiable risk factors in periodontal disease: epigenetic regulation of gene expression in the inflammatory response. Periodontol 2000. 2014;64:95–110. doi: 10.1111/prd.12000. [DOI] [PubMed] [Google Scholar]
- 15.Barth J, Jacob T, Daha I, Critchley JA. Psychosocial interventions for smoking cessation in patients with coronary heart disease. Cochrane Database Syst Rev. 2015:Cd006886. doi: 10.1002/14651858.CD006886.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belinsky SA, Palmisano WA, Gilliland FD, Crooks LA, Divine KK, Winters SA, Grimes MJ, Harms HJ, Tellez CS, Smith TM, Moots PP, Lechner JF, Stidley CA, Crowell RE. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002;62:2370–2377. [PubMed] [Google Scholar]
- 17.Benowitz NL. The biology of nicotine dependence: from the 1988 Surgeon General's Report to the present and into the future. Nicotine Tob Res. 1999;1(Suppl 2):S159–163. doi: 10.1080/14622299050012001. [DOI] [PubMed] [Google Scholar]
- 18.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 19.Bergstrom J. Influence of tobacco smoking on periodontal bone height Long-term observations and a hypothesis. J Clin Periodontol. 2004;31:260–266. doi: 10.1111/j.1600-051X.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 20.Bergstrom J. Tobacco smoking and chronic destructive periodontal disease. Odontology. 2004;92:1–8. doi: 10.1007/s10266-004-0043-4. [DOI] [PubMed] [Google Scholar]
- 21.Bergstrom J, Bostrom L. Tobacco smoking and periodontal hemorrhagic responsiveness. J Clin Periodontol. 2001;28:680–685. doi: 10.1034/j.1600-051x.2001.028007680.x. [DOI] [PubMed] [Google Scholar]
- 22.Bergstrom J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000;71:1338–1347. doi: 10.1902/jop.2000.71.8.1338. [DOI] [PubMed] [Google Scholar]
- 23.Bernzweig E, Payne JB, Reinhardt RA, Dyer JK, Patil KD. Nicotine and smokeless tobacco effects on gingival and peripheral blood mononuclear cells. J Clin Periodontol. 1998;25:246–252. doi: 10.1111/j.1600-051x.1998.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 24.Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667–675. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 25.Bostrom L, Linder LE, Bergstrom J. Smoking and cervicular fluid levels of IL-6 and TNF-alpha in periodontal disease. J Clin Periodontol. 1999;26:352–357. doi: 10.1034/j.1600-051x.1999.260604.x. [DOI] [PubMed] [Google Scholar]
- 26.Bostrom L, Linder LE, Bergstrom J. Smoking and GCF levels of IL-1beta and IL-1ra in periodontal disease. J Clin Periodontol. 2000;27:250–255. doi: 10.1034/j.1600-051x.2000.027004250.x. [DOI] [PubMed] [Google Scholar]
- 27.Chai L, Song YQ, Zee KY, Leung WK. Single nucleotide polymorphisms of complement component 5 and periodontitis. J Periodontal Res. 2010;45:301–308. doi: 10.1111/j.1600-0765.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 28.Chantarangsu S, Sura T, Mongkornkarn S, Donsakul K, Torrungruang K. Vitamin D receptor gene polymorphism and smoking in the risk of chronic periodontitis. J Periodontol. 2016:1–13. doi: 10.1902/jop.2016.160222. [DOI] [PubMed] [Google Scholar]
- 29.Chen LS, Horton A, Bierut L. Pathways to precision medicine in smoking cessation treatments. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corberand J, Laharrague P, Nguyen F, Dutau G, Fontanilles M, Gleizes B, Gyrard E. In vitro effect of tobacco smoke components on the functions of normal human polymorphonuclear leukocytes. Infect Immun. 1980;30:649–655. doi: 10.1128/iai.30.3.649-655.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couch ET, Darius E, Walsh MM, Chaffee BW. Smokeless tobacco decision-making among rural adolescent males in California. J Community Health. 2016 doi: 10.1007/s10900-016-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'aiuto F, Parkar M, Brett PM, Ready D, Tonetti MS. Gene polymorphisms in pro-inflammatory cytokines are associated with systemic inflammation in patients with severe periodontal infections. Cytokine. 2004;28:29–34. doi: 10.1016/j.cyto.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Darby IB, Hodge PJ, Riggio MP, Kinane DF. Clinical and microbiological effect of scaling and root planing in smoker and non-smoker chronic and aggressive periodontitis patients. J Clin Periodontol. 2005;32:200–206. doi: 10.1111/j.1600-051X.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 35.Darby IB, Hodge PJ, Riggio MP, Kinane DF. Microbial comparison of smoker and non-smoker adult and early-onset periodontitis patients by polymerase chain reaction. J Clin Periodontol. 2000;27:417–424. doi: 10.1034/j.1600-051x.2000.027006417.x. [DOI] [PubMed] [Google Scholar]
- 36.De Heens GL, Kikkert R, Aarden LA, Van Der Velden U, Loos BG. Effects of smoking on the ex vivo cytokine production in periodontitis. J Periodontal Res. 2009;44:28–34. doi: 10.1111/j.1600-0765.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 37.De Heens GL, Van Der Velden U, Loos BG. Cigarette smoking enhances T cell activation and a Th2 immune response; an aspect of the pathophysiology in periodontal disease. Cytokine. 2009;47:157–161. doi: 10.1016/j.cyto.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich T, Maserejian NN, Joshipura KJ, Krall EA, Garcia RI. Tobacco use and incidence of tooth loss among US male health professionals. J Dent Res. 2007;86:373–377. doi: 10.1177/154405910708600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Difranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, Mcneill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- 40.Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, Lange EM, Moss K, Barros SP, Weyant RJ, Liu Y, Newman AB, Beck JD, Offenbacher S. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. 2013;22:2312–2324. doi: 10.1093/hmg/ddt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dye BA. The relationship between periodontitis and alcohol use is not clear. J Evid Based Dent Pract. 2010;10:225–227. doi: 10.1016/j.jebdp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994;5:112–141. doi: 10.1111/j.1600-0757.1994.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 43.El-Zaatari ZM, Chami HA, Zaatari GS. Health effects associated with waterpipe smoking. Tob Control. 2015;24(Suppl 1):31–i43. doi: 10.1136/tobaccocontrol-2014-051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erdemir EO, Duran I, Haliloglu S. Effects of smoking on clinical parameters and the gingival crevicular fluid levels of IL-6 and TNF-alpha in patients with chronic periodontitis. J Clin Periodontol. 2004;31:99–104. doi: 10.1111/j.0303-6979.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 45.Faveri M, Rebello A, De Oliveira Dias R, Borges-Junior I, Duarte PM, Figueiredo LC, Feres M. Clinical and microbiologic effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized chronic periodontitis: smokers versus non-smokers. J Periodontol. 2014;85:581–591. doi: 10.1902/jop.2013.130278. [DOI] [PubMed] [Google Scholar]
- 46.Feres M, Bernal M, Matarazzo F, Faveri M, Duarte PM, Figueiredo LC. Subgingival bacterial recolonization after scaling and root planing in smokers with chronic periodontitis. Aust Dent J. 2015;60:225–232. doi: 10.1111/adj.12225. [DOI] [PubMed] [Google Scholar]
- 47.Fiore MJC, Baker Tb, Bailey Wc, Bennett G, Benowitz Nl, Christiansen Ba, Connell M, Curry Sj, Dorfman Sf, Fraser D, Froelicher Es, Goldstein Mg, Hasselblad V, Healton Cg, Heishman S, Henderson Pn, Heyman Rb, Huten C, Koh Hk, Kottke Te, Lando Ha, Leitzke C, Mecklenburg Re, Mermelstein Rj, Morgan G, Mullen Pd, Murray Ew, Orleans Ct, Piper Me, Robinson L, Stitzer Ml, Theobald W, Tommasello Ac, Villejo L, Wewers Me, Williams C. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Kottke TE, Lando HA. Treating tobacco use and dependence: quick reference guide for clinicians. Rockville, MD: US Department of Health and Human Services The Public Health Service; 2000. [Google Scholar]
- 49.Fisher MA, Taylor GW, Tilashalski KR. Smokeless tobacco and severe active periodontal disease, NHANES III. J Dent Res. 2005;84:705–710. doi: 10.1177/154405910508400804. [DOI] [PubMed] [Google Scholar]
- 50.Forrester K, Biglan A, Severson HH, Smolkowski K. Predictors of smoking onset over two years. Nicotine Tob Res. 2007;9:1259–1267. doi: 10.1080/14622200701705357. [DOI] [PubMed] [Google Scholar]
- 51.Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS One. 2011;6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fredriksson MI, Figueredo CM, Gustafsson A, Bergstrom KG, Asman BE. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol. 1999;70:1355–1360. doi: 10.1902/jop.1999.70.11.1355. [DOI] [PubMed] [Google Scholar]
- 53.Garcia RI, Compton R, Dietrich T. Risk assessment and periodontal prevention in primary care. Periodontol 2000. 2016;71:10–21. doi: 10.1111/prd.12124. [DOI] [PubMed] [Google Scholar]
- 54.Garcia RI, Nunn ME, Dietrich T. Risk calculation and periodontal outcomes. Periodontol 2000. 2009;50:65–77. doi: 10.1111/j.1600-0757.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 55.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 56.Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol. 2003;30:145–153. doi: 10.1034/j.1600-051x.2003.300201.x. [DOI] [PubMed] [Google Scholar]
- 57.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 58.Grady D, Greene J, Daniels TE, Ernster VL, Robertson PB, Hauck W, Greenspan D, Greenspan J, Silverman S., Jr Oral mucosal lesions found in smokeless tobacco users. J Am Dent Assoc. 1990;121:117–123. doi: 10.14219/jada.archive.1990.0139. [DOI] [PubMed] [Google Scholar]
- 59.Grossi SG, Genco RJ, Machtei EE, Ho AW, Koch G, Dunford R, Zambon JJ, Hausmann E. Assessment of risk for periodontal disease II. Risk indicators for alveolar bone loss. J Periodontol. 1995;66:23–29. doi: 10.1902/jop.1995.66.1.23. [DOI] [PubMed] [Google Scholar]
- 60.Grossi SG, Goodson JM, Gunsolley JC, Otomo-Corgel J, Bland PS, Doherty F, Comiskey J. Mechanical therapy with adjunctive minocycline microspheres reduces red-complex bacteria in smokers. J Periodontol. 2007;78:1741–1750. doi: 10.1902/jop.2007.070118. [DOI] [PubMed] [Google Scholar]
- 61.Grossi SG, Zambon J, Machtei EE, Schifferle R, Andreana S, Genco RJ, Cummins D, Harrap G. Effects of smoking and smoking cessation on healing after mechanical periodontal therapy. J Am Dent Assoc. 1997;128:599–607. doi: 10.14219/jada.archive.1997.0259. [DOI] [PubMed] [Google Scholar]
- 62.Gursoy UK, Kononen E, Pradhan-Palikhe P, Tervahartiala T, Pussinen PJ, Suominen-Taipale L, Sorsa T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol. 2010;37:487–493. doi: 10.1111/j.1600-051X.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- 63.Hach M, Holm-Pedersen P, Adegboye AR, Avlund K. The effect of alcohol consumption on periodontitis in older Danes. Int J Dent Hyg. 2015;13:261–267. doi: 10.1111/idh.12121. [DOI] [PubMed] [Google Scholar]
- 64.Haffajee AD, Socransky SS. Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol. 2001;28:377–388. doi: 10.1034/j.1600-051x.2001.028005377.x. [DOI] [PubMed] [Google Scholar]
- 65.Hanes PJ, Schuster GS, Lubas S. Binding, uptake, and release of nicotine by human gingival fibroblasts. J Periodontol. 1991;62:147–152. doi: 10.1902/jop.1991.62.2.147. [DOI] [PubMed] [Google Scholar]
- 66.Hartmann-Boyce J, Mcrobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:Cd010216. doi: 10.1002/14651858.CD010216.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hatsukami DK, Le CT, Zhang Y, Joseph AM, Mooney ME, Carmella SG, Hecht SS. Toxicant exposure in cigarette reducers versus light smokers. Cancer Epidemiol Biomarkers Prev. 2006;15:2355–2358. doi: 10.1158/1055-9965.EPI-06-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 69.Helme DW, Cohen EL, Parrish AJ. Health, masculinity and smokeless tobacco use among college-aged men. Health Commun. 2012;27:467–477. doi: 10.1080/10410236.2011.610257. [DOI] [PubMed] [Google Scholar]
- 70.Herrmann JM, Meyle J. Neutrophil activation and periodontal tissue injury. Periodontol 2000. 2015;69:111–127. doi: 10.1111/prd.12088. [DOI] [PubMed] [Google Scholar]
- 71.Holla LI, Jurajda M, Fassmann A, Dvorakova N, Znojil V, Vacha J. Genetic variations in the matrix metalloproteinase-1 promoter and risk of susceptibility and/or severity of chronic periodontitis in the Czech population. J Clin Periodontol. 2004;31:685–690. doi: 10.1111/j.1600-051X.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 72.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42:241–249. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hujoel PP, Del Aguila MA, Derouen TA, Bergstrom J. A hidden periodontitis epidemic during the 20th century? Community Dent Oral Epidemiol. 2003;31:1–6. doi: 10.1034/j.1600-0528.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 74.Hujoel PP, Drangsholt M, Spiekerman C, Derouen TA. Periodontitis-systemic disease associations in the presence of smoking--causal or coincidental? Periodontol 2000. 2002;30:51–60. doi: 10.1034/j.1600-0757.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 75.Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med. 2017;177:87–95. doi: 10.1001/jamainternmed.2016.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isaza-Guzman DM, Hernandez-Viana M, Bonilla-Leon DM, Hurtado-Cadavid MC, Tobon-Arroyave SI. Determination of NLRP3 (rs4612666) and IL-1B (rs1143634) genetic polymorphisms in periodontally diseased and healthy subjects. Arch Oral Biol. 2016;65:44–51. doi: 10.1016/j.archoralbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 77.Johannsen A, Susin C, Gustafsson A. Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontol 2000. 2014;64:111–126. doi: 10.1111/j.1600-0757.2012.00456.x. [DOI] [PubMed] [Google Scholar]
- 78.Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol 2000. 2007;44:178–194. doi: 10.1111/j.1600-0757.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 79.Johnson GK, Hill M. Cigarette smoking and the periodontal patient. J Periodontol. 2004;75:196–209. doi: 10.1902/jop.2004.75.2.196. [DOI] [PubMed] [Google Scholar]
- 80.Johnson JD, Houchens DP, Kluwe WM, Craig DK, Fisher GL. Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: a review. Crit Rev Toxicol. 1990;20:369–395. doi: 10.3109/10408449009089870. [DOI] [PubMed] [Google Scholar]
- 81.Jorenby DE, Fiore MC. The Agency for Health Care Policy and Research smoking cessation clinical practice guideline: basics and beyond. Prim Care. 1999;26:513–528. doi: 10.1016/s0095-4543(05)70115-9. [DOI] [PubMed] [Google Scholar]
- 82.Joshi V, Matthews C, Aspiras M, De Jager M, Ward M, Kumar P. Smoking decreases structural and functional resilience in the subgingival ecosystem. J Clin Periodontol. 2014;41:1037–1047. doi: 10.1111/jcpe.12300. [DOI] [PubMed] [Google Scholar]
- 83.Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down. J Periodontol. 1996;67:103–108. doi: 10.1902/jop.1996.67.2.103. [DOI] [PubMed] [Google Scholar]
- 84.Kamma JJ, Giannopoulou C, Vasdekis VG, Mombelli A. Cytokine profile in gingival crevicular fluid of aggressive periodontitis: influence of smoking and stress. J Clin Periodontol. 2004;31:894–902. doi: 10.1111/j.1600-051X.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 85.Kamma JJ, Nakou M, Baehni PC. Clinical and microbiological characteristics of smokers with early onset periodontitis. J Periodontal Res. 1999;34:25–33. doi: 10.1111/j.1600-0765.1999.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 86.Katono T, Kawato T, Tanabe N, Tanaka H, Suzuki N, Kitami S, Morita T, Motohashi M, Maeno M. Effects of nicotine and lipopolysaccharide on the expression of matrix metalloproteinases, plasminogen activators, and their inhibitors in human osteoblasts. Arch Oral Biol. 2009;54:146–155. doi: 10.1016/j.archoralbio.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 87.Kaufmann A, Hitsman B, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, Gariti P, Tyndale RF, Schnoll RA. Rate of nicotine metabolism and smoking cessation outcomes in a community-based sample of treatment-seeking smokers. Addict Behav. 2015;51:93–99. doi: 10.1016/j.addbeh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kazor C, Taylor GW, Loesche WJ. The prevalence of BANA-hydrolyzing periodontopathic bacteria in smokers. J Clin Periodontol. 1999;26:814–821. doi: 10.1111/j.1600-051x.1999.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 89.Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15:68–74. [PubMed] [Google Scholar]
- 90.Kim HS, Son JH, Yi HY, Hong HK, Suh HJ, Bae KH. Association between harmful alcohol use and periodontal status according to gender and smoking. BMC Oral Health. 2014;14:73. doi: 10.1186/1472-6831-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim JS, Park JY, Chung WY, Choi MA, Cho KS, Park KK. Polymorphisms in genes coding for enzymes metabolizing smoking-derived substances and the risk of periodontitis. J Clin Periodontol. 2004;31:959–964. doi: 10.1111/j.1600-051X.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 92.Kinane DF, Radvar M. The effect of smoking on mechanical and antimicrobial periodontal therapy. J Periodontol. 1997;68:467–472. doi: 10.1902/jop.1997.68.5.467. [DOI] [PubMed] [Google Scholar]
- 93.King BA, Tynan MA, Dube SR, Arrazola R. Flavored-little-cigar flavored-cigarette use among U.S. middle and high school students. J Adolesc Health. 2014;54:40–46. doi: 10.1016/j.jadohealth.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knox P. Blown Up By An E-cig: Vaper shares shocking pictures after suffering second-degree burns and losing seven teeth when e-cigarette exploded. The Sun. 2016 Available from: https://www.thesun.co.uk/news/2628586/vaper-shares-shocking-pictures-after-suffering-second-degree-burns-and-losing-seven-teeth-when-e-cigarette-exploded/
- 95.Kraal JH, Chancellor MB, Bridges RB, Bemis KG, Hawke JE. Variations in the gingival polymorphonuclear leukocyte migration rate in dogs induced by chemotactic autologous serum and migration inhibitor from tobacco smoke. J Periodontal Res. 1977;12:242–249. doi: 10.1111/j.1600-0765.1977.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 96.Krall EA, Dietrich T, Nunn ME, Garcia RI. Risk of tooth loss after cigarette smoking cessation. Prev Chronic Dis. 2006;3:A115. [PMC free article] [PubMed] [Google Scholar]
- 97.Krall EA, Garvey AJ, Garcia RI. Alveolar bone loss and tooth loss in male cigar and pipe smokers. J Am Dent Assoc. 1999;130:57–64. doi: 10.14219/jada.archive.1999.0029. [DOI] [PubMed] [Google Scholar]
- 98.Kumar PS, Matthews CR, Joshi V, De Jager M, Aspiras M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun. 2011;79:4730–4738. doi: 10.1128/IAI.05371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lages EJ, Costa FO, Cortelli SC, Cortelli JR, Cota LO, Cyrino RM, Lages EM, Nobre-Franco GC, Brito JA, Gomez RS. Alcohol consumption and periodontitis: quantification of periodontal pathogens and cytokines. J Periodontol. 2015;86:1058–1068. doi: 10.1902/jop.2015.150087. [DOI] [PubMed] [Google Scholar]
- 100.Lamster IB, Kaufman E, Grbic JT, Winston LJ, Singer RE. Beta-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J Periodontol. 2003;74:353–359. doi: 10.1902/jop.2003.74.3.353. [DOI] [PubMed] [Google Scholar]
- 101.Launay JM, Del Pino M, Chironi G, Callebert J, Peoc'h K, Megnien JL, Mallet J, Simon A, Rendu F. Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS One. 2009;4:e7959. doi: 10.1371/journal.pone.0007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee M, Choi YH, Sagong J, Yu S, Kim Y, Lee D, Kim S. The interactive association of smoking and drinking levels with presence of periodontitis in South Korean adults. BMC Oral Health. 2016;16:80. doi: 10.1186/s12903-016-0268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, Benowitz N. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 104.Li QY, Zhao HS, Meng HX, Zhang L, Xu L, Chen ZB, Shi D, Feng XH, Zhu XL. Association analysis between interleukin-1 family polymorphisms and generalized aggressive periodontitis in a Chinese population. J Periodontol. 2004;75:1627–1635. doi: 10.1902/jop.2004.75.12.1627. [DOI] [PubMed] [Google Scholar]
- 105.Little SJ, Stevens VJ, Lachance PA, Severson HH, Bartley MH, Lichtenstein E, Leben JR. Smokeless tobacco habits and oral mucosal lesions in dental patients. J Public Health Dent. 1992;52:269–276. doi: 10.1111/j.1752-7325.1992.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 106.Lod S, Johansson T, Abrahamsson KH, Larsson L. The influence of epigenetics in relation to oral health. Int J Dent Hyg. 2014;12:48–54. doi: 10.1111/idh.12030. [DOI] [PubMed] [Google Scholar]
- 107.Lu D, Meng H, Xu L, Lu R, Zhang L, Chen Z, Feng X, Shi D, Tian Y, Wang X. New attempts to modify periodontal risk assessment for generalized aggressive periodontitis: a retrospective study. J Periodontol. 2013;84:1536–1545. doi: 10.1902/jop.2013.120427. [DOI] [PubMed] [Google Scholar]
- 108.Macfarlane GD, Herzberg MC, Wolff LF, Hardie NA. Refractory periodontitis associated with abnormal polymorphonuclear leukocyte phagocytosis and cigarette smoking. J Periodontol. 1992;63:908–913. doi: 10.1902/jop.1992.63.11.908. [DOI] [PubMed] [Google Scholar]
- 109.Mager DL, Haffajee AD, Socransky SS. Effects of periodontitis and smoking on the microbiota of oral mucous membranes and saliva in systemically healthy subjects. J Clin Periodontol. 2003;30:1031–1037. doi: 10.1046/j.0303-6979.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 110.Majeed BA, Sterling KL, Weaver SR, Pechacek TF, Eriksen MP. Prevalence harm perceptions of hookah smoking among U.S. adults, 2014–2015. Addict Behav. 2017;69:78–86. doi: 10.1016/j.addbeh.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matthews JB, Chen FM, Milward MR, Wright HJ, Carter K, Mcdonagh A, Chapple IL. Effect of nicotine, cotinine and cigarette smoke extract on the neutrophil respiratory burst. J Clin Periodontol. 2011;38:208–218. doi: 10.1111/j.1600-051X.2010.01676.x. [DOI] [PubMed] [Google Scholar]
- 112.Mcneill A, Brose LS, Calder R, Hitchman SC, Hajek P, Mcrobbie H. E-cigarettes: the need for clear communication on relative risks. Lancet. 2015;386:1237. doi: 10.1016/S0140-6736(15)00079-3. [DOI] [PubMed] [Google Scholar]
- 113.Meisel P, Schwahn C, Gesch D, Bernhardt O, John U, Kocher T. Dose-effect relation of smoking and the interleukin-1 gene polymorphism in periodontal disease. J Periodontol. 2004;75:236–242. doi: 10.1902/jop.2004.75.2.236. [DOI] [PubMed] [Google Scholar]
- 114.Meisel P, Siegemund A, Grimm R, Herrmann FH, John U, Schwahn C, Kocher T. The interleukin-1 polymorphism, smoking, and the risk of periodontal disease in the population-based SHIP study. J Dent Res. 2003;82:189–193. doi: 10.1177/154405910308200308. [DOI] [PubMed] [Google Scholar]
- 115.Miller WrRS. Motivational interviewing: preparing people for change. New York: NY: The Guilford Press; 2002. [Google Scholar]
- 116.Mooney J, Hodge PJ, Kinane DF. Humoral immune response in early-onset periodontitis: influence of smoking. J Periodontal Res. 2001;36:227–232. doi: 10.1034/j.1600-0765.2001.036004227.x. [DOI] [PubMed] [Google Scholar]
- 117.Nile CJ, Sherrabeh S, Ramage G, Lappin DF. Comparison of circulating tumour necrosis factor superfamily cytokines in periodontitis patients undergoing supportive therapy: a case-controlled cross-sectional study comparing smokers and non-smokers in health and disease. J Clin Periodontol. 2013;40:875–882. doi: 10.1111/jcpe.12134. [DOI] [PubMed] [Google Scholar]
- 118.Nishida N, Tanaka M, Hayashi N, Nagata H, Takeshita T, Nakayama K, Morimoto K, Shizukuishi S. Association of ALDH(2) genotypes and alcohol consumption with periodontitis. J Dent Res. 2004;83:161–165. doi: 10.1177/154405910408300215. [DOI] [PubMed] [Google Scholar]
- 119.Novak MJ, Johns LP, Miller RC, Bradshaw MH. Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe, generalized, chronic periodontitis. J Periodontol. 2002;73:762–769. doi: 10.1902/jop.2002.73.7.762. [DOI] [PubMed] [Google Scholar]
- 120.Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 121.Ozcaka O, Bicakci N, Pussinen P, Sorsa T, Kose T, Buduneli N. Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis. Oral Dis. 2011;17:68–76. doi: 10.1111/j.1601-0825.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- 122.Ozcaka O, Nalbantsoy A, Kose T, Buduneli N. Plasma osteoprotegerin levels are decreased in smoker chronic periodontitis patients. Aust Dent J. 2010;55:405–410. doi: 10.1111/j.1834-7819.2010.01261.x. [DOI] [PubMed] [Google Scholar]
- 123.Paquette D, Oringer R, Lessem J, Offenbacher S, Genco R, Persson GR, Santucci EA, Williams RC. Locally delivered minocycline microspheres for the treatment of periodontitis in smokers. J Clin Periodontol. 2003;30:787–794. doi: 10.1034/j.1600-051x.2003.00375.x. [DOI] [PubMed] [Google Scholar]
- 124.Park JB, Han K, Park YG, Ko Y. Association between alcohol consumption and periodontal disease: the 2008 to 2010 Korea National Health and Nutrition Examination Survey. J Periodontol. 2014;85:1521–1528. doi: 10.1902/jop.2014.130782. [DOI] [PubMed] [Google Scholar]
- 125.Pauletto NC, Liede K, Nieminen A, Larjava H, Uitto VJ. Effect of cigarette smoking on oral elastase activity in adult periodontitis patients. J Periodontol. 2000;71:58–62. doi: 10.1902/jop.2000.71.1.58. [DOI] [PubMed] [Google Scholar]
- 126.Payne JB, Johnson GK, Reinhardt RA, Dyer JK, Maze CA, Dunning DG. Nicotine effects on PGE2 and IL-1 beta release by LPS-treated human monocytes. J Periodontal Res. 1996;31:99–104. doi: 10.1111/j.1600-0765.1996.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 127.Payne JB, Johnson GK, Reinhardt RA, Maze CR, Dyer JK, Patil KD. Smokeless tobacco effects on monocyte secretion of PGE2 and IL-1 beta. J Periodontol. 1994;65:937–941. doi: 10.1902/jop.1994.65.10.937. [DOI] [PubMed] [Google Scholar]
- 128.Persson L, Bergstrom J, Gustafsson A. Effect of tobacco smoking on neutrophil activity following periodontal surgery. J Periodontol. 2003;74:1475–1482. doi: 10.1902/jop.2003.74.10.1475. [DOI] [PubMed] [Google Scholar]
- 129.Persson L, Bergstrom J, Ito H, Gustafsson A. Tobacco smoking and neutrophil activity in patients with periodontal disease. J Periodontol. 2001;72:90–95. doi: 10.1902/jop.2001.72.1.90. [DOI] [PubMed] [Google Scholar]
- 130.Pitiphat W, Merchant AT, Rimm EB, Joshipura KJ. Alcohol consumption increases periodontitis risk. J Dent Res. 2003;82:509–513. doi: 10.1177/154405910308200704. [DOI] [PubMed] [Google Scholar]
- 131.Polk DE, Wang X, Feingold E, Shaffer JR, Weeks DE, Weyant RJ, Crout RJ, Mcneil DW, Marazita ML. Effects of smoking and genotype on the PSR index of periodontal disease in adults aged 18–49. Int J Environ Res Public Health. 2012;9:2839–2850. doi: 10.3390/ijerph9082839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Preber H, Bergstrom J, Linder LE. Occurrence of periopathogens in smoker and non-smoker patients. J Clin Periodontol. 1992;19:667–671. doi: 10.1111/j.1600-051x.1992.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 133.Prochaska JO, Velicer WF, Diclemente CC, Fava J. Measuring processes of change: applications to the cessation of smoking. J Consult Clin Psychol. 1988;56:520–528. doi: 10.1037//0022-006x.56.4.520. [DOI] [PubMed] [Google Scholar]
- 134.Quinn SM, Zhang JB, Gunsolley JC, Schenkein HA, Tew JG. The influence of smoking and race on adult periodontitis and serum IgG2 levels. J Periodontol. 1998;69:171–177. doi: 10.1902/jop.1998.69.2.171. [DOI] [PubMed] [Google Scholar]
- 135.Quist-Paulsen P, Bakke PS, Gallefoss F. Predictors of smoking cessation in patients admitted for acute coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:472–477. doi: 10.1097/01.hjr.0000183914.90236.01. [DOI] [PubMed] [Google Scholar]
- 136.Raulin LA, Mcpherson JC, 3rd, Mcquade MJ, Hanson BS. The effect of nicotine on the attachment of human fibroblasts to glass and human root surfaces in vitro. J Periodontol. 1988;59:318–325. doi: 10.1902/jop.1988.59.5.318. [DOI] [PubMed] [Google Scholar]
- 137.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Razzouk S, Termechi O. Host genome, epigenome, and oral microbiome interactions: toward personalized periodontal therapy. J Periodontol. 2013;84:1266–1271. doi: 10.1902/jop.2012.120531. [DOI] [PubMed] [Google Scholar]
- 139.Ribeiro MS, Pacheco RB, Fischer RG, Macedo JM. Interaction of IL1B and IL1RN polymorphisms, smoking habit, gender, and ethnicity with aggressive and chronic periodontitis susceptibility. Contemp Clin Dent. 2016;7:349–356. doi: 10.4103/0976-237X.188560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robertson PB, Walsh M, Greene J, Ernster V, Grady D, Hauck W. Periodontal effects associated with the use of smokeless tobacco. J Periodontol. 1990;61:438–443. doi: 10.1902/jop.1990.61.7.438. [DOI] [PubMed] [Google Scholar]
- 141.Rollnick S, Miller WR, Butler CC, Aloia MS. Motivational interviewing in health care: helping patients change behavior. Taylor & Francis; 2008. [Google Scholar]
- 142.Rollnick SMW, Butler Cc. Motivational Interviewing in Healthcare: Helping Patients Change Behavior. New York, NY: The Guildford Press; 2008. [Google Scholar]
- 143.Ryder MI. Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol 2000. 2010;53:124–137. doi: 10.1111/j.1600-0757.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 144.Ryder MI. The influence of smoking on host responses in periodontal infections. Periodontol 2000. 2007;43:267–277. doi: 10.1111/j.1600-0757.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- 145.Ryder MI, Fujitaki R, Johnson G, Hyun W. Alterations of neutrophil oxidative burst by in vitro smoke exposure: implications for oral and systemic diseases. Ann Periodontol. 1998;3:76–87. doi: 10.1902/annals.1998.3.1.76. [DOI] [PubMed] [Google Scholar]
- 146.Ryder MI, Pons B, Adams D, Beiswanger B, Blanco V, Bogle G, Donly K, Hallmon W, Hancock EB, Hanes P, Hawley C, Johnson L, Wang HL, Wolinsky L, Yukna R, Polson A, Carron G, Garrett S. Effects of smoking on local delivery of controlled-release doxycycline as compared to scaling and root planing. J Clin Periodontol. 1999;26:683–691. doi: 10.1034/j.1600-051x.1999.261008.x. [DOI] [PubMed] [Google Scholar]
- 147.Ryder MI, Saghizadeh M, Ding Y, Nguyen N, Soskolne A. Effects of tobacco smoke on the secretion of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta from peripheral blood mononuclear cells. Oral Microbiol Immunol. 2002;17:331–336. doi: 10.1034/j.1399-302x.2002.170601.x. [DOI] [PubMed] [Google Scholar]
- 148.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.U.S. Department of Health and Human Services. Published e-cigarette use among youth and young adults. A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; Atlanta, GA: 2016. [Google Scholar]
- 150.Severson HH, Forrester KK, Biglan A. Use of smokeless tobacco is a risk factor for cigarette smoking. Nicotine Tob Res. 2007;9:1331–1337. doi: 10.1080/14622200701705209. [DOI] [PubMed] [Google Scholar]
- 151.Shariff JA, Ahluwalia KP, Papapanou PN. Relationship between frequent recreational cannabis (marijuana and hashish) use and periodontitis in adults in the United States: Nhanes 2011–12. J Periodontol. 2016:1–12. doi: 10.1902/jop.2016.160370. [DOI] [PubMed] [Google Scholar]
- 152.Sharma M, Bairy I, Pai K, Satyamoorthy K, Prasad S, Berkovitz B, Radhakrishnan R. Salivary IL-6 levels in oral leukoplakia with dysplasia and its clinical relevance to tobacco habits and periodontitis. Clin Oral Investig. 2011;15:705–714. doi: 10.1007/s00784-010-0435-5. [DOI] [PubMed] [Google Scholar]
- 153.Shepherd S. Alcohol consumption a risk factor for periodontal disease. Evid Based Dent. 2011;12:76. doi: 10.1038/sj.ebd.6400808. [DOI] [PubMed] [Google Scholar]
- 154.Shimazaki Y, Saito T, Kiyohara Y, Kato I, Kubo M, Iida M, Yamashita Y. Relationship between drinking and periodontitis: the Hisayama Study. J Periodontol. 2005;76:1534–1541. doi: 10.1902/jop.2005.76.9.1534. [DOI] [PubMed] [Google Scholar]
- 155.Soder B. Neutrophil elastase activity, levels of prostaglandin E2, and matrix metalloproteinase-8 in refractory periodontitis sites in smokers and non-smokers. Acta Odontol Scand. 1999;57:77–82. doi: 10.1080/000163599428940. [DOI] [PubMed] [Google Scholar]
- 156.Soder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J Clin Periodontol. 2002;29:384–391. doi: 10.1034/j.1600-051x.2002.290502.x. [DOI] [PubMed] [Google Scholar]
- 157.Souto GR, Queiroz-Junior CM, Costa FO, Mesquita RA. Effect of smoking on immunity in human chronic periodontitis. Immunobiology. 2014;219:909–915. doi: 10.1016/j.imbio.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 158.Souto GR, Queiroz-Junior CM, Costa FO, Mesquita RA. Smoking effect on chemokines of the human chronic periodontitis. Immunobiology. 2014;219:633–636. doi: 10.1016/j.imbio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 159.Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:Cd008286. doi: 10.1002/14651858.CD008286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2015:Cd009670. doi: 10.1002/14651858.CD009670.pub3. [DOI] [PubMed] [Google Scholar]
- 161.Stein SH, Green BE, Scarbecz M. Augmented transforming growth factor-beta1 in gingival crevicular fluid of smokers with chronic periodontitis. J Periodontol. 2004;75:1619–1626. doi: 10.1902/jop.2004.75.12.1619. [DOI] [PubMed] [Google Scholar]
- 162.Stoltenberg JL, Osborn JB, Pihlstrom BL, Herzberg MC, Aeppli DM, Wolff LF, Fischer GE. Association between cigarette smoking, bacterial pathogens, and periodontal status. J Periodontol. 1993;64:1225–1230. doi: 10.1902/jop.1993.64.12.1225. [DOI] [PubMed] [Google Scholar]
- 163.Strietzel FP, Reichart PA, Kale A, Kulkarni M, Wegner B, Kuchler I. Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis. J Clin Periodontol. 2007;34:523–544. doi: 10.1111/j.1600-051X.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 164.Suls JM, Luger TM, Curry SJ, Mermelstein RJ, Sporer AK, An LC. Efficacy of smoking-cessation interventions for young adults: a meta-analysis. Am J Prev Med. 2012;42:655–662. doi: 10.1016/j.amepre.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sundar IK, Javed F, Romanos GE, Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget. 2016;7:77196–77204. doi: 10.18632/oncotarget.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Susin C, Wagner MC, Haas AN, Oppermann RV, Albandar JM. The association between alcohol consumption and periodontitis in southern Brazilian adults. J Periodontal Res. 2015;50:622–628. doi: 10.1111/jre.12242. [DOI] [PubMed] [Google Scholar]
- 167.Tanur E, Mcquade MJ, Mcpherson JC, Al-Hashimi IH, Rivera-Hidalgo F. Effects of nicotine on the strength of attachment of gingival fibroblasts to glass and non-diseased human root surfaces. J Periodontol. 2000;71:717–722. doi: 10.1902/jop.2000.71.5.717. [DOI] [PubMed] [Google Scholar]
- 168.Tappia PS, Troughton KL, Langley-Evans SC, Grimble RF. Cigarette smoking influences cytokine production and antioxidant defences. Clin Sci (Lond) 1995;88:485–489. doi: 10.1042/cs0880485. [DOI] [PubMed] [Google Scholar]
- 169.Tezal M, Grossi SG, Ho AW, Genco RJ. Alcohol consumption periodontal disease The Third National Health and Nutrition Examination Survey. J Clin Periodontol. 2004;31:484–488. doi: 10.1111/j.1600-051X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 170.Thomson WM, Poulton R, Broadbent JM, Moffitt TE, Caspi A, Beck JD, Welch D, Hancox RJ. Cannabis smoking and periodontal disease among young adults. Jama. 2008;299:525–531. doi: 10.1001/jama.299.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Toker H, Akpinar A, Aydin H, Poyraz O. Influence of smoking on interleukin-1beta level, oxidant status and antioxidant status in gingival crevicular fluid from chronic periodontitis patients before and after periodontal treatment. J Periodontal Res. 2012;47:572–577. doi: 10.1111/j.1600-0765.2012.01468.x. [DOI] [PubMed] [Google Scholar]
- 172.Tsigarida AA, Dabdoub SM, Nagaraja HN, Kumar PS. The influence of smoking on the peri-implant microbiome. J Dent Res. 2015;94:1202–1217. doi: 10.1177/0022034515590581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tymkiw KD, Thunell DH, Johnson GK, Joly S, Burnell KK, Cavanaugh JE, Brogden KA, Guthmiller JM. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol. 2011;38:219–228. doi: 10.1111/j.1600-051X.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Van Der Velden U, Varoufaki A, Hutter JW, Xu L, Timmerman MF, Van Winkelhoff AJ, Loos BG. Effect of smoking and periodontal treatment on the subgingival microflora. J Clin Periodontol. 2003;30:603–610. doi: 10.1034/j.1600-051x.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 175.Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, Serhan CN. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. 2015;94:148–156. doi: 10.1177/0022034514557331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vaz P, Gallas MM, Braga AC, Sampaio-Fernandes JC, Felino A, Tavares P. IL1 gene polymorphisms and unsuccessful dental implants. Clin Oral Implants Res. 2012;23:1404–1413. doi: 10.1111/j.1600-0501.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 177.Vernillo AT, Ramamurthy NS, Golub LM, Rifkin BR. The nonantimicrobial properties of tetracycline for the treatment of periodontal disease. Curr Opin Periodontol. 1994:111–118. [PubMed] [Google Scholar]
- 178.Walsh MM, Ellison JA. Treatment of tobacco use and dependence: the role of the dental professional. J Dent Educ. 2005;69:521–537. [PubMed] [Google Scholar]
- 179.Wang CW, Colas RA, Dalli J, Arnardottir HH, Nguyen D, Hasturk H, Chiang N, Van Dyke TE, Serhan CN. Maresin 1 Biosynthesis and proresolving anti-infective functions with human-localized aggressive periodontitis leukocytes. Infect Immun. 2015;84:658–665. doi: 10.1128/IAI.01131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang J, Lv J, Wang W, Jiang X. Alcohol consumption and risk of periodontitis: a meta-analysis. J Clin Periodontol. 2016;43:572–583. doi: 10.1111/jcpe.12556. [DOI] [PubMed] [Google Scholar]
- 181.Williams GC, Mcgregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, Deci EL. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychology. 2006;25:91. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 182.Williams RC, Paquette DW, Offenbacher S, Adams DF, Armitage GC, Bray K, Caton J, Cochran DL, Drisko CH, Fiorellini JP, Giannobile WV, Grossi S, Guerrero DM, Johnson GK, Lamster IB, Magnusson I, Oringer RJ, Persson GR, Van Dyke TE, Wolff LF, Santucci EA, Rodda BE, Lessem J. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol. 2001;72:1535–1544. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 183.Wu L, Zhou Y, Zhou Z, Liu Y, Bai Y, Xing X, Wang X. Nicotine induces the production of IL-1beta and IL-8 via the alpha7 nAChR/NF-kappaB pathway in human periodontal ligament cells: an in vitro study. Cell Physiol Biochem. 2014;34:423–431. doi: 10.1159/000363011. [DOI] [PubMed] [Google Scholar]
- 184.Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67:1050–1054. doi: 10.1902/jop.1996.67.10s.1050. [DOI] [PubMed] [Google Scholar]