Abstract
Objective
The aim of this study was to investigate the diagnostic performance of serum 1,3-β-D-gluan as biomarker for invasive fungal infection through meta-analysis.
Methods
The electronic databases of Medline, Cochrane, Embase, Web of Science, OVID and CNKI were systematic searched to identified the case-control or Cohort studies relevant to diagnostic efficacy of serum 1,3-β-D-glucan for invasive fungal infection. The data of true positive (tp), false positive (fp), false negative (fn) and true negative (tn) patients number were extracted from each of the original included studies. The diagnostic sensitivity, specificity and systematic receiver operating characteristic (SROC) curve were calculated and pooled through random or fixed effect method. The publication bias was evaluated by the Deek’s funnel plot.
Results
Thirty-seven relevant studies were fulfilled the inclusion criteria and included in our present meta-analysis. The combined sensitivity, specificity, positive likely hood ratio (+lr), negative likely hood ratio (-lr) and diagnostic odds ratio(dor) for 1,3-β-D-glucan in diagnosis of invasive fungal infectionwere 0.83 (95%CI:0.38-0.61), 0.81 (95%CI:0.80-0.82), 5.13 (95%CI:3.98-6.62), 0.23 (95%CI:0.18-0.30), and 29.68 (95%CI:18.94-46.52) respectively. The pooled area under the ROC curve (AUC) was 0.91.The Deek’s funnel plot asymmetry test showed there was no publication bias for 1,3-β-D-glucan in diagnosis of invasive fungal infection of the included 37 studies.
Conclusion
Serum 1,3-β-D-glucan assay was a promising biomarker for invasive fungal infection diagnosis.
Keywords: 1,3-β-D-glucan; invasive fungal infection; meta-analysis; diagnosis
1. Introduction
Invasive fungal infections (IFIs) are serious kinds of fungal diseases that invade the body, grow and reproduce in body tissues, organs and blood, and cause inflammation and tissue damage [1]. Due to the application of high strength, immunosuppressive agents and high-dose chemotherapy for organ transplantation, hematopoietic stem cell transplantation, a variety of catheter in interventional, indwelling intubation ventilator have been widely carried out and the increase of AIDS infection, the incidence of IFI increased significantly [2]. However, the diagnosis of invasive fungal infection was not easy in clinical practice [3]. Generally, invasive fungal infection often requires fungal culture or histopathological examination to confirm the diagnosis. However, fungal culture usually takes 2 to 4 days to obtain results, while histological examination is difficult to achieve in most cases. Therefore, it is important to make a rapid and accurate diagnosis of IFI with a simple and convenient method. 1,3-β-D-glucan is a fungal-cell-wall polysaccharide that can released into the peripheral blood in patients with IFIs. And serum 1,3-β-D-glucan were used as serum biomarker for IFIs diagnosis. However, the diagnostic performance of 1,3-β-D-glucan was quite different according to the previously published studies because of different 1,3-β-D-glucan assays and cutoff value [4]. Therefore, we performed this meta-analysis and pooled the diagnostic efficacy to further assess it clinical value.
2. Methods
2.1. Publication search strategy
The electronic databases of Medline, Cochrane, Embase, Web of Science, OVID and CNKI were systematic searched to identified the case-control or Cohort studies relevant to diagnostic efficacy of serum 1,3-β-D-glucan for invasive fungal infection. The below terms were used for electronic databases searching. (1) invasive fungal infection/IFI; (2) invasive fungal disease/IFD; (3) 1,3-β-D-glucan/BG/BDG. The language was limited to English and Chinese. In order to identify additional relevant publications, the reference of the included studies were also screened to find potential suitable studies. For studies without enough data to make the meta-analysis, the corresponding authors were contacted by e-mail to obtain further information, if necessary.
2.2. Data and information extraction
Hu Caibao and Zhao Tian independently read the whole paper and extracted the data and information. Any disagreement was consulted to another investigator (Chen Changqin) for consensus. The extracted data and information included (1) The study type (case-control or Cohort); (2) The language of the publication (English or Chinese); (3) The journal name of the paper published; (4) The year of the paper published; (5) The first and the corresponding authors; (6) The diagnosis of invasive fungal infectionreference standard; (7) The cutoff value of serum 1,3-β-D-glucan in each study; (8) The number of true positive, false positive, false negative and true negative; (9) Invasive fungal infection detection methods; (10) The region the study performed. And the above detail information and data were by Zhao Tian and Chen Changqin respectively and cross checked.
2.3. Publication quality evaluation
The general method quality of the included 37 studies was evaluated through the QUADAS tool byTingyu and Hu Caibao independently with eight questionnaires [5, 6]. For each of the question, there was 3 status “yes”, “no” and “unclear”. “yes” represents high quality, “no” represents low quality and “unclear” represents moderate quality.
3. Statistical analysis
The diagnostic efficacy for 1,3-β-D-glucan for invasive fungal infection was pooled by MetaDiSc 1.4 software by the equation of sensitivity=true positive/(true positive+false negative), specificity=true negative/( true negative+ false positive). Subgroup analysis was performed according to different serum 1,3-β-D-glucan cutoff value. The diagnostic parameters were pooled by mixed or random effect model according to the statistical heterogeneity across theincluded studies. The SROC curve was drawn by Stata12.0SE software and the AUC was calculated through sensitivity vs specificity for 1,3-β-D-glucan in diagnosis of invasive fungal infection. P< 0.05 was considered statistical significant.
4. Results
4.1. Main characteristic of the individual 37 publications
As a result of the related electronic databases systematic searching, finally 37 relevant studies were fulfilled the inclusion criteria and included in our present meta-analysis (Figure 1). Among the 37 publications, 15 studies were Cohort designed prospective study and other 22 are case-control publications. The cutoff value for 1,3-β-D-glucan in diagnosis of invasive fungal infection ranged from 7 (pg/mL) to 140 (pg/mL). The general characteristics of the 37 publications were demonstrated in Table 1.
Figure 1.

The flowchart of publication searching and studies inclusion
Table 1.
The main characters of the recruited 37 publications
| Study | Year | Country | Study type | Method | Cutoff value (pg/mL) | Reference | tp | fp | fn | tn |
|---|---|---|---|---|---|---|---|---|---|---|
| Miyazaki [7] | 1997 | Japan | Case-control | Fungitec G-test | 10 | Micobiological culture | 17 | 0 | 7 | 36 |
| Obayashi [8] | 1995 | Japan | Case-control | Fungitec G-test | 20 | Autopsy | 37 | 0 | 4 | 153 |
| Kawazu [9] | 2004 | Japan | Cohort | Wako | 11 | EORTC/MSG | 6 | 2 | 5 | 123 |
| Kondori [10] | 2004 | Sweden | Case-control | Fungitec G-test | 20 | Micobiological culture | 14 | 0 | 0 | 19 |
| Odabasi [11] | 2004 | U.S | Cohort | Fungitell | 80 | EORTC/MSG | 18 | 15 | 2 | 248 |
| Ostrosky-Zeichner [12] | 2005 | U.S | Case-control | Fungitell | 60 | EORTC/MSG | 95 | 22 | 22 | 148 |
| Pazos [13] | 2005 | Spain | Cohort | Fungitell | 120 | EORTC/MSG | 7 | 3 | 1 | 26 |
| Pickering [14] | 2005 | U.S | Case-control | Fungitell | 60 | Histopathologic examination | 31 | 10 | 1 | 22 |
| Fujita [15] | 2006 | Japan | Case-control | Wako | 11 | Micobiological culture | 72 | 28 | 4 | 147 |
| Alam [16] | 2007 | Kuwait | Case-control | Fungitell | 80 | EORTC/MSG | 14 | 0 | 13 | 26 |
| Akamatsu [17] | 2007 | Japan | Cohort | Fungitec G-test | 40 | EORTC/MSG | 12 | 26 | 7 | 130 |
| Obayashi [18] | 2008 | Japan | Case-control | Fungitec G-test | 30 | Autopsy | 39 | 9 | 2 | 98 |
| Senn [19] | 2008 | Switzerland | Cohort | Wako | 7 | EORTC/MSG | 20 | 28 | 10 | 85 |
| Persat [20] | 2008 | France | Case-control | Fungitell | 80 | EORTC/MSG | 70 | 39 | 26 | 123 |
| Ellis [21] | 2008 | UAE | Cohort | Fungitell | 80 | EORTC/MSG | 36 | 23 | 2 | 19 |
| Leon [22] | 2009 | Europe | Cohort | Fungitell | 75 | Histopathologic examination | 14 | 105 | 4 | 117 |
| Lunel [23] | 2009 | Netherlands | Cohort | Fungitell | 60 | Micobiological culture | 16 | 12 | 5 | 18 |
| Hachem [24] | 2009 | U.S | Cohort | Fungitell | 80 | EORTC/MSG | 29 | 2 | 16 | 18 |
| Koo [25] | 2009 | U.S | Case-control | Fungitell | 80 | EORTC/MSG | 50 | 124 | 23 | 635 |
| Presterl [26] | 2009 | Austria | Cohort | Fungitell | 40 | Micobiological culture | 12 | 14 | 11 | 44 |
| Racil [27] | 2010 | Czech Republic | Case-control | Fungitell | 80 | EORTC/MSG | 8 | 55 | 1 | 27 |
| Alexander [28] | 2010 | U.S | Cohort | Fungitell | 60 | EORTC/MSG | 8 | 54 | 3 | 5 |
| Hirata [29] | 2010 | Japan | Cohort | Wako | 8.9 | EORTC/MSG | 8 | 2 | 2 | 196 |
| Li J [30] | 2010 | China | Case-control | Fungitec G-test | 20 | Micobiological culture | 37 | 16 | 1 | 26 |
| Zuo XH (1)[31] | 2010 | China | Case-control | Fungitec G-test | 20 | EORTC/MSG | 35 | 4 | 5 | 31 |
| Zuo XH (2) [31] | 2010 | China | Case-control | Fungitec G-test | 50 | EORTC/MSG | 29 | 3 | 11 | 32 |
| De Vlieger [32] | 2011 | Belgium | Cohort | Fungitell | 140 | EORTC/MSG | 12 | 10 | 2 | 33 |
| Posteraro [33] | 2011 | Italy | Cohort | Fungitell | 80 | EORTC/MSG | 15 | 5 | 1 | 74 |
| Acosta [34] | 2011 | Spain | Cohort | Fungitell | 80 | EORTC/MSG | 7 | 7 | 2 | 31 |
| Jiang ZM [35] | 2011 | China | Case-control | Fungitec G-test | 20 | Micobiological culture | 44 | 21 | 2 | 45 |
| Yang HQ [36] | 2011 | China | Case-control | Fungitec G-test | 20 | Micobiological culture | 423 | 355 | 34 | 1027 |
| Jin X [37] | 2011 | China | Case-control | Fungitec G-test | 10 | EORTC/MSG | 40 | 1 | 5 | 39 |
| Metan [38] | 2012 | Turkery | Case-control | Fungitell | 80 | EORTC/MSG | 33 | 19 | 17 | 59 |
| Liu CH [39] | 2012 | China | Case-control | Fungitec G-test | 20 | EORTC/MSG | 36 | 6 | 7 | 51 |
| Ding C [40] | 2012 | China | Case-control | Fungitec G-test | 20 | Micobiological culture | 42 | 1 | 6 | 29 |
| Wang QF [41] | 2012 | China | Case-control | Fungitec G-test | 20 | Micobiological culture | 47 | 7 | 2 | 75 |
| Yang D [42] | 2013 | China | Case-control | Fungitec G-test | 20 | Micobiological culture | 26 | 16 | 9 | 87 |
| Zeng WX [43] | 2016 | China | Case-control | Fungitec G-test | 20 | Micobiological culture | 38 | 3 | 39 | 478 |
4.2. Quality assessment of the included studies
Eight items (questions) original from QUADAS (Quality Assessment of Diagnostic Accuracy Studies) were used to assessed the general quality of the included studies. For the QUADAS quality assessment, most of the 37 studies fulfilled the items of “Clear description of study selection criteria “and” acceptable reference standard”. However, most of the included studies didn’t clearly addressed the item of “ withdraw reports”. The generally quality of the included 37 publications were showed in Figure 2.
Figure 2.

The general quality of the included 37 studies evaluated by QUADAS
4.3. The pooled diagnostic sensitivity
The I2 test of the 37-publication indicated significant heterogeneity (I2=83.5%). The diagnostic sensitivity was pooled by random effect model. The combined sensitivity for 1,3-β-D-glucan in diagnosis of invasive fungal infection was 0.83 (95%CI:0.38-0.61), Figure 3.
Figure 3.

Pooled forest plot of sensitivity of 1,3-β-D-glucan in diagnosis of invasive fungal infection.
4.4. The pooled diagnostic specificity
Significant statistical heterogeneity was found in the aspect of pooling the specificity through the I2 test (I2=95.5%). The pooled diagnostic specificity was 0.81 (95%CI:0.80-0.82) by random effect model, Figure 4.
Figure 4.

Pooled forest plot of specificity of 1,3-β-D-glucan in diagnosis of invasive fungal infection
4.5. The pooled +lr and –lr
The statistical heterogeneity for effect size +lr and –lr were evaluated through I2 test. And the results indicated statistical significant heterogeneity (P=0.000). Therefore, the data was pooled through random effect. The pooled +lr and –lr were 5.13 (95%CI:3.98-6.62), Figure 5 and 0.23 (95%CI:0.18-0.30), Figure 6.
Figure 5.

Pooled forest plot of +lr for 1,3-β-D-glucan in diagnosis of invasive fungal infection
Figure 6.

Pooled forest plot of -lr for 1,3-β-D-glucan in diagnosis of invasive fungal infection
4.6. Pooled diagnostic odds ratio(dor)
Because of significant statistical heterogeneity across the included 37 studies (I2=80.9%), the dor was calculated through random effect model. The combined dor was 29.68 (95%CI:18.94-46.52), Figure 7.
Figure 7.

Pooled forest plot of dor for 1,3-β-D-glucan in diagnosis of invasive fungal infection
4.7. The pooled summary ROC and AUC
The combined receiver operating characteristic (ROC) curve was calculated by Stata12.0SE software (Figure 8). The area under the ROC curve was 0.91.
Figure 8.

The AUC of the SROC for 1,3-β-D-glucan in diagnosis of invasive fungal infection
4.8. Diagnostic efficacy changes according to cutoff value
The diagnostic sensitivity, specificity, +lr, -lr and odds ratio changes according to cutoff value were demonstrated in Figure 9. The diagnostic sensitivity, specificity and –lrdid not change a lot for different cutoff value of serum 1,3-β-D-glucan (Figure 9a, Figure 9b, Figure 9d).
Figure 9.
The scatter plot of diagnostic efficacy changes according to cutoff value
However, the +lr and dor changed significantly for different cutoff value especially for <20 (pg/mL) group (Figure 9c and Figure 9e).
4.9. Subgroup analysis
In order to minimize the clinical heterogeneity, we performed subgroup analysis according to invasive fungal infection detection method, study type and gold diagnostic reference. However, the diagnostic efficacy didn’t significant changed for different subgroups (Table 2).
Table 2.
The subgroup analysis of 1,3-β-D-glucan for invasive fungal infection
| Subgroup | No. of study | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| Method | ||||
| Fungitell | 18 | 0.76(0.72-0.79) | 0.76(0.74-0.78) | 0.86 |
| Fungitec G-test | 16 | 0.87(0.84-0.89) | 0.83(0.82-0.85) | 0.95 |
| Wako | 3 | 0.83(0.76-0.89) | 0.90(0.88-0.92) | 0.93 |
| Study type | ||||
| Cohort | 14 | 0.75(0.70-0.80) | 0.79(0.77-0.81) | 0.85 |
| Case-control | 23 | 0.84(0.82-0.86) | 0.82(0.81-0.83) | 0.93 |
| Reference | ||||
| EORTC/MSG | 21 | 0.76(0.73-0.79) | 0.83(0.81-0.84) | 0.87 |
| Micobiological culture | 12 | 0.87(0.84-0.89) | 0.81(0.80-0.83) | 0.94 |
| Others | 4 | 0.92(0.86-0.96) | 0.76(0.82-0.80) | 0.97 |
4.10. Publication bias evaluation
The publication bias was evaluate by the Deek’s funnel plot (Figure 10) and line regression test. The Deek’s funnel plot asymmetry test showed there was no publication bias for 1,3-β-D-glucan in diagnosis of invasive fungal infection of the included 37 studies.
Figure 10.
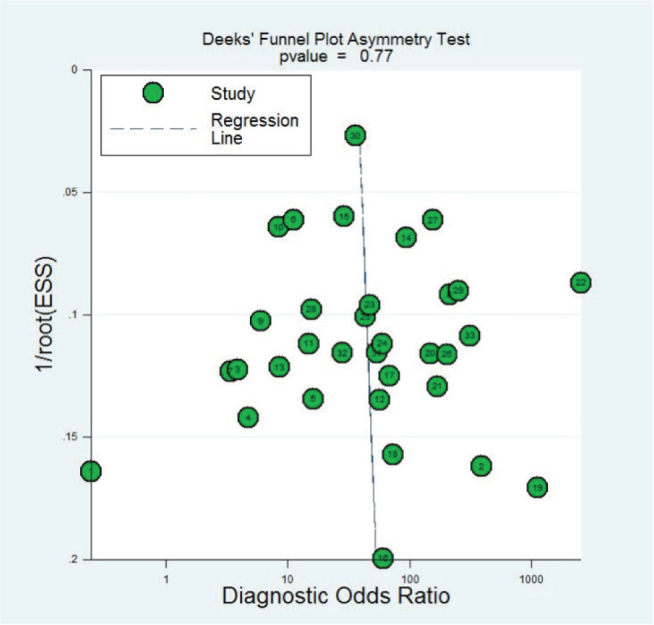
The funnel plot of publication bias for 1,3-β-D-glucan in diagnosis of invasive fungal infection
5. Discussion
Statistical studies demonstrated that invasive fungal infection(IFI) has significantly increased because of the extensive application of high-dose chemotherapy, glucocorticoids, broad-spectrum antibiotics and immunosuppressive agents, as well as the extensive development of solid organ transplantation and hematopoietic stem cell transplantation [44, 45]. Generally, appropriate clinical antifungal treatment is often not timely, which leading to deterioration of the patient’s condition, thereafter resulting in high mortality. Therefore, rapid and accurate diagnosis of IFI is particularly important for patients with IFI.
1,3-β-D-glucan is a polysaccharide component of fungal cell wall, which is fungi specific and can’t find in other microorganisms infection disease such as bacteria, viruses and mycoplasma.1,3-β-D-glucan assay was widely used clinically for IFI diagnosis with good clinical practice value. Previously studies showed 1,3-β-D-glucan was continuously released into the peripheral blood in patients with IFI. Generally, 1,3-β-D-glucan concentration was very low in serum of healthy people, usually less than 10 pg /mL. However, it serum level elevated significant when invasive fungal infection occurred in patients, which was generally greater than 20 pg /mL. Theoretically, 1,3-β-D-glucan detection may be the ideal method for rapid diagnosis of invasive fungal infections in early stage. However, the sensitivity and specificity of these findings are not completely consistent according to the previously published studies. In order to explicated its diagnostic efficacy, we performed this meta-analysis with 37 case-control or cohort studies and pooled the diagnostic efficacy. In our present, meta-analysis, we included 37 relevant studies with the combined sensitivity, specificity, positive likelihood ratio(+lr), negative likelyhood ratio(-lr) and diagnostic odds ratio(dor) of 0.83 (95%CI:0.38-0.61), 0.81 (95%CI:0.80-0.82), 5.13 (95%CI:3.98-6.62), 0.23 (95%CI:0.18-0.30), and 29.68 (95%CI:18.94-46.52). And the pooled AUC was 0.91. The results indicated that serum 1,3-β-D-glucan assay was a promising biomarker for invasive fungal infection diagnosis.
ROC curve is an accurate and comprehensive method for evaluation diagnostic tests. According to the results of Swets [46], if the area under the ROC curve (AUC) less than 0.5, there is no diagnostic value. The diagnostic value of low with limited clinical value when the AUC between 0.5 ~ 0.7. However, the diagnostic accuracy is high when the AUC is more than 0.7. In this present meta-analysis, we found the pooled AUC was 0.91 which indicated that the diagnostic accuracy is high for serum 1,3-β-D-glucan assay in diagnosis of IFIs.
However, there are several limitations in this study. Firstly, there are some clinical heterogeneity which can lead to unstable results. Secondly, statistical heterogeneity was also existed in pooling the data, which may decrease the statistical power. Thirdly, BDG is present in numerous fungi and is therefore non-specific. However, it could probably be a helpful tool for ruling out an IFI.
6. Conclusion
Serum 1,3-β-D-glucan assay was a promising biomarker for invasive fungal infection diagnosis. However, because of the non-specificity and heterogeneity across the included studies, it should be further evaluated by high quality multicenter prospective diagnostic studies which can provided more strong evidence.
Footnotes
Conflict of interest: The authors report no conflicts of interest in this work.
References
- [1].Martino R, Lopez R, Sureda A, Brunet S, Domingo-Albós A.. Risk of reactivation of a recent invasive fungal infection in patients with hematological malignancies undergoing further intensive chemo-radiotherapy. A single-center experience and review of the literature. Haematologica. 1997;82:297–304. [PubMed] [Google Scholar]
- [2].García-Ruiz JC, Amutio E, Pontón J.. Invasive fungal infection in immunocompromised patients. Rev Iberoam Micol. 2004;21:55–62. [PubMed] [Google Scholar]
- [3].Ibáñez-Martínez E, Ruiz-Gaitán A, Pemán-García J.. Update on the diagnosis of invasive fungal infection. Rev Esp Quimioter. 2017;30(1):16–21. [PubMed] [Google Scholar]
- [4].Onishi A, Sugiyama D, Kogata Y, Saegusa J, Sugimoto T, Kawano S, Morinobu A, Nishimura K, Kumagai S.. Diagnostic accuracy of serum 1, 3-β-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50:7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].He S, Hang JP, Zhang L, Wang F, Zhang DC, Gong FH.. A systematic review and meta-analysis of diagnostic accuracy of serum 1, 3-β-D-glucan for invasive fungal infection: Focus on cutoff levels. J Microbiol Immunol Infect. 2015;48:351–361. doi: 10.1016/j.jmii.2014.06.009. [DOI] [PubMed] [Google Scholar]
- [6].Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J.. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(25) doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K, Ishikawa N, Hara K.. Plasma (1-->3)-beta-D-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J Clin Microbiol. 1995;33:3115–3118. doi: 10.1128/jcm.33.12.3115-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A.. Plasma (1-->3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet. 1995;345:17–20. doi: 10.1016/s0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- [9].Kawazu M, Kanda Y, Nannya Y, Aoki K, Kurokawa M, Chiba S, Motokura T, Hirai H, Ogawa S.. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1-->3)-beta-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J Clin Microbiol. 2004;42:2733–2741. doi: 10.1128/JCM.42.6.2733-2741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kondori N, Edebo L, Mattsby-Baltzer I.. Circulating beta (1-3) glucan and immunoglobulin G subclass antibodies to Candida albicans cell wall antigens in patients with systemic candidiasis. Clin Diagn Lab Immunol. 2004;11:344–350. doi: 10.1128/CDLI.11.2.344-350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L.. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodys-plastic syndrome. Clin Infect Dis. 2004;39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- [12].Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH.. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–659. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- [13].Pazos C, Pontón J, Del PA.. Contribution of (1->3)-beta-D-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J Clin Microbiol. 2005;43:299–305. doi: 10.1128/JCM.43.1.299-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL.. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fujita S, Takamura T, Nagahara M, Hashimoto T.. Evaluation of a newly developed down-flow immunoassay for detection of serum mannan antigens in patients with candidaemia. J Med Microbiol. 2006;55:537–543. doi: 10.1099/jmm.0.46314-0. [DOI] [PubMed] [Google Scholar]
- [16].Alam FF, Mustafa AS, Khan ZU.. Comparative evaluation of (1, 3)-beta-D-glucan, mannan and anti-mannan antibodies, and Candida species-specific snPCR in patients with candidemia. BMC Infect Dis. 2007;7(103) doi: 10.1186/1471-2334-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Akamatsu N, Sugawara Y, Kaneko J, Tamura S, Makuuchi M.. Preemptive treatment of fungal infection based on plasma (1 --> 3)beta-D-glucan levels after liver transplantation. Infection. 2007;35:346–351. doi: 10.1007/s15010-007-6240-7. [DOI] [PubMed] [Google Scholar]
- [18].Obayashi T, Negishi K, Suzuki T, Funata N.. Reappraisal of the serum (1-->3)-beta-D-glucan assay for the diagnosis of invasive fungal infections--a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864–1870. doi: 10.1086/588295. [DOI] [PubMed] [Google Scholar]
- [19].Senn L, Robinson JO, Schmidt S, Knaup M, Asahi N, Satomura S, Matsuura S, Duvoisin B, Bille J, Calandra T, Marchetti O.. 1, 3-Beta-D-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin Infect Dis. 2008;46:878–885. doi: 10.1086/527382. [DOI] [PubMed] [Google Scholar]
- [20].Persat F, Ranque S, Derouin F, Michel-Nguyen A, Picot S, Sulahian A.. Contribution of the (1-->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2008;46:1009–1013. doi: 10.1128/JCM.02091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ellis M, Al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, Klingspor L.. Assessment of the clinical utility of serial beta-D-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol. 2008;57:287–295. doi: 10.1099/jmm.0.47479-0. [DOI] [PubMed] [Google Scholar]
- [22].León C, Ruiz-Santana S, Saavedra P, Galván B, Blanco A, Castro C, Balasini C, Utande-Vázquez A, de Molina FJ G, Blasco-Navalproto MA, López MJ, Charles PE, Martín E, Hernández-Viera MA.. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37:1624–1633. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]
- [23].Lunel FM, Mennink-Kersten MA, Ruegebrink D, van der Lee HA, Donnelly JP, Blijlevens NM, Verweij PE.. Value of Candida serum markers in patients with invasive candidiasis after myeloablative chemotherapy. Diagn Microbiol Infect Dis. 2009;64:408–415. doi: 10.1016/j.diagmicrobio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [24].Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I.. Utility of galactomannan enzyme immunoassay and (1, 3) beta-D-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol. 2009;47:129–133. doi: 10.1128/JCM.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koo S, Bryar JM, Page JH, Baden LR, Marty FM.. Diagnostic performance of the (1-->3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis. 2009;49:1650–1659. doi: 10.1086/647942. [DOI] [PubMed] [Google Scholar]
- [26].Presterl E, Parschalk B, Bauer E, Lassnigg A, Hajdu S, Graninger W.. Invasive fungal infections and (1, 3)-beta-D-glucan serum concentrations in long-term intensive care patients. Int J Infect Dis. 2009;13:707–712. doi: 10.1016/j.ijid.2008.10.013. [DOI] [PubMed] [Google Scholar]
- [27].Racil Z, Kocmanova I, Lengerova M, Weinbergerova B, Buresova L, Toskova M, Winterova J, Timilsina S, Rodriguez I, Mayer J.. Difficulties in using 1, 3-{beta}-D-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies--high frequency of false-positive results and their analysis. J Med Microbiol. 2010;59:1016–1022. doi: 10.1099/jmm.0.019299-0. [DOI] [PubMed] [Google Scholar]
- [28].Alexander BD, Smith PB, Davis RD, Perfect JR, Reller LB.. The (1, 3){beta}-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol. 2010;48:4083–4088. doi: 10.1128/JCM.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hirata Y, Yokote T, Kobayashi K, Nakayama S, Oka S, Miyoshi T, Akioka T, Hiraoka N, Iwaki K, Takayama A, Nishimura Y, Makino J, Takubo T, Tsuji M, Hanafusa T.. Antifungal prophylaxis with micafungin in neutropenic patients with hematological malignancies. Leuk Lymphoma. 2010;51:853–859. doi: 10.3109/10428191003682726. [DOI] [PubMed] [Google Scholar]
- [30].Li Jun, Wang Junji, Chen Wei, Li Min, Deng Shaoli, Chen Ming. Diagnostic value of plasma (1, 3)-β-D-glucan detection for invasive fungal infection. Laboratory Medicine and Clinic. 2010;071807:1804–1805. [Google Scholar]
- [31].Zuo Xianghua, Chen Kejian, Yu Nong, Jin Xin, Yin Xiuyun, Song Shiping, Huang Yuan, Du Yu, Zhu Xiaohua, Zeng Lijun, Wang Mial. The clinical value of measuring the 1, 3-β-D-glucan among the patients with invasive fungal infections. International Journal of Laboratory Medicine. 2010;31:220–221. 223. [Google Scholar]
- [32].De Vlieger G, Lagrou K, Maertens J, Verbeken E, Meersseman W, Van Wijngaerden E.. Beta-D-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: an autopsy-based study. J Clin Microbiol. 2011;49:3783–3787. doi: 10.1128/JCM.00879-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Posteraro B, De Pascale G, Tumbarello M, Torelli R, Pennisi MA, Bello G, Maviglia R, Fadda G, Sanguinetti M, Antonelli M.. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1→3)-β-D-glucan assay, Candida score, and colonization index. Crit Care. 2011;15:R249. doi: 10.1186/cc10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Acosta J, Catalan M, del PA, Lora D, Montejo JC, Cuetara MS, Moragues MD, Ponton J, del PA.. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1→3)-β-d-glucan chromogenic assay in serum samples. Clin Microbiol Infect. 2011;17:1053–1060. doi: 10.1111/j.1469-0691.2010.03357.x. [DOI] [PubMed] [Google Scholar]
- [35].Jiang Zuiming, Gu Min, Chen Jingqun, Liu Jiaqiang, Li Shunwu. Application of Serum (1, 3) β-D Glucan Detection in Early Diagnosis of Deep Fungal Infection. Practical Preventive Medicine. 2011;18:1979–1980. [Google Scholar]
- [36].Yang Huiqin Mei Yanying. Clinical value of(1, 3)-β-D-glucan in plasma on the diagnosis of deep fungal infection. Chinese Journal of Mycology. 2011;06:136–140. [Google Scholar]
- [37].JJin X, Chen JK, Yu N, Yin XY, Zuo XH, Song Sp, Tong LW, Xu XY, Tian SG.. Clinical significance of 1, 3-β-D-glucan in diagnosis of invasive fungal infection. Hebei Medical Journal. 2011;33:378–379. [Google Scholar]
- [38].Metan G, Koç AN, Atalay A, Kaynar LG, Ozturk A, Alp E, Eser B.. What should be the optimal cut-off of serum 1, 3-β-D-glucan for the detection of invasive pulmonary aspergillosis in patients with haematological malignancies. Scand J Infect Dis. 2012;44:330–336. doi: 10.3109/00365548.2011.638319. [DOI] [PubMed] [Google Scholar]
- [39].Liu CH.. Diagnostic value of serum 1, 3-β-D-glucan in diagnosis of invasive fungal infection. Medical Laboratory Science and Clinics. 2012;10:147–148. [Google Scholar]
- [40].Ding C SJ, Xu YL.. Diagnostic efficacy of serum 1, 3-β-D-glucan in diagnosis of invasive fungal infection. Medical Laboratory Science and Clinics. 2012;23:81–82. [Google Scholar]
- [41].Wang QF.. Research on (1-3)-β-D-glucan detection in plasma to diagnose invasive fungal infection. Chinese Journal of Clinical Rational Drug Use. 2012;05:39–40. [Google Scholar]
- [42].Yang D, Ma DY, He X, Chi S.. Value of (1-3)-β-D-glucan assay combined with fungal culture in diagnosis of invasive fungal infections. Chinese Journal of Nosocomiology. 2013;23:2252–2254. [Google Scholar]
- [43].Zeng WX, Huang Y, Deng Y, Wen my, Han YL, Zhong WH, Zeng HK.. Clinical evaluation of the (1, 3)-β-D-glucan assay as an aid to diagnosis of fungal infections in severe pneumonia patients. Chinese Journal of Emergency Medicine. 2016;25:659–662. [Google Scholar]
- [44].Fisher BT, Robinson PD, Lehrnbecher T, Steinbach WJ, Zaoutis TE, Phillips B, Sung L.. Risk Factors for Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review. J Pediatric Infect Dis Soc. 2017 doi: 10.1093/jpids/pix030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sai SPV, Azim A.. Risk factors for early invasive fungal disease in critically ill patients. Indian J Crit Care Med. 2016;20(750) doi: 10.4103/0972-5229.195723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Swets JA.. ROC analysis applied to the evaluation of medical imaging techniques. Invest Radiol. 1979;14:109–121. doi: 10.1097/00004424-197903000-00002. [DOI] [PubMed] [Google Scholar]



