Abstract
Background
The pathophysiology of late-life depression (LLD) is complex and heterogeneous, with age-related processes implicated in its pathogenesis. This study examined the cross-sectional and longitudinal association between depressive symptoms and a baseline multibiomarker algorithm of biological age (BA) that aggregates indicators of inflammatory, metabolic, cardiovascular, lung, liver, and kidney functioning.
Method
Data were analyzed from 2,776 men and women from the prospective observational Health Aging and Body Composition Study, who had both evaluable chronological age (CA) and BA. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression (CES-D) scale.
Results
A covariate-adjusted regression model showed that BA (B = 0.03, p = .0471) but not CA (B = −0.01, p = .7185) is associated with baseline CES-D scores. The mean baseline BA for individuals with a CES-D ≥ 10 was 1.28 years greater than in those with a CES-D < 10. Comparatively, there is only a 0.05-year difference in mean CA between the two depression groups. A covariate-adjusted longitudinal model found that baseline BA predicts CES-D score at follow-up (B = 0.04, p = .0058), whereas CA does not (B = 0.03, p = .4125). Additionally, an older BA significantly predicted a CES-D ≥ 10 (B = 0.02, p = .032) over a 10-year period.
Conclusions
A multibiomarker index of an older adult’s BA outperformed their CA in predicting subsequent increased and clinically significant depressive symptoms. This result supports the evolving view of LLD as a brain disorder resulting from deleterious age-associated changes across numerous physiological systems.
Keywords: Biological age, Chronological age, Depression, Cognitive impairment, Longitudinal, Healthy aging
Depression in later life (LLD) is a severe public health problem due to its prevalence, chronicity, and association with mortality (1,2). The presence of LLD is associated with twice the functional impairment compared to individuals without LLD and high rates of completed suicide (3,4). Compounding this burden, LLD is recurrent, can become chronic, and is often difficult to treat (5,6). Residual symptoms place patients at increased risk of suicide, and cardiovascular morbidity and mortality (7,8).
The pathophysiology of LLD is complex and heterogeneous, with distinct, age-related, etiologic pathways capable of producing symptoms (9,10). LLD is associated with small cerebrovascular infarcts appearing as white matter hyperintensities (WMH) on magnetic resonance imaging scans (11). WMH accumulate with increasing age, perhaps due to aging-associated arterial thickening and stiffening due to calcium deposition, endothelial dysfunction, reduction in capillary number, and decreased elasticity of small vessels (12). Prospective studies demonstrate that greater WMH burden is associated with comorbid cognitive dysfunction and portends poor antidepressant therapeutic response (13,14). Progression of WMH and concomitant cognitive decline may reflect worsening of the underlying vascular disease, predict a poor course of depression, and confer treatment resistance (15).
Recent studies have implicated other age-related processes in the pathogenesis of LLD. Inflammaging observed in older adults may cause depression by altering levels and functionality of mood-related neurotransmitter systems such as dopamine (16). Proinflammatory cytokines reduce dopaminergic transmission by a variety of mechanisms including decreasing dopamine synthesis and glutamate-dependent dopamine signaling (17). These deleterious changes are compounded by concomitant aging of cellular components such as mitochondria, which results in maximal energy expenditure (VO2 max) decreasing by approximately 10% annually starting in the third decade of life (18). Deficits in mitochondria functioning manifest clinically as decreased energy capacity and fatigue, which is not only a cardinal feature of depression but also of frailty, a biological syndrome that is ubiquitous in depressed older adults (18–20).
Since treatments for LLD are limited in efficacy, it would be advantageous to develop methods of identifying individuals at risk of LLD and instituting therapies that prevent the full-blown (and potentially less treatable) syndrome of LLD. Most of the known pathophysiologic contributors to LLD increase with advancing age (eg, vascular damage, inflammation, cellular senescence), suggesting that LLD might be considered an adverse outcome of unhealthy aging. Recently, methods of quantifying unhealthy aging (ie, biological age [BA]) have been developed by aggregating indicators of the integrity of important bodily systems (eg, cardiovascular, immune) (21). Even in younger adults, BA can be quantified, related to current functioning, and used to predict important health outcomes (22). Multibiomarker algorithms such as the U.S. National Health and Nutrition Survey (NHANES)-based measure of BA have been shown to outperform chronological age (CA) in predicting mortality and physical decline by multiple groups in multiple datasets (22–24).
The aim of this study was to utilize the NHANES-based multibiomarker algorithm for BA (22,25), that aggregates measures of inflammatory, metabolic, cardiovascular, lung, liver, and kidney functioning, to predict incident LLD among healthy older adults enrolled in the Health Aging and Body Composition (Health ABC) Study. We hypothesized that greater BA would be associated with both increased depressive symptoms and the development of clinically significant depressive symptoms over a 10-year period. Moreover, to test the specificity of the differences in the relationship between BA and CA and LLD, we examined the utility of BA compared to CA in predicting changes in cognition over time. We chose cognition as our specificity marker due to its strong association with increasing CA.
Methods
Study Participants
The Health ABC Study is a National Institute on Aging project launched in 1997 to characterize the extent of change in body composition in older adults over time and examine the impact of these changes. Data were obtained in November 2013 and comprise 3,075 persons aged 70–79 years at baseline. Included participants were free of difficulty in activities of daily living and had no baseline functional limitation. Details regarding selection criteria and Health ABC methodology were previously published (26,27).
Assessments
Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression (CES-D) scale at baseline, and years 4, 6, 8, and 10 after baseline (28). Scores range from 0 to 60 with a cutoff of ≥ 10 used to denote a dichotomous variable for significant depressive symptoms, a level associated with poor prognosis (1,19). Cognition was assessed using the modified Mini-Mental Status Examination (3MS) at baseline, and years 3, 5, 7, 9, and 10 after baseline (29). Scores range from 0 to 100 with higher scores indicating better cognition. A severity cutoff of ≤78 was used to denote significant cognitive impairment (30). Primary analyses utilized the continuous scores for each measure.
The eight baseline biomarkers include C-reactive protein (CRP), creatinine, total cholesterol, Hemoglobin A1C (HgbA1c), albumin, alkaline phosphatase, forced expiratory volume in 1st second (FEV), and systolic blood pressure. Bloods were drawn after an 8-hour fast and stored at −70°C until they were assayed at the study laboratory (University of Vermont). Creatinine, total cholesterol, and albumin were measured on a Johnson & Johnson VITROS 950 Chemistry Analyzer (Johnson & Johnson, New Brunswick, NJ) (31,32). HgbA1c was measured using Tosoh 2.2 Plus (Tosoh Bioscience, Tokyo, Japan), a fully automated glycosylated hemoglobin analyzer that uses nonporous ion-exchange high-performance liquid chromatography for separation of HgbA1c (33). Baseline CRP serum concentrations were measured in duplicate by enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN) (34). FEV and systolic blood pressure were measured in year 1 (35), with the latter the average of two seated, at-rest measurements by a conventional mercury sphygmomanometer (36).
Covariates included gender, education (less than high school, high school graduate, secondary education), race (Black, Caucasian), and baseline CA. Medical comorbidities were also included when investigating baseline BA and CA. Dichotomous comorbidity variables were created from questionnaires and grouped into categories: physical (arthritis, osteoporosis), respiratory (asthma, chronic bronchitis, emphysema, chronic obstructive pulmonary disease), vascular (hypertension, diabetes mellitus, and current smoker, a vascular risk factor), cardiovascular (myocardial infarction, angina pectoris, congestive heart failure, coronary bypass/coronary artery bypass grafting), cerebrovascular (transient ischemic attack, stroke/cerebrovascular accident), and cancer (any type) (37).
Quantification of BA
Quantification of baseline BA was based on an algorithm (25) that outperformed several other multibiomarker algorithms in predicting mortality in 9,389 NHANES III participants (21). We calculated BA in this investigation using eight biomarkers in the Health ABC data set. Details regarding the quantification of BA are included in the Supplementary Material.
Statistical Analyses
Associations of baseline BA and CA with demographics, comorbidities, CES-D and 3MS scores, and death status were evaluated using t tests for correlations between continuous variables and two-sample t tests for categorical variables. Follow-up CES-D data were available at 4, 6, 8, and 10 years postbaseline, with at least one follow-up available for 89.7% (n = 2,489) of the analytic sample (n = 2,776). The remaining 5.5% died (n = 153) before the first follow-up (year 4) or were lost to follow-up for unspecified reasons (4.8%, n = 134). Follow-up 3MS data were available at 3, 5, 7, 9, and 10 years postbaseline, with at least one follow-up available for 88.1% (n = 2,447) of the sample. The remaining 3.0% died (n = 82) before the first follow-up or were lost to follow-up (8.9%, n = 247). Longitudinal mixed effect models of CES-D scores at years 4, 6, 8, and 10 were fit, predicted by baseline CES-D score, BA, CA, years in study, and demographics. A random intercept and random slopes for years in the study for each individual was included to control for repeated measures and to allow for varying slopes of CES-D scores across individuals across time. Specifically, CES-Dit = b0i + b1i × years in study + b2 × CES-Di0 + b3 × BAi + b4 × CAi + b5 × demographics + eit for i = 1 to 2,489, where b0i and b1i are the random intercept and slope respectively with expected fixed value b0 and b1. Because models control for baseline CES-D and overall population slope change of CES-D over time, the estimated coefficients for BA (ie, b3) indicates the expected change in CES-D scores compared to average change across follow-up for each 1-year increase in BA at baseline holding CA and demographics constant. An additional model was tested including interactions of BA and CA with years in study; neither interaction was significant for CES-D, hence the results are not shown. Mixed effects models treat missing CES-D as missing at random. To assess the robustness of BA and CA coefficient results to possible nonignorable missing, we performed sensitivity analyses by implementing a pattern mixture model additionally controlling for categorical groups defined by the different missing data patterns. The coefficient for the overall main effect of BA and CA remained very similar and there was a trend such that subjects with more missing data showed even stronger associations between BA and CES-D. Similar longitudinal mixed effect models of follow-up 3MS scores were fit using the same methods. An additional 3MS model was tested including interactions of BA and CA with years in the study; while the CA interaction was significant, the BA interaction was not. As BA is the focus of this investigation, these results are not shown.
To examine the effects of BA directly on both death as well as incident depression, we performed a survival analysis with time to event outcome of either CES-D ≥ 10 or death. Subjects with baseline CES-D ≥ 10 were excluded from these analyses. Cox proportional hazards regression included baseline CES-D score, BA, CA, and demographics. All analyses were conducted in SAS software (version 9.4, 2013; SAS, Inc., Cary, NC). Statistical significance was set at α less than 0.05.
Results
Sample Characteristics
The final analytic sample included 2,776 (of the total 3,075) subjects who had evaluable CA and BA data. Subjects (n = 299) were not included if they were missing at least one biomarker used to model BA, with the most commonly missing biomarker (n = 212) being FEV; no other biomarker was missing in more than 80 subjects (HgbA1c). The sample was 51.2% female, 40.7% Black, and had mean CA of 73.6 ± 2.9 years (Table 1).
Table 1.
Baseline Characteristics from the Health Aging and Body Composition Study by Chronological and Biological Age
| Total | Chronological Age | Biological Age | |||||
|---|---|---|---|---|---|---|---|
| N | % | Mean ± SD | Stand Diff ± SE | Mean ± SD | Stand Diff ± SE | ||
| Gender | Male | 1,356 | 48.8 | 73.75 ± 2.85 | 0.115 ± 0.038* | 68.48 ± 7.80 | −0.507 ± 0.039** |
| Female | 1,420 | 51.2 | 73.42 ± 2.86 | 72.51 ± 7.59 | |||
| Education | <High school grad | 669 | 24.1 | 73.58 ± 2.92 | −0.003 ± 0.051a | 72.43 ± 8.01 | 0.160 ± 0.051a,* |
| High school grad | 922 | 33.2 | 73.59 ± 2.86 | 0.007 ± 0.044b | 71.16 ± 7.80 | 0.275 ± 0.044b,** | |
| Secondary | 1,177 | 42.4 | 73.57 ± 2.82 | 0.003 ± 0.048c | 68.97 ± 7.73 | 0.435 ± 0.049c,** | |
| Race | Caucasian | 1,645 | 59.3 | 73.71 ± 2.84 | 0.112 ± 0.039* | 68.46 ± 7.18 | −0.643 ± 0.040** |
| Mortality | Alive by year 10 | 2,013 | 72.5 | 73.36 ± 2.78 | −0.283 ± 0.043** | 69.72 ± 7.59 | −0.375 ± 0.043** |
| Dead by year 10 | 763 | 27.5 | 74.17 ± 2.97 | 72.70 ± 8.47 | |||
| Baseline CES-D | <10 | 2,366 | 85.2 | 73.58 ± 2.87 | −0.017 ± 0.055 | 70.35 ± 7.92 | −0.161 ± 0.055* |
| ≥10 | 386 | 13.9 | 73.63 ± 2.80 | 71.63 ± 7.81 | |||
| Baseline 3MS | >78 | 2,523 | 90.9 | 73.54 ± 2.84 | −0.168 ± 0.067 | 70.16 ± 7.81 | −0.513 ± 0.067** |
| ≤78 | 247 | 8.9 | 74.02 ± 3.02 | 74.24 ± 8.39 | |||
| Vascular | Yes | 1,590 | 57.3 | 73.49 ± 2.87 | −0.073 ± 0.039 | 72.30 ± 7.80 | 0.540 ± 0.040** |
| No | 1,081 | 38.9 | 73.70 ± 2.84 | 68.01 ± 7.45 | |||
| Physical | Yes | 1,525 | 54.9 | 73.69 ± 2.90 | 0.091 ± 0.039 | 70.96 ± 8.13 | 0.120 ± 0.039* |
| No | 1,145 | 41.2 | 73.43 ± 2.80 | 70.01 ± 7.63 | |||
| Respiratory | Yes | 481 | 17.3 | 73.38 ± 2.75 | −0.084 ± 0.050 | 72.11 ± 7.68 | 0.243 ± 0.051** |
| No | 2,158 | 77.7 | 73.62 ± 2.88 | 70.18 ± 7.94 | |||
| Cardio | Yes | 586 | 21.1 | 73.80 ± 2.88 | 0.105 ± 0.047 | 71.59 ± 8.65 | 0.164 ± 0.047** |
| No | 2,118 | 76.3 | 73.50 ± 2.84 | 70.29 ± 7.70 | |||
| Cerebro | Yes | 203 | 7.3 | 74.20 ± 3.00 | 0.234 ± 0.073* | 72.48 ± 9.03 | 0.265 ± 0.073** |
| No | 2,566 | 92.4 | 73.53 ± 2.84 | 70.37 ± 7.83 | |||
| Cancer | Yes | 128 | 4.6 | 73.77 ± 2.82 | 0.066 ± 0.091 | 69.46 ± 8.17 | −0.136 ± 0.091 |
| No | 2,478 | 89.2 | 73.58 ± 2.86 | 70.54 ± 7.90 | |||
Note: Data is for baseline visit with the exception of mortality. CES-D = Center for Epidemiologic Studies Depression scale; Stand Diff = Standardized difference; SE = Standard error; 3MS = Teng Modified Mini-Mental Status Exam; Education defined as 1=less than high school, 2=high school graduation, 3=secondary education.
aCompares < high school education versus high school graduate; bCompares high school graduate versus secondary education; cCompares < high school education versus secondary education.
*p < .01. **p < .001.
Mean baseline CES-D score was 4.7 ± 5.3, and 13.9% of subjects at baseline reported a CES-D ≥ 10. CES-D scores significantly increased at a rate of 0.43 (standard error [SE] = 0.02) points per year (t = 24.4, df = 2,485, p < .0001), with the prevalence of subjects who experienced a CES-D ≥ 10 by the end of the study increasing to 29.3% (Table 2). Mean baseline 3MS score was 90.2 ± 8.4, and 8.9% of subjects at baseline had a 3MS ≤ 78. 3MS scores significantly decreased at a rate of 0.41 points (SE = 0.02) per year (t = −17.23, df = 2,444, p < .0001), with the prevalence of subjects with a 3MS ≤ 78 by the end of the study increasing to 13.2% (Table 2).
Table 2.
Longitudinal Characteristics from the Health Aging and Body Composition Study for Depressive Symptoms, Cognitive Functioning, and Death Status
| Cumulative Mortality % (n) | CES-D | 3MS | |||||
|---|---|---|---|---|---|---|---|
| N Followed | Total Symptom Mean ± SD | CES-D ≥ 10 % (n) | N Followed | Total Score Mean ± SD | 3MS ≤ 78 % (n) | ||
| Baseline (Year 1) | 2,752 | 4.66 ± 5.27 | 13.90 (386) | 2,770 | 90.16 ± 8.41 | 8.92 (247) | |
| Year 2 | 0.97 (27) | ||||||
| Year 3 | 2.95 (82) | 2,306 | 90.08 ± 8.81 | 9.32 (215) | |||
| Year 4 | 5.51 (153) | 2,422 | 6.35 ± 6.35 | 24.07 (583) | |||
| Year 5 | 7.96 (221) | 2,134 | 90.32 ± 9.06 | 9.42 (201) | |||
| Year 6 | 11.60 (322) | 2,235 | 7.51 ± 6.97 | 29.40 (657) | |||
| Year 7 | 15.96 (443) | 681 | 90.62 ± 8.49 | 7.93 (54) | |||
| Year 8 | 19.38 (538) | 1,501 | 7.37 ± 6.57 | 28.98 (435) | |||
| Year 9 | 23.45 (651) | 600 | 90.15 ± 9.53 | 11.00 (66) | |||
| Year 10 | 27.49 (763) | 1,322 | 7.52 ± 6.64 | 29.27 (387) | 1,466 | 88.82 ± 10.54 | 13.17 (193) |
Note: The full 20-item CES-D was assessed at baseline, and years 4, 6, 8, or 10 and prevalence rates were based on a cutoff score of ≥ 10; The 3MS was assessed at baseline, and years 3, 5, 7, 9, or 10 and prevalence rates are based on a cutoff score of ≤ 78. CES-D = Center for Epidemiologic Studies Depression scale; 3MS = Teng Modified Mini-Mental Status Exam.
BA
Mean BA calculated for study subjects was 70.5 ± 7.9 years (range 50–115). BA was correlated with CA (r = .24, p < .0001) and each included biomarker in the expected direction (CRP: r = .20, p < .0001; FEV1: r = −.55, p < .0001; cholesterol: = .30, p < .0001; creatinine: r = .20, p < .0001; HgbA1c: r = .36, p < .0001; systolic blood pressure: r = .71, p < .0001; albumin: r = −.073, p = .0001; alkaline phosphatase: r = .31, p < .0001).
As shown in Table 1, BA was lower in men versus women and in White versus Black subjects, despite both men and white subjects being older chronologically than women and Black subjects, respectively. BA was lower in more versus less educated subjects, counter to the CA of subjects, which did not differ across education levels. BA was greater with the presence of each medical comorbidity, with the exception of cancer, while CA was only greater in subjects with physical disabilities, and cardiovascular and cerebrovascular diseases.
In a regression model predicting BA with CA, demographics and medical comorbidities, participants being female (B = 3.8, p < .0001), Black (B = 3.9, p < .0001), and with less than postsecondary education (B = 1.06, p = .0032) predicted higher BA. To examine the relationship between demographic traits and BA, bivariate correlations between demographic variables and each biomarker in the BA index were observed. Higher education was negatively correlated (p < .001) with CRP, HgbA1c, blood pressure, and alkaline phosphatase levels and positively correlated with lung function and albumin levels. White race was negatively correlated (p < .05) with CRP, cholesterol, creatinine, HgbA1c, and blood pressure levels and positively correlated with lung function and albumin levels. Compared to men, women had higher (p < .01) CRP and cholesterol and lower lung function and albumin levels.
Association of Late-Life Depression with BA
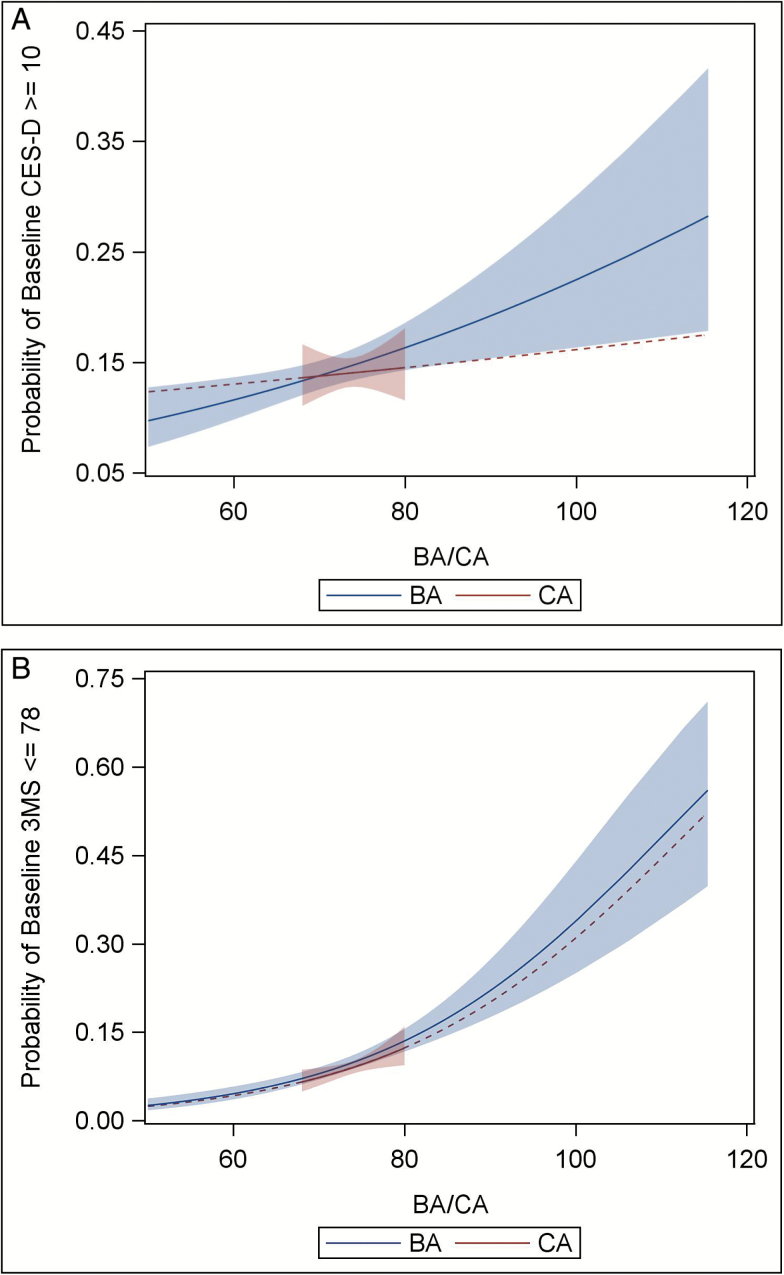
Baseline CES-D scores were associated with baseline BA (p < .001) but not CA (p = .955). The mean baseline BA for individuals with a CES-D ≥ 10 was 1.28 years greater (SE = 0.43) than that of individuals with a CES-D < 10, compared to the 0.05 year difference (SE = 0.16) in mean CA between the two depression groups (see Table 1). Moreover, a regression of baseline CES-D on both baseline BA and CA adjusting for demographic variables showed BA (B = 0.03, p = .0471) but not CA (B = −0.01, p = .7185) was associated with baseline CES-D scores. This effect is evident in Figure 1a, which depicts the bivariate association between the predicted prevalence of significant depressive symptoms at baseline and increasing BA. In contrast, only a weak relationship exists between the prevalence of significant depressive symptoms at baseline and CA.
Figure 1.
The predicted prevalence of significant depressive symptoms and cognitive impairment at baseline as a function of baseline biological and chronological age. (a) Significant depressive symptoms (CES-D ≥ 10). (b) Significant cognitive impairment (3MS ≤ 78). Note: Depicted is the predicted prevalence of experiencing significant depressive symptoms (Figure 1a; CES-D ≥ 10) and significant cognitive impairment (Figure 1b; 3MS ≤ 78) at baseline with the 95% confidence intervals plotted against BA and CA. The regression line for CA was extended beyond the baseline age range (68–80 years; reflected as a dashed line) for ease of comparison between BA and CA. BA = Biological age; CA = Chronological age; CES-D = Center for Epidemiologic Studies Depression scale.
Results from a longitudinal model indicated that having a higher baseline BA was associated with a worse CES-D score at follow-up after accounting for baseline CES-D score, demographics and years in study, whereas CA was not (Table 3). Being female and having lower educational attainment were associated with higher CES-D scores at follow-up. The final model explained 23.8% of the variance in CES-D score at follow-up, with no additional variance explained by the addition of CA.
Table 3.
Longitudinal Models Predicting Worsening Depressive Symptoms and Cognitive Impairment with Biological Age, Chronological Age, Demographics, Years in Study, and Baseline CES-D and 3MS Score
| Predictors | Outcome: CES-D at Follow-up Years 4, 6, 8, and 10 | |||
|---|---|---|---|---|
| B weights | SE | t Value | p Value | |
| Biological age at baseline | 0.03965 | 0.01437 | 2.76 | .0058 |
| Chronological age at baseline | 0.02918 | 0.03561 | 0.82 | .4125 |
| Sex (male vs female) | −0.6106 | 0.2036 | −3.00 | .0027 |
| Race (Caucasian vs Black) | −0.4102 | 0.2204 | −1.86 | .0628 |
| Education | ||||
| Less than HS vs postsecondary | 1.4404 | 0.2674 | 5.39 | <.0001 |
| HS graduate vs postsecondary | 0.4561 | 0.2229 | 2.05 | .0409 |
| Year in study | 0.2924 | 0.02461 | 11.88 | <.0001 |
| CES-D (baseline) | 0.5909 | 0.01874 | 31.52 | <.0001 |
| Outcome: 3MS at follow-up years 3, 5, 7, 9, or 10 | ||||
| Predictors | B weights | SE | t Value | p Value |
| Biological age at baseline | −0.00464 | 0.01540 | −0.30 | .7631 |
| Chronological age at baseline | −0.2284 | 0.03848 | −5.93 | <.0001 |
| Sex (male vs female) | 0.02471 | 0.2192 | 0.11 | .9103 |
| Race (Caucasian vs Black) | 1.6463 | 0.2446 | 6.73 | <.0001 |
| Education | ||||
| Less than HS vs postsecondary | −3.1120 | 0.3115 | −9.99 | <.0001 |
| HS graduate vs postsecondary | −0.7418 | 0.2408 | −3.08 | .0021 |
| Year in study | −0.4790 | 0.02656 | −18.03 | <.0001 |
| 3MS at baseline | 0.7316 | 0.01565 | 46.75 | <.0001 |
Note: Education defined as 1=less than high school, 2=high school graduation, 3=secondary education; Reference groups are female for sex, Black for race, and postsecondary for education. CES-D = Center for Epidemiologic Studies Depression scale; HS = High school; 3MS = Teng Modified Mini-Mental Status Exam.
To investigate the robustness of these results, we modeled the event of CES-D ≥ 10 or death over a 9-year period among subjects with CES-D < 10 at baseline using survival analysis. Baseline BA predicted the development of CES-D ≥ 10 or death during follow-up (hazard ratio for 1 year increase in BA is 1.08, 95% confidence interval 1.011–1.026) when adjusting for baseline CA, demographics, and baseline CES-D score.
Association of Cognitive Impairment with BA
Baseline 3MS scores were associated with both BA (p < .0001) and CA (p < .0001). The mean baseline BA for individuals with a 3MS ≤ 78 was 4.08 years greater (SE = 0.52) than that of individuals with a baseline 3MS > 78, compared to the 0.48-year difference (SE = 0.19) in mean CA between the two cognitive groups (Table 1). A covariate-adjusted regression showed both BA (B = −0.06, p = .0009) and CA (B = −0.21, p < .0001) associated with baseline 3MS, with the similarity of the effect of both BA and CA on the predicted probability of experiencing significant cognitive impairment at baseline depicted in Figure 1b.
Results from a longitudinal model found that BA at baseline did not predict 3MS score at follow-up after accounting for all other variables (p = .7631), whereas CA did (p < .0001; Table 3). Having lower educational attainment was associated with lower 3MS scores at follow-up. Furthermore, baseline BA did not predict the development of 3MS ≤ 78 over a 9-year period using a survival analysis (B = 0.001, p = .7298), but did predict the event of 3MS ≤ 78 or death (B = 0.025, p < .0001).
Discussion
A multibiomarker index of an older adult’s BA (22,25) outperformed their CA in predicting the incidence of depressive symptoms over long-term follow-up. BA, but not CA, was positively associated with a greater worsening of depressive symptoms and the incidence of clinically significant depressive symptoms and mortality over the 10-year study period. Significantly, our results speak to the specificity of the link between BA and LLD. It does not appear to be the case that more advanced BA is simply a general predictor of illness in later life, as BA was not a significant predictor of changes in cognitive impairment over the 10-year study period after accounting for CA.
This result supports the evolving view of LLD as resulting from deleterious age-associated changes such as increases in cardiovascular risk factors, adverse endocrinological developments, and declining functionality of important tissues and organ systems (10). Applications of multibiomarker algorithms have the potential to aid in the identification of individuals at high biological risk of developing LLD and to facilitate targeting these individuals with prevention and/or treatment interventions. Moreover, the BA index provides specific therapeutic targets for which efficacious interventions already exist, such as blood pressure, serum lipids, and blood glucose. Thus, far from being an inevitable result of aging with its concomitant physiologic decline and/or psychosocial losses, LLD can more accurately be viewed as a preventable illness. By targeting these individual markers, we can decrease mortality risk and the risk for the development of LLD, thereby improving health trajectories of our older patients. The theory underlying BA, however, is that by aggregating indicators for the integrity of important bodily systems, we can detect molecular changes that cause the multisystem physiologic dysregulation that is the hallmark of aging-related morbidity (22,25). It is unknown however whether targeting these individual therapeutic markers will subsequently alter the underlying BA of the individual. This question is in need of further research.
Our analyses also highlighted interesting social and demographic differences in BA values. Subjects who were White and more educated tended to have lower BA scores compared to Black and less educated subjects. These associations appear to be due to both Black race (38) and lower educational attainment being associated with specific components of BA (increased inflammation, cholesterol, creatinine, HgbA1c, and blood pressure and decreased lung function and albumin). These results suggest that individuals who are either Black or who have high school or less level of educational attainment should be targeted for vigorous control of cardiovascular risk factors, blood glucose, and lung/kidney function. Disparities in access to health care among these groups should be assessed and addressed with appropriate outreach efforts if present. Interestingly, female sex was associated with higher BA (39), which was unexpected given generally reduced life expectancies for men compared to women. These results may be explained by a selection bias according to which relatively less healthy cohorts of men were removed prior to study initiation, since excess cardiovascular mortality in men typically occurs prior to the 70-year-old age threshold recruited into the Health ABC study.
The above results should be considered in light of limitations, including the fact that the CES-D is a screening instrument rather than a diagnostic tool for depressive illness, and the cutoff does not denote depressive illness (40). Additionally, quantification of BA included only 8 of 10 biomarkers used previously, and extrapolated outside the age-range of the original study, as baseline ages ranged from 68 to 80 years in the Health ABC study compared with 30–75 years in NHANES III (22). Furthermore, although only baseline HgbA1c values are used in this investigation, HgbA1c levels tended to be higher at baseline than in subsequent years in Health ABC. The samples are suitable for these analyses however as they were consistent across the entire baseline sample and are not compared to subsequent HgbA1c levels. Finally, it should be noted that this investigation cannot speak specifically to the trajectories of BA so much as provide a snapshot association between BA at a certain time point and the potential development of clinical correlates such as depression, cognition, or mortality.
Conclusions
A multibiomarker index of an older adult’s BA outperformed their CA in predicting subsequent increased and clinically significant depressive symptoms by study endpoint. The BA model is based upon common and inexpensive biological and behavioral variables that can be utilized to identify older adults at risk for subsequent development of LLD. This identification can lead to intervention strategies that may dramatically improve the clinical trajectories of these individuals. Furthermore, the relationship between decrements in BA to specific demographic subgroups highlights the need for outreach in these at-risk populations in the hopes of intervening at an earlier time point.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This research was supported by National Institute on Aging (N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459) and the National Institute for Mental Health (K23-MH099097), as well as by the Intramural Research Program of the National Institute on Aging.
Conflict of Interest
B.R.R. reports receiving consulting fees from Pfizer. Other authors have no conflicts to report. This article has not been previously presented.
Author Contributions
P.J.B., M.M.W., S.P.R., K.Y., B.R.R., and C.C. had full access to the data and take responsibility for its integrity and the accuracy of the analysis.
Supplementary Material
References
- 1. Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129:126–142. doi:10.1016/j.jad.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothschild AJ. The diagnosis and treatment of late-life depression. J Clin Psychiatry. 1996;57(suppl 5):5–11. [PubMed] [Google Scholar]
- 3. Callahan CM, Wolinsky FD, Stump TE, Nienaber NA, Hui SL, Tierney WM. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13:746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conwell Y, Lyness JM, Duberstein P et al. Completed suicide among older patients in primary care practices: a controlled study. J Am Geriatr Soc. 2000;48:23–29. [DOI] [PubMed] [Google Scholar]
- 5. Alexopoulos GS, Meyers BS, Young RC et al. Recovery in geriatric depression. Arch Gen Psychiatry. 1996;53:305–312. [DOI] [PubMed] [Google Scholar]
- 6. Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry. 2008;16:65–73. doi:10.1097/JGP.0b013e3181 256b1d [DOI] [PubMed] [Google Scholar]
- 7. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80:135–144. doi:10.1016/S0165-0327(03)00054-5 [DOI] [PubMed] [Google Scholar]
- 8. Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. [DOI] [PubMed] [Google Scholar]
- 9. Brown PJ, Rutherford BR, Yaffe K et al. The depressed frail phenotype: the clinical manifestation of increased biological aging. Am J Geriatr Psychiatry. 2016;24:1084–1094. doi:10.1016/j.jagp.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutherford BR, Taylor WD, Brown PJ, Sneed JR, Roose SP. Biological aging and the future of geriatric psychiatry. J Gerontol A Biol Sci Med Sci 2017;72:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi:10.1176/ajp.154.4.497 [DOI] [PubMed] [Google Scholar]
- 12. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi:10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheline YI, Pieper CF, Barch DM et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi:10.1001/archgenpsychiatry.2009.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. doi:10.1176/ajp.154.4.562 [DOI] [PubMed] [Google Scholar]
- 15. Taylor WD, Steffens DC, MacFall JR et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi:10.1001/archpsyc.60.11.1090 [DOI] [PubMed] [Google Scholar]
- 16. Franceschi C, Bonafè M, Valensin S et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 17. Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33:315–327. doi:10.1016/j.yfrne.2012.09. 003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, Molina AJ. Respirometric profiling of muscle mitochondria and blood cells are associated with differences in gait speed among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2015;70:1394–1399. doi:10.1093/gerona/glu096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown PJ, Roose SP, Fieo R et al. Frailty and depression in older adults: a high-risk clinical population. Am J Geriatr Psychiatry. 2014;22:1083–1095. doi:10.1016/j.jagp.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 21. Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi:10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 22. Belsky DW, Caspi A, Houts R et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. doi:10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103:14158–14163. doi:10.1073/pnas.0606215103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen AA, Milot E, Li Q et al. Detection of a novel, integrative aging process suggests complex physiological integration. PLoS One. 2015;10:e0116489. doi:10.1371/journal.pone.0116489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age?J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi:10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cesari M, Kritchevsky SB, Penninx BW et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi:10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 27. Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM; Health ABC Collaborative Research Group Walking performance and cardiovascular response: associations with age and morbidity–the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;58:715–720. [DOI] [PubMed] [Google Scholar]
- 28. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. [DOI] [PubMed] [Google Scholar]
- 29. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 30. Canadian study of health and aging: study methods and prevalence of dementia. CMAJ 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- 31. Minev E, Unruh M, Shlipak MG et al. ; Health ABC Study Association of cystatin C and depression in healthy elders: the health, aging and body composition study. Nephron Clin Pract. 2010;116:c241–c246. doi:10.1159/000317205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nettiksimmons J, Ayonayon H, Harris T et al. ; Health ABC Study Development and validation of risk index for cognitive decline using blood-derived markers. Neurology. 2015;84:696–702. doi:10.1212/WNL.0000000000001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipska KJ, Inzucchi SE, Van Ness PH et al. ; Health ABC Study Elevated HbA1c and fasting plasma glucose in predicting diabetes incidence among older adults: are two better than one?Diabetes Care. 2013;36:3923–3929. doi:10.2337/dc12-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsu FC, Kritchevsky SB, Liu Y et al. ; Health ABC Study Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol A Biol Sci Med Sci. 2009;64:581–589. doi:10.1093/gerona/glp005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Juthani-Mehta M, De Rekeneire N, Allore H et al. ; Health ABC Study Modifiable risk factors for pneumonia requiring hospitalization of community-dwelling older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2013;61:1111–1118. doi:10.1111/jgs.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu C, Shlipak MG, Stawski RS et al. ; Health ABC Study. Visit-to-visit blood pressure variability and mortality and cardiovascular outcomes among older adults: the Health, Aging, and Body Composition Study. Am J Hypertens. 2017;30:151–158. doi:10.1093/ajh/hpw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown PJ, Roose SP, Zhang J et al. Inflammation, depression, and slow gait: a high mortality phenotype in later life. J Gerontol A Biol Sci Med Sci. 2016;71:221–227. doi:10.1093/gerona/glv156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levine ME, Crimmins EM. Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med. 2014;118:27–32. doi:10.1016/j.socscimed.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Draper TW, Abrahamson H. Developmental timing of the male-female health-survival paradox. Percept Mot Skills. 2014;119:655–660. doi:10.2466/10.PMS.119c25z8 [DOI] [PubMed] [Google Scholar]
- 40. Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.