Abstract
Multimorbidity is a common and burdensome condition that may affect quality of life, increase medical needs, and make people live more years of life with disability. Negative outcomes related to multimorbidity occur beyond what we would expect from the summed effect of single conditions, as chronic diseases interact with each other, mutually enhancing their negative effects, and eventually leading to new clinical phenotypes. Moreover, multimorbidity mirrors an accelerated global susceptibility and a loss of resilience, which are both hallmarks of aging. Due to the complexity of its assessment and definition, and the lack of clear evidence steering its management, multimorbidity represents one of the main current challenges for clinicians, researchers, and policymakers. The authors of this article recently reflected on these issues during two twin international symposia at the 2016 European Union Geriatric Medicine Society (EUGMS) meeting in Lisbon, Portugal, and the 2016 Gerontological Society of America (GSA) meeting in New Orleans, USA. The present work summarizes the most relevant aspects related to multimorbidity, with the ultimate goal to identify knowledge gaps and suggest future directions to approach this condition.
Keywords: Multimorbidity, Comorbidity, Chronic diseases, Guidelines, Frailty
Multimorbidity, defined as the co-occurrence of multiple diseases in one same person, affects a large proportion of older people and represents a distinctive hallmark of aging (1,2). Aging and chronic diseases fuel one another in a vicious circle, each accelerating the progression of the other (3–5). Further, aging with multimorbidity means experiencing a poorer quality of life, having higher medical needs and spending more years of life with disability (1). Negative outcomes related to multimorbidity occur beyond what we would expect from the summed effect of single conditions, as chronic diseases interact with each other leading to enhanced negative effects, and eventually to new clinical phenotypes (eg, geriatric syndromes) (6,7). Health practitioners strive to provide adequate care to seniors with multimorbidity, but their actions are hampered by traditional clinical constructs based on a reductionist single-disease view of medicine. Such constructs collide with the widely advocated holistic approach to older people’s health, where not only diseases but also non-nosological conditions and the environment may lead to a state of frailty (8,9). Due to the complexity of its assessment and definition, and the lack of clear evidence steering its management, multimorbidity represents today one of the major challenges for clinicians, researchers and policymakers (8,10).
The authors of this article recently reflected on these issues during two twin international symposia at the 2016 European Union Geriatric Medicine Society (EUGMS) meeting in Lisbon, Portugal, and the 2016 Gerontological Society of America (GSA) meeting in New Orleans, USA. In the present work, we summarize the most relevant aspects discussed during these two events, with the ultimate goal to identify knowledge gaps and suggest future directions to approach multimorbidity: the big elephant in the room of our health care systems.
Epidemiology of Multimorbidity: Some Methodological Considerations
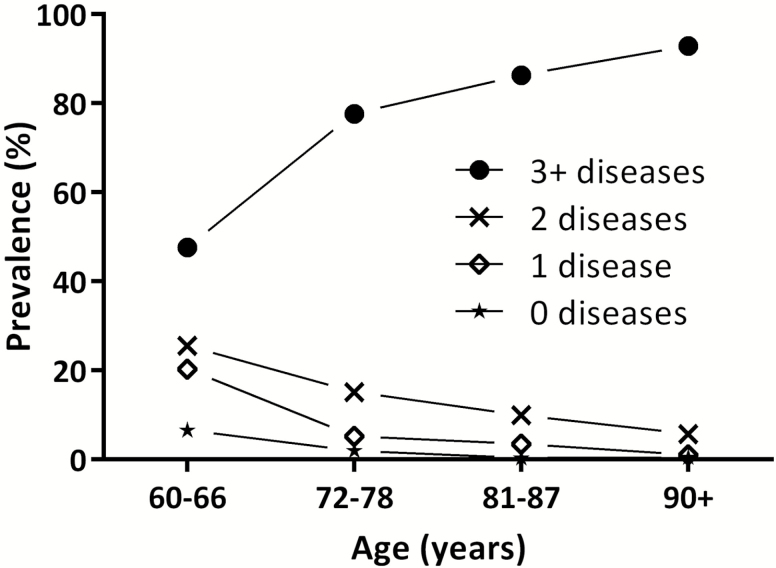
With a prevalence spanning from 55% to 98%, multimorbidity is the most common feature characterizing older adults (Figure 1) (1). Older age, female sex, and low socioeconomic status are the strongest determinants of multimorbidity. Living in deprived areas may anticipate the onset of multimorbidity by 10–15 years (8). Still, variability in the prevalence of multimorbidity is not entirely explained by the sociodemographic profile. Being able to adequately operationalize the measurement of multimorbidity in the population represents the basis to fine-tune guidelines and care models to people with multimorbidity. However, results are vitiated by: (i) the lack of a universal definition of chronic disease (11); (ii) the use of heterogeneous lists of chronic diseases, ranging from few to more than a hundred different nosological entities (12); (iii) the aggregation of diseases according to different levels of specificity; and (iv) the use of different cutoffs for the definition of multimorbidity (11). Recently, in an attempt to face the above-mentioned limitations, a proposal for the operationalization of multimorbidity in older people was suggested by Calderón-Larrañaga and colleagues (11). In this work, 918 chronic diseases (coded using the International Classification of Diseases [ICD] 10th revision) were identified and grouped into 60 homogeneous categories by an international team of geriatricians and general practitioners. Once widely validated, this tool could provide the common language needed for comparisons across different countries, settings, and research groups.
Figure 1.
Number of chronic diseases by age groups in the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K; N = 3,363).
There is consistent evidence that chronic diseases tend to aggregate in one same individual according to specific patterns (13). So far, many clusters of chronic diseases have been identified, and those with highest consistency across the literature include cardiometabolic, neuropsychiatric, and musculoskeletal disorders. Shared pathophysiological pathways and risk factors partially explain the systematic clustering of diseases. However, several associations still remain unexplained, raising question about the consistency of the associations itself. Studying how and why diseases appear together might help to detect homogeneous groups of people sharing analogous needs, prognosis and health trajectories. This could eventually contribute to improve the effectiveness of our models of care and better tailoring interventions for complex older adults (13,14).
Are Health Care Systems Ready to Deal With Multimorbidity?
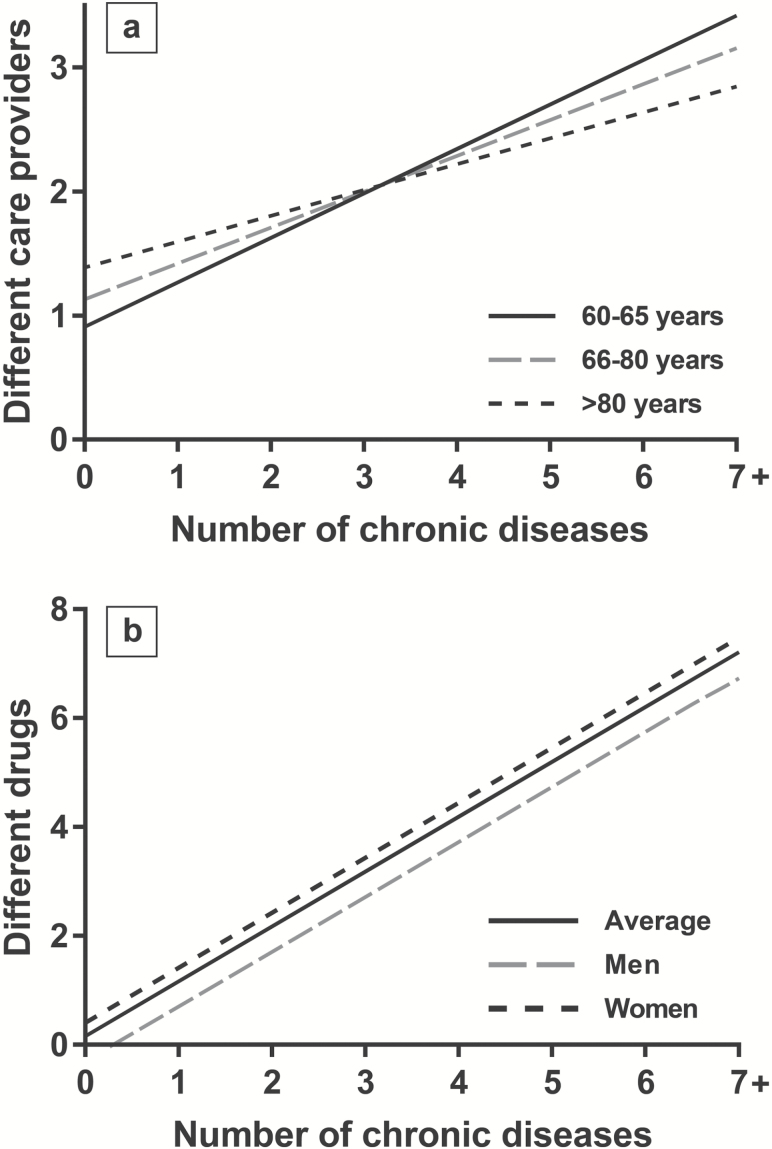
Although multimorbidity is one of the strongest predictors of health care services utilization (Figure 2a), the capacity of many health care systems to address frail patients affected by multiple diseases still lacks consistency and is often not rooted on scientific knowledge (15). As a consequence, in spite of a continued increase in health care spending, the auspicated changes for better addressing the clinical needs as well as improving patients’ satisfaction and quality of life are still unattended. Underlying this disconnection is a triadic crisis overarching health systems, medicine, and health professionals (16). Health systems are still designed to address acute health problems, even if older people’s health and social needs tend to span over a spectrum of areas of functioning and to wax and wane over time. Modern medicine prioritizes technology and hyper-specialization, and the emphasis on a strong primary and highly coordinated care, which is needed more than ever before, is progressively fading. Last, while many physicians recognize that effective care of older persons affected by multiple diseases and interacting social and financial constrains requires a “whole human” approach, the distance between health professionals and patients seems to gradually widen. All of this has led to health care models that are often expensive, burdensome, of unclear benefit and potential harm, and economically unsustainable (17).
Figure 2.
Association of the number of chronic diseases with (a) number of drugs and (b) number of different providers involved in the care process of older people from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K; N = 3,363).
Changes are needed. First, quality and performance measurements should be developed and implemented. They should not be merely focused on the cure of specific diseases but primarily capture the individual’s functions in order to prioritize care preferences and provide added value to treatments and interventions (18). With this regard, the multidimensional assessment of multimorbid patients may help to detect social, psychological, and environmental factors that are crucial determinants of the effectiveness of care. Second, innovations in health care delivery should guarantee integration and coordination across different clinicians and care settings (19). Ensuring that each patient has a clearly designated primary care doctor and nurse is critical for care continuity (20). Third, since in the presence of multimorbidity (and especially when associated with frailty), most medical decisions are not supported by robust scientific evidence, it is imperative that physicians share the decision-making process with their patients, incorporating their values and priorities in the care plan (21). Fourth, the identification and treatment of symptoms and diseases upon which medical education and training is exclusively based should be complemented with other nonmedical processes, such as shared decision-making, team-based care, and/or use of information technology (22). Last, patients and citizens need to become familiar with the concepts of priorities and tradeoffs, understand the balance between harms and benefits of interventions, and be willing to participate in shared decision making (23).
Clinical Guidelines for the Management of Multimorbidity
In the past few years, a number of articles have highlighted that disease-specific guidelines are difficult to apply in patients affected by multiple chronic diseases, making their effectiveness questionable (19,24,25). The reasons for such discrepancy rely on both the way disease-specific guidelines are developed and their applicability in real life. First, disease-specific guidelines are usually based on findings from randomized clinical trials that have systematically excluded frail persons affected by multiple coexisting diseases (26). Second, few guidelines acknowledge that target patients can also suffer from other diseases for which they might also receive additional medications (27).
Recently, there have been attempts to develop guidelines for persons affected by multimorbidity, some of them are summarized in Table 1. It is noteworthy that the primary goal of such guidelines is no longer to increase survival or to accomplish a disease-specific target (eg, lowering cholesterol or blood pressure values), but rather to maximize quality of life. These principles are aimed at health care professionals, patients with multimorbidity, and their caregivers, and have the potential for high external validity.
Table 1.
Examples of Guidelines and Recommendations for the Care of People with Multimorbidity
| Guidelines, Year | Description |
|---|---|
| Guiding principles for the care of older adults with multimorbidity, 2012 (23) | Consensus document by the American Geriatrics Society (AGS) Expert Panel on the Care of Older Adults with Multimorbidity. |
| It offers expert-based guidance on patient preferences, interpreting the evidence, prognosis, clinical feasibility, and optimizing therapies and care plans. | |
| The principles are relevant across settings and types of clinicians. | |
| Managing multiple chronic conditions (MCC): a strategic framework for improving health outcomes and quality of life, 2011 (53) | Action-oriented framework developed by the U.S. Department of Health and Human Services. |
| One of its goals is to provide better tools and information to health care, public health, and social services workers who deliver care to individuals with MCC. | |
| It includes key objectives and strategies that can be used to address MCC. | |
| Minimally Disruptive Medicine Care Model for patients with multiple chronic conditions, 2015 (54) | Theory-based approach to care that focuses on achieving patient goals for life and health while imposing the smallest possible treatment burden on patients’ lives. |
| It focuses on how to identify the right care; and how to make it happen. | |
| Ariadne principles to handle multimorbidity in primary care consultations, 2014 (55) | Guiding principles aimed at sharing realistic treatment goals by physicians and patients in primary care. |
| The principles result from: (i) an interaction assessment of the patient’s health and context; (ii) the prioritization of health problems taking into account patient preferences; and (iii) individualized management considering the best options of diagnostics, treatment, and prevention. | |
| Guideline for the comprehensive clinical care of multimorbid chronic patients, 2017 (29) | Elaborated by the Joint Action on CHROnic DISeases and promoting healthy ageing across the life cycle (JA-CHRODIS) funded by the European Commission. |
| The overall aim is to describe sixteen key components for an optimum care model for multimorbid patients. | |
| Guideline for the clinical assessment and management of multimorbidity, 2016 (28) | Elaborated by the National Institutet of Health and Care Excellence (NICE). |
| It aims to improve quality of life by promoting shared decisions based on what is important to each person in terms of treatments, health priorities, lifestyle, and goals. | |
| It is targeted to people with multimorbidity, their families, and caregivers. |
Despite having been elaborated by two different groups of experts, the two most recent guidelines issued by the NICE and the JA-CHRODIS present several commonalities (28,29). First, they make a serious attempt to identify people who might benefit from their application, for example, frail individuals. Second, they support the flexible use of disease-specific guidelines with special focus on treatment burden. Third, both emphasize the need to assure care coordination and the identification of a health care provider that is responsible for patients’ global care process. Fourth, they encourage shared decision making to agree on an individualized care plan. Fifth, both provide recommendations for patients and families to improve self-management and self-efficacy.
Challenges in the Field of Pharmacological Treatment
The presence of multimorbidity presents numerous therapeutic challenges (30). The pharmacological treatment of multiple chronic conditions often determines polypharmacy (Figure 2b) and increased risk of iatrogenic illness (25). The direct application of disease-specific guidelines in persons with multimorbidity leads to several drug-disease interactions (27). Indeed, the effect of a medication for a given condition might be substantially different when observed in an individual only with that disease compared to a frail one characterized by multimorbidity (31). The involvement of clinical pharmacists and the use of technologies (eg, computerized prescription support systems) in the medication review of multimorbid and frail older adults might be useful for improving the quality of the prescribing process (32). The effectiveness of physician training on prescription appropriateness and shared decision making is also being tested in two ongoing European trials (33,34).
Patients with multimorbidity usually present higher levels of complexity and consequent increased risk of iatrogenic events due to functional, cognitive and social problems as well as the presence of geriatric syndromes (eg, urinary incontinence, delirium, falls) (35). Moreover, these patients may not properly adhere to treatment protocols, with the risk of incurring in errors that may cause harm (eg, antihypertensive treatment and orthostatic hypotension) and/or function loss (eg, cognitive impairment due to anticholinergic drugs). In order to improve the appropriateness of treatments, all the elements potentially affecting effectiveness and adherence should be timely identified and weighted during the prescription process. In this regard, the comprehensive geriatric assessment might help to draw a clinical, functional and social profile of complex patients that can help in the prioritization of specific areas of intervention (36). When potential harms of a treatment outweigh its benefits, especially in the context of frailty and social disadvantages, medication deprescribing should be considered in order to improve quality of life and reduce treatment burden (37).
Multimorbidity and Frailty: Two Complementary Measures of Biological Aging
For decades, geriatric medicine has constantly been looking for useful and reliable models able to help: (i) clinicians, to better predict the prognosis of complex patients and tailor more appropriate treatments; (ii) researchers, to properly understand the mechanisms underlying the aging process; and (iii) health systems, to effectively address the needs and optimize the care of complex older adults. Frailty together with multimorbidity are emerging as promising biomarkers of aging. Although closely related, these two constructs have rarely been investigated together (38).
Frailty is defined as a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function, increasing an individual’s vulnerability to dependency and/or death (39). This construct is intended to capture both clinical and subclinical deficits that accumulate during the aging process, and it may represent a proxy measure of the biological health of the individual. Frailty well discriminates people with higher versus lower risk of adverse events and is a potentially powerful tool for assessing complexity and prognosis (40).
Under this perspective, frailty and multimorbidity largely overlap. In fact, multimorbidity might be considered as the accumulation of biological abnormalities that are clinically relevant and define disease diagnoses (35,41). The rapid accumulation of chronic diseases may accelerate the development of frailty and vice versa (5). Disease-related inflammation has been suggested as the process shared by multimorbidity and frailty (2). If inflammation is a causal element or only a proxy for unmeasurable biological dysfunctions remains to be clarified. Indeed, an important accumulation of damage can occur below the threshold of disease definition, or in domains currently not counted as diseases. With this regard, frailty is able to capture multiple biological and functional deficits regardless of clinical thresholds or the severity of diseases themselves, usually not accounted for in most multimorbidity measures.
Although frailty and multimorbidity are overlapping and complementary concepts, they remain separate entities since patients with multimorbidity may or may not be frail, and vice versa. Frailty might be used to distinguish patients with multimorbidity that could benefit from traditional treatment from those who might better benefit from a comprehensive and integrated intervention (28). Given their increased vulnerability, patients with multimorbidity and frailty could respond negatively to a rigid guidelines-based treatment and, for these patients, the development of an individualized care plan might be recommended (28). On the other hand, patients with multimorbidity but without frailty are more similar to those included in randomized clinical trials, making the application of clinical guidelines more reasonable in this population.
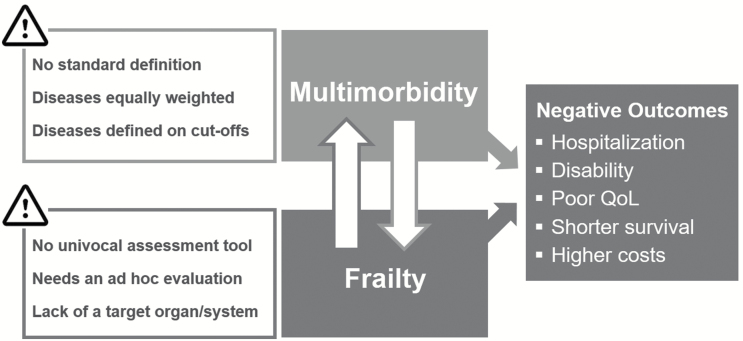
Even if both concepts adequately describe the consequences of the aging process and are useful predictors of negative outcomes, none of them is free from limitations (Figure 3), raising the need for further empirical investigations: how can these constructs be incorporated into routine clinical practice in order to improve the care of older adults? Can they be combined in order to maximize their clinical and epidemiological applicability?
Figure 3.
Multimorbidity and frailty: two constructs with close relationship, similar consequences and equal challenges.
Discussion
Identified gaps and suggested future perspectives are summarized in Table 2. Standardization is a first mandatory step: we need univocal ways to measure and collect data on multimorbidity. The advantage when it comes to diseases is that these are routinely collected during clinical practice, allowing researchers to study the epidemiology of multimorbidity in real world-settings (eg, primary care) (7,42). However, structural differences concerning disease-coding systems, the idiosyncrasy of electronic health record use and availability, and/or the potential for among health care-level data integration still hinder comparability. Until a common language is agreed upon, it will be difficult to draw conclusions regarding how and for whom multimorbidity should be incorporated into clinical decision making for screening and prognostic purposes (11). Moreover, the use of a cutoff (eg, 2+ diseases) for the definition of multimorbidity has little discriminative power in older adults, encompassing people with highly varying levels of health status (43). Even more challenging might be the assessment and management of multimorbidity in low-income countries and rural areas, where the poor development and limited access to health services strongly affects the validity of the concept of disease itself. Similarly but in the opposite direction, over-medicalization and excessive recourse to diagnostic tests, more frequent in high-income countries, could derive in a clinically irrelevant phenomenon underlying multimorbidity.
Table 2.
Knowledge Gaps and Future Perspectives in the Study of Multimorbidity
| Knowledge Gaps | Future Perspectives |
|---|---|
| RESEARCH | |
| Standardization The lack of standardization in the assessment of multimorbidity leads to incomparable and sometimes contrasting results. |
A clinically-driven prespecified list of chronic conditions needs to be agreed upon in order to guarantee comparable assessments of multimorbidity across different countries, settings and research groups. |
| Considering their prevalence and clinical/functional impact in the population, geriatric symptoms and syndromes must be part of the definition of multimorbidity. | |
| The use of a cutoff to define multimorbidity has low discriminative power in older adults; studying it as a continuous grading scale of medical health problems or as clusters of chronic diseases should be further explored. | |
| Longitudinal approach People age at varying speed which may lead to different trajectories of disease development. In addition, risk and protective factors of multimorbidity have a life-long impact. |
Longitudinal studies are required to detect differences in the speed of disease accumulation, as a biomarker of the progression of biological aging. |
| Life-long observations may help identify environmental, behavioral and biological determinants of trajectories of multimorbidity development. | |
| Cellular and molecular pathways stemming from the aging process and responsible for the development of age-related chronic diseases need to be untangled in order to consider new therapeutic targets to prevent the development of multimorbidity. | |
| Link with frailty Multimorbidity and frailty emerge as two complementary biomarkers of aging. |
The chronological relationship between multimorbidity and frailty should be further investigated. |
| The pooled significance of multimorbidity and frailty for screening, risk stratification and prognosis in older adults should be addressed in dedicated studies. | |
| CLINICAL PRACTICE | |
| Management of multimorbid patients Guidelines for the treatment of chronic diseases rely on clinical trials excluding complex multimorbid patients. |
Albeit recent attempts to issue guidelines for the assessment and management of persons with multimorbidity, more initiatives are required to provide practitioners with reliable and effective guidance. |
| Pragmatic randomized controlled trials and qualitative studies need to be performed to test the applicability and effectiveness of the guidelines in real-world practice. | |
| Pharmacological treatment The lack of guidelines generates uncertainty regarding the safety and effectiveness of prescriptions to multimorbid patients. |
Computerized prescription support systems are essential to identify potential drug– drug and drug–disease interactions, improve prescribing and reduce adverse drug reactions. |
| Factors influencing the treatment’s effectiveness must be promptly identified to guarantee the development of personalized regimes that balance benefits and harms. | |
| Patients should be actively involved in the decisions affecting their treatment, discussing goals and prioritizing interventions. | |
| HEALTH POLICY AND PUBLIC HEALTH | |
| Health services organization Older people’s health care needs are not met by current medical and social care services. |
The organization of primary care needs to adapt to the reality of patients with multimorbidity, in terms of human resources, information technology, physician performance assessment and financial incentives. |
| The coordination across conditions and between care providers should be facilitated, as well as the continuity of care by the physician having the primary responsibility for helping multimorbid patients make decisions. | |
| Such interventions need to be assessed through large scale pragmatic cluster randomized trials, including detailed process evaluation and cost-effectiveness analyses, and using outcome measures that are relevant for patients and their caregivers. | |
| Prevention of multimorbidity There is substantial lack of evidence regarding effective measures to prevent multimorbidity. |
The design of nonpharmacological trials based on behavioral and multidomain interventions in adults and older persons aimed at preventing multimorbidity might pave the way to implement effective policies to reduce the burden of chronic diseases. |
| Public awareness Patients, their relatives and caregivers are familiar with single and common diseases as diabetes and hypertension but not with multimorbidity. |
Making patients and their families aware of the definition, consequences and challenges of multimorbidity may facilitate guideline implementation, care coordination, and promote self-management in patients themselves. |
| Primary care physicians and geriatricians, ideally prepared to deal with multimorbid patients and their families, are pivotal in this process and should be involved in the translation of research to practice. |
Another important step forward is the study of multimorbidity across long observation periods. The description of multimorbidity trajectories depending of older people's disease accumulation rate could shed light on the mechanisms of accelerated aging supporting the design of future preventive interventions (5,14). In parallel, the study of cellular and molecular senescence (ie, geroscience) could help researchers detect new targets for therapeutic interventions to delay the development of chronic diseases and multimorbidity (44). Under this perspective, multidomain intervention trials aimed at preventing multimorbidity and its consequences are warmly encouraged (45). Interestingly, the Targeting Aging with Metformin (TAME) trial, the first randomized clinical trial testing a drug to slow the aging process, will consider the occurrence of several age-related diseases as the primary endpoint (46).
Multimorbidity generates uncertainty at different levels during the care process, from care planning to goal definition and therapeutic strategies. This is particularly true in the presence of frailty and/or social and environmental disadvantages. The guidelines recently issued for the management of multimorbid patients provide, for the first time, a framework for the assessment and treatment of multimorbid complex persons, moving the focus from the disease to the patient (28,47). However, the feasibility and effectiveness of the implementation of these guidelines need to be tested through mixed methodologies. Another work package of the JA-CHRODIS has precisely the aim of designing pragmatic trials to test the guidelines in real-world conditions (48).
The reorganization and reinforcement of primary care represents a key step to optimize resource utilization and to reduce the burden exerted by multimorbidity on the patient. Nowadays, care delivered to people with multimorbidity generates multiple specialist referrals, frequent emergency room and hospital admissions, and invasive (and often inappropriate) diagnostic procedures (49–51). This represents a source of stress per se affecting, ultimately, patients’ quality of life.
Finally, patients and their families should be informed of the consequences and challenges posed by multimorbidity (52). Patient empowerment is pivotal in the implementation of coordinated health plans, and even more so in contexts where the risk of care fragmentation is high. This can be accomplished by strengthening their self-management and self-efficacy, such as through explaining their diagnoses, diseases, and medical conditions, as well as by providing information on medication use. In turn, patients may learn to use medical devices, supportive aids, and health monitoring tools correctly. This might also facilitate multimorbidity guideline implementation and help in achieving a fully shared decision-making process. Furthermore, public health policies aimed at raising population awareness may also aid in implementation. Yet, well-prepared primary care physicians and nurses are key communicators, particularly in reaching older adults affected by multimorbidity and their families.
Funding
This work was supported by the funders of the Swedish National study on Aging and Care, SNAC: the Ministry of Health and Social Affairs, Sweden, the participating County Councils and Municipalities, and the Swedish Research Council.
Conflict of Interests
None reported.
References
- 1. Marengoni A, Angleman S, Melis R et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi:10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 2. Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65:407–413. doi:10.1093/gerona/glp181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yokota RT, Van der Heyden J, Nusselder WJ et al. Impact of chronic conditions and multimorbidity on the disability burden in the older population in Belgium. J Gerontol A Biol Sci Med Sci. 2016;71:903–909. doi:10.1093/gerona/glv234 [DOI] [PubMed] [Google Scholar]
- 4. Fabbri E, An Y, Schrack JA et al. Energy metabolism and the burden of multimorbidity in older adults: results from the baltimore longitudinal study of Aging. J Gerontol A Biol Sci Med Sci. 2015;70:1297–1303. doi:10.1093/gerona/glu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fabbri E, An Y, Zoli M et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70:63–70. doi:10.1093/gerona/glu127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gassmann D, Cheetham M, Siebenhuener K et al. The multimorbidity interaction severity index (MISI): a proof of concept study. Medicine (Baltimore). 2017;96:e6144. doi:10.1097/MD.0000000000006144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Angelantonio E, Kaptoge S, Wormser D et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015; 314: 52–60. doi:10.1001/jama.2015.7008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi:10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 9. Bierman AS, Tinetti ME. Precision medicine to precision care: managing multimorbidity. Lancet. 2016;388:2721–2723. doi:10.1016/S0140-6736(16)32232-2 [DOI] [PubMed] [Google Scholar]
- 10. Prince MJ, Wu F, Guo Y et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi:10.1016/S0140-6736(14)61347-7 [DOI] [PubMed] [Google Scholar]
- 11. Calderon-Larranaga A, Vetrano DL, Onder G et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2016. doi:10.1093/gerona/glw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases–a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–311. doi:10.1093/gerona/glq208 [DOI] [PubMed] [Google Scholar]
- 13. Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67:254–266. doi:10.1016/j.jclinepi.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 14. Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc. 2015;16:640–647. doi:10.1016/j.jamda.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization (WHO). World Report on Ageing and Health. 2015. http://apps.who.int/iris/bitstream/10665/186463/ 1/9789240694811_eng.pdf [Google Scholar]
- 16. Labrador Repullo JR. Medicina, Médicos y Sistema ¿Triple crisis?Médicos y patientes 2010. http://www.medicosypacientes.com/articulo/medicina-m%C3%A9dicos-y-sistema-%C2%BFtriple-crisis [Google Scholar]
- 17. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. [DOI] [PubMed] [Google Scholar]
- 18. Giovannetti ER, Dy S, Leff B et al. Performance measurement for people with multiple chronic conditions: conceptual model. Am J Manag Care. 2013;19:e359–e366. [PMC free article] [PubMed] [Google Scholar]
- 19. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. JAMA. 2012;307:2493–2494. doi:10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salisbury C. Multimorbidity: time for action rather than words. Br J Gen Pract. 2013;63:64–5.doi: 10.3399/bjgp13X661020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heath I. How medicine has exploited rationality at the expense of humanity: an essay by Iona Heath. BMJ (Clinical research ed). 2016;355:i5705. doi:10.1136/bmj.i5705 [DOI] [PubMed] [Google Scholar]
- 22. Boult C, Wieland GD. Comprehensive primary care for older patients with multiple chronic conditions: “Nobody rushes you through”. JAMA. 2010;304:1936–1943. doi:10.1001/jama.2010.1623 [DOI] [PubMed] [Google Scholar]
- 23. American Geriatrics Society Expert Panel on the Care of Older Adults with M. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1–E25. doi:10.1111/j.1532-5415.2012.04188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi:10.1056/NEJMsb042458 [DOI] [PubMed] [Google Scholar]
- 25. Marengoni A, Onder G. Guidelines, polypharmacy, and drug-drug interactions in patients with multimorbidity. BMJ (Clinical research ed). 2015;350:h1059. doi:10.1136/bmj.h1059 [DOI] [PubMed] [Google Scholar]
- 26. Crome P, Lally F, Cherubini A et al. Exclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT study. Drugs Aging. 2011;28:667–677. doi:10.2165/11591990-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 27. Dumbreck S, Flynn A, Nairn M et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ (Clinical research ed). 2015;350:h949. doi:10.1136/bmj.h949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. NICE. National Institute for Health and Care Excellence (NICE). Multimorbidity: Clinical Assessment and Management; 2016. [Google Scholar]
- 29. Palmer K, Marengoni A, Forjaz MJ et al. Onder on behalf of the joint action on Chronic Diseases and Promoting Healthy Ageing Across the Life Cycle (JA-CHRODIS). Multimorbidity care model: recommendations from the consensus meeting of the Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS). Accepted for publication in Health Policy. doi:10.1016/j.healthpol.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 30. Onder G, Palmer K, Navickas R et al. ; Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS) Time to face the challenge of multimorbidity. A European perspective from the joint action on chronic diseases and promoting healthy ageing across the life cycle (JA-CHRODIS). Eur J Intern Med. 2015;26:157–159. doi:10.1016/j.ejim.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 31. Banerjee S. Multimorbidity--older adults need health care that can count past one. Lancet (London, England). 2015; 385:587–589. doi:10.1016/S0140-6736(14)61596–8 [DOI] [PubMed] [Google Scholar]
- 32. Marengoni A, Pasina L, Concoreggi C et al. Understanding adverse drug reactions in older adults through drug-drug interactions. Eur J Intern Med. 2014;25:843–846. doi:10.1016/j.ejim.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 33. Muth C, Harder S, Uhlmann L et al. Pilot study to test the feasibility of a trial design and complex intervention on PRIoritising MUltimedication in Multimorbidity in general practices (PRIMUMpilot). BMJ Open. 2016;6:e011613. doi:10.1136/bmjopen-2016-011613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prados-Torres A, Del Cura-González I, Prados-Torres D et al. ; Multi-PAP Group Effectiveness of an intervention for improving drug prescription in primary care patients with multimorbidity and polypharmacy: study protocol of a cluster randomized clinical trial (Multi-PAP project). Implement Sci. 2017;12:54. doi:10.1186/s13012-017-0584-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Onder G, van der Cammen TJ, Petrovic M, Somers A, Rajkumar C. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing. 2013;42:284–291. doi:10.1093/ageing/aft038 [DOI] [PubMed] [Google Scholar]
- 36. Bernabei R, Landi F, Onder G, Liperoti R, Gambassi G. Second and third generation assessment instruments: the birth of standardization in geriatric care. J Gerontol A Biol Sci Med Sci. 2008;63:308–313. [DOI] [PubMed] [Google Scholar]
- 37. Scott IA, Hilmer SN, Reeve E et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827–834. doi:10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 38. Cesari M, Pérez-Zepeda MU, Marzetti E. Frailty and multimorbidity: different ways of thinking about geriatrics. J Am Med Dir Assoc. 2017;18:361–364. doi:10.1016/j.jamda.2016.12.086 [DOI] [PubMed] [Google Scholar]
- 39. Morley JE, Vellas B, van Kan GA et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14: 392–397. doi:10.1016/ j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vermeiren S, Vella-Azzopardi R, Beckwée D et al. ; Gerontopole Brussels Study group Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17:1163.e1–1163.e17. doi:10.1016/j.jamda.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 41. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:10.1016/S0140- 6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rocca WA, Boyd CM, Grossardt BR et al. The prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and ethnicity. Mayo Clin Proc. 2014;89:1336–1349. doi:10.1016/j.mayocp.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marengoni A, Angleman S, Meinow B et al. Coexisting chronic conditions in the older population: variation by health indicators. Eur J Intern Med. 2016;31:29–34. doi:10.1016/j.ejim.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 44. Ferrucci L. Commentary: life course epidemiology embraces geroscience. Int J Epidemiol. 2016;45:1015–1019. doi:10.1093/ije/dyw104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Espeland MA, Crimmins EM, Grossardt BR et al. ; Multimorbidity Clinical Trials Consortium Clinical trials targeting aging and age-related multimorbidity. J Gerontol A Biol Sci Med Sci. 2017;72:355–361. doi:10.1093/gerona/glw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi:10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. CHRODIS - Addressing Chronic Diseases & Healthy Ageing Across The Life Cycle. 2016. http://chrodis.eu/. [Google Scholar]
- 48. Thai M, Hilmer S, Pearson SA, Reeve E, Gnjidic D. Prevalence of potential and clinically relevant statin-drug interactions in frail and robust older inpatients. Drugs Aging. 2015;32:849–856. doi:10.1007/s40266- 015-0302-9 [DOI] [PubMed] [Google Scholar]
- 49. Mondor L, Maxwell CJ, Hogan DB et al. Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: a retrospective analysis of a population-based cohort. PLoS Med. 2017;14:e1002249. doi:10.1371/journal.pmed.1002249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bowling CB, Plantinga L, Phillips LS et al. Association of multimorbidity with mortality and healthcare utilization in chronic kidney disease. J Am Geriatr Soc. 2016;65:704–711. doi:10.1111/jgs.14662 [DOI] [PubMed] [Google Scholar]
- 51. Geersing GJ, de Groot JA, Reitsma JB, Hoes AW, Rutten FH. The impending epidemic of chronic cardiopulmonary disease and multimorbidity: the need for new research approaches to guide daily practice. Chest. 2015;148:865–869. doi:10.1378/chest.14-3172 [DOI] [PubMed] [Google Scholar]
- 52. Marengoni A, Fratiglioni L, Onder G. Improving public awareness of multimorbidity. J Am Med Dir Assoc. 2017;18:372–373. doi:10.1016/j.jamda.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 53. Parekh AK, Goodman RA, Gordon C, Koh HK; HHS Interagency Workgroup on Multiple Chronic Conditions Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126:460–471. doi:10.1177/003335491112600403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3:50–63. doi:10.3390/healthcare3010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muth C, van den Akker M, Blom JW et al. The Ariadne principles: how to handle multimorbidity in primary care consultations. BMC Med. 2014;12:223. doi:10.1186/s12916-014-0223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]