Abstract
BACKGROUND
Hypertensive disorders of pregnancy (HDP) are linked to excessive maternal weight and frequent snoring. However, pathways between maternal excessive weight, pregnancy-onset snoring, and HDP are only partially estimated. We examined and quantified the total and direct associations between excessive maternal weight and incident HDP and their indirect pathway through pregnancy-onset snoring.
METHODS
Third trimester pregnant women enrolled from prenatal clinics of a large tertiary medical center. Sleep data were collected through a questionnaire. Demographic and pregnancy information and first trimester maternal weight were abstracted from medical charts. After exclusion of women with prepregnancy hypertension and/or chronic snoring, causal mediation analysis was used to estimate the total and direct association between maternal weight and incident HDP and their indirect association through pregnancy-onset snoring. The proportion of the mediated association through pregnancy-onset snoring from the total association of maternal weight and HDP was also quantified.
RESULTS
After excluding those with chronic hypertension and/or snoring, the final sample included 1,333 pregnant women. In adjusted analysis, excessive maternal weight was directly associated with incident HDP; odds ratio (OR) = 1.87 (95% confidence interval (CI) 1.30, 2.70). Pregnancy-onset snoring significantly mediated the association between maternal weight and incident HDP; OR = 1.08 (95% CI 1.01, 1.17). The mediated pathway accounted for 15% of the total association between maternal weight and incident HDP.
CONCLUSIONS
Pregnancy-onset snoring mediates the association between maternal weight and incident HDP in women without prepregnancy snoring or hypertension. These findings demonstrate the relative contributions of excessive maternal weight and pregnancy-onset snoring to incident HDP.
Keywords: blood pressure, body mass index, gestational hypertension, habitual snoring, hypertension, hypertensive disorders of pregnancy, maternal obesity, pre-eclampsia, pregnancy snoring, sleep-disordered breathing
BACKGROUND
Hypertensive disorders of pregnancy (HDP) are a group of conditions marked by elevated blood pressure, systolic ≥140 mm Hg and diastolic ≥90 mm Hg, with or without proteinuria.1 Chronic hypertension, gestational hypertension, and pre-eclampsia, collectively defined as HDP, complicate 5–10% of US pregnancies2 and contribute to maternal and neonatal morbidity and mortality.3,4
Frequent or habitual snoring, defined as snoring at least 3 days/week, is the hallmark symptom of sleep-disordered breathing (SDB), a respiratory dysfunction with associated airway obstruction. Previously, frequent snoring has been linked to adverse pregnancy outcomes, including HDP, gestational diabetes mellitus, preterm birth, and small newborn size.5–12 In particular, frequent snoring is associated with a 2-fold increased risk of HDP.5,7,8,13,14 Among pregnant women, with chronic or incident snoring, the prevalence of gestational hypertension ranges from 2- to 3-fold compared with nonsnorers.8,13 Conversely, pregnant women with hypertension have a much higher snoring prevalence compared with normotensive women.15,16
Obesity is an established risk factor for snoring in the general population.17 Recent data suggest that more than two-thirds of reproductive-age women are overweight or obese18 and that excessive maternal weight increases the risk of HDP.19 It is plausible that excessive maternal weight may induce or exacerbate snoring that, in turn, increases the risk for HDP. Further, “bi-directional links” have been proposed between obesity and snoring20,21 and snoring and hypertension.22,23 Nonetheless, the reported bi-directionality in these associations from cross-sectional studies could mask their temporal aspect. In addition to limitations of small sample sizes and inadequate control for confounding, prior studies had near-universal adjustment for maternal weight in studies on snoring and HDP association rather than investigation of the potential role of pregnancy-onset snoring in the pathway between maternal weight and HDP.
To disentangle the complex associations of maternal snoring, excessive weight, and HDP, this study sought to (i) examine whether pregnancy-onset snoring mediates the temporal pathway between maternal weight and incident HDP and (ii) quantify the total and direct associations between maternal weight and incident HDP and their indirect pathway of association through pregnancy-onset snoring. We hypothesized that pregnancy-onset snoring mediates the association of maternal weight and incident HDP and that the total, direct, and indirect effects—through pregnancy-onset snoring—would be significant.
METHODS
Study population
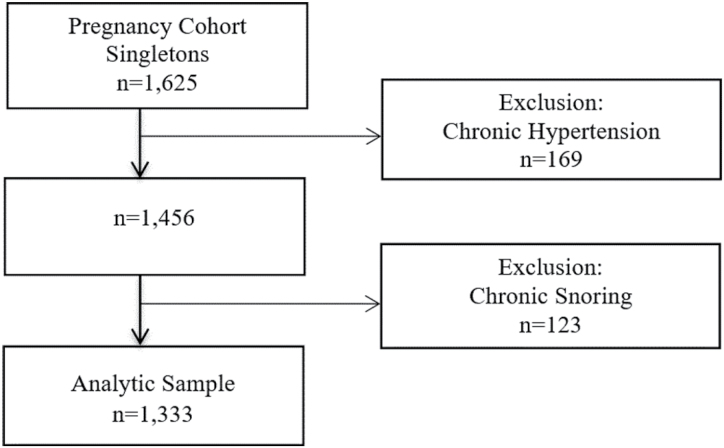
Third trimester pregnant women receiving care at prenatal clinics of a large tertiary medical center were recruited to this prospective cohort study between March 2008 and December 2010. Women were eligible to be included in this study if they were ≥18 years old and ≥28 weeks pregnant with a single fetus. To account for temporality, we excluded women with chronic, prepregnancy hypertension, and/or chronic, prepregnancy snoring (Figure 1). After all exclusions, the final analytic sample included 1,333 pregnant women.
Figure 1.
Flowchart of participants in the Sleep Pregnancy Cohort: 2008–2010.
The study obtained approval from the Institutional Review Board.
Exposure: maternal weight
Maternal weight and height were recorded in medical charts at baseline prenatal care visit during the first trimester. We used these data to calculate body mass index (BMI) (kg/m2) and then classify women into 2 groups by their baseline weight: (i) underweight or normal weight (BMI <25 kg/m2) and (ii) overweight or obese (BMI ≥ 25 kg/m2).
Mediator: pregnancy-onset snoring
Snoring data were collected via a questionnaire administered to pregnant women with questions about the frequency of their snoring during pregnancy. Possible responses were “almost daily,” “3–4 times per week,” “1–2 times per week,” “1–2 times per month,” or “never.” Women were defined as “snorers” if they reported snoring in pregnancy and “nonsnorers” if they never or rarely snored. Furthermore, in a subsequent question, women reported the timing of their snoring onset, whether prepregnancy or pregnancy-induced. To examine the temporal relationship between pregnancy-onset snoring and gestational hypertension, women with prepregnancy snoring were excluded from the sample.
Outcome: HDP
Gestational hypertension and pre-eclampsia diagnoses were obtained from medical charts using the International Classification of Diseases, 9th edition (ICD-9). Blood pressure measures were collected from pregnant women receiving care at the University of Michigan prenatal clinics. During their prenatal care visit, pregnant women sat in a quiet room before their blood pressure was taken by trained medical assistants. In all women, blood pressure was measured once using the same protocol. However, if blood pressure reading was abnormal, the medical assistant repeated the measurement to ensure that the reading was valid. Women with a diagnosis of chronic, prepregnancy hypertension were excluded.
Covariates
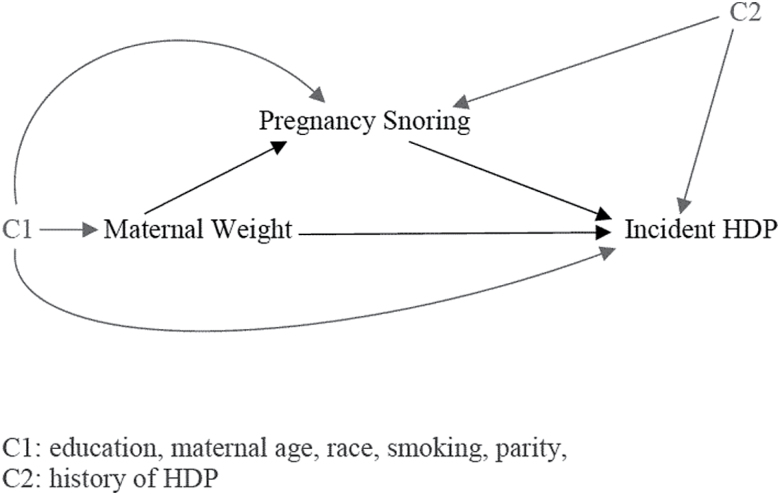
We selected potential confounders for the exposure, mediator, and outcome, based on prior literature24–27 and with the use of a directed acyclic graph,28 a visual representation of the examined associations among maternal weight, pregnancy snoring, and HDP, in addition to their potential confounders (Figure 2).
Figure 2.
Causal diagram representing direct and indirect (mediated) pathways of associations between maternal weight, pregnancy snoring, and incident hypertensive disorders of pregnancy (HDP).
Maternal characteristics were abstracted from medical charts. Maternal age was analyzed as a categorical variable (<25, 26–29, 30–34, ≥35). Women were classified into 2 racial groups (White, non-White) and 4 education groups (“less than high school,” “high school,” “some college,” and “Bachelor’s degree or higher”). Parity, smoking, and history of HDP were binary variables (yes/no).
Statistical analysis
We conducted bivariate analysis to examine potential predictors of the mediator and the outcome, pregnancy-onset snoring, and incident HDP, respectively. In this bivariate analysis, we used a Poisson regression with a log link and robust error variance to calculate risk ratios and 95% confidence intervals (CIs) of incident HDP and pregnancy-onset snoring by respective, potential predictors.
To disentangle associations between excessive maternal weight, pregnancy-onset snoring, and incident HDP, we used a causal diagram to represent potential pathways to incident HDP (Figure 2). Based on the hypothesized causal diagram, we conducted causal mediation analysis using the Valeri and Vanderweele approach,29 which allows computation of total and direct effects of maternal weight and incident HDP, in addition to their indirect effects through the pathway of pregnancy-onset snoring. We specified logistic regression model to account for the dichotomous exposure, mediator, and outcome (maternal weight, pregnancy-onset snoring, and incident HDP). In adjusted analysis, we concurrently controlled for all potential confounders on the pathways between exposure-outcome, exposure-mediator, and mediator-outcome (Figure 2). We calculated the total, direct, and indirect effects and the 95% CI for those effects. If the indirect effects were statistically significant at P <0.05, we also computed the proportion of the total effect that was mediated by pregnancy-onset snoring. Finally, we tested for the presence of an interaction between maternal weight and pregnancy-onset snoring in relation to incident HDP. In the absence of significant interaction between exposure and mediator, we did not specify interaction in the mediation formula.
We performed sensitivity analysis to estimate and quantify the mediated pathway between maternal weight and incident HDP through frequent snoring. In this analysis, we created an exclusive group to frequent snorers and classified occasional snorers and nonsnorers as controls. All statistical analyses were performed in SAS 9.4 (Cary, NC).
RESULTS
Of the 1,625 pregnant women enrolled, 169 and 123 women with chronic hypertension and/or chronic snoring were excluded, resulting in a final sample with 1,333 pregnant women. In bivariate analyses, risk of incident HDP was associated with prepregnancy BMI, pregnancy-onset snoring, parity, and history of HDP (Table 1). Pregnancy-onset snoring was associated with maternal weight (Table 2).
Table 1.
Risk of incident HDP by maternal characteristics
| HDP | |||
|---|---|---|---|
| Maternal and pregnancy characteristics | N | N (%) | RR (95% CI) |
| HDP | 140 (11) | — | |
| Age | |||
| <25 | 268 | 33 (12) | 1.32 (0.83, 2.10) |
| 25–29 | 344 | 38 (11) | 1.19 (0.76, 1.86) |
| 30–34 | 419 | 39 (9) | Reference |
| ≥35 | 302 | 30 (10) | 1.07 (0.66, 1.72) |
| BMI (categorical) | |||
| <25 | 752 | 51 (7) | Reference |
| 25–29.9 | 302 | 44 (15) | 2.15 (1.44, 3.22) |
| ≥30 | 269 | 42 (16) | 2.30 (1.53, 3.46) |
| Pregnancy-onset snoring frequency | |||
| Habitual/frequent | 293 | 44 (15) | 1.85 (1.26, 2.73) |
| Occasional/infrequent | 281 | 31 (11) | 1.36 (0.88, 2.10) |
| Nonsnorers | 740 | 60 (8) | Reference |
| Gestational diabetes | |||
| Yes | 208 | 21 (10) | 0.95 (0.60, 1.52) |
| No | 1,125 | 119 (11) | Reference |
| Parity | |||
| 0 | 591 | 86 (15) | Reference |
| ≥1 | 736 | 54 (7) | 0.50 (0.36, 0.71) |
| Education | |||
| Less than high school | 107 | 11 (10) | 1.24 (0.65, 2.37) |
| High school | 265 | 35 (13) | 1.60 (1.05, 2.44) |
| Some college | 266 | 30 (11) | 1.36 (0.87, 2.13) |
| Bachelor’s degree or higher | 665 | 55 (8) | Reference |
| Race/ethnicity | |||
| White | 961 | 107 (11) | Reference |
| Non-White | 372 | 33 (9) | 0.80 (0.54, 1.18) |
| Smokers | |||
| Yes | 146 | 20 (14) | 1.36 (0.85, 2.19) |
| No | 1185 | 119 (10) | Reference |
| History of HDP | |||
| Yes | 1272 | 122 (10) | 3.18 (1.94, 5.22) |
| No | 59 | 18 (31) | Reference |
Abbreviations: BMI = body mass index; CI = confidence interval; HDP = hypertensive disorders of pregnancy; Non-White = African-American, Asian, or Hispanic; RR = relative risk.
Table 2.
Risk of pregnancy-onset snoring by maternal characteristics
| Pregnancy-onset snoring | |||
|---|---|---|---|
| Maternal and pregnancy characteristics | N | N (%) | RR (95% CI) |
| Pregnancy-onset snoring | 574 (44) | — | |
| Age | |||
| <25 | 263 | 102 (39) | 0.86 (0.39, 1.10) |
| 25–29 | 337 | 134 (40) | 0.88 (0.68, 1.10) |
| 30–34 | 415 | 187 (45) | Reference |
| ≥35 | 299 | 151 (33) | 1.12 (0.68, 1.39) |
| BMI (categorical) | |||
| <25 | 745 | 268 (36) | Reference |
| 25–29.9 | 296 | 149 (50) | 1.40 (1.15, 1.71) |
| ≥30 | 264 | 156 (59) | 1.64 (1.35, 2.00) |
| Gestational diabetes | |||
| Yes | 205 | 95 (46) | 1.07 (0.86, 1.34) |
| No | 1,109 | 479 (43) | Reference |
| Parity | |||
| 0 | 591 | 249 (42) | Reference |
| ≥1 | 736 | 324 (44) | 1.04 (0.88, 1.23) |
| Education | |||
| Less than high school | 105 | 42 (40) | 0.93 (0.67, 1.29) |
| High school | 259 | 114 (44) | 1.03 (0.83, 1.28) |
| Some college | 263 | 120 (46) | 1.06 (0.86, 1.32) |
| Bachelor’s degree or higher | 658 | 282 (43) | Reference |
| Race/ethnicity | |||
| White | 947 | 421 (44) | Reference |
| Non-White | 367 | 153 (42) | 0.94 (0.78, 1.13) |
| Smokers | |||
| Yes | 141 | 68 (48) | 1.12 (0.87, 1.44) |
| No | 1,172 | 505 (43) | Reference |
Abbreviations: BMI = body mass index; CI = confidence interval; Non-White = African-American, Asian, or Hispanic; RR = relative risk.
In unadjusted regression models, excessive maternal weight (BMI ≥ 25) had nearly 2-fold direct and significant association with incident HDP. The magnitude of this association remained unchanged after adjusting for maternal age, race, education, parity, smoking, and history of HDP: OR = 1.87 (95% CI 1.30, 2.70). Pregnancy-onset snoring significantly mediated the pathway between maternal weight and incident HDP: adjusted OR = 1.08 (95% CI 1.01, 1.17). The total associations, i.e., combined direct and indirect associations, of maternal weight and incident HDP through pregnancy-onset snoring pathway were significant: OR = 2.03 (95% CI 1.41, 2.91). Pregnancy-onset snoring mediated 15% of the total association between maternal weight and incident HDP (Table 3).
Table 3.
Mediation by pregnancy-onset snoring of the association between maternal weight and HDP
| OR (95% CI) | ||||
|---|---|---|---|---|
| Mediator | Total association | Direct association | Indirect association through mediator | Percentage of association mediated % |
| Pregnancy snoring | ||||
| Unadjusted | 2.07 (1.47, 2.89) | 1.93 (1.37, 2.72) | 1.07 (1.00, 1.15) | 13 |
| Adjusteda | 2.03 (1.41, 2.91) | 1.87 (1.30, 2.70) | 1.08 (1.01, 1.17) | 15 |
Abbreviations: BMI = body mass index; CI = confidence interval; HDP = hypertensive disorders of pregnancy; OR = odds ratio.
aAdjusted for maternal age, race, education, parity, smoking, and history of hypertension.
The sensitivity analysis produced unchanged effect estimates; direct association between maternal weight and HDP (OR = 1.89, 95% CI 1.31, 2.72); indirect association between maternal weight and HDP, through frequent snoring (OR = 1.08, 95% CI 1.00, 1.16). Frequent, pregnancy-onset snoring mediated 14% of the total association between maternal weight and incident HDP.
DISCUSSION
In this large cohort of pregnant women without chronic hypertension and prepregnancy snoring, we have shown significant associations between maternal weight and incident HDP with direct and indirect pathways. Alternative to the direct association pathway from maternal weight to incident HDP, an indirect and significant pathway exists through pregnancy-onset snoring. Furthermore, if induced in pregnancy, maternal snoring—a key symptom of SDB—accounts for about 15% of the total association between maternal weight and incident HDP in pregnant women.
Prior reports have suggested a greater than a 2-fold association between snoring and HDP.7,13,30 A meta-analysis, associated symptoms of SDB—mostly snoring—with HDP [pooled OR 3.11 (95% CI 2.28, 4.25)].31 When objectively measured with polysomnography, SDB had a 2-fold association with HDP [pooled OR 2.25 (95% CI 1.13, 4.52)].31 Similarly, BMI has been linked independently to frequent snoring32 and also to HDP.33,34 Frequent snoring and hypertension are known consequence of excessive weight, and hypertension has also been linked to frequent snoring. However, no study, thus far, has examined potential, alternative pathways—beyond direct associations—of maternal weight, pregnancy-onset snoring, and incident HDP. Rather, these studies examined “partial pathways” between (i) maternal weight and pregnancy snoring, (ii) maternal weight and gestational hypertension, or (iii) pregnancy snoring and gestational hypertension, with near-universal control for BMI.
In this study, we used causal mediation analysis to examine the direct association of maternal weight early in pregnancy and incident HDP and their indirect association through pregnancy-onset snoring in a restricted cohort of women without chronic hypertension or prepregnancy snoring. This approach accounts for the temporal aspect of these associations and their 3 potential pathways: (i) maternal weight and HDP, (ii) maternal weight and pregnancy-onset snoring, and (iii) pregnancy-onset snoring and incident HDP. Furthermore, with causal mediation analyses, we are able to quantify the fraction of each pathway—direct and indirect—from the total association. Estimation of the magnitude of these associations may inform and identify priorities for clinical interventions.
Excessive weight is one of the strongest risk factors for frequent snoring. As fat tissue in the neck accumulates, it may cause airway narrowing and resistance that increases the risk of airway obstruction during sleep.35 Furthermore, excessive weight has also been linked to pre-eclampsia and gestational hypertension. Obesity is characterized with metabolic abnormalities, increased inflammation, and oxidative stress, each of these represents a potential pathway to HDP.36,37 Similarly, frequent snoring may cause sleep fragmentation and intermittent hypoxia that, in turn, can lead to hypertension through pathways of increased sympathetic activity, oxidative stress, or activation of inflammatory processes.30
This large cohort study elucidated the associations among maternal weight, pregnancy-onset snoring, and incident HDP by estimation and quantification of their total, direct, and indirect associations. In addition, we controlled for potential confounders in each of the pathways between maternal weight, pregnancy-onset snoring, and incident HDP. Furthermore, by excluding women with chronic, prepregnancy snoring, or hypertension, this study has provided a temporal perspective to its findings.
As a potential limitation, snoring frequency was self-reported through questionnaires and may have resulted in misclassification of some cases. However, as a key symptom of obstructive sleep apnea, subjective snoring measures in “nonpregnant” populations have been well validated against polysomnography38,39 but not in “pregnant women”. New data show that patient-reported snoring questions are strongly and reliably associated with apnea/hypopnea index (number of apneic events per hour of sleep, apnea/hypopnea index indicates the presence and severity of obstructive sleep apnea).40 According to this study, patient-reported snoring has 73% sensitivity and 77% specificity with apnea/hypopnea index in pregnancy. Prior studies have shown that symptoms predict outcomes as well as objective measures,41 and no study, thus far, has failed to associate snoring with objective measures of SDB from polysomnography. Furthermore, validation of SDB-screening tools in pregnancy has not provided significantly better measure of obstructive sleep apnea vs. symptoms alone. Most scales emphasize weight, which in pregnancy will be necessarily high, while some rely on male gender, irrelevant to a study of pregnant women. Finally, the use of questionnaires in this study is similar to symptom-based screening in clinical settings and is affordable and quick to administer to large-scale sample compared with an overnight sleep study.
Another limitation is related to the inclusion of pregnant women with frequent or occasional snoring in 1 study group. Ideally, we would classify women to 3 categories of “frequent snorers,” “occasional snorers,” and controls. However, current statistical packages for causal mediation are limited to analysis of binary or continuous mediators. Nonetheless, to estimate and quantify indirect pathway between maternal weight and HDP through frequent snoring, we performed sensitivity analysis by creating an exclusive group to frequent snorers and including occasional snorers and nonsnorers in the control group. This sensitivity analysis produced unchanged effect estimates, suggesting that frequent snoring drives the indirect association between maternal weight and incident HDP.
Finally, in addition to maternal weight in early pregnancy, gestational weight gain may also predict pregnancy-onset snoring and incident HDP. However, data on maternal weight gain were not available, and their link to incident HDP was beyond the aims of this study.
In conclusion, maternal weight is associated with incident HDP, directly and indirectly, through pregnancy-onset snoring in women without chronic, prepregnancy snoring, or hypertension. These findings demonstrate the role of maternal weight and pregnancy-onset snoring in incident HDP. Along with clinical recommendations related to excessive weight control in pregnancy and prior to conception, screening for maternal snoring may identify pregnant women at-risk for incident HDP.
DISCLOSURE
Dr L.M.O. has received equipment support from Philips Respironics Inc. and Itamar Medical. Other authors declared no conflict of interest.
ACKNOWLEDGMENTS
We wish to thank the women who participated in this study. We also thank Dr Erica C. Jansen for providing technical assistance. G.L.D was supported by a T32 Grant from the National Institute of Neurological Disorders and Stroke (NIH/NINDS T32 NS007222) and by an F32 National Research Service Award from the National Institute of Child Health and Development (NIH/NICHD F32 HD091938); L.M.O. was supported by the Gene and Tubie Gilmore Fund for Sleep Research, by the University of Michigan Institute for Clinical and Health Research (MICHR) grant UL1TR000433, by MICHR seed pilot grant F021024, and by the National Heart, Lung, and Blood Institute (R21 HL089918). During the course of this study, Dr L.M.O. was also supported by a career grant from the National Heart, Lung, and Blood Institute (K23 HL095739) and in part by R21 HL087819. None of the funding sources had any role in study design; collection, analysis, interpretation of data; writing of the report; or the decision to submit the report for publication.
REFERENCES
- 1. Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, Militello M, Pedata R. Hypertensive disorders of pregnancy. J Prenat Med 2009; 3:1–5. [PMC free article] [PubMed] [Google Scholar]
- 2. Wagner SJ, Barac S, Garovic VD. Hypertensive pregnancy disorders: current concepts. J Clin Hypertens (Greenwich) 2007; 9:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 2013; 347: f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, Jia C, Megson IL, Wei J, Zhang W. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One 2014; 9:e100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding XX, Wu YL, Xu SJ, Zhang SF, Jia XM, Zhu RP, Hao JH, Tao FB. A systematic review and quantitative assessment of sleep-disordered breathing during pregnancy and perinatal outcomes. Sleep Breath 2014; 18:703–713. [DOI] [PubMed] [Google Scholar]
- 6. Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, Chatzi L. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology 2011; 22:738–744. [DOI] [PubMed] [Google Scholar]
- 7. Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J 2010; 36:849–855. [DOI] [PubMed] [Google Scholar]
- 8. O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, Kalbfleisch JD, Chervin RD. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol 2012; 207:487.e1–487.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, Kalbfleisch JD, Chervin RD. Snoring during pregnancy and delivery outcomes: a cohort study. Sleep 2013; 36:1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma SK, Nehra A, Sinha S, Soneja M, Sunesh K, Sreenivas V, Vedita D. Sleep disorders in pregnancy and their association with pregnancy outcomes: a prospective observational study. Sleep Breath 2016; 20:87–93. [DOI] [PubMed] [Google Scholar]
- 11. Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health 2010; 10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol 2010; 203:142.e1–142.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franklin KA, Holmgren PA, Jönsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest 2000; 117:137–141. [DOI] [PubMed] [Google Scholar]
- 14. Ursavas A, Karadag M, Nalci N, Ercan I, Gozu RO. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration 2008; 76:33–39. [DOI] [PubMed] [Google Scholar]
- 15. O’Brien LM, Bullough AS, Chames MC, Shelgikar AV, Armitage R, Guilleminualt C, Sullivan CE, Johnson TR, Chervin RD. Hypertension, snoring, and obstructive sleep apnoea during pregnancy: a cohort study. BJOG 2014; 121:1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reid J, Skomro R, Cotton D, Ward H, Olatunbosun F, Gjevre J, Guilleminault C. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep 2011; 34:1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bloom JW, Kaltenborn WT, Quan SF. Risk factors in a general population for snoring. Importance of cigarette smoking and obesity. Chest 1988; 93:678–683. [DOI] [PubMed] [Google Scholar]
- 18. Hillemeier MM, Weisman CS, Chuang C, Downs DS, McCall-Hosenfeld J, Camacho F. Transition to overweight or obesity among women of reproductive age. J Womens Health (Larchmt) 2011; 20:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frederick IO, Rudra CB, Miller RS, Foster JC, Williams MA. Adult weight change, weight cycling, and prepregnancy obesity in relation to risk of preeclampsia. Epidemiology 2006; 17:428–434. [DOI] [PubMed] [Google Scholar]
- 20. Quan SF, Budhiraja R, Parthasarathy S. Is there a bidirectional relationship between obesity and sleep-disordered breathing?J Clin Sleep Med 2008; 4:210–211. [PMC free article] [PubMed] [Google Scholar]
- 21. Hargens TA, Kaleth AS, Edwards ES, Butner KL. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep 2013; 5:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med 2013; 1:329–338. [DOI] [PubMed] [Google Scholar]
- 23. Jhamb M, Unruh M. Bidirectional relationship of hypertension with obstructive sleep apnea. Curr Opin Pulm Med 2014; 20:558–564. [DOI] [PubMed] [Google Scholar]
- 24. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25:391–403. [DOI] [PubMed] [Google Scholar]
- 25. Balserak BI. Sleep disordered breathing in pregnancy. Breathe 2015; 11:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grandner MA, Petrov ME, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med 2013; 9:897–905; 905A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med 1994; 154:2219–2224. [PubMed] [Google Scholar]
- 28. Suttorp MM, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant 2015; 30:1418–1423. [DOI] [PubMed] [Google Scholar]
- 29. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013; 18:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dunietz GL, Chervin RD, O’Brien LM. Sleep-disordered breathing during pregnancy: future implications for cardiovascular health. Obstet Gynecol Surv 2014; 69:164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol 2014; 210:52.e1–52.e14. [DOI] [PubMed] [Google Scholar]
- 32. Olivarez SA, Ferres M, Antony K, Mattewal A, Maheshwari B, Sangi-Haghpeykar H, Aagaard-Tillery K. Obstructive sleep apnea screening in pregnancy, perinatal outcomes, and impact of maternal obesity. Am J Perinatol 2011; 28:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology 2003; 14:368–374. [DOI] [PubMed] [Google Scholar]
- 34. Ursavas A, Karadag M, Nalci N, Ercan I, Gozu RO. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration 2008; 76:33–39. [DOI] [PubMed] [Google Scholar]
- 35. Leinum CJ, Dopp JM, Morgan BJ. Sleep-disordered breathing and obesity: pathophysiology, complications, and treatment. Nutr Clin Pract 2009; 24:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts JM, Bodnar LM, Patrick TE, Powers RW. The role of obesity in preeclampsia. Pregnancy Hypertens 2011; 1:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J Hypertens 2011; 29:937–944. [DOI] [PubMed] [Google Scholar]
- 38. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 39. Bliwise DL, Nekich JC, Dement WC. Relative validity of self-reported snoring as a symptom of sleep apnea in a sleep clinic population. Chest 1991; 99:600–608. [DOI] [PubMed] [Google Scholar]
- 40. Smith J, Triebwasser JE, Langen E, O’Brien LM. 818: How best to screen for obstructive sleep apnea in pregnancy?Am J Obstet Gynecol 2018; 218:S487–S488. [Google Scholar]
- 41. Gottlieb DJ, Yao Q, Redline S, Ali T, Mahowald MW. Does snoring predict sleepiness independently of apnea and hypopnea frequency?Am J Respir Crit Care Med 2000; 162:1512–1517. [DOI] [PubMed] [Google Scholar]