Highlights
-
•
We examined the role of TRPV1 in the generation of spontaneous APs in NGF-treated cultured DRG neurons of rats.
-
•
Spontaneous firing in the on-cell configuration was abolished by TRPV1 antagonists capsazepine and BCTC.
-
•
Chronic treatment with NGF induced capsazepine- and BCTC-sensitive cation conductance.
-
•
NGF-induced cation conductance through TRPV1 causes spontaneous firing.
Keywords: Action potential, Neuropathic pain, Patch clamp, Sensory neuron
Abstract
Dorsal root ganglion (DRG) neurons cultured in the presence of nerve growth factor (NGF, 100 ng/ml) often show a spontaneous action potential. Underlying mechanisms of this spontaneous firing were examined using the patch clamp technique. The spontaneous firing in the on-cell configuration was abolished by a decrease in the Na+ concentration and by the TRPV1 antagonists capsazepine (10 μM) and BCTC (1 μM). These responses were accompanied by hyperpolarization of the resting potential. The holding current observed in neurons voltage clamped at –60 mV in the whole-cell configuration was significantly larger in the neurons that fired spontaneously, indicating that these neurons had an additional cation conductance that caused depolarization and triggered action potentials. The holding current in the firing neurons was decreased by extracellular Na+ reduction, capsazepine and BCTC. The amplitudes of the capsazepine- or BCTC-sensitive component of the holding current in the spontaneously firing neurons were ten times as large as those recorded in the other neurons showing no spontaneous firing. However, the amplitudes of the current responses to capsaicin (1 μM) were not different regardless of the presence of spontaneous firing or treatment with NGF. These results indicate that chronic NGF treatment of cultured DRG neurons in rats induces a constitutively active cation conductance through TRPV1, which depolarizes the neurons and triggers spontaneous action potentials in the absence of any stimuli. Since NGF in the DRG is reported to increase after nerve injury, this NGF-mediated regulation of TRPV1 may be a cause of the pathogenesis of neuropathic pain.
1. Introduction
Peripheral nerve injury often causes an abnormal pain that continues after relief of the injury itself. Patients feel pain in response to weak stimuli that usually cause no pain (allodynia, hyperalgesia) or in the absence of any stimuli (chronic pain). This condition is called neuropathic pain, and it is well known that neuropathic pain is poorly responsive to usual analgesic treatments, including the administration of narcotic drugs such as morphine. At present, effective therapy for neuropathic pain is lacking, and neuropathic pain spoils the quality of life of many patients. The pathogenic mechanisms of neuropathic pain are complicated and not well understood, which may be a reason that we have no effective treatment for neuropathic pain. It is reported that artificial injury of peripheral nerves in rats induces neuropathic symptoms (Bennett and Xie, 1988; Seltzer et al., 1990; Kim and Chung, 1992; Decosterd and Woolf, 2000; Winkelstein et al., 2001; Barrot, 2012), and abnormal spontaneous firing has been observed in sensory neurons of some animal models with nerve injury, both in vivo (Burchiel, 1984; Devor et al., 1992; Eide, 1998; Liu et al., 2000; Sun et al., 2005) and in vitro (Petersen et al., 1996; Study and Kral, 1996; Amir et al., 1999; Devor, 1999; Liu et al., 1999). Such abnormal firing is considered to be a cause of spontaneous pain.
Nerve growth factor (NGF) is known as one of the mediators that cause neuropathic pain because NGF induces hyperalgesia in rats (Lewin et al., 1993; Woolf et al., 1994; Andreev et al., 1995) and because the expression level of NGF in the dorsal root ganglion (DRG) rises after nerve injury (Herzberg et al., 1997; Shen et al., 1999). Therefore, trials using anti-NGF agents to cure neuropathic pain conditions have been conducted (Cattaneo, 2010; Ossipov, 2011; McKelvey et al., 2013). Actions of NGF in the pathogenesis of neuropathic pain are complicated: NGF seems to have effects on both peripheral tissues and the central nervous system (Lewin et al., 1994; Hao et al., 2000). It is also reported that increased NGF in the DRG causes an extension of sympathetic nerves that make synapses onto DRG neurons and transmit excitatory signals by releasing noradrenaline (Zhang and Tan, 2011). On the other hand, it was reported that DRG neurons that were isolated from adult rats and cultured in the presence of NGF generated action potentials (APs) spontaneously (Kitamura et al., 2005); from these neurons, spontaneous APs were recorded in the on-cell configuration without intracellular dialysis with an artificial solution, and spontaneous action currents (named “Isp”) were recorded even under the voltage-clamped condition in the whole-cell configuration (Kayano et al., 2013). Based on the evidence that Isp was blocked by tetrodotoxin (a blocker of the voltage-gated Na+ channel), it is concluded that Isp reflects spontaneous discharges occurring in loosely voltage-clamped areas of the cell membrane. Chronic treatment of DRG neurons with NGF seemed to activate an intrinsic mechanism, which caused the hyperexcitability, within the membrane of the soma of DRG neurons because the Isp was also recorded from the outside-out patch membranes excised from the soma (Kayano et al., 2013).
The essential factors for neurons to generate an AP are (1) a resting membrane potential that is polarized below the threshold potential for the generation of the AP and (2) an ion conductance that drives membrane potentials to a potential above the threshold of the AP. We hypothesized that NGF induces some additional ionic conductance, which is constitutively active in the absence of any stimuli, in cultured DRG neurons and that this constitutively active conductance makes neurons hyperexcitable. One of the typical ion channels that confers such conductance to neurons is a non-selective cation channel belonging to the transient receptor potential (TRP) superfamily. Among these channels, TRP vanilloid 1 (TRPV1) plays very important roles in nociception (Caterina et al., 1997). It is reported that NGF increases the expression level and activity of TRPV1 in trigeminal neurons (Price et al., 2005), DRG neurons (Ji et al., 2002; Stein et al., 2006; Eskander et al., 2015) and the heterologous expression system (Zhang et al., 2005; Stein et al., 2006). Therefore, we examined the role of TRPV1 in the generation of spontaneous APs in NGF-treated cultured DRG neurons of rats in the present study and found that chronic treatment with NGF induces an additional cation conductance through TRPV1, which triggers spontaneous firing.
2. Experimental procedures
2.1. Cell isolation and culture
All animal experiments were performed in accordance with the guidelines of Tottori University, and this study was approved by the Institutional Animal Care and Use Committee, Tottori University. DRG neurons were isolated from male Wistar rats (7–12 weeks old) using a conventional enzymatic procedure. The rats were sacrificed by decapitation under anesthesia with isoflurane, and all efforts were made to minimize the suffering of the rats. Ganglia were dissected from the entire length of the vertebral column. The collected ganglia were incubated at 37 °C in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing collagenase type IV (200–400 U/ml; Worthington Biochemicals, Lakewood, NJ, USA), DNase I (1–50 μg/ml; Sigma, St Louis, MO, USA) and bovine serum albumin (BSA, 1 mg/ml, Sigma) for 2 h and then rinsed with PBS to remove collagenase. Next, ganglia were incubated in PBS containing trypsin (Gibco trypsin 1:250; 0.25%; Life Technologies, Carlsbad, CA, USA) and BSA (1 mg/ml) for 10 min. After the enzymatic digestion, tissues were gently agitated with a silicon-coated Pasteur pipette and centrifuged to remove the enzymes. The isolated cells were suspended in Dulbecco’s-modified Eagle medium (DMEM, Life Technologies) containing 4.5 g/l of glucose and cultured on coverslips coated with poly-D-lysine (Sigma). Cells were cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2 until use. DMEM was supplemented with 10% fetal bovine serum (MP Biochemicals, Irvine, CA, USA), 100 U/ml penicillin (Sigma), 100 ng/ml streptomycin (Sigma), and 5 μM cytosine arabinoside (Sigma). The culture medium was changed every 2–3 days. NGF-7S (100 ng/ml, Sigma) was added to the medium 3–5 days after the isolation of neurons. Neurons were used in the electrophysiological experiments after 4–8 days of culture.
2.2. Electrophysiology
The on-cell and whole-cell recording was made at room temperature (22–24 °C). Heat-polished glass electrodes with a tip resistance of 2.5–4 MΩ were used. DRG neurons with a small diameter (10–30 μm), which had been reported to be largely C-neurons (Harper and Lawson, 1985a, 1985b), were used to record membrane potentials and currents. Neurons with these sizes are well known to express TRPV1 (Luo et al., 2004; Yu et al., 2008) and we confirmed TRPV1 expression by recording capsaicin-evoked current responses in this study. A standard bath solution consisted of 150 NaCl, 6 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 D-glucose and 10 HEPES (in mM), and the pH was adjusted to 7.4 with Tris. When an effect of a decrease in the Na+ concentration was examined, Na+ was replaced with N-methyl-D-glucamine chloride (NMDG-Cl; Merck, Darmstadt, Germany). The pipette solution consisted of 130 K-gluconate, 10 Na-gluconate, 4.5 MgCl2, 0.74 CaCl2, 10 EGTA-2K and 10 HEPES (in mM), and the pH was adjusted to 7.3 with Tris or gluconate. The liquid junction potential between the pipette solution and the bath solution was not corrected because it was recorded to be smaller than 2 mV. Neurons were continuously perfused with the bath solution at a flow rate of 1 ml/min throughout the experiments. Current responses under the voltage-clamped condition were measured with Axopatch 200 A (Molecular Devices, Sunnyvale, CA, USA) or EPC-10 (HEKA, Lambrecht/Pfalz, Germany), and potential changes under the current-clamp condition were measured by EPC-10 (HEKA). Cell capacitances were determined by integrating the area under a capacity transient current elicited by a 5-mV voltage step from the holding potential, and a series resistance was also calculated from the capacity transient and the cell capacitance. Voltage errors at the holding potential caused by a series resistance were smaller than 1 mV and were not corrected. To estimate basal levels of resting membrane potentials or holding currents between spontaneous spikes, we calculated a moving average during 10 s, in which the influence of rapid responses such as APs was minimized. In the figures, the moving average during 10 s is shown as the basal level of the membrane potentials and holding currents.
2.3. Drugs
A concentrated stock solution of NGF-7S at 10 μg/ml was made by dissolution in DMEM and stored at –30 °C until use. Concentrated stock solutions of 4-(3-chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl) phenyl]-1- piperazinecarboxamide (BCTC, 10 mM, Sigma), 5’-iodoresiniferatoxin (5’-IRTX; 200 μM; Alomone Labs, Jerusalem, Israel), capsaicin (10 mM, Sigma), capsazepine (10 mM, Sigma), icilin (50 mM, Sigma) and SB366791 (30 mM, Sigma) were made by dissolution in DMSO and stored at –30 °C until use. All other chemicals used were of analytical grade.
2.4. Data analysis and statistics
Data acquisition was performed at a sampling frequency of 40–100 kHz throughout the experiments by a personal computer (Macintosh; Apple, Cupertino, CA, USA) in conjunction with an analog/digital converter (Power Lab; AD Instruments, Castle Hill, NSW, Australia). Data were analyzed with AxoGraph (AxoGraph Scientific, Sydney, NSW, Australia), Patch Master (HEKA), Lab Chart (AD Instruments), IGOR Pro (WaveMetrics, Lake Oswego, OR, USA) and Excel (Microsoft, Redmond, WA, USA). Data are presented as the mean values ± SEM (n = the number of observations). Statistical significance was assessed by Student’s t-test or ANOVA with post-hoc Tukey’s HSD test. Differences were considered statistically significant if P < 0.05.
3. Results
DRG neurons cultured in the presence of NGF showed spontaneous firing in the on-cell recording, and it was reported that this firing was abolished by a decrease in the concentration of extracellular Na+ (Kitamura et al., 2005). To know whether the resting membrane potential was affected by the Na+ reduction in the neurons showing the spontaneous firing, a moving average of the holding current for 10 s was calculated under the condition in which the pipette potential was clamped at 0 mV in the on-cell configuration (Fig. 1). In the neuron shown in Fig. 1A, the AP was abolished when the Na+ concentration was decreased to 100 mM. The basal level of the holding current (red lines in Fig. 1A) shifted positively with a decrease in the Na+ concentration. Fig. 1B represents the expanded trace of the current response shown in Fig. 1A. Changes in the membrane potential at each Na+ concentration were estimated based on differences in the amplitudes of the holding current in the presence and absence of a voltage command and are shown in Fig. 1C. The neurons were hyperpolarized by the reduction of Na+, and the amplitude of the hyperpolarization increased with a decrease in the Na+ concentration in the extracellular solution.
Fig. 1.
Effects of the reduction in the extracellular Na+ concentrations on spontaneous firing and basal levels of the membrane potential. (A) A current trace recorded from the DRG neuron cultured in the presence of NGF in the on-cell configuration in the presence of decreasing concentrations of Na+ is shown. Time expanded traces at two time points (a, b) are drawn by the black lines in the lower panel. Spontaneous action potentials were observed in the presence of 150 mM Na+ and disappeared with the reduction of Na+ to lower than 100 mM. The command pulses (5 mV, 50 ms) were applied to neurons every 10 s to calibrate changes in current amplitudes to changes in membrane potentials. Changes in the basal levels of holding currents were estimated by calculating 10-s moving averages of the recorded currents and are represented by red lines. (B) An expanded trace of the basal level of the membrane potential in the neuron shown in A. (C) Summarized data of estimated changes in the membrane potential are plotted against concentrations of Na+. The difference between the membrane potentials in the presence of a decreased concentration of Na+ and that in the presence of 150 mM Na+ (ΔPotential) was calculated (n = 4).
The result that the reduction of the extracellular Na+ caused the hyperpolarization of neurons suggests that an influx of Na+ contributes to setting the resting membrane potential. To assess how much Na+ influx affects membrane potentials, effects of the Na+ reduction on the amplitudes of the holding current in the whole-cell configuration were examined in the NGF-treated neurons (Fig. 2). The neurons were voltage clamped at –60 mV because the resting membrane potential of C-neurons in the DRG of adult rats has been reported to be approximately –60 mV (Caffrey et al., 1992; Jeftinija, 1994). We found two types of neurons among NGF-treated DRG neurons: some showed spontaneous firing in the on-cell recording, and the others did not. We analyzed the current responses of both types of neurons in the on-cell and whole-cell configurations. From the neurons that fired spontaneously in the on-cell recording, spontaneous action currents (called Isp) were often recorded even in the voltage-clamped condition in the whole-cell configuration. It has been reported that Isp reflects spontaneous discharges occurring in loosely voltage-clamped areas on the somatic membrane of the neuron (Kayano et al., 2013). In the neuron shown in Fig. 2A, Isp was observed and was abolished by the reduction of extracellular Na+ to 25 mM. The basal level of the holding current was estimated by calculating the moving average during 10 s periods and is represented by a red line in Fig. 2A. The expanded traces of the basal level of the holding current in the neuron shown in Fig. 2A and another neuron that showed no spontaneous firing in the on-cell configuration are shown in Fig. 2B and C, respectively. Negative holding currents were observed in both types of neurons, and the density of the holding current was significantly higher in the firing neurons than in the non-firing neurons (Fig. 2D). In the firing neurons, reduction of Na+ from 150 mM to 25 mM clearly and rapidly reduced the holding current and abolished Isp (Fig. 2A). Assuming that the decrease in the holding current resulted simply from the decrease in the driving force of Na+, the resting Na+ conductance of the neurons in these two groups was estimated (Fig. 2E). The estimated Na+ conductance was much larger in the spontaneously firing neurons (“+” in Fig. 2D and E) than in the silent neurons (“–” in Fig. 2D and E).
Fig. 2.
Effects of the Na+ reduction on the current recorded under the voltage-clamped condition in the whole-cell configuration. (A) The current in the whole-cell configuration was recorded from the NGF-treated DRG neuron showing the spontaneous firing in the on-cell configuration. The neuron was voltage clamped at –60 mV. The presented neuron showed the spontaneous action current (Isp), which were abolished by the reduction of Na+ to 25 mM. The red line indicates the 10-s moving average of current estimated as the basal level of the holding current. (B) An expanded trace of the basal level of the holding current in the neuron shown in A. (C) A typical trace of the basal level of the holding current at –60 mV in the NGF-treated DRG neurons showing no spontaneous firing in the on-cell configuration. (D) Amplitudes of the density of the holding current are compared between the NGF-treated neurons with (Firing (+), n = 63) and without (Firing (–), n = 27) the spontaneous firing in the on-cell configuration. ***, P < 0.01 by Student’s t-test. (E) The estimated Na+ conductance is compared between the NGF-treated neurons with and without the spontaneous firing in the on-cell configuration. Conductance was calculated by dividing changes in the density of the holding current by changes in the driving force of Na+ between 150 and 25 mM. ***, P < 0.01 by Student’s t-test.
It is reported that NGF regulates the expression and the activity of one of the cation channels, TRPV1, in DRG neurons (Ji et al., 2002; Stein et al., 2006). Activity of TRPV1 may contribute to the constitutive Na+ conductance in the hyperexcitable neurons treated with NGF. To examine this hypothesis, effects of some antagonists of TRPV1 on the spontaneous firing in the on-cell configuration and the basal Na+ conductance in the whole-cell configuration were examined. Several agents have been reported to have an inhibitory effect on TRPV1, but it is uncertain which antagonist inhibits TRPV1 channels in cultured DRG neurons of rats. To determine which is an effective antagonist in this study, we first examined the effects of capsazepine (Dickenson and Dray, 1991), BCTC (Valenzano et al., 2003), 5′-IRTX (Wahl et al., 2001) and SB366791 (Gunthorpe et al., 2004) on current responses to capsaicin in the whole-cell configuration (Fig. 3). In this series of experiments, NGF-treated DRG neurons showing no spontaneous firing were used. Neurons were voltage clamped at –60 mV and exposed to 1 μM capsaicin. Capsaicin was applied for 2 min in each trial and 3 times with an interval of 5 min in each neuron. The amplitude of the inward current responses to capsaicin decreased during repetitive stimulation because of the desensitizing properties of TRPV1 (Touska et al., 2011). The 2nd stimulation was applied in the presence or absence of the 4 antagonists. Each antagonist was applied from 1 min prior to the stimulations with capsaicin. Capsaicin-evoked current responses disappeared in the presence of capsazepine (10 μM, Fig. 3B) or BCTC (1 μM, Fig. 3C) and did not recover for 5 min after washing out of the antagonists. On the other hand, capsaicin responses were evident in the presence of SB366791 (10 μM, Fig. 3D) or 5’-IRTX (0.2 μM, Fig. 3E). These data are summarized in Fig. 3F. Capsazepine and BCTC almost abolished the capsaicin currents. On the other hand, SB366791 and 5’-IRTX did not inhibit the capsaicin currents significantly. Therefore, we concluded that capsazepine and BCTC are suitable antagonists to investigate the activity of TRPV1 in rat DRG neurons. It is reported that capsazepine and BCTC also inhibits TRPM8 in addition to TRPV1 (Behrendt et al., 2004; Weil et al., 2005; Xing et al., 2007). To determine whether TRPM8 is involved in the action of capsazepine and BCTC, effects of these agents on the current responses to icilin, an agonist for TRPM8 and TRPA1 were examined. Similar to the experiments with capsaicin, icilin at 10 μM was applied 3 times, and the 2nd stimulation was applied in the presence or absence of the antagonists. Both capsazepine (10 μM) and BCTC (1 μM) did not significantly affect the amplitudes of the current responses to icilin, significantly (data not shown). Capsazepine and BCTC were thus unlikely to have inhibitory effects on TRPM8 or TRPA1 in rat DRG neurons.
Fig. 3.
Effects of TRPV1 antagonists on the capsaicin-evoked current. Capsaicin at 1 μM was applied for 2 min for 3 times with an interval of 5 min. (A) A typical trace of the capsaicin-evoked current when capsaicin was applied in the absence of any antagonist. (B–E) The second stimulation with capsaicin was applied in the presence of capsazepine (Capz, 10 μM, B), BCTC (1 μM, C), SB366791 (SB, 10 μM, D) and 5′-IRTX (0.2 μM, E). Each antagonist was applied 1 min prior to the capsaicin application. (F) Summarized data of the amplitudes of current responses to 3 repetitive applications of capsaicin as shown in A–E. Columns and the vertical bars indicate the averages of the current density of capsaicin responses. The second application of capsaicin was made in the presence or absence of the TRPV1 antagonists. N = 5 (control), 5 (capsazepine), 7 (BCTC), 4 (SB366791) and 3 (5′-IRTX). **, P < 0.01 vs the corresponding responses observed in the neurons in the control group by ANOVA with post-hoc Tukey’s HSD test.
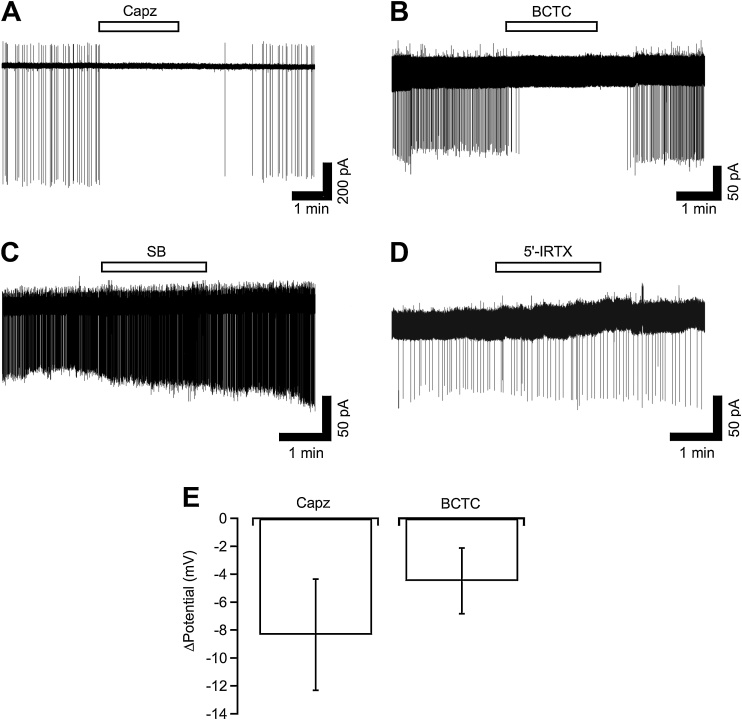
Effects of the TRPV1 antagonists on the spontaneous firing in the on-cell configuration were examined in NGF-treated neurons. Capsazepine (10 μM), BCTC (1 μM), SB366791 (10 μM) and 5’-IRTX (0.2 μM) were applied for 2 min. Capsazepine (Fig. 4A) and BCTC (Fig. 4B) abolished the repetitive APs, and they recovered after removal of the antagonists (n = 4 each). On the other hand, similar to the effects on the capsaicin-evoked current responses, SB366791 and 5’-IRTX did not inhibit the spontaneous firing in the on-cell configuration (n = 4 each). Effects of these antagonists on the basal levels of the holding current were also examined. Basal levels were estimated by calculating the 10-s moving average as shown in Fig. 1. Capsazepine and BCTC slightly but not significantly changed the holding current from 8.4 ± 1.8 pA to 11.4 ± 2.3 pA (n = 12, P = 0.12 by paired t-test) and from 4.9 ± 2.2 pA to 7.9 ± 2.8 pA (n = 10, P = 0.09 by paired t-test), respectively. The estimated amplitudes of the hyperpolarization are shown in Fig. 4E.
Fig. 4.
Effects of TRPV1 antagonists on the spontaneous firing and basal level of the membrane potential in the on-cell configuration. (A–D) Typical current traces recorded from the NGF-treated DRG neurons in the on-cell configuration are shown. Capsazepine (Capz, 10 μM, A), BCTC (1 μM, B), SB366791 (SB, 10 μM, C) and 5′-IRTX (0.2 μM, D) were applied for 2 min to the neurons showing the spontaneous firing. (E) Summarized data of the change in the basal level of the membrane potential by capsazepine and BCTC are shown by columns. The change in the membrane potential was estimated from the difference in the averaged holding current between the presence and absence of the antagonists.
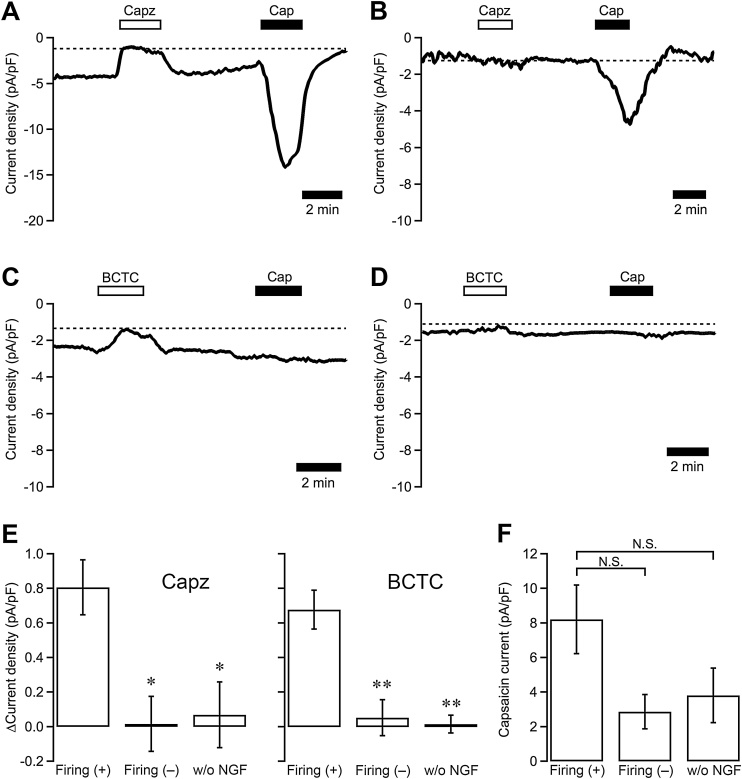
Using the antagonists that were effective on TRPV1 in rat DRG neurons, we examined whether TRPV1 contributed to basal Na+ conductance in NGF-treated DRG neurons. Neurons were voltage clamped at –60 mV in the whole-cell configuration, and the effects of capsazepine (10 μM) and BCTC (1 μM) on basal levels of holding currents were examined. Both capsazepine and BCTC applied for 2 min reduced the amplitude of inward holding currents reversibly in the NGF-treated neurons, which showed spontaneous firing in the on-cell configuration (Fig. 5A, C), but not in the NGF-treated neurons showing no spontaneous firing (Fig. 5B, D). The density of the inward holding currents reduced by capsazepine and BCTC was significantly higher in the neurons with spontaneous firing than in the neurons without spontaneous firing and in the neurons not treated with NGF (Fig. 5E). Moreover, to examine the expression density of functional TRPV1 channels in each neuron, current responses to capsaicin (1 μM), which causes the maximum response in individual neurons, were recorded. Capsaicin-evoked currents were observed in the neurons pretreated with capsazepine but could not be observed after the application of BCTC. Amplitudes of the capsaicin-evoked current after the removal of capsazepine in the neurons of the 3 categories are summarized in Fig. 5F. The average of the amplitudes seemed to be higher in the neurons with spontaneous firing than those in the neurons in the other categories, but these values were not significantly different from each other.
Fig. 5.
Effects of capsazepine and BCTC on the current recorded under the voltage-clamped condition in the whole-cell configuration. (A–D) The current in the whole-cell configuration was recorded from the NGF-treated DRG neuron. Neurons were voltage clamped at –60 mV. Typical traces of the 10-s moving averages of the holding current in the neurons with (A, C) and without (B, D) the on-cell spontaneous firing are shown. Capsazepine (Capz; 10 μM; A, B) and BCTC (1 μM; C, D) were applied for 2 min. Capsaicin (Cap, 1 μM, 2 min) was applied 5 min after the removal of the TRPV1 antagonists. (E) Summarized data of the amplitude of the density of the basal holding current inhibited by capsazepine and BCTC are shown. Columns indicate the data in the NGF-treated neurons with (Firing (+), n = 22 for Capz and 7 for BCTC) and without (Firing (–); n = 4 and 6, respectively) the on-cell spontaneous firing, and in the control neurons not treated with NGF (w/o NGF; n = 6 and 5, respectively). *, P < 0.05; **, P < 0.01 vs Firing (+) by ANOVA with post-hoc Tukey’s HSD test. (F) The amplitudes of capsaicin-evoked responses recorded after the application of capsazepine are summarized. The columns indicate the sum of the amplitudes of the capsazepine-sensitive component and the capsaicin-evoked responses in each neuron. N = 9, 4 and 4, respectively. N.S., not significant by ANOVA with post-hoc Tukey’s HSD test.
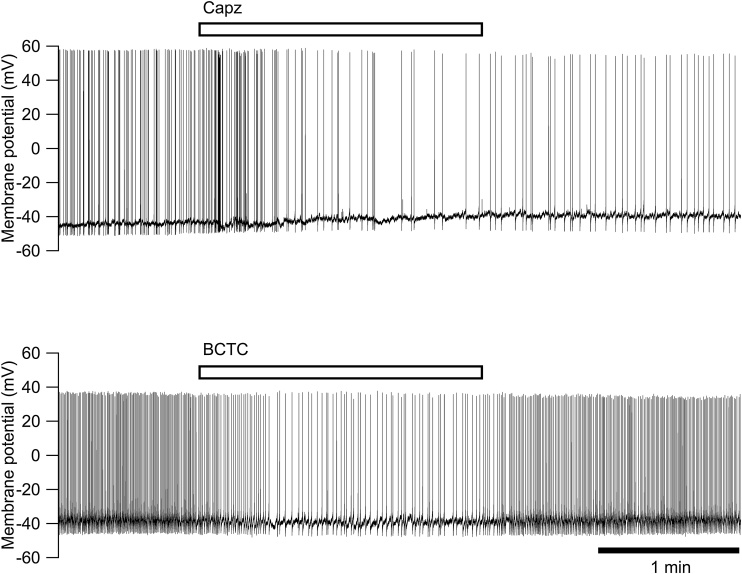
Effects of capsazepine and BCTC on the spontaneous firing and the resting membrane potential were also examined in the current clamp mode of the whole-cell configuration (Fig. 6). Capsazepine (10 μM) and BCTC (1 μM) were applied to the NGF-treated neurons showing spontaneous firing in the current clamp mode. Capsazepine and BCTC applied for 2 min reduced the firing frequency from 1.86 ± 0.29 Hz to 1.24 ± 0.35 Hz (n = 15, P < 0,05 by paired t-test) and from 1.88 ± 0.34 Hz to 1.03 ± 0.27 Hz (n = 12, P < 0,05 by paired t-test), respectively. The resting membrane potential between APs was decreased by capsazepine (from –41.6 ± 1.2 mV to –37.6 ± 1.8 mV, n = 15, P < 0.001 by paired t-test) but was not affected by BCTC (from –43.7 ± 1.1 mV to –42.8 ± 1.3 mV, n = 12, P > 0.05 by paired t-test).
Fig. 6.
Effects of capsazepine and BCTC on the membrane potential recorded under the current clamp condition in the whole-cell configuration. Typical traces of the membrane potential in the NGF-treated neurons showing the spontaneous action potentials are shown. Capsazepine (Capz, 10 μM) and BCTC (1 μM) were applied for 2 min, as indicated by the bars.
4. Discussion
It is reported that NGF acutely acts on C-neurons isolated from the DRG of adult rats and gains sensitivity of TRPV1 to capsaicin (Shu and Mendell, 2001; Bonnington and McNaughton, 2003; Zhu et al., 2004). It is also reported that NGF that is injected intrathecally or intradermally increases the expression level of TRPV1 in DRG neurons (Ji et al., 2002; Zhu and Oxford, 2011; Eskander et al., 2015), and this upregulation of TRPV1 is thought to be a cause of the noxious hypersensitivity. Moreover, it has been reported that chronic treatment of cultured DRG neurons with NGF induces the hyperexcitability that triggers spontaneous firing and this action of NGF was considered to be one cause of the pathogenesis of neuropathic and inflammatory pain (Kitamura et al., 2005). In the present study, we examined the contribution of TRPV1 activity to the spontaneous firing in NGF-treated DRG neurons.
We examined effects of the four TRPV1 antagonists and found that capsazepine and BCTC abolished the capsaicin-evoked current but that SB366791 and 5’-IRTX, both of which are used as selective antagonists of TRPV1 (Wahl et al., 2001; Gunthorpe et al., 2004), did not inhibit the capsaicin-evoked current in rat DRG neurons. Although we do not know the reasons why SB366791 and 5’-IRTX were ineffective in our preparations of rat DRG neurons, we judged that SB366791 and 5’-IRTX were not suitable antagonists to investigate the roles of TRPV1 activity in rat DRG neurons and focused on the effects of capsazepine and BCTC in this study. In addition to the inhibitory effect on TRPV1, both capsazepine and BCTC have been reported to inhibit menthol-evoked responses (Behrendt et al., 2004; Weil et al., 2005; Xing et al., 2007). TRPM8 is reported to be expressed in approximately 10% of DRG neurons (McKemy et al., 2002; Peier et al., 2002; Okazawa et al., 2004; Abe et al., 2005). It has also been reported that cultured DRG neurons sensitive to both capsaicin and icilin, an agonist for TRPM8 and TRPA1, respond to NGF and become hyperexcitable (Kayano et al., 2010). We confirmed the inhibitory effect of capsazepine and BCTC on TRPM8 and/or TRPA1 and found that they did not inhibit icilin-evoked responses in cultured rat DRG neurons of the rat, indicating that TRPM8 and TRPA1 are unlikely to be involved in the capsazepine- and BCTC-sensitive currents recorded in this study.
DRG neurons cultured in the presence of NGF often show spontaneous action currents (Isp) even in the voltage-clamped condition in the whole-cell configuration. It was concluded that Isp reflected spontaneous discharges occurring in loosely voltage-clamped regions of the membrane of the soma of DRG neurons, because the Isp was recorded even in patch membranes excised from soma membranes (Kayano et al., 2013). Although Isp is an event in the unclamped region, levels of holding currents recorded in the voltage clamp mode are considered to mainly reflect the conductance of voltage-clamped areas of cell membranes. The density of the inward holding current recorded at –60 mV was significantly higher in the firing neurons than in the silent neurons. This inward holding current is likely to depend on the constitutively active Na+ conductance, suggesting that chronic exposure of DRG neurons to NGF induces an additional cation conductance that causes additional inward currents.
Similar to the effect of the Na+ reduction, capsazepine and BCTC also inhibited the spontaneous firing in the on-cell configuration and the Isp in the whole-cell configuration. Simultaneously, the amplitudes of the inward holding currents recorded in the whole-cell configuration were reduced by capsazepine and BCTC. These inhibitory effects were observed only in the firing neurons treated with NGF, indicating that capsazepine- and BCTC-sensitive ion channels contributed the additional cation conductance induced by NGF, and these channels are considered to be TRPV1. Capsazepine- and BCTC-caused hyperpolarizing changes observed in the on-cell configuration are consistent with the reduction in the inward cation current of the whole-cell current. Although the frequency of spontaneous firing was reduced, the hyperpolarizing effect of capsazepine and BCTC was not detected under the current clamp condition. This situation may be caused by the limitation of the spatiotemporal ability of the current clamp technique, and it is possible that small changes in the clamp potential may not be detected. Based on the evidence obtained in the voltage clamp recording in the on-cell and whole-cell configurations, we conclude that the chronic treatment of DRG neurons with NGF induced the additional cation currents through TRPV1 that caused depolarization and triggered spontaneous APs.
The amplitudes of the current responses to capsaicin were not different between the neurons with and without the spontaneous firing. However, since the mean amplitude of the capsaicin currents in the firing neurons was approximately twice as large as that in the silent neurons, we consider it possible that NGF raises the expression level of TRPV1, similar to the previous report in which NGF treatment caused upregulation of the expression of TRPV1 in vivo (Ji et al., 2002; Eskander et al., 2015) and in vitro (Stein et al., 2006). TRPV1 is reported to be activated by the lowering of the extracellular pH below 5.9 (Caterina et al., 1997; Tominaga et al., 1998) and a rise in temperature above 43 °C (Caterina et al., 1997; Caterina and Julius, 2001). All of the experiments in this study were performed at room temperature (22–24 °C) and at neutral pH (7.4 outside and 7.3 inside of the cells). TRPV1 is not normally activated under these conditions. The amplitude of the holding current reduced by the Na+ reduction, capsazepine and BCTC in the firing neurons was ten times as large as those in the silent neurons. Even though NGF raised the expression level of TRPV1, it is not usually considered that the increase in the expression density of TRPV1 simply results in the constitutively active cation conductance that evokes depolarization of neurons. It may be necessary that NGF stimulates a certain regulatory mechanism that activates TRPV1 at neutral pH and room temperature in the DRG neuron. It has been reported that NGF acutely sensitizes TRPV1 depending on mitogen-activated kinase (MAPK), phosphatidylinositol-3-kinase (PI3K), protein kinase C (PKC) and calcium-calmodulin dependent protein kinase II (CaMKII) in mouse DRG neurons (Bonnington and McNaughton, 2003). On the other hand, it has been reported that a similar sensitizing effect of NGF on TRPV1 depends on the protein kinase A (PKA) pathway in small neurons (< 30 μm in soma diameter) isolated from the rat DRG (Shu and Mendell, 2001). Recently, it was reported that NGF increased concentrations of oxidized lipid metabolites that activate TRPV1, resulting in the persistent activation of TRPV1 in vivo (Eskander et al., 2015). It is not clear which mechanism listed above contributed to the NGF-induced constitutive activity of TRPV1 observed in this study. Moreover, several other mechanisms that regulate the activity of TRPV1 have also been reported (Rosenbaum and Simon, 2007). One example is that phosphatidylinositol 4,5-diphosphate (PIP2) inhibits TRPV1 and that reduction of the PIP2 concentration in the cell membrane causes activation of TRPV1 (Chuang et al., 2001; Prescott and Julius, 2003; Cao et al., 2013). It is also possible that TRPV1 is usually inactivated by membrane PIP2, upregulation of TRPV1 causes a shortage of PIP2, and a part of TRPV1 becomes constitutively active. Future study may clarify the underlying mechanisms.
We conclude that NGF acts on the DRG neuron and induces the constitutively active cation conductance through TRPV1. The cation current flowing into the neuron through TRPV1 causes depolarization and triggers spontaneous APs. Since NGF is reported to increase in the DRG after nerve injury, this action of NGF is one potential cause of the pathogenesis of neuropathic pain.
Author contributions
Study concept and design: NK. Data acquisition: NK, EN, YM, and TK. Data analysis and interpretation: NK, EN, YM, and TK. Drafting of the manuscript: NK. Review and revision of the manuscript: NK, YM and IS. Statistical analyses: NK, EN and TK. Study supervision: NK.
Funding sources
Research reported in this publication was partially supported by KAKENHI (Grant # 16K08073 to IS, 17H03933 to NK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Abe J., Hosokawa H., Okazawa M., Kandachi M., Sawada Y., Yamanaka K., Matsumura K., Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Mol. Brain Res. 2005;136:91–98. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Amir R., Michaelis M., Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J. Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev N., Dimitrieva N., Koltzenburg M., McMahon S.B. Peripheral administration of nerve growth factor in the adult rat produces a thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurones. Pain. 1995;63:109–115. doi: 10.1016/0304-3959(95)00024-M. [DOI] [PubMed] [Google Scholar]
- Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi: 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Behrendt H.J., Germann T., Gillen C., Hatt H., Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G.J., Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bonnington J.K., McNaughton P.A. Signalling pathways involveed in the sensitisation of mouse nociceptive neurones by nerve growth factor. J. Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchiel K.J. Effects of electrical and mechanical stimulation on two foci of spontaneous activity which develop in primary afferent neurons after peripheral axotomy. Pain. 1984;18:249–265. doi: 10.1016/0304-3959(84)90820-0. [DOI] [PubMed] [Google Scholar]
- Caffrey J.M., Eng D.L., Black J.A., Waxman S.G., Kocsis J.D. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Cao E., Cordero-Morales J.F., Liu B., Qin F., Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J., Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann. Rev. Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cattaneo A. Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Curr. Opin. Mol. Ther. 2010;12:94–106. [PubMed] [Google Scholar]
- Chuang H.H., Prescott E.D., Kong H., Shields S., Jordt S.E., Basbaum A.I., Chao M.V., Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Decosterd I., Woolf C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain Suppl. 1999;6:S27–35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P.D., Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992;48:261–268. doi: 10.1016/0304-3959(92)90067-L. [DOI] [PubMed] [Google Scholar]
- Dickenson A.H., Dray A. Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. Br. J. Pharmacol. 1991;104:1045–1049. doi: 10.1111/j.1476-5381.1991.tb12547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide P.K. Pathophysiological mechanisms of central neuropathic pain after spinal cord injury. Spinal Cord. 1998;36:601–612. doi: 10.1038/sj.sc.3100737. [DOI] [PubMed] [Google Scholar]
- Eskander M.A., Ruparel S., Green D.P., Chen P.B., Por E.D., Jeske N.A., Gao X., Flores E.R., Hargreaves K.M. Persistent nociception triggered by nerve growth factor (NGF) Is mediated by TRPV1 and oxidative mechanisms. J. Neurosci. 2015;35:8593–8603. doi: 10.1523/JNEUROSCI.3993-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe M.J., Rami H.K., Jerman J.C., Smart D., Gill C.H., Soffin E.M., Luis Hannan S., Lappin S.C., Egerton J., Smith G.D., Worby A., Howett L., Owen D., Nasir S., Davies C.H., Thompson M., Wyman P.A., Randall A.D., Davis J.B. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Hao J., Ebendal T., Xu X., Wiesenfeld-Hallin Z., Eriksdotter Jönhagen M. Intracerebroventricular infusion of nerve growth factor induces pain-like response in rats. Neurosci. Lett. 2000;286:208–212. doi: 10.1016/s0304-3940(00)01107-1. [DOI] [PubMed] [Google Scholar]
- Harper A.A., Lawson S.N. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J. Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A.A., Lawson S.N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J. Physiol. 1985;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U., Eliav E., Dorsey J.M., Gracely R.H., Kopin I.J. NGF involvement in pain induced by chronic constriction injury of the rat sciatic nerve. Neuroreport. 1997;8:1613–1618. doi: 10.1097/00001756-199705060-00012. [DOI] [PubMed] [Google Scholar]
- Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibers. Brain Res. 1994;639:125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Samad T.A., Jin S.X., Schmoll R., Woolf C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Kayano T., Kitamura N., Moriya T., Tsutsumi A., Ozaki Y., Dayanithi G., Shibuya I. Chronic treatment with NGF induces spontaneous fluctuations of intracellular Ca2+ in icilin-sensitive dorsal root ganglion neurons of the rat. J. Vet. Med. Sci. 2010;72:1531–1538. doi: 10.1292/jvms.10-0196. [DOI] [PubMed] [Google Scholar]
- Kayano T., Kitamura N., Moriya T., Kuwahara T., Komagiri Y., Toescu E.C., Shibuya I. Chronic NGF treatment induces somatic hyperexcitability in cultured dorsal root ganglion neurons of the rat. Biomed. Res. 2013;34:329–342. doi: 10.2220/biomedres.34.329. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Chung J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Konno A., Kuwahara T., Komagiri Y. Nerve growth factor-induced hyperexcitability of rat sensory neuron in culture. Biomed. Res. 2005;26:123–130. doi: 10.2220/biomedres.26.123. [DOI] [PubMed] [Google Scholar]
- Lewin G.R., Ritter A.M., Mendell L.M. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J. Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G.R., Rueff A., Mendell L.M. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur. J. Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Liu C.N., Amir R., Devor M. Effect of age and nerve injury on cross-excitation among sensory neurons in rat dorsal root ganglia. Neurosci. Lett. 1999;259:95–98. doi: 10.1016/s0304-3940(98)00909-4. [DOI] [PubMed] [Google Scholar]
- Liu C.N., Wall P.D., Ben-Dor E., Michaelis M., Amir R., Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Luo H., Cheng J., Han J.S., Wan Y. Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund’s adjuvant in rats. Neuroreport. 2004;15:655–658. doi: 10.1097/00001756-200403220-00016. [DOI] [PubMed] [Google Scholar]
- McKelvey L., Shorten G.D., O’Keeffe G.W. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J. Neurochem. 2013;124:276–289. doi: 10.1111/jnc.12093. [DOI] [PubMed] [Google Scholar]
- McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Okazawa M., Inoue W., Hori A., Hosokawa H., Matsumura K., Kobayashi S. Noxious heat receptors present in cold-sensory cells in rats. Neurosci. Lett. 2004;359:33–36. doi: 10.1016/j.neulet.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Ossipov M.H. Growth factors and neuropathic pain. Curr. Pain Headache Rep. 2011;15:185–192. doi: 10.1007/s11916-011-0183-5. [DOI] [PubMed] [Google Scholar]
- Peier A.M., Moqrich A., Hergarden A.C., Reeve A.J., Andersson D.A., Story G.M., Earley T.J., Dragoni I., McIntyre P., Bevan S., Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Petersen M., Zhang J., Zhang J.M., LaMotte R.H. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–397. doi: 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- Prescott E.D., Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Price T.J., Louria M.D., Candelario-Soto D., Dussor G.O., Jeske N.A., Patwardhan A.M., Diogenes A., Trott A.A., Hargreaves K.M., Flores C.M. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum T., Simon S.A. TRPV1 receptors and signal transduction. In: Liedtke W.B., Heller S., editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL); CRC Press/Taylor & Francis: 2007. https://www.ncbi.nlm.nih.gov/books/NBK5260/ [PubMed] [Google Scholar]
- Seltzer Z., Dubner R., Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Shen H., Chung J.M., Chung K. Expression of neurotrophin mRNAs in the dorsal root ganglion after spinal nerve injury. Mol. Brain Res. 1999;64:186–192. doi: 10.1016/s0169-328x(98)00314-3. [DOI] [PubMed] [Google Scholar]
- Shu X., Mendell L.M. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J. Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Stein A.T., Ufret-Vincenty C.A., Hua L., Santana L.F., Gordon S.E. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study R.E., Kral M.G. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Sun Q., Tu H., Xing G.-G., Han J.-S., Wan Y. Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats with spinal nerve ligation. Exp. Neurol. 2005;191:128–136. doi: 10.1016/j.expneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Tominaga M., Caterina M.J., Malmberg A.B., Rosen T.A., Gilbert H., Skinner K., Raumann B.E., Basbaum A.I., Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Touska F., Marsakova L., Teisinger J., Vlachova V. A "cute" desensitization of TRPV1. Curr. Pharm. Biotechnol. 2011;12:122–129. doi: 10.2174/138920111793937826. [DOI] [PubMed] [Google Scholar]
- Valenzano K.J., Grant E.R., Wu G., Hachicha M., Schmid L., Tafesse L., Sun Q., Rotshteyn Y., Francis J., Limberis J., Malik S., Whittemore E.R., Hodges D. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. In vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- Wahl P., Foged C., Tullin S., Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Weil A., Moore S.E., Waite N.J., Randall A., Gunthorpe M.J. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol. Pharmacol. 2005;68:518–527. doi: 10.1124/mol.105.012146. [DOI] [PubMed] [Google Scholar]
- Winkelstein B.A., Rutkowski M.D., Weinstein J.N., DeLeo J.A. Quantification of neural tissue injury in a rat radiculopathy model: comparison of local deformation, behavioral outcomes, and spinal cytokine mRNA for two surgeons. J. Neurosci. Methods. 2001;111:49–57. doi: 10.1016/s0165-0270(01)00445-9. [DOI] [PubMed] [Google Scholar]
- Woolf C.J., Safieh-Garabedian B., Ma Q.P., Crilly P., Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Xing H., Chen M., Ling J., Tan W., Gu J.G. TRPM8 mechanism of cold allodynia after chronic nerve injury. J. Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Yang F., Luo H., Liu F.Y., Han J.S., Xing G.G., Wan Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4:61. doi: 10.1186/1744-8069-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Tan Y. Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience. 2011;193:72–79. doi: 10.1016/j.neuroscience.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Zhang X., Huang J., McNaughton P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Oxford G.S. Differential gene expression of neonatal and adult DRG neurons correlates with the differential sensitization of TRPV1 responses to nerve growth factor. Neurosci. Lett. 2011;500:192–196. doi: 10.1016/j.neulet.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Galoyan S.M., Petruska J.C., Oxford G.S., Mendell L.M. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J. Neurophysiol. 2004;92:3148–3152. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]