Abstract
Background
Malnutrition after hip fracture is associated with increased rehabilitation time, complications, and mortality. We assessed the effect of intensive 3 month nutritional intervention in elderly after hip fracture on length of stay (LOS).
Methods
Open-label, randomized controlled trial. Exclusion criteria: age < 55 years, bone disease, life expectancy < 1 year, bedridden, using oral nutritional supplements (ONS) before hospitalization, and cognitive impairment. Intervention: weekly dietetic consultation, energy-protein–enriched diet, and ONS (400 mL per day) for 3 months. Control: usual nutritional care. Primary outcome: total LOS in hospital and rehabilitation clinic, including readmissions over 6 months (Cox regression adjusted for confounders); hazard ratio (HR) < 1.0 reflects longer LOS in the intervention group. Secondary outcomes: nutritional and functional status, cognition, quality of life, postoperative complications (6 months); subsequent fractures and all-cause mortality (1 and 5 years). Effect modification by baseline nutritional status was also tested.
Results
One hundred fifty-two patients were randomized (73 intervention, 79 control). Median total LOS was 34.0 days (range 4–185 days) in the intervention group versus control 35.5 days (3–183 days; plogrank = .80; adjusted hazard ratio (adjHR): 0.98; 95% CI: 0.68–1.41). Hospital LOS: 12.0 days (4–56 days) versus 11.0 days (3–115 days; p = .19; adjHR: 0.75; 95% CI: 0.53–1.06) and LOS in rehabilitation clinics: 19.5 days (0–174 days) versus 18.5 days (0–168 days; p = .82; adjHR: 1.04; 95% CI: 0.73–1.48). The intervention improved nutritional intake/status at 3, but not at 6 months, and did not affect any other outcome. No difference in intervention effect between malnourished and well-nourished patients was found.
Conclusions
Intensive nutritional intervention after hip fracture improved nutritional intake and status, but not LOS or clinical outcomes. Paradigms underlying nutritional intervention in elderly after hip fracture may have to be reconsidered.
Keywords: Oral nutritional supplementation, Dietetic counselling, Length of stay, Postoperative complications, Nutritional status
Hip fractures are among the most common reasons for admission to hospital and nursing facilities in elderly in Western countries (1). As only 37% of the patients return to prefracture functional status (2), this leads to high health care and social costs (3). At hospital admission, hip fracture patients often have a poor nutritional status (4–7), which can deteriorate further during hospitalization because of inadequate food intake or surgical trauma (4, 7). Malnutrition in these patients is associated with functional disability and loss of independence (6, 8–11), impaired cognitive function (10), higher postoperative complication rate (8), prolonged rehabilitation time (4), and increased mortality (6, 8, 10–12). In view of the aging population and rising health care costs, it is important to achieve rapid and full recovery of elderly after hip fracture, to return to the prefracture state, and, if possible, to live independently.
In previous studies, oral nutritional supplementation (ONS) after hip fracture reduced length of stay (LOS) in hospital and/or rehabilitation clinic (5, 13, 14), number of postoperative complications (5, 13, 15, 16) and mortality (5), and improved daily living (17). However, other studies failed to show such effects (14–16, 18, 19). Possible factors underlying these heterogeneous results are low adherence and short duration of the intervention (20). In a recently updated Cochrane review (21), it was concluded that the evidence for the effectiveness of oral multinutrient supplements after hip fracture remains weak, that adequately size randomized trials with robust methodology are required to overcome the defects of the reviewed studies (particularly inadequate size and trial methods), and that the role of adequate dietetic assistance requires further evaluation.
Therefore, the primary aim of the present study was to assess the effect of intensive nutritional intervention, comprising ONS and regular dietetic counselling, on total LOS in hospital and rehabilitation clinics in elderly after hip fracture. We hypothesized that nutritional intervention would reduce total LOS and improve dietary intake and nutritional status, 6 month postoperative complications, 1 and 5 year fractures rates and mortality, functional disability, cognitive status, psychological distress, fatigue, and health–related quality of life (QoL). We also investigated whether screening for malnutrition at baseline could contribute to targeting the intervention, that is, whether the nutritional intervention would be more effective in patients with (risk of) malnutrition.
Methods
Participants
Eligible were elderly patients admitted for surgical treatment of hip fracture (22). Exclusion criteria were as follows: age < 55 years, pathological or periprosthetic fracture, diseases of bone metabolism (eg Paget′s disease, primary/secondary bone tumors, hyperparathyroidism, M. Kahler), life expectancy < 1 year due to underlying disease (eg cancer), using ONS before hospital admission, inability to speak Dutch, living outside the region, being bedridden before the hip fracture, dementia or cognitive impairment (score < 7 on Abbreviated Mental Test, AMT) (23), or unavailable for follow-up. A daily inventory was made of all hip fracture patients admitted to the surgical and orthopedic wards of three hospitals in the region of South-Limburg, The Netherlands: Maastricht University Medical Center and Zuyderland Medical Center, Heerlen and Sittard-Geleen. A total sample size of 150 patients was calculated to be sufficient to detect a clinically relevant reduction in total LOS in hospital and rehabilitation clinics of 31 per cent (SD 59 per cent) with a power of 90 per cent and a two-tailed α of 0.05 (5, 13, 24, 25).
Trial Design
Study design and methods were previously published (22). Briefly, the study was an open-label, randomized controlled, multicenter trial. Masked telephone randomization was performed immediately following baseline measurements, based on a computer-generated random-number sequence list (block size: 4) with prestratification for center, sex, and age (55–74 vs ≥75 years). Patients allocated to the intervention group received dietetic counseling and ONS for 3 months following surgery. The control group received usual nutritional care (ie ONS only if prescribed by the medical doctor in charge). All patients in both groups received physical and exercise therapy daily during hospitalization and after discharge.
Patients were enrolled within 5 days after surgical treatment of hip fracture, and baseline measurements are performed immediately after enrolment. Outcome measurements were performed at the patient’s home at 3 and 6 months following hip fracture. The study was approved by the Medical Ethical Committee of Maastricht University Hospital and Maastricht University (06-3-098) and conducted according to the Declaration of Helsinki. All participants gave written informed consent (22).
Nutritional Intervention
The nutritional intervention comprised regular counselling by a trained study dietician and consumption of a multinutrient ONS for 3 months postsurgery, that is, starting during hospitalization and continuing in the rehabilitation center and/or, if applicable, at home. The dietician had ten contacts with each patient: two during hospitalization and eight thereafter (three face-to-face and five telephone calls). Throughout the study, a patient was supervised by the same dietician. Dietary advice, based on individual energy and protein requirements, comprised an energy- and protein-rich diet as well as recommendations on choice, quantity, and timing of foods. Energy requirement after hip fracture was calculated according to Harris–Benedict (26), with 20 per cent surcharge for metabolic stress and, if indicated, a surcharge for activity or desired body weight increase (maximum total surcharge: 40 per cent). Patients received 400 mL per day ONS (two bottles of Cubitan, N.V. Nutricia, Zoetermeer, The Netherlands), providing 2.1 MJ per day (500 kcal per day) and 40 g per day protein, to be consumed in-between meals. Patients consumed one bottle of ONS in the morning and one in the afternoon, either at once or divided over the day; spreading over the day was adapted individually to achieve the target ONS dose per day. To allow feedback by the dietician, all patients kept an ONS diary showing amount and time of ONS throughout the intervention period. When a patient’s food intake was sufficient to meet individual energy and protein requirements (27, 28), the ONS was stopped. For further details, see Ref. 22.
Adherence to dietary recommendations and ONS was evaluated by repeated 24-hour recalls and reported by early (days 1–10) and late (days 11–90) postoperative period. Patients were considered adherent to the ONS in the concerned period if they used ≥75 per cent of the advised amount and adherent to dietary advice if they followed this advice in ≥75 per cent of the 24 hour recalls collected by the dietician during patient contacts (visits and telephone calls). Adherence is reported as the proportion of patients in the intervention group who were adherent according to the above definition.
Usual Care
Control patients received usual nutritional care as provided in the hospital, rehabilitation clinic, or at home, that is, dietetic advice or ONS were only provided if prescribed by the medical doctor in charge.
Primary and Secondary Endpoints
Primary endpoint
Primary endpoint was total LOS in hospital and rehabilitation clinics until 6 months postoperatively. Hospital LOS was calculated as the sum (ie number of days) of all separate hospital stays until 6 months postoperatively. Dates of admission and discharge were obtained from medical charts.
Secondary endpoints
Nutritional outcomes included energy and nutrient intake, body weight and height, midupper arm circumference, biceps, triceps, suprailiacal and subscapular skinfolds, and handgrip strength; body mass index and midupper arm muscle area were also calculated. Functional outcomes were assessed using validated questionnaires. Data on postoperative complications (until 6 months), incidence of subsequent fractures, and mortality (until 5 years) were derived from medical charts and the Dutch national Basic Register of Persons. For details on nutritional assessment and questionnaires, see Supplementary Material.
Confounders
Nutritional status at baseline was assessed by the 18-item Mini Nutritional Assessment (29). Since LOS as the primary outcome was similar for the categories “at risk” and “malnutrition,” and as only ten patients in the total population were in the MNA category “malnutrition,” we combined the categories “at risk” and “malnutrition” into one category (further called “malnourished”). Medical history, type of fracture, American Society of Anesthesiologists (ASA) score before surgery, and duration of surgery were derived from medical charts.
Statistical Analysis
SPSS 23.0 for Windows (IBM Corporation, Armonk, NY) was used. Results were analyzed by intention-to-treat. Intervention effects on LOS and mortality were analyzed by Kaplan–Meier plots, the logrank test, and Cox proportional hazards models. A hazard ratio (HR) < 1.0 for LOS reflects a lower rate of discharge (ie longer LOS) in the intervention group (unfavorable), and a HR < 1.0 for mortality reflects a lower mortality rate, that is, longer survival, in the intervention group (favorable). Chi-square was used for testing proportions. Intervention effect on secondary endpoints was defined as difference in change from baseline between intervention and control group, and appraised by analysis of covariance for continuous outcomes and by logistic regression for dichotomous outcomes, adjusting for stratification variables (center, sex, and age), and for baseline value of the concerned outcome parameter (model 1, partially adjusted). Multivariable adjusted models (model 2) were fitted with additional adjustment for fracture type, diseases of the nervous system, chronic obstructive pulmonary disease (COPD) and asthma, cerebrovascular diseases, ASA classification, and risk of malnutrition (yes/no). Adjusted results, where not mentioned otherwise, refer to model 2. Additional analyses included, amongst others, per protocol analyses and a check for effect modification by baseline nutritional status (MNA). p < .05 was considered statistically significant.
Results
Trial Population
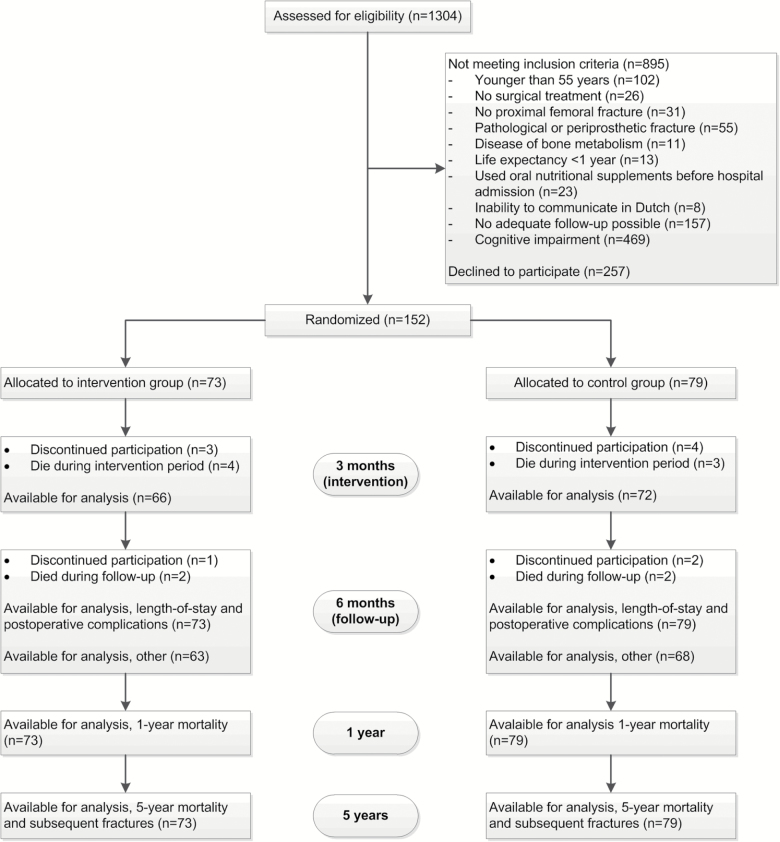
The flowchart of the study is shown in Figure 1. Between July 2007 and December 2009, 1304 hip fracture patients were assessed for eligibility, and 895 (69 per cent) did not meet inclusion and exclusion criteria. Of the 409 eligible patients, 257 declined participation, resulting in 152 patients (37 per cent) who gave informed consent. Of these, 73 were randomly allocated to the intervention group and 79 to the control group. A total of 138 patients (66 intervention, 72 control) completed the 3 month intervention period, and 131 patients (63 intervention, 68 control) completed the 6 month follow-up. For LOS, complications, mortality, and subsequent fractures, data were available for all 152 patients.
Figure 1.
Flow chart. Patients were excluded based on the exclusion criteria in the order given in the figure, so subsequent exclusion criteria were not checked.
Baseline data are given in Table 1 and Supplementary Tables 1–2. In both groups, over 80 per cent of patients were admitted from their home situation, and ~40 per cent were at risk of malnutrition or malnourished. Both groups were comparable in age, sex, blood parameters, time between hospital admission and surgery, and duration of surgery, but slightly differed in comorbidities, ASA classification, and fracture type; these factors were adjusted for in all statistical analyses.
Table 1.
Baseline Characteristics of the Study Population
| Intervention | Control | |||
|---|---|---|---|---|
| (n = 73) | (n = 79) | |||
| Female | 54 | (74.0) | 54 | (68.4) |
| Sex | n | (%) | n | (%) |
| Mean | (SEM) | Mean | (SEM) | |
| Age (y) | 77 | (1.2) | 76 | (1.1) |
| Type of residence before fracture | n | (%) | n | (%) |
| Home | 63 | (86.3) | 66 | (83.5) |
| Home for the elderly | 8 | (11.0) | 7 | (8.9) |
| Nursing home | 2 | (2.7) | 4 | (5.1) |
| Rehabilitation clinic/hospital | 0 | (0.0) | 2 | (2.5) |
| Medical history | ||||
| Cardiovascular diseases | 40 | (54.8) | 43 | (54.4) |
| Heart diseases | 20 | (27.4) | 26 | (32.9) |
| Cerebrovascular diseases | 2 | (2.7) | 8 | (10.1) |
| Diabetes mellitus | 16 | (21.9) | 14 | (17.7) |
| Diseases of the nervous system | 8 | (11.0) | 18 | (22.8) |
| Diseases of muscoskeletal system and connective tissue | 28 | (38.4) | 26 | (32.9) |
| Rheumatoid arthritis | 6 | (8.2) | 5 | (6.3) |
| Arthrosis | 5 | (6.8) | 9 | (11.4) |
| Osteoporosis | 8 | (11.0) | 8 | (10.1) |
| Hernia nuclei pulposi | 7 | (9.6) | 5 | (6.3) |
| Other | 13 | (17.8) | 15 | (19.0) |
| Chronic obstructive pulmonary disease and asthma | 3 | (4.1) | 8 | (10.1) |
| ASA classification | ||||
| I | 9 | (12.5) | 9 | (11.4) |
| II | 49 | (68.1) | 48 | (60.8) |
| III | 14 | (19.4) | 21 | (26.6) |
| IV | 0 | (0.0) | 1 | (1.3) |
| Biochemical parameters† | Mean | (SEM) | Mean | (SEM) |
| Hemoglobin (mmol/L) | 8.2 | (0.12) | 8.0 | (0.09) |
| Hematocrit (mmol/L) | 0.39 | (0.01) | 0.38 | (0.01) |
| Leucocytes (10E9/L) | 9.7 | (0.40) | 11.8 | (1.4) |
| Creatinine (µmol/L) | 95.8 | (4.7) | 90.7 | (3.8) |
| INR | 1.1 | (0.05) | 1.1 | (0.04) |
| Fracture type | n | (%) | n | (%) |
| Medial neck | 36 | (49.3) | 45 | (57.0) |
| Pertrochanteric | 32 | (43.8) | 33 | (41.8) |
| Subtrochanteric | 5 | (6.8) | 1 | (1.3) |
| Mean | (SEM) | Mean | (SEM) | |
| Time between admission and surgery (h) | 20.3 | (1.8) | 19.8 | (2.4) |
| Duration of surgery (min) | 67.6 | (2.9) | 68.5 | (3.5) |
| MNA‡ | n | (%) | n | (%) |
| No malnutrition | 46 | (63.0) | 41 | (51.9) |
| At risk of malnutrition or malnourished | 27 | (37.0) | 38 | (48.1) |
Notes: Numbers may not add up to total because of missing values.
ASA = American Society of Anesthesiologists; INR = International Normalized Ratio; MNA = Mini-Nutritional Assessment; SEM = standard error of mean.
†Measured preoperatively.
‡Categories malnutrition and risk of malnutrition combined.
Intervention and adherence
After randomization, all intervention patients, except one, started with ONS. The median period of ONS use was 76 days (3–91 days). During the early postoperative period (days 1–10), adherence was 78 per cent for ONS and 66 per cent for dietary advice given by the dietician; during the late postoperative period (days 11–90), adherence was 80 and 73 per cent, respectively. The contribution of ONS to total energy intake in the intervention group was 21 per cent at 1 week, 5 per cent at 3 months, and 1 per cent at 6 months. For protein, this was 33 per cent at 1 week, 8 per cent at 3 months, and 1 per cent at 6 months.
Seven control patients (9 per cent) received ONS on medical indication at any time during the first 3 months postoperatively (ie three during hospitalization, and four during rehabilitation or at home). The contribution of ONS to energy and protein intake in the control group was <1 per cent.
Primary Outcome
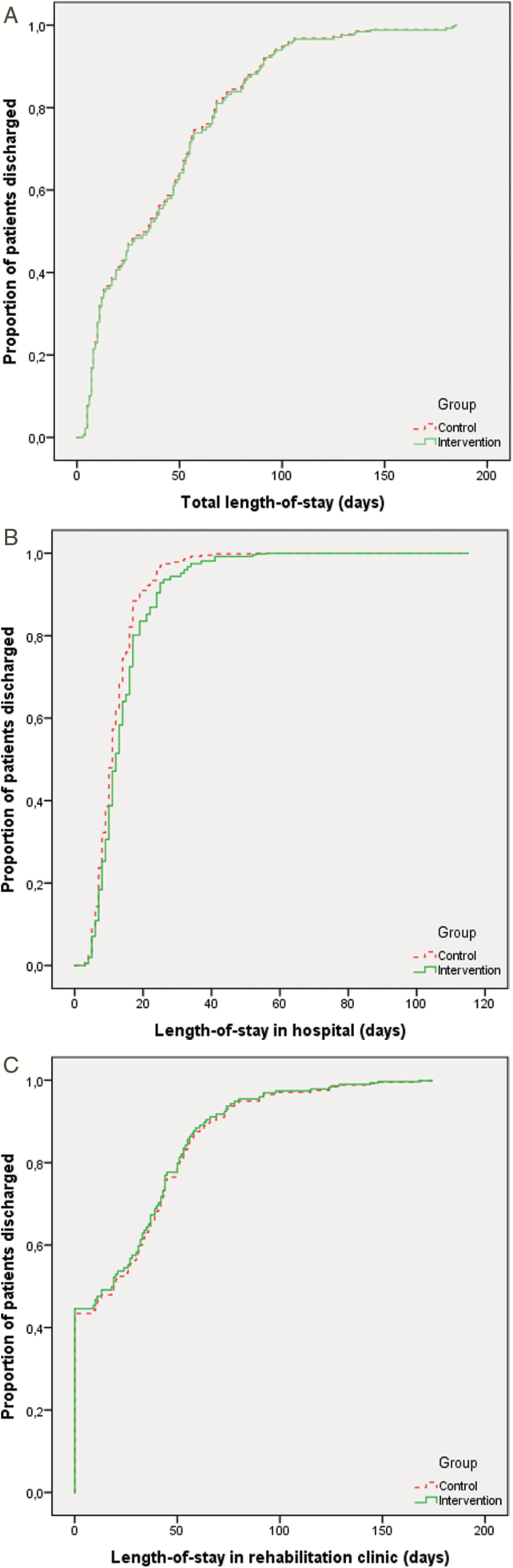
Figure 2 depicts Kaplan–Meier plots by group for total LOS in hospital and rehabilitation combined (A), as well as for LOS in hospital (B) and rehabilitation clinic (C) separately, including hospital readmissions until 6 months postoperatively. Median total LOS was 34.0 (range: 4–185) days in the intervention group and 35.5 (3–183) days in the control group [plogrank = .80, adjusted hazard ratio (adjHR): 0.98, 95% confidence interval (95% CI): 0.68–1.41; HR < 1 reflects lower rate of discharge (ie longer LOS) in the intervention group]. Median LOS in hospital was 12.0 (4–56) days and 11.0 (3–115) days, respectively (plogrank = .19, adjHR: 0.75, 95% CI: 0.53–1.06), and median LOS in rehabilitation clinics 19.5 (0–174) days and 18.5 (0–168) days, respectively (plogrank = .82, adjHR: 1.04, 95% CI: 0.73–1.48). Of the patients discharged alive from hospital, 42 intervention (59 per cent) and 42 control (53 per cent) patients were discharged to a rehabilitation clinic and the remaining patients to their home situation.
Figure 2.
Kaplan-Meier plots for intervention and control group of total length of stay (LOS) in hospital and rehabilitation clinics (A), LOS in hospital (B), and rehabilitation clinics (C) separately. Hospital readmissions until six months postoperatively are included. The X-axis represents LOS (in days), the Y-axis represents the cumulative proportion of patients discharged from hospital and/or rehabilitation clinic. A hazard ratio <1.0 reflects a lower rate of discharge (i.e. longer LOS) in the intervention group.
Secondary Outcomes
Nutritional intake and status
At 1 week postoperatively, a significant positive intervention effect was found on intake of energy and all nutrients in the fully adjusted models (Model 2, Table 2). At 3 months, a positive intervention effect was seen for total fat and calcium. At 6 months, no significant differences were found for any nutrient. At 1 week, 76 per cent of intervention patients versus 55 per cent of control patients consumed ≥80 per cent of energy requirements (p = .009); at 3 months, these proportions were 69 and 68 per cent (p = .88) and at 6 months, 78 and 69 per cent (p = .23), respectively.
Table 2.
Energy and Nutrient Intake in Intervention and Control Group, at Baseline, 1 Week, 3 and 6 Months Postoperatively, as Well as Intervention Effect at Different Time Points
| Baseline | 1 week | 3 months | 6 months | Intervention effect† | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 week | 3 months | 6 months | ||||||||||||||
| Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | Mean | (SEM) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | ||||||
| Energy (kcal) | I | 1268 | (66.6) | 1703 | (59.5) | 1699 | (58.0) | 1763 | (65.5) | M1‡ | 249 | (115–384)*** | 117 | (−40–275) | 48 | (−129–226) |
| C | 1304 | (65.1) | 1488 | (50.3) | 1602 | (60.7) | 1762 | (72.0) | M2$ | 261 | (117–404)*** | 90 | (−76–257) | 54 | (−130–239) | |
| Protein (g) | I | 54.2 | (2.8) | 81.2 | (3.1) | 66.0 | (2.5) | 70.1 | (3.0) | M1 | 22.6 | (15.9–29.3)*** | 3.8 | (−2.9–10.4) | 2.2 | (−6.2–10.6) |
| C | 53.0 | (2.9) | 60.2 | (2.2) | 62.4 | (2.3) | 68.9 | (3.2) | M2 | 23.8 | (15.7–29.8)*** | 2.6 | (−4.4–9.6) | 0.7 | (−8.1–9.6) | |
| Fat (g) | I | 45.6 | (2.9) | 60.7 | (2.5) | 70.1 | (3.3) | 70.9 | (3.4) | M1 | 6.0 | (−0.6–12.6) | 11.3 | (3.0–19.5)** | 4.0 | (−5.5–13.5) |
| C | 48.4 | (3.0) | 55.8 | (2.4) | 60.3 | (2.9) | 69.1 | (3.5) | M2 | 7.3 | (0.3–14.3)* | 11.3 | (2.6–19.9)* | 4.6 | (−5.3–14.5) | |
| Carbohydrate (g) | I | 160 | (8.8) | 207 | (8.0) | 195 | (7.4) | 200 | (8.3) | M1 | 26.9 | (8.2–45.6)** | 2.1 | (−19.5–23.6) | −4.3 | (−26.8–18.2) |
| C | 164 | (8.6) | 184 | (7.2) | 194 | (8.4) | 209 | (8.7) | M2 | 26.6 | (6.7–46.5)** | −2.7 | (−25.5-20.1) | −1.5 | (−24.8–21.8) | |
| Calcium (mg) | I | 633 | (45.0) | 1243 | (63.0) | 853 | (47.7) | 814 | (44.8) | M1 | 563 | (435–692)*** | 161 | (43–279)** | 7 | (−135–150) |
| C | 647 | (46.9) | 709 | (34.4) | 690 | (37.0) | 815 | (58.7) | M2 | 558 | (425–690)*** | 144 | (21–268)* | 11 | (−138–159) | |
| Vitamin D (µg) | I | 2.3 | (0.18) | 6.7 | (0.42) | 4.1 | (0.36) | 3.7 | (0.32) | M1 | 3.76 | (2.87–4.66)*** | 0.39 | (−0.62–1.39) | 0.07 | (−0.97–1.12) |
| C | 2.5 | (0.25) | 3.0 | (0.20) | 3.8 | (0.35) | 4.0 | (0.42) | M2 | 3.91 | (2.99–4.82)*** | 0.49 | (−0.59–1.57) | 0.00 | (−1.11–1.05) | |
Numbers printed in bold indicate a significant intervention effect (i.e. a significant difference in the intervention group).Notes: I = intervention group; C = control group; SEM = standard error of mean; 95% CI = 95% confidence interval.
†Intervention effect: difference in change from baseline to 1 wk, 3 or 6 mo postoperatively between intervention and control group.
‡M1 = partially adjusted model: adjusted for stratification variables, i.e., center, sex, and age, and for baseline value of the concerned variable.
§M2 = fully adjusted model: adjusted for center, sex age, baseline value of the concerned variable, diseases of the nervous system, chronic obstructive pulmonary disease and asthma, cerebrovascular diseases, ASA score, and risk of malnutrition according to the Mini-Nutritional Assessment.
*p < 0.05; **p < .01; ***p < .001.
At 3 months, body weight had increased from 69.1 to 70.4 kg in the intervention group and decreased from 67.6 to 67.3 kg in the control group (intervention effect: 1.9 kg (95% CI: 0.6–3.3 kg, p < .01); BMI and plasma vitamin C and 5-methyl-tetrahydrofolate also showed a positive intervention effect (Supplementary Table 1). By 6 months, all differences had disappeared. No consistent intervention effects were seen for any other anthropometric or biochemical parameters, cognition, fatigue, functional disability, psychological distress, or health-related QoL (Supplementary Tables 1–2).
Postoperative complications
No significant difference in incidence of total postoperative complications, infectious complications, cardiovascular complications, pressure ulcers, delirium, or anemia was detected (Table 3). However, overall surgical complications as well as dislocation of the hip implant and surgical revision of osteosynthesis material occurred in a significantly higher proportion of intervention patients than control patients in fully adjusted analyses.
Table 3.
Postoperative Complications in Intervention and Control Group Over 6 Months Postoperatively [Number of Patients (%)]
| Intervention (n=73) | Control (n=79) | P value | |||||
|---|---|---|---|---|---|---|---|
| Postoperative complications | n | (%) | n | (%) | Unadj.† | Part.adj.‡ | Fully adj.§ |
| Total number of patients with ≥1 complication tion | 32 | (43.8) | 35 | (44.3) | 0.95 | 0.99 | 0.49 |
| Surgical complications | 19 | (26.0) | 11 | (13.9) | 0.06 | 0.04 | 0.03 |
| Surgical bleeding | 3 | (4.1) | 2 | (2.5) | 0.67 | 0.54 | 0.90 |
| Dislocation of the hip implant | 8 | (11.0) | 1 | (1.3) | 0.02 | 0.04 | 0.05 |
| Surgical revision of osteosynthesis material | 13 | (17.8) | 6 | (7.6) | 0.06 | 0.05 | 0.02 |
| Wound infection | 5 | (6.8) | 3 | (3.8) | 0.48 | 0.32 | 0.31 |
| Infectious complications | 10 | (13.7) | 11 | (13.9) | 0.97 | 0.87 | 0.90 |
| Urinary tract infection | 5 | (6.8) | 8 | (10.1) | 0.47 | 0.47 | 0.40 |
| Pulmonary infection | 2 | (2.7) | 2 | (2.5) | 1.00 | 0.72 | 0.99 |
| Sepsis | 1 | (1.4) | 0 | (0.0) | 0.48 | 0.99 | 0.99 |
| Other infections | 5 | (6.8) | 2 | (2.5) | 0.26 | 0.19 | 0.20 |
| Cardiovascular complications | 2 | (2.7) | 4 | (5.1) | 0.68 | 0.46 | 0.63 |
| Myocardial infarction | 1 | (1.4) | 1 | (1.3) | 1.00 | 0.46 | 1.00 |
| Congestive heart failure | 1 | (1.4) | 0 | (0.0) | 0.48 | 1.00 | 1.00 |
| Cerebrovascular accident | 0 | (0.0) | 1 | (1.3) | 1.00 | 1.00 | 1.00 |
| Pulmonary embolism | 0 | (0.0) | 2 | (2.5) | 0.50 | 1.00 | 0.98 |
| Pressure ulcers | 4 | (5.5) | 2 | (2.5) | 0.43 | 0.34 | 0.33 |
| Delirium | 5 | (6.8) | 5 | (6.3) | 1.00 | 0.79 | 0.56 |
| Anemia | 10 | (13.7) | 13 | (16.5) | 0.64 | 0.50 | 0.39 |
†Unadjusted p value of difference between intervention and control group (chi square).
‡Partially adjusted model: adjusted for stratification variables, i.e., center, sex, and age.
§Fully adjusted model: adjusted for center, sex, age, fracture type, diseases of the nervous system, chronic obstructive pulmonary disease and asthma, cerebrovascular diseases, ASA score, and risk of malnutrition according to the Mini-Nutritional Assessment.
Subsequent fractures
No difference in fracture incidence was observed over 1 and 5 years post-hip fracture (Supplementary Table 3). Overall fracture rate at 1 year was 1 per cent in intervention patients and 3 per cent in control patients (p = .80). At 5 years, overall fracture rate was 16 per cent in the intervention group and 17 per cent in the control group (p = 1.00). Also, the type of subsequent fractures did not significantly differ between groups.
Mortality
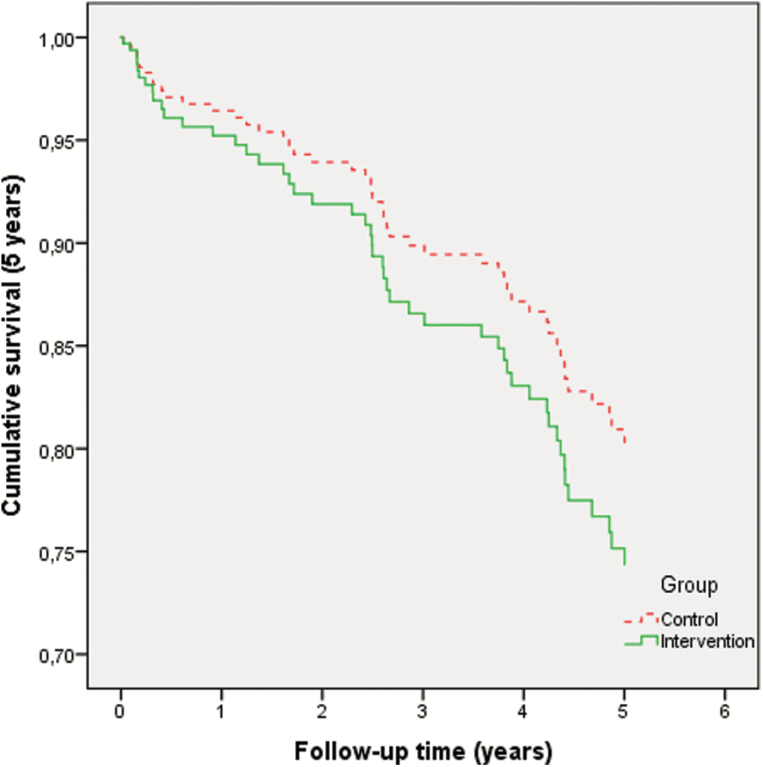
Overall 1-year mortality was seven intervention patients (10 per cent) versus six control patients (8 per cent; adjHR: 2.10, 95% CI: 0.53–8.39). Overall 5-year mortality was 21 intervention patients (29 per cent) versus 27 control patients (34 per cent; adjHR: 1.35, 95% CI: 0.70–2.61; Figure 3).
Figure 3.
Kaplan-Meier plot of 5 year mortality in intervention and control group. The X-axis represents follow-up time in years, the Y-axis represents the proportion of patients still alive. A hazard ratio <1.0 reflects a lower mortality rate (i.e. longer survival) in the intervention group.
Additional Analyses
Association between nutritional status at baseline and clinical outcome (regardless of intervention/control group)
At baseline, as expected, malnourished patients had significantly lower weight, BMI, muscle/fat mass, and handgrip strength and had significantly worse scores on mobility and self-care, depression, cognition, and health-related QoL than well-nourished patients. Overall, malnourished patients had longer LOS in hospital (median 14.0 days) than well-nourished patients (median 10.0 days; p = .002), higher rates of hospital readmissions (29 vs 9 per cent, p = .002) and postoperative complications (55 vs 36 per cent, p = .015), and higher 1- and 5-year mortality [1 year, 15 vs 3 per cent (p = .009); 5 years: 48 vs 20 per cent (p < .001)].
Intervention effect in patients who were malnourished at baseline
In patients who were malnourished at baseline according to the MNA, the intervention effect on energy and nutrient intake was similar to the total study population. For instance, energy intake at 1 week in malnourished intervention patients increased to 1686 ± 121 (mean ± SEM) versus 1456 ± 68 kcal per day in malnourished control patients [n = 38; intervention effect: 194 (95% CI: −78–465) kcal per day]. At 3 months, energy intake in malnourished intervention patients was 1713 ± 103 versus 1528 ± 90 kcal per day in malnourished control patients and intervention effect 183 (−104–469) kcal per day. For protein intake, the intervention effect in malnourished patients was 19.8 (7.2–32.2) g per day (p < .01) at 1 week and 9.3 (−3.0–21.5) g per day at 3 months. Also for most other nutrients, dietary intake during the intervention increased to a similar extent in the malnourished patients as in the total group. Baseline nutritional status did not modify either the nutritional intake and status, nor clinical outcome for LOS, complications, mortality, or any other secondary outcome (p-interaction > 0.10), indicating that in patients who were malnourished at baseline, the intervention effect was similar in malnourished and well-nourished patients for all outcomes.
Association between level of nutritional intake and clinical outcome, regardless of intervention versus control group
To further explore the association between nutritional intake and clinical outcome, we examined the association of nutritional intake level at 1 week and 3 months postoperatively (regardless of control/intervention group) with different outcomes. Results showed that higher intake of energy was not associated with better clinical outcome for LOS (total LOS, hospital, or rehabilitation), postoperative complications, functional status, QoL, 5 year subsequent fracture rate, and mortality.
Per protocol analysis
A per protocol analysis was performed in which we included only intervention patients who had been fully adherent to ONS and dietary advice at all times of the intervention (n = 44) and only control patients who did not receive ONS or dietary advice at any time (n = 59). In this per protocol analysis, total and hospital LOS tended to be longer in the intervention group than in the control group (total LOS: adjHR 0.68, 95% CI 0.43–1.07, p = .09; hospital LOS: adjHR 0.62, 95% CI 0.40–0.96, p = 0.03; rehabilitation LOS: adjHR 0.87, 95% CI 0.56–1.34, p = 0.52).
Analysis excluding patients with implant dislocation and need for surgical revision
To check whether the between-group difference in number of implant dislocations and need for surgical revision could have affected the results of the trial, we reanalyzed the data excluding these patients (analysed patients: intervention group, n = 60; control group, n = 73). Results for nutritional status, LOS (total, hospital, or rehabilitation), postoperative complications, functional status, QoL, 5 year subsequent fracture rate, and mortality remained unchanged compared with the results in the total study population.
Discussion
The present RCT can be considered as an important contribution to the existing scientific evidence on the efficacy of nutritional intervention in elderly after hip fracture. The study was based on a daily inventory of hip fracture patients admitted to three major hospitals, covering virtually all hip fracture patients in a geographically confined region in the Netherlands. Validity of data was safeguarded by prospective collection of all information, following GCP standards. All data analyses were performed as crude and confounder-adjusted analyses, without any substantial differences. The intervention entailed an energy- and protein-rich diet and daily ONS, with regular counselling by trained dieticians over 3 months after hip fracture, based on individual assessment of energy and protein requirements. A process evaluation including views of caregivers and participants in our study (30) showed high appreciation of the intervention by the participants. The tailor-made dietary advice, the frequent personal coaching of participants by skilled dieticians, and the continuity of care (monitoring, personnel, and type of advice) are likely to have contributed to the high adherence to the dietetic advice and the ONS, which was also corroborated by a positive intervention effect on intake of energy and nutrients, body weight, and a number of biochemical parameters at 3 months (end of intervention).
Nevertheless, we did not find any tangible effect of the intervention on LOS or any secondary clinical and functional endpoints including postoperative complications, functional status, health-related QoL, subsequent fractures, and mortality. This lack of clinical effectiveness was even more unexpected as hip fracture patients in our study who were malnourished at baseline and had a markedly impaired prognosis compared with well-nourished patients, confirming many earlier observational reports (6, 8–12). In additional analyses, we found that the effect of the nutritional intervention was not modified by nutritional status at baseline, confirming that, even in malnourished patients, the intervention did not have any effect on clinical outcome, despite short-term amelioration of nutritional status. Also a per protocol analysis (excluding intervention patients with <80 per cent compliance and control patients receiving ONS) did not alter the results; in fact, in this analysis LOS in hospital was significantly longer in the intervention group, which would be consistent with an unfavorable intervention effect. The higher frequency of surgical complications in the intervention group may be a chance finding due to imbalance in randomization, as it is unlikely that, for example, dislocation of hip implant is causally linked to nutritional intervention.
According to a Cochrane review (21), previous studies were methodologically flawed; thus, earlier reports suggesting potential positive effects of nutritional intervention on LOS (5, 13, 14, 24), postoperative complication rate (5, 13, 15, 16), mortality rate (5, 13), or functional ability (17) might be biased. Moreover, some of the positive studies were performed several decades ago (5, 13, 24), limiting their applicability to present-day health care. But also, the majority of previous studies was limited in size (≤100 patients) and/or duration (≤4 weeks), or had only moderate compliance, all of which could explain previous negative reports (14–16, 18, 19). All of these issues were carefully taken care of in the present study.
Some limitations of this study should be mentioned. First, the study was not blinded, which could have induced performance, drop-out, and/or detection bias. All three are contradicted by our findings; moreover, for detection of LOS, complications, fractures, and survival, blinding is irrelevant. Second, the moment of hospital discharge is partly influenced by space limitations in rehabilitation clinics and by home conditions. However, this does not explain the lack of effect on any other clinical endpoint in our study. Third, the need for informed consent could have led to selecting patients with the best prognosis, thereby attenuating a potential benefit of nutritional intervention. However, in a satellite study, we showed that the nutritional status of our study population was representative for the total population of patients admitted with hip fracture (data not shown). Fourth, for giving informed consent, patients had to be fully recovered from anesthesia so that the nutritional intervention could start only several days after hip fracture surgery. And finally, the results of the study apply to elderly with good cognition, who have lived independently prior to the hip fracture (~95 per cent of our study population), that is, a clinically highly relevant target group. The results do not apply to patients with cognitive decline, who had to be excluded from our study both for ethical reasons (informed consent) and to safeguard optimal trial participation of participants (adherence, outcome assessment). However, there is no obvious reason why the physiological effect of the same nutritional intervention would have been any better in cognitively impaired patients.
In conclusion, despite a positive short-term effect on dietary intake and nutritional status nutritional status, an intensive 3 month nutritional intervention combining dietetic counselling and ONS in elderly after hip fracture had no effect on LOS, postoperative complications, or functional parameters, nor on 1 and 5 year fracture and mortality rates. The intervention also lacked clinical effectiveness in malnourished patients. Our findings indicate that paradigms underlying nutritional intervention in elderly may have to be reconsidered. Types of nutritional intervention other than ONS might be needed to improve clinical outcome, but first of all, better understanding of causes underlying undernutrition in elderly and factors determining the effects of nutritional intervention on clinical outcome is needed, as was also suggested recently by the Health Council of the Netherlands (31).
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The Netherlands Organization for Health Research and Development (ZonMw, Grant/Award Number: 80-007022-98-07510 / 94507510) financed the study after extensive peer-review of the study protocol. The study was strictly performed according to this protocol. The study was registered prior to starting enrolment at http://www.clinicaltrials.gov as NCT00523575 and its design and methods were previously published (22). Nutricia Research (Utrecht, The Netherlands) provided the oral nutritional supplement. Neither The Netherlands Organization for Health Research and Development, nor Nutricia Research had any influence on design, collection, analysis, or interpretation of data, on writing of the publication, nor on the decision to submit the paper for publication.
Conflict of interest statement
None declared.
References
- 1. Cumming RG, Klineberg R, Katelaris A. Cohort study of risk of institutionalisation after hip fracture. Aust N Z J Public Health. 1996;20:579–582. doi:10.1111/j.1467-842X.1996.tb01069.x [DOI] [PubMed] [Google Scholar]
- 2. Foster MR, Heppenstall RB, Friedenberg ZB, Hozack WJ. A prospective assessment of nutritional status and complications in patients with fractures of the hip. J Orthop Trauma. 1990;4:49–57. [DOI] [PubMed] [Google Scholar]
- 3. Hernlund E, Svedbom A, Ivergård M et al. . Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8:136. doi:10.1007/s11657-013-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lumbers M, New SA, Gibson S, Murphy MC. Nutritional status in elderly female hip fracture patients: comparison with an age-matched home living group attending day centres. Br J Nutr. 2001;85:733–740. doi:10.1079/BJN2001350 [DOI] [PubMed] [Google Scholar]
- 5. Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet. 1990;335:1013–1016. doi:10.1016/0140-6736(90)91073-J [DOI] [PubMed] [Google Scholar]
- 6. Bell JJ, Bauer JD, Capra S, Pulle RC. Concurrent and predictive evaluation of malnutrition diagnostic measures in hip fracture inpatients: a diagnostic accuracy study. Eur J Clin Nutr. 2014;68:358–362. doi:10.1038/ejcn.2013.276 [DOI] [PubMed] [Google Scholar]
- 7. Eneroth M, Olsson UB, Thorngren KG. Insufficient fluid and energy intake in hospitalised patients with hip fracture. A prospective randomised study of 80 patients. Clin Nutr. 2005;24:297–303. doi:10.1016/ j.clnu.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 8. Patterson BM, Cornell CN, Carbone B, Levine B, Chapman D. Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. J Bone Joint Surg Am. 1992;74:251–260. [PubMed] [Google Scholar]
- 9. Lumbers M, Driver LT, Howland RJ, Older MW, Williams CM. Nutritional status and clinical outcome in elderly female surgical orthopaedic patients. Clin Nutr. 1996;15:101–107. [DOI] [PubMed] [Google Scholar]
- 10. Koren-Hakim T, Weiss A, Hershkovitz A et al. . The relationship between nutritional status of hip fracture operated elderly patients and their functioning, comorbidity and outcome. Clin Nutr. 2012;31:917–921. doi:10.1016/j.clnu.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 11. Gumieiro DN, Rafacho BP, Gonçalves AF et al. . Mini nutritional assessment predicts gait status and mortality 6 months after hip fracture. Br J Nutr. 2013;109:1657–1661. doi:10.1017/S0007114512003686 [DOI] [PubMed] [Google Scholar]
- 12. Bell JJ, Pulle RC, Crouch AM, Kuys SS, Ferrier RL, Whitehouse SL. Impact of malnutrition on 12-month mortality following acute hip fracture. ANZ J Surg. 2016;86:157–161. doi:10.1111/ans.13429 [DOI] [PubMed] [Google Scholar]
- 13. Tkatch L, Rapin CH, Rizzoli R et al. . Benefits of oral protein supplementation in elderly patients with fracture of the proximal femur. J Am Coll Nutr. 1992;11:519–525. [DOI] [PubMed] [Google Scholar]
- 14. Myint MW, Wu J, Wong E et al. . Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: a single blind randomised controlled trial. Age Ageing. 2013;42:39–45. doi:10.1093/ageing/afs078 [DOI] [PubMed] [Google Scholar]
- 15. Anbar R, Beloosesky Y, Cohen J et al. . Tight calorie control in geriatric patients following hip fracture decreases complications: a randomized, controlled study. Clin Nutr. 2014;33:23–28. doi:10.1016/j.clnu.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 16. Espaulella J, Guyer H, Diaz-Escriu F, Mellado-Navas JA, Castells M, Pladevall M. Nutritional supplementation of elderly hip fracture patients. A randomized, double-blind, placebo-controlled trial. Age Ageing. 2000;29:425–431. [DOI] [PubMed] [Google Scholar]
- 17. Tidermark J, Ponzer S, Carlsson P et al. . Effects of protein-rich supplementation and nandrolone in lean elderly women with femoral neck fractures. Clin Nutr. 2004;23:587–596. doi:10.1016/j.clnu.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 18. Botella-Carretero JI, Iglesias B, Balsa JA, Zamarrón I, Arrieta F, Vázquez C. Effects of oral nutritional supplements in normally nourished or mildly undernourished geriatric patients after surgery for hip fracture: a randomized clinical trial. JPEN J Parenter Enteral Nutr. 2008;32:120–128. doi:10.1177/0148607108314760 [DOI] [PubMed] [Google Scholar]
- 19. Bruce D, Laurance I, McGuiness M, Ridley M, Goldswain P. Nutritional supplements after hip fracture: poor compliance limits effectiveness. Clin Nutr. 2003;22:497–500. doi:10.1016/S0261-5614(03)00050-5 [DOI] [PubMed] [Google Scholar]
- 20. Duncan DG, Beck SJ, Hood K, Johansen A. Using dietetic assistants to improve the outcome of hip fracture: a randomised controlled trial of nutritional support in an acute trauma ward. Age Ageing. 2006;35: 148–153. doi:10.1093/ageing/afj011 [DOI] [PubMed] [Google Scholar]
- 21. Avenell A, Smith TO, Curtain JP, Mak JC, Myint PK. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2016;11:CD001880. doi:10.1002/14651858.CD001880.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wyers CE, Breedveld-Peters JJ, Reijven PL et al. . Efficacy and cost-effectiveness of nutritional intervention in elderly after hip fracture: design of a randomized controlled trial. BMC Public Health. 2010;10:212. doi:10.1186/1471-2458-10-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swain DG, Nightingale PG. Evaluation of a shortened version of the abbreviated mental test in a series of elderly patients. Clin Rehabil. 1997;11:243–248. doi:10.1177/026921559701100308 [DOI] [PubMed] [Google Scholar]
- 24. Schürch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–809. [DOI] [PubMed] [Google Scholar]
- 25. Bastow MD, Rawlings J, Allison SP. Benefits of supplementary tube feeding after fractured neck of femur: a randomised controlled trial. Br Med J (Clin Res Ed). 1983;287:1589–1592. [PMC free article] [PubMed] [Google Scholar]
- 26. Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40:168–182. [DOI] [PubMed] [Google Scholar]
- 27. Dietary Reference Intakes: Calcium, Vitamin D, Thiamin, Riboflavin, Niacin, Pantothenic Acid, and Biotin. The Hague: Health Council of the Netherlands; 2000. [Google Scholar]
- 28. Guidelines for a Healthy Diet 2006. The Hague: Health Council of the Netherlands; 2006. [Google Scholar]
- 29. Vellas B, Guigoz Y, Garry PJ et al. . The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116–122. [DOI] [PubMed] [Google Scholar]
- 30. Breedveld-Peters JJ, Reijven PL, Wyers CE et al. . Integrated nutritional intervention in the elderly after hip fracture. A process evaluation. Clin Nutr. 2012;31:199–205. doi:10.1016/j.clnu.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 31. Netherlands HCot. Undernutrition in the Elderly. The Hague: Health Council of the Netherlands; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.