Abstract
Muscle weakness in the elderly has been linked to recurrent falls and morbidity; therefore, elucidating the mechanisms contributing to the loss of muscle function and mobility with advancing age is critical. To this aim, we comprehensively examined skeletal muscle metabolic function and hemodynamics in 11 young (23 ± 2 years), 11 old (68 ± 2 years), and 10 oldest-old (84 ± 2 years) physical activity–matched participants. Specifically, oxidative stress markers, mitochondrial function, and the ATP cost of contraction as well as peripheral hemodynamics were assessed during dynamic plantar flexion exercise at 40 per cent of maximal work rate (WRmax). Both the PCr recovery time constant and the peak rate of mitochondrial ATP synthesis were not significantly different between groups. In contrast, the ATP cost of dynamic contractions (young: 1.5 ± 1.0, old: 3.4 ± 2.1, oldest-old: 6.1 ± 3.6 mM min−1 W−1) and systemic markers of oxidative stress were signficantly increased with age, with the ATP cost of contraction being negatively correlated with WRmax (r = .59, p < .05). End-of-exercise blood flow per Watt rose significantly with increasing age (young: 37 ± 20, old: 82 ± 68, oldest-old: 154 ± 93 mL min−1 W−1). These findings suggest that the progressive deterioration of muscle contractile efficiency with advancing age may play an important role in the decline in skeletal muscle functional capacity in the elderly.
Keywords: Aging, 31P-MRS, ATP cost of contraction
Most of the developed world is experiencing a rapid growth of the older population, with those considered to be the oldest-old (>80 years) exhibiting an even faster rate of increase, and this cohort is predicted to reach nearly 10 per cent of the population worldwide by 2050. Despite this increase in longevity, the decline in functional capacity with advancing age is still clearly evident, highlighted now by the apparently accelerated physical deterioration of the oldest-old (1,2). As muscle weakness in the elderly has been linked to recurrent falls and is a major cause of morbidity and mortality, elucidating the physiological mechanisms contributing to the loss of muscle function and mobility in, not only the typically old (65 to 70 years), but also the oldest-old has, therefore, become a topic of major scientific and clinical importance.
Although there is a growing body of evidence suggesting that the loss of muscle mass (3) and strength (4) is exaggerated in the oldest-old and is accompanied by blunted muscle plasticity in response to physical training (5,6), there is limited information regarding skeletal muscle metabolic function in this cohort. Furthermore, in the studies that have examined a wide spectrum of age, there is no consensus. For example, a recent study reported preserved muscle respiratory capacity in permeabilized muscle fibers from the vastus lateralis of oldest-old (~82 years) in comparison to old (~70 years) and young (~24 years) participants (7), whereas, in contrast, another study, using 31P-magnetic resonance spectroscopy (31P-MRS), documented that phosphocreatine (PCr) recovery time constant, measured by 31P-MRS, an index of muscle mitochondrial capacity (8), was slower in the plantar flexor muscles of the oldest-old (~80 years) compared with young (~25 years) and old (~67 years) participants (9). It should be noted, however, that since this latter study and the recognition that advancing age may alter skeletal muscle blood flow and modulate PCr recovery kinetics (10), such in vivo metabolic studies, should, when possible, also assess peripheral hemodynamics. Therefore, it is still unclear whether the skeletal muscle of the oldest-old exhibits a preserved mitochondrial phosphorylation capacity in vivo.
Impaired muscle efficiency is another potential mechanism which may contribute to muscle dysfunction and weakness with age. Indeed, our group recently documented an exaggerated ATP cost of contraction, potentially mediated by an oxidative stress–induced increase in the energy demand from noncontractile processes (ionic pump) in the locomotor muscles of old individuals (~70 years) (11,12). Interestingly, however, pulmonary O2 consumption during cycle exercise has been documented to be noticeably lower in centenarians, actually implying an improved mechanical efficiency in these people of exceptional age (13). These apparently contrasting findings raise the intriguing question as to whether these efficient metabolic characteristics are limited to those exceptional people who live to be centenarians or is this a universal compensatory mechanism that occurs later in life (eg >80 years) (14).
Therefore, in activity-matched young (<25 years), old (65 to 70 years), and oldest-old (>80 years) participants, this study sought to comprehensively examine the impact of advancing age on redox balance, peripheral hemodynamics, mitochondrial function, and the ATP cost of dynamic contractions. Specifically, with the tenet that attenuated physical activity accounts for many age-related metabolic adaptations, we hypothesized that, despite elevated oxidative stress, the plantar flexor muscles of the oldest-old participants would exhibit preserved skeletal muscle mitochondrial capacity, but a greater ATP cost of contraction in comparison to their younger counterparts. Additionally, we hypothesized that skeletal muscle hemodynamics would be preserved in the physical activity matched oldest-old compared with their younger counterparts.
Methods
Participants
Following informed consent procedures, 11 young (<25 years, 5 males, 6 females), 11 old (65 to 70 years, 5 males, 6 females), and 10 oldest-old (>80 years, 6 males, 4 females) volunteers participated in this study (Table 1). Participants were matched for physical activity, by both questionnaire and accelerometry. All participants were nonsmokers, free of diabetes, and known cardiovascular, peripheral vascular, neuromuscular, or pulmonary disease. Although, generally, in excellent health for their age, several of the oldest-old were taking medications for high blood pressure (n = 3), hyperuricemia (n = 1), hypercholesterolemia (n = 2), and early signs of Alzheimer’s disease (n = 1). Additionally, one participant from the older group was taking a statin medication. The premenopausal women were studied during days 1–7 of their menstrual cycle to standardize the influence of female hormones. Women taking hormone replacement therapy were excluded from the study. The study was approved by the Human Research Protection Programs of the University of Utah and the Salt Lake City Veterans Affairs Medical Center.
Table 1.
Participant Characteristics
| n (female/male) | Young | Old | Oldest Old |
|---|---|---|---|
| 11 (6/5) | 11 (6/5) | 10 (4/6) | |
| Age (y) | 23 ± 2 | 68 ± 2† | 84 ± 2‡ |
| Height (cm) | 171 ± 7 | 168 ± 9 | 168 ± 10 |
| Weight (kg) | 67 ± 11 | 76 ± 14 | 71 ± 16 |
| BMI (kg/m2) | 23 ± 4 | 27 ± 4 | 25 ± 5 |
| Muscle Volume (dL) | 19 ± 5 | 22 ± 5 | 19 ± 6 |
| Peak work rate (W) | 12 ± 6 | 11 ± 5 | 6 ± 3‡ |
| Step (count/day) | 5,830 ± 1,986 | 6,249 ± 1,592 | 5,100 ± 3,768 |
| Physical activity (count/min) | 135 ± 45 | 144 ± 43 | 105 ± 53 |
| Glucose (mg/dL) | 72 ± 8 | 79 ± 13 | 75 ± 16 |
| Cholesterol (mg/dL) | 185 ± 48 | 195 ± 25 | 195 ± 36 |
| Triglycerides (mg/dL) | 121 ± 90 | 129 ± 78 | 126 ± 67 |
| HDL (mg/dL) | 53 ± 12 | 52 ± 13 | 56 ± 13 |
| LDL (mg/dL) | 116 ± 39 | 126 ± 23 | 118 ± 26 |
| WBC (K/μL) | 5.6 ± 0.9 | 5.5 ± 1.4 | 5.9 ± 0.9 |
| RBC (M/μL) | 5.1 ± 0.5 | 4.8 ± 0.2 | 4.7 ± 0.2 |
| Haemoglobin (g/dL) | 15.4 ± 1.6 | 14.7 ± 0.8 | 14.7 ± 1.0 |
| Hematocrit (%) | 45.0 ± 3.6 | 43.9 ± 2.2 | 43.5 ± 2.6 |
| Neutrophil (K/μL) | 3.0 ± 0.8 | 3.1 ± 1.0 | 3.8 ± 0.8‡ |
| Lymphocyte (K/μL) | 2.0 ± 0.5 | 1.7 ± 0.5 | 1.5 ± 0.4 |
| Monocyte (K/μL) | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 |
Notes: Values expressed as mean ± SD.
BMI = Body mass index; HDL = High-density lipoprotein; LDL = Low-density lipoprotein; RBC = Red blood cells; WBC = White blood cells.
† p < .05 significantly different from OLD.
‡ p < .05 significantly different from OLD and young.
Exercise Protocol
After familiarization, WRmax was determined during an incremental dynamic plantar flexion exercise to exhaustion (frequency of 1 Hz, 1 minute stages). On separate days, participants performed constant-load dynamic submaximal plantar flexion at ~40 per cent of WRmax both in the whole body magnetic resonance (MR) scanner (TimTrio 2.9T Siemens Medical Systems, Erlangen, Germany), to assess metabolic responses, and outside the scanner, to measure lower limb blood flow and microvascular oxygenation using Doppler ultrasound and near-infrared spectroscopy (NIRS). The ergometer used to perform plantar flexion exercise within the confines of a clinical whole body magnetic resonance imaging (MRI) system was constructed from nonferrous materials to be MR compatible and consisted of a foot plate against which participants pushed down within a range of motion imposed by the ergometer (~13 cm) while a cable-pulley system allowed varying weights to provide resistance to the movement. The displacement of the weight was recorded using a displacement transducer (3590s, Bourns, Mexico) placed on the pulley, analog-to-digital converted (MP150, Biopac Syst Inc., USA) with a sample frequency of 100 Hz, and recorded on a personal computer (Acknowledge, Biopac Syst Inc., USA) to calculate power output every 6 seconds during the exercise, ensuring that the work rate was constant. In addition, a member of the research team monitored the movement of the weight to ensure that participants exercised within the full range of motion throughout the exercise. For the vascular tests, outside the magnet, a similar plantar flexion setup, in which displacement and force applied to the footplate were directly measured and controlled (MLP, Transducer Techniques, USA), was employed. After 1 minute of data collection at rest, participants exercised for 5 minutes followed by 5 minutes of recovery. Prior to initiation of the protocol outside the MR scanner, blood samples were collected for a complete blood cell count and lipid panel. The sequence of testing inside and outside of the MR scanner was balanced to minimize any potential ordering effects and each protocol was separated by at least 72 hours to minimize the potential confounding effects of exercise-induced muscle injury. All experimental trials were performed in a thermoneutral environment with participants in an overnight fasted state.
31P-magnetic resonance spectroscopy
MRS was performed using a clinical 2.9T MR scanner (Tim-Trio, Siemens Medical Solutions, Erlangen, Germany) operating at 49.9 MHz for 31P resonance. 31P MRS data were acquired with a 31P-1H dual surface coil with linear polarization (Rapid biomedical GmbH, Rimpar, Germany) positioned around the calf at its maximum diameter. The 31P single-loop coil diameter was 125 mm surrounding a 110 mm 1H coil loop. After a three plane scout proton image, advanced localized volume shimming was performed. Before each experiment, two fully relaxed spectra were acquired at rest with 3 averages per spectrum and a repetition time of 30 seconds. Then, MRS data acquisition was performed throughout the rest-exercise-recovery protocol using a FID (free-induction-decay) pulse sequence with a 2.56 millisecond adiabatic-half-passage excitation RF pulse and the following parameters (repetition time = 2 seconds, receiver bandwidth = 5 kHz, 1024 data points, and 3 averages per spectrum). Saturation factors were quantified by the comparison between fully relaxed (TR = 30 seconds) and partially relaxed spectra (TR = 2 seconds).
Relative concentrations of phosphocreatine [PCr], inorganic phosphate [Pi], and [ATP] were obtained by a time-domain fitting routine using the AMARES algorithm incorporated into the CSIAPO software (15). Intracellular pH was calculated from the chemical shift difference between the Pi and PCr signals. The free cytosolic [ADP] was calculated from [PCr] and pH using the creatine kinase equilibrium constant (KCK = 1.66 × 109 M−1) and the assumption that phosphocreatine represents 85 per cent of the total creatine content. The resting concentrations were calculated from the average peak areas of the two relaxed spectra recorded at rest and assuming an 8.2 mM [ATP] under these conditions. Changes in pH and in the concentrations of phosphorus metabolites during contraction were used to calculate the ATP cost of contraction. At the onset of exercise, ATP generated from oxidative pathways is negligible such that anaerobic ATP synthesis accounts for, essentially, all ATP synthesis (16). The corresponding amount of ATP scaled to power output provides a reliable estimation of the initial rate of total ATP synthesis. Muscle mitochondrial capacity for ATP synthesis (Vmax in mM min−1) was calculated using the initial rate of PCr synthesis (ViPCr) during the recovery period and [ADP] obtained at the end of exercise,
,
in which Km (the [ADP] at half-maximal oxidation rate) is 30 µM in skeletal muscle. Of note, unlike the PCr recovery time constant, Vmax has been documented to be independent of pH or PCr changes (17,18) and therefore provides a reliable assessment of muscle mitochondrial function in conditions with different end-exercise metabolic states (19,20). The initial rate of ATP demand (mM min−1) at the offset of the exercise was determined from the initial PCr resynthesis rate during the first 6 seconds of the recovery, as ATP synthesis is almost entirely oxidative at this point (21).
Popliteal Artery Blood Flow
Measurements of popliteal artery blood velocity and vessel diameter were performed in the popliteal fossa of the exercising leg, proximal to the branching of the medial inferior genicular artery, with a Logic 7 Doppler ultrasound system (General Electric Medical Systems, Milwaukee, WI). The ultrasound system was equipped with a linear transducer operating at an imaging frequency of 10 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was measured using the same transducer with a frequency of 5 MHz. All blood velocity measurements were obtained with the probes appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel. Arterial diameter was measured off-line every 12 seconds using automated edge-detection software (Medical Imaging Applications, Coralville, IA), and mean velocity (Vmean) (angle corrected, and intensity-weighted area under the curve) was automatically calculated beat by beat (Logic 7). Using arterial diameter and Vmean, blood flow in the popliteal artery was calculated as blood flow = Vmean ∙ π (vessel diameter/2)2 ∙ 60, where blood flow is in milliliters per minute.
Microvascular Oxygenation
Microvascular oxygenation was assessed using the NIRS technique, which provides continuous, noninvasive measurements of oxygenated (HbO2), deoxygenated (HHb), and total (Hbtot) hemoglobin levels as well as microvascular oxygenation (TOI, ie, HbO2/Hbtot). Due to identical spectral characteristics, hemoglobin and myoglobin are not separated using NIRS; however, considering the ratio of Mb to Hb concentrations in human muscle, the signal is usually accepted to be predominantly derived from Hb (22). In the present study, changes in muscle microvascular oxygenation of the right gastrocnemius muscle were continuously monitored at 2 Hz using a near-infrared frequency–resolved spectroscopy oximeter (Oxiplex TS, ISS Inc., Illinois, USA). The probe was positioned at the level of the largest circumference of the medial gastrocnemius and secured with Velcro straps and biadhesive tape. NIRS uses intensity-modulated light with the probe consisting, in this case, of eight infrared light sources (four emitting at 690 nm and four emitting at 830 nm, emitter–detector distance ranging from to 2.0 to 4.0 cm) and one detection channel including a selected light detector (photomultiplier tube), providing a measurement of absorption and scattering coefficient of the tissue.
Oxidative Stress, Antioxidant Assays, and Free Radicals
Blood samples were spun down and plasma stored at −80°C until analysis. Lipid and protein peroxidation, as markers of oxidant damage, were assessed by malondialdehyde (MDA) (Bioxytech LPO-586, Foster City, CA) and protein carbonyl (Biocell Corporation, Papatoetoe, New Zealand) levels, respectively. Total antioxidant capacity was evaluated by determining the ferric reducing ability of plasma (FRAP), using the method described by Benzie and Strain (23). Plasma ascorbate concentration (CosmoBio, Carlsbad, CA), free radical scavenging potential by superoxide dismutase (SOD) and catalase activity, was also assayed in the plasma (Cayman Chemical Company, Ann Arbor, MI). C-reactive protein concentration, a marker of inflammation, was determined by immunoassay in the plasma (R&D systems, Minneapolis, MN).
Electron paramagnetic resonance (EPR) spectroscopy was performed on whole blood samples to directly assess concentration of free radicals, as described previously (24). Briefly, 1.5 mL of venous blood was collected into a vacutainer containing 0.5 mL of the spin trap α-phenyl-tert-butylnitrone (PBN) (0.0140 mol/L). The PBN adduct was snap frozen in liquid nitrogen and stored at −80°C. After thawing, the PBN adduct (1000 μL) was pipetted into a glass tube and extracted with 500 µL toluene. After centrifugation, 300 µL of the toluene/PBN extract was pipetted into a precision-bore quartz EPR sample tube (Wilmad, Vineland, NJ). EPR spectroscopy was then performed at 21°C using an EMX X-band spectrometer (Bruker, MA) and commercially available software (version 1.1b.51, Bruker Xenon System), which was also used to calculate the area under the curve of the EPR spectroscopy signal by double integration.
Lower leg volume
Lower leg volume was calculated based on lower leg circumference (three sites: distal, middle, and proximal), lower leg length, and skinfold measurements. This method has recently been confirmed to provide a valid estimate for muscle volume across a spectrum of individuals with normal muscle mass and severe muscle atrophy (25).
Physical activity level
Physical activity level was assessed using both a subjective physical activity level recall questionnaire and objective accelerometer data. The physical activity level questionnaire included items regarding the average type, frequency, intensity, and duration of physical activity in any given week. After receiving standardized operating instructions, participants wore an accelerometer (GT1M; Actigraph, Pensacola, FL, USA) for at least 7 days, with adherence automatically assessed. Average daily physical activity was expressed as both steps per day and total accelerometer counts per minute.
Data Analysis
The PCr recovery kinetics were determined by fitting the PCr time-dependent changes during the recovery period to a single exponential curve described by the following equation:
,
where [PCr]end is the concentration of [PCr] measured at end-of-exercise and [PCr]res refers to the amount of PCr resynthesized during the recovery period. The initial rate of PCr resynthesis (ViPCr) was calculated as follows:
,
in which [PCr]res indicates the amount of PCr resynthesized during the recovery period and the rate constant, k = 1/τ.
Model variables were determined with an iterative process by minimizing the sum of squared residuals (RSS) between the fitted function and the observed values. Goodness of fit was assessed by visual inspection of the residual plot and the frequency plot distribution of the residuals, chi-square values, and the coefficient of determination (r2) calculated as follows:
,
with SSreg representing the sum of squares of the residuals from the fit and SStot representing the sum of squares of the residuals from the mean.
Statistical Analysis
The assessment of differences among young, old, and the oldest-old was performed with a one-way ANOVA (Statsoft, version 5.5; Statistica, Tulsa, Oklahoma). The kinetics of PCr, Pi, ADP, and pH during exercise were analyzed using a two-way ANOVA with repeated measures. A Duncan test was used for post hoc comparisons. For the popliteal blood flow, cumulative area under the curve (AUC) was calculated during the first 180 seconds of recovery and used to identify how differences over time were affected by the age-group. Significance was accepted at p < .05. Results are presented as mean ± SD in tables and mean ± SEM in the figures, for clarity.
Results
Plantar Flexion Exercise Testing and Muscle Volume
Plantar flexion WRmax was lower in the oldest-old compared with the old and young groups (Table 1, p < .05) such that the corresponding absolute power output during constant-load exercise (40% of WRmax) was also lower in the oldest-old (young: 5.5 ± 3.2 W; old: 4.8 ± 1.9 W; oldest-old: 2.3 ± 1.1; p < .05 from old and young). Muscle volume was not significantly different between groups (Table 1). Similarly, subcutaneous adipose tissue thickness in the oldest-old (8.1 ± 3.2 mm) was not significantly different from the old (8.4 ± 2.8 mm) and young (7.5 ± 2.0 mm, p > 0.05).
Intracellular Phosphate Compound, pH, and the ATP Cost of Contraction
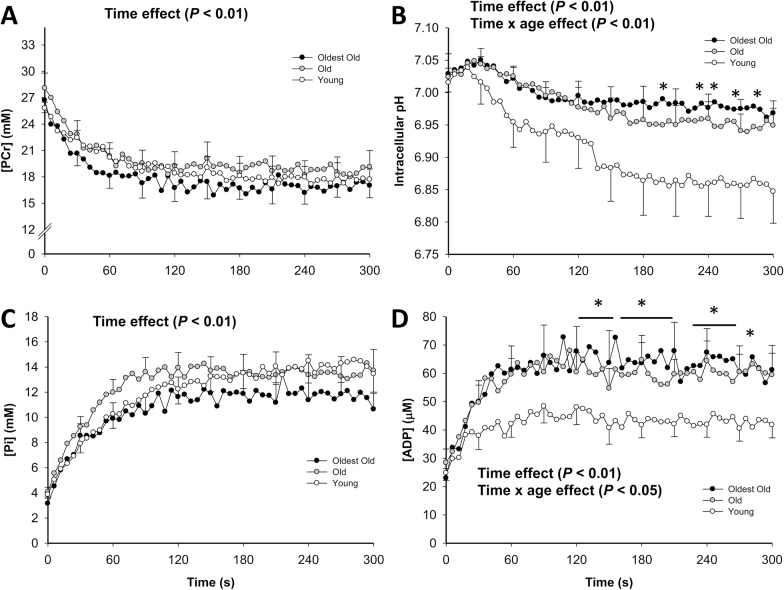
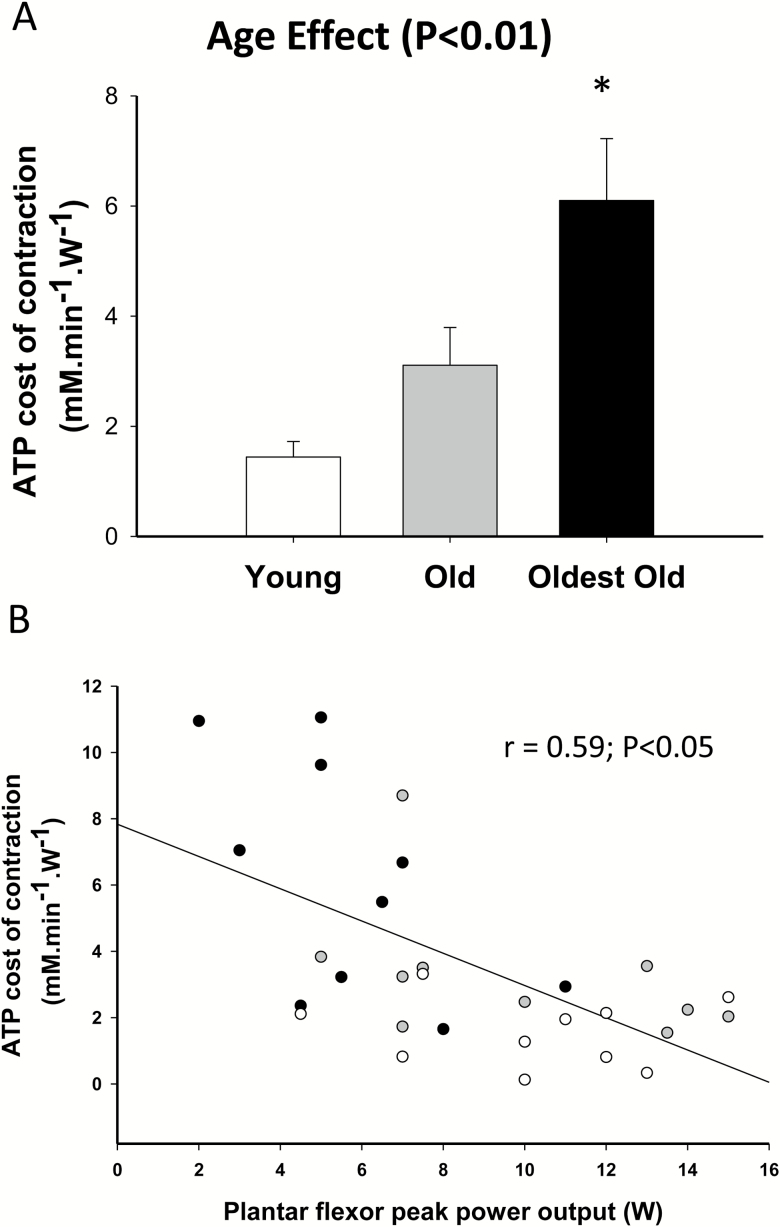
Figure 1 illustrates the changes in muscle metabolites and pH during the constant-load plantar flexion exercise protocol at 40 per cent of WRmax, assessed by 31MRS, in the young, old, and oldest-old. Although PCr consumption and Pi accumulation were not different between groups (p > 0.05), intracellular pH and [ADP] were higher in the oldest-old compared with the young (p < .05), but not the old. As illustrated in Figure 2A, there was a significant age effect on the ATP cost of contraction (Main effect, p < .05), with the oldest-old exhibiting a greater cost of contraction than both the old and young (p < .05). Interestingly, the ATP cost of contraction was negatively correlated with plantar flexion WRmax (r = .59, p < .05) (Figure 2B). In a secondary analysis, we tested the effect of gender on the ATP cost of contraction, which was not significant (p = .82). However, due to the limited sample size for this type of comparison, we cannot completely rule out a potential role for gender in influencing the ATP cost of contraction with advancing age.
Figure 1.
Changes in phosphocreatine (PCr) (A), intracellular pH (B), inorganic phosphate (Pi) (C), and adenosine diphosphate (ADP) (B) with respect to time at the onset of plantar flexion exercise in young (empty circles, female = 6; male = 5; n = 11), old (gray circles, female = 6; male = 5; n = 11), and oldest-old (black circles, female = 4; male = 6; n = 10). Values are presented as means ± SEM. *Significantly different from young at the same time point (p < .05).
Figure 2.
(A) ATP cost of contraction during submaximal plantar flexion exercise in the young, old, and oldest-old participants, (B) the relationship between the maximal work rate reached during an incremental plantar flexion exercise and the ATP cost of contraction during submaximal plantar flexion exercise [young (empty circles, female = 6; male = 5; n = 11), old (gray circles, female = 6; male = 5; n = 11), and oldest old (black circles, female = 4; male = 6; n = 10)]. Values are presented as mean ± SEM. *p < .05, significantly different from the old and young.
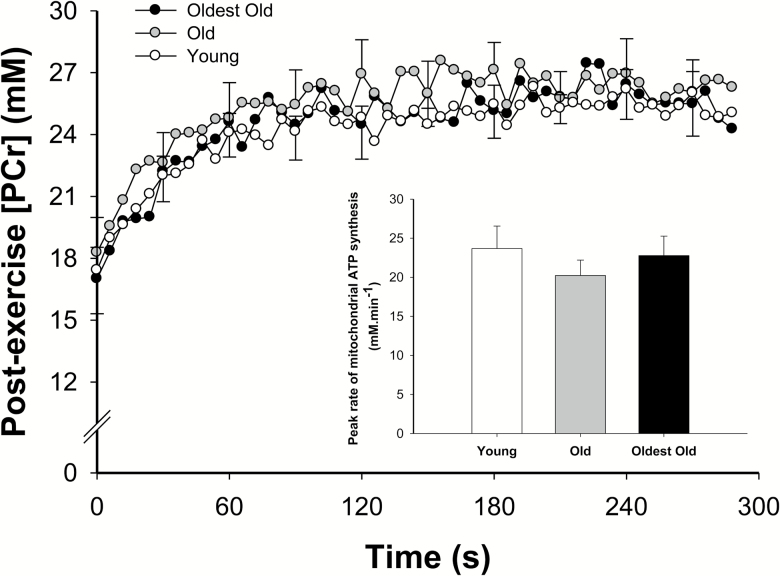
PCr Kinetics at the Offset of Exercise and Muscle Mitochondrial Capacity
The PCr dynamics during the post-exercise recovery period are displayed in Figure 3. Specifically, the PCr recovery time constant was 35 ± 19 seconds in the young, 33 ± 13 seconds in the old, and 35 ± 11 seconds in the oldest-old (p > 0.05). The peak rate of mitochondrial ATP synthesis (Vmax) was also not significantly different between the groups (p > 0.05, Figure 3, inlay). The 31P-MRS data collected in one old participant were not included in the analyses due to poor signal-noise ratio during the recovery.
Figure 3.
PCr changes with respect to time at the offset of plantar flexion exercise in the young (empty circles, female = 6; male = 5; n = 11), old (gray circles, female = 6; male = 5; n = 11), and oldest-old (black circles, female = 4; male = 6; n = 10). Values are presented as mean ± SEM. The figure inset provides the mean of the estimated peak rate of mitochondrial ATP synthesis. There was no significant difference between groups (p > 0.05).
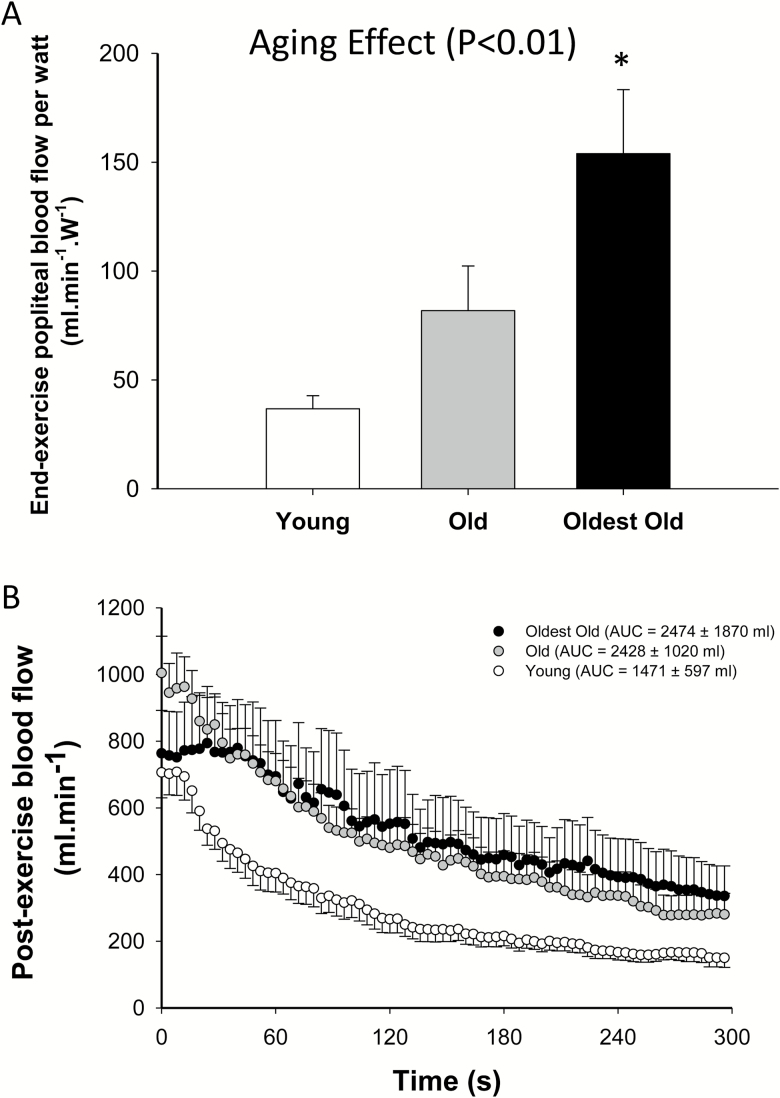
Skeletal Muscle Hemodynamics and Microvascular Oxygenation
As illustrated in Figure 4A, initial post-exercise blood flow per Watt of plantar flexion work, which reflect end-exercise hemodynamics, revealed an age effect on blood flow (p < .05) with the oldest-old exhibiting a significantly higher blood flow/Watt than both the old and young (p < .05). Following the cessation of the constant-load plantar flexion exercise, although blood flow AUC appeared to be higher in the older groups, this did not reach significance (p = .11, Figure 4B). At the end of the exercise and throughout the recovery period, oxy-, deoxy-hemoglobin, and microvascular oxygenation indices were not different between groups (p > 0.05). The blood flow data collected in one old and one young participant were not included in the analyses due to poor velocity spectra during the recovery.
Figure 4.
(A) Mean of the initial blood flow per watt; (B) blood flow changes with respect to time at the offset of plantar flexion exercise in the young (empty circles, female = 6; male = 5; n = 11), old (gray circles, female = 6; male = 5; n = 11), and oldest-old (black circles, female = 4; male = 6; n = 10). *p < .05, significantly different from both the young and old. Values are presented as mean ± SEM.
Free Radicals, Oxidative Stress, Antioxidants, and Inflammation
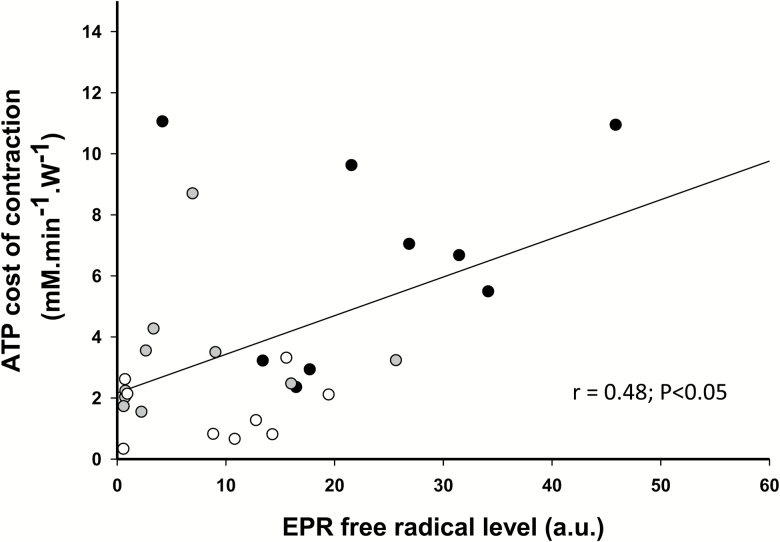
Free-radical concentration, measured by EPR, was significantly elevated in the oldest-old (p < 0.05) compared with the young (Table 2) and was significantly correlated with the ATP cost of contraction of the plantar flexor muscles (Figure 5). MDA levels, a marker of lipid peroxidation, was significantly elevated in both the oldest-old and the old in comparison to the young (p < .05) (Table 2). Protein carbonyls, a marker of protein peroxidation, were significantly higher only in the old compared with the young (p < .05) (Table 2). In terms of antioxidants, with the exception of catalase, which was significantly lower in the oldest-old compared with the young (p < .05), the enzymatic and nonenzymatic antioxidants were not significantly different between groups (p > 0.05) (Table 2). Inflammation, as assessed by C-reactive protein, was significantly greater (2.7 fold) in the oldest-old compared with the young (p < .05) (Table 2).
Table 2.
Markers of Inflammation and Oxidative Stress
| Young | Old | Oldest-old | |
|---|---|---|---|
| Pro-oxidant markers | |||
| malondialdehyde (µm mL−1) | 0.6 ± 0.3 | 1.0 ± 0.4† | 0.9 ± 0.3† |
| Protein Carbonyls (ηm mg−1) | 0.155 ± 0.028 | 0.178 ± 0.020† | 0.167 ± 0.018 |
| EPR free radical level (a.u.) | 8.5 ± 7.2 | 5.8 ± 8.0 | 27.4 ± 17.0† |
| Antioxidant markers | |||
| FRAP (mM mL−1) | 2.2 ± 0.5 | 2.1 ± 0.5 | 2.3 ± 0.5 |
| Plasma ascorbate (µg mL−1) | 17.2 ± 5.0 | 22.5 ± 27.4 | 27.6 ± 16.7 |
| Catalase (ηm/mL−1) | 48.7 ± 25.5 | 35.6 ± 11.6 | 28.7 ± 11.4† |
| Superoxide dismutase (IU mL−1) | 15.8 ± 11.3 | 11.9 ± 8.2 | 11.4 ± 4.7 |
| Marker of inflammation | |||
| C-reactive protein (ηg mL−1) | 908 ± 1,402 | 1,511 ± 730 | 2,459 ± 1,637† |
Notes: Values expressed as mean ± SD.
EPR = Electron paramagnetic spectroscopy; FRAP = Ferrous reducing ability of plasma.
† p < .05 significantly different from young.
Figure 5.
The relationship between the level of free radicals measured by electron paramagnetic resonance spectroscopy (EPR) and the ATP cost of contraction during submaximal plantar flexion exercise [young (empty circles, female = 5; male = 3; n = 9), old (gray circles, female = 5; male = 5; n = 10), and oldest-old (black circles, female = 4; male = 5; n = 9)].
Discussion
The deterioration of muscle function and mobility with advancing age has been linked to recurrent falls and morbidity. Therefore, utilizing an integrative approach, this study sought to elucidate the mechanisms contributing to this decline by examining skeletal muscle metabolic function and hemodynamics during exercise in young, old, and oldest-old activity–matched participants. Consistent with our hypotheses, muscle mitochondrial capacity for ATP synthesis, assessed in vivo, was similar across all age groups. In contrast, the ATP cost of contraction signficantly increased with age, being the highest in the oldest-old compared with both the old and the young participants. Interestingly, likely as a result of the age-related decrease in muscle efficiency, end-exercise blood flow normalized for work rate was also highest in the oldest-old compared with both the old and the young participants. In addition, muscle microvascular oxygenation was similar between groups, indicative of an appropriate hemodynamic adjustment. As anticipated, systemic free radicals, markers of oxidative stress, and inflammation were augmented with advancing age, whereas endogenous antioxidants generally exhibited signs of attenuation in the oldest-old. Independent of the decline in physical activity, these findings suggest that deteriorating muscle contractile efficiency with advancing age may play an important role in the decline in skeletal muscle functional capacity and altered hemodynamics in the elderly.
Mitochondrial Capacity for ATP Synthesis and Blood Flow With Advancing Age
Cognizant of the confounding role that physical activity (26) and mitochondrial isolation procedures (27) may have played in the initial studies investigating the impact of age on mitochondrial function (9,28,29), there is growing evidence that mitochondrial density, electron transport chain activity, and muscle mitochondrial capacity for ATP synthesis are not affected by chronological age in 60- to 70-year-old individuals (30–32). The current results, assessed in vivo, are consistent with the concept of a preserved muscle mitochondrial capacity for ATP synthesis with age when physical activity is taken into account and, unique to this investigation, extend these findings to the oldest-old. Indeed, both the PCr recovery time constant and the inferred peak rate of mitochondrial ATP synthesis (Figure 3) were not significantly different between activity-matched participant groups with an average age of ~25 to ~84 years. In agreement with these findings, but performed in vitro, a recent study reported preserved muscle respiratory capacity in permeabilized muscle fibers from the vastus lateralis of the oldest-old (~82 years) in comparison to old (~70 years) and young (~24 years) participants (7). Of note, these results, conflict with a previous in vivo study documenting a slower PCr recovery time constant in the plantar flexor muscles of the oldest-old (~70 seconds) compared with the old (~50 seconds) and young (~40 seconds) (9). However, methodological limitations and not controlling for physical activity likely had some bearing on the interpretation of the results from this prior study and may have contributed to the different conclusions. Interestingly, it has previously been suggested that, with advancing age, maintaining muscle respiratory capacity requires a higher mitochondrial content (32). Although mitochondrial content was not directly assessed in the present investigation, it is noteworthy that two longitudinal studies conducted over a period of 7 and 11 years in older men, from ~69 to ~80 years, did not report any changes in mitochondrial content (4,33). Together, these findings lend support to the concept that both mitochondrial content and intrinsic mitochondrial capacity for ATP synthesis in vivo are preserved up to the ninth decade of life.
It is well known that mitochondria play a central role in cellular bioenergetics by generating ATP. However, mitochondria are also involved in numerous other biological processes, such as calcium signaling (34), cell cycle regulation, and apoptosis (35), as well as redox homeostasis (36). Therefore, although the present findings indicate that the energetic function of the mitochondria may well be preserved in the oldest-old, this does not rule out the possibility that, for instance, mitochondrial reactive oxygen species generation might be elevated, as previously observed in somewhat younger individuals (~67 years) (37). In this regard, the oldest-old exhibited an elevated systemic level of free radicals measured by EPR (Table 2), which likely predominantly originated from the skeletal muscle (38). Interestingly, in addition to a greater level of free radicals, a reduced activity of some of the enzymes responsible for free radical scavenging (catalase, which converts hydrogen peroxide (H2O2) into water and O2) combined with elevated markers of oxidative damage (MDA, a lipid peroxidation marker) was evident in the oldest-old, thus implying chronic oxidative stress (a chronic imbalance between free radical generation and intracellular antioxidant defenses) (Table 2). Specifically, the finding of a lower catalase activity in the face of a greater level of free radicals in the oldest-old suggests higher intracellular concentration of H2O2 as SOD (which catalyzes the dismutation of ion superoxide into H2O2) was not significantly different from that found in their younger counterparts. This finding adds to the growing body of evidence suggesting a central role for H2O2 in disrupting redox-regulated signaling mechanisms by shifting the redox balance toward a more oxidized state, eventually leading to irreversible protein damage and the development of muscle dysfunction with advancing age (39–41). It should, however, be recognized that, besides catalase, other enzymes (eg glutathione peroxidase, peroxiredoxine) can also scavenge H2O2 and were not investigated here, which, without being too speculative, limits the interpretation of these findings. The present results are also in accordance with previous studies demonstrating significant age-associated increases in oxidative damage to human DNA, proteins, and lipids (42,43) and suggest a further exacerbation at an age of older than 80 years. It is important to recognize that 31P-MRS measurements of mitochondrial function, performed in vivo, integrate the contribution from muscle mitochondrial density, the intrinsic function of the respiratory chain complexes, and can, in some scenarios, be influenced by muscle O2 availability (44). In this regard, the present finding of actually augmented absolute limb blood flow and increased blood flow normalized for work rate as well as unchanged muscle microvascular oxygenation in the calf of this oldest-old cohort implies that O2 delivery/availability likely did not influence the current interpretation of mitochondrial function using 31P-MRS.
Decreased nitric oxide (NO) bioavailability, secondary to elevated free radicals, has previously been implicated in the attenuation of blood flow with age (45). Therefore, although not the primary focus of this study, it is interesting to note that the finding of adequate blood flow in the face of higher levels of reactive oxygen species (ROS, Table 2) in the oldest-old, suggests compensatory vasodilatory mechanisms potentially involving recognized dilators such as prostaglandins (46), endothelium-derived hyperpolarization factor, and/or hydrogen peroxide facilitating the maintenance of O2 delivery to O2 demand matching in the skeletal muscle of the oldest-old. In support of this inference, it has recently been suggested that the capacity for resistance artery endothelial cells to release Ca2+ and hyperpolarize adjacent smooth muscle cells is augmented with advancing age to compensate for lower NO bioavailability (47). In addition, there is emerging evidence suggesting that ROS may also play a compensatory role in vasodilation of the aging microvasculature (48). For instance, scavenging of H2O2 alone, or in combination with ion superoxide, has been documented to attenuate flow-induced dilatation in skeletal muscle feed arteries of older rats (49) and an antioxidant cocktail (vitamins C, E, and α-lipoic acid) attenuated both free radical levels and brachial artery vasdodilation in young healthy participants (24). These findings implicate an important role for ROS in the regulation of vessel function. Additional studies are, therefore, warranted to determine whether ROS signaling and endothelium-derived hyperpolarization can fully compensate for the loss of NO-mediated vasodilation in the oldest-old.
ATP Cost of Contraction With Advancing Age
A novel finding of this study was the observation that the ATP cost of dynamic contractions signficantly increased with advancing age, being the highest in the oldest-old compared with both the old and the young participants (Figure 2A). These results, therefore, extend our previous findings of an age-related increase in the ATP cost of contraction during dynamic (12) or intermittent isometric contractions (11) of locomotor muscles to the oldest-old. In addition, there was a correlation between the ATP cost of contraction and peak power attained during the incremental plantar flexion exercise (Figure 2B), which confirms our previous exploratory analysis revealing a similar correlation between the ATP cost of contraction of the plantar flexor muscles and exercise capacity during incremental cycling exercise (12). Together, these findings underline that the age-related decline in muscle efficiency appears to be further accelerated in the oldest-old, and this impairment likely plays a key role in the decline in functional capacity with advancing age.
Initially, these findings might appear somewhat at odds with a recent study documenting a decreased pulmonary O2 cost or evidence of improved mechanical efficiency, during cycling exercise in centenarians (13). In addition, a study using pump-perfused rat hind limb preparation found that although muscle efficiency was impaired in late middle-aged rats (28–29 months old), senescent rats (36 months old) actually exhibited an improved muscle efficiency (14). However, unlike the present study, it is interesting to note that, in these prior studies, both the centenarians and the senescent rats exhibited significant muscle atrophy and impaired muscle respiratory capacity. Thus, taken together, these findings suggest that in the somewhat “early” stages of aging muscle efficiency is impaired, elicited, perhaps, by an increased ATP cost of contraction and/or mitochondrial uncoupling (50). However, in cases of “exceptional aging,” muscle efficiency seems to be improved when muscle is atrophied and aerobic capacity is greatly compromised. The mechanisms responsible for such adaptations are still unclear, but some evidence suggests that, in sarcopenic participants, the “surviving” population of fibers are hypertrophied and demonstrate better contractile performance (5,51,52), perhaps to compensate for the loss of motor units and muscle cells.
Potential Mechanisms Responsible for the Age-Related Increase in ATP Cost of Contraction
As previously discussed (11,12), multiple mechanisms may underlie the observed age-related increase in the ATP cost of muscle contraction including an increase energy demand from the noncontractile processes of ion transport (Ca2+ ATPase and Na+-K+ ATPase) (11), a slowing of the contractile properties (53), altered structural elastic properties of skeletal muscle (54,55) or neural adaptations (56). In terms of the relative ATP use by noncontractile processes, it is important to recognize that this can represent 30% to 50% of the total energy consumed by contracting muscle in humans (57). Therefore, the age-related impairment in Ca2+ sequestration by the sarcoplasmic reticulum (58,59) could be partly responsible for the exaggerated metabolic demand with age during an exercise requiring repeated contraction-relaxation cycle. The sarcoplasmic reticulum Ca2+ ATPase (SERCA) is primarily responsible for muscle relaxation by transporting cytosolic Ca2+ into the lumen of the sarcoplasmic reticulum coupled to ATP hydrolysis. Chronic oxidative stress, which is commonly associated with aging, could mediate impaired Ca2+ sequestration. Indeed, SERCA proteins are sensitive to muscle redox state as these proteins are typically characterized by a relatively long half-life, with turnover being even more prolonged with age (60). Accordingly, nitrotyrosine, a marker of oxidative stress, has previously been reported to accumulate on SERCA in an age-dependent manner (61). In the present study, both the old and oldest-old groups exhibited apparent signs of oxidative stress, being more pronounced in the oldest-old (Table 2). In addition, we observed a significant correlation between the levels of free radicals measured by EPR and the ATP cost of contraction of the plantar flexor muscles (Figure 5). C-reactive proteins were also progressively augmented with increasing age, documenting a concomitant inflammatory state (Table 2). Together, these findings lend support to the concept that the accumulation of oxidative damage on cell membranes and SERCA may compromise Ca2+ pumping and, in turn, progressively increase ATP demand with advancing age.
With regard to the age-related loss of motor neurons, this appears to be compensated for, at least to some extent, by the dynamic process of fiber re-innervation through collateral sprouting, thus preserving muscle activation in the typically old (<80 years) (53,62,63). However, at a more advanced age, in the oldest-old, the remodeling of motor units may no longer be able to compensate for motor unit loss (64). The subsequent reduction of large motor units would, in turn, affect neural activation of the agonist muscles (65,66), motor output variability, and impair movement coordination, thus further exacerbating the increased metabolic demand in the oldest-old. In support of this explanation, the oldest-old in this study demonstrated a twofold higher ATP cost of dynamic contraction compared with the, already elevated, cost in the old (Figure 2 and Refs. 11 and 12). Given that the neuromuscular adaptation to aging was not investigated in this study, further research is warranted to clarify the link between the change in the ATP cost of contraction and the loss of motor units with advancing age.
Experimental Considerations
It is noteworthy that muscle volume was quantified using the anthropometric method. Although we have recently confirmed that this method provides a valid estimate for muscle volume across a large spectrum of individuals with normal muscle mass and severe muscle atrophy (25), it should be acknowledged that this method does not provide the same sensitivity or accuracy than quantitative MR imaging methods (e.g., three point Dixon (67)) to detect small, but functionally relevant (68) changes in muscle volume and fat infiltration in the muscle.
Conclusion
As both the PCr recovery time constant and the peak rate of mitochondrial ATP synthesis were not significantly different with advancing age in physical activity–matched participants, mitochondrial capacity appears to be resistant to aging per se. In contrast, the ATP cost of dynamic contractions was signficantly increased with age and significantly correlated with WRmax. Post-exercise blood flow per Watt rose with increasing age, likely as consequence of this increased energy demand and, thus, muscle oxygenation was not different between groups. These findings suggest that the progressive deterioration of muscle contractile efficiency, potentially mediated by elevated oxidative stress with advancing age, may play an important role in the decline in skeletal muscle functional capacity and altered hemodynamics in the elderly.
Funding
This study was funded, in part, by NIH Heart, Lung, and Blood Institute (K99HL125756, HL-091830), the Flight Attendant Medical Research Institute (FAMRI), the Veterans Affairs Rehabilitation Research and Development Service (Merit Awards: E6910-R, E1697-R), Senior Research Career Scientist (Grant: E9275-L), Career Development award (IK2RX001215), and the American Heart Association (1850039).
Acknowledgements
The authors would like to express their gratitude to participants who gave their time and effort so generously to partake in this study.
Conflict of interest
None declared.
References
- 1. Grimby G, Danneskiold-Samsøe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78-81 year old men and women. Acta Physiol Scand. 1982;115:125–134. doi:10.1111/j.1748-1716.1982.tb07054.x [DOI] [PubMed] [Google Scholar]
- 2. Weiss EP, Spina RJ, Holloszy JO, Ehsani AA. Gender differences in the decline in aerobic capacity and its physiological determinants during the later decades of life. J Appl Physiol (1985). 2006;101:938–944. doi:10.1152/japplphysiol.01398.2005 [DOI] [PubMed] [Google Scholar]
- 3. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 4. Aniansson A, Grimby G, Hedberg M. Compensatory muscle fiber hypertrophy in elderly men. J Appl Physiol (1985). 1992;73:812–816. doi:10.1152/jappl.1992.73.3.812 [DOI] [PubMed] [Google Scholar]
- 5. Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R273–R280. doi:10.1152/ajpregu.00093.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson DL, Raue U, Slivka DR, Trappe S. Resistance exercise, skeletal muscle FOXO3A, and 85-year-old women. J Gerontol A Biol Sci Med Sci. 2010;65:335–343. doi:10.1093/gerona/glq005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spendiff S, Vuda M, Gouspillou G et al. . Denervation drives mitochondrial dysfunction in skeletal muscle of octogenarians. J Physiol. 2016;594: 7361–7379. doi:10.1113/JP272487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanza IR, Bhagra S, Nair KS, Port JD. Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging. 2011;34:1143–1150. doi:10.1002/jmri.22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCully KK, Forciea MA, Hack LM et al. . Muscle metabolism in older subjects using 31P magnetic resonance spectroscopy. Can J Physiol Pharmacol. 1991;69:576–580. [DOI] [PubMed] [Google Scholar]
- 10. Wray DW, Nishiyama SK, Monnet A et al. . Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009;297:H1870–H1875. doi:10.1152/ajpheart.00709.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Layec G, Hart CR, Trinity JD, Le Fur Y, Jeong EK, Richardson RS. Skeletal muscle work efficiency with age: the role of non-contractile processes. Clin Sci (Lond). 2015;128:213–223. doi:10.1042/CS20140274 [DOI] [PubMed] [Google Scholar]
- 12. Layec G, Trinity JD, Hart CR et al. . In vivo evidence of an age-related increase in ATP cost of contraction in the plantar flexor muscles. Clin Sci (Lond). 2014;126:581–592. doi:10.1042/CS20130442 [DOI] [PubMed] [Google Scholar]
- 13. Venturelli M, Schena F, Scarsini R, Muti E, Richardson RS. Limitations to exercise in female centenarians: evidence that muscular efficiency tempers the impact of failing lungs. Age (Dordr). 2013;35:861–870. doi:10.1007/s11357-011-9379-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hepple RT, Hagen JL, Krause DJ, Baker DJ. Skeletal muscle aging in F344BN F1-hybrid rats: II. Improved contractile economy in senescence helps compensate for reduced ATP-generating capacity. J Gerontol A Biol Sci Med Sci. 2004;59:1111–1119. [DOI] [PubMed] [Google Scholar]
- 15. Le Fur Y, Nicoli F, Guye M, Confort-Gouny S, Cozzone PJ, Kober F. Grid-free interactive and automated data processing for MR chemical shift imaging data. MAGMA. 2010;23:23–30. doi:10.1007/s10334-009-0186-y [DOI] [PubMed] [Google Scholar]
- 16. Kemp GJ, Thompson CH, Barnes PR, Radda GK. Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med. 1994;31:248–258. [DOI] [PubMed] [Google Scholar]
- 17. Roussel M, Bendahan D, Mattei JP, Le Fur Y, Cozzone PJ. 31P magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: the issue of intersubject variability. Biochim Biophys Acta. 2000;1457:18–26. [DOI] [PubMed] [Google Scholar]
- 18. Lodi R, Kemp GJ, Iotti S, Radda GK, Barbiroli B. Influence of cytosolic pH on in vivo assessment of human muscle mitochondrial respiration by phosphorus magnetic resonance spectroscopy. MAGMA. 1997;5:165–171. [DOI] [PubMed] [Google Scholar]
- 19. Layec G, Bringard A, Le Fur Y et al. . Comparative determination of energy production rates and mitochondrial function using different 31P MRS quantitative methods in sedentary and trained subjects. NMR Biomed. 2011;24:425–438. doi:10.1002/nbm.1607 [DOI] [PubMed] [Google Scholar]
- 20. Layec G, Bringard A, Le Fur Y et al. . Reproducibility assessment of metabolic variables characterizing muscle energetics in vivo: a 31P-MRS study. Magn Reson Med. 2009;62:840–854. doi:10.1002/mrm.22085 [DOI] [PubMed] [Google Scholar]
- 21. Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J. 1993;291:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koga S, Kano Y, Barstow TJ et al. . Kinetics of muscle deoxygenation and microvascular PO(2) during contractions in rat: comparison of optical spectroscopy and phosphorescence-quenching techniques. J Appl Physiol (1985). 2012;112:26–32. doi:10.1152/japplphysiol.00925.2011 [DOI] [PubMed] [Google Scholar]
- 23. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239: 70–76. doi:10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 24. Richardson RS, Donato AJ, Uberoi A et al. . Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol. 2007;292:H1516–H1522. doi:10.1152/ajpheart.01045.2006 [DOI] [PubMed] [Google Scholar]
- 25. Layec G, Venturelli M, Jeong EK, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum: from able-bodied adults to individuals with a spinal cord injury. J Appl Physiol (1985). 2014;116:1142–1147.doi:10.1152/japplphysiol.01120.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age?Sports Med. 2004;34:221–229. [DOI] [PubMed] [Google Scholar]
- 27. Picard M, Ritchie D, Wright KJ et al. . Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9:1032–1046. doi:10.1111/j.1474-9726.2010.00628.x [DOI] [PubMed] [Google Scholar]
- 28. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol. 2003;38:877–886. [DOI] [PubMed] [Google Scholar]
- 30. Fitzgerald LF, Christie AD, Kent JA. Heterogeneous effects of old age on human muscle oxidative capacity in vivo: a systematic review and meta-analysis. Appl Physiol Nutr Metab. 2016;41:1137–1145. doi:10.1139/apnm-2016-0195 [DOI] [PubMed] [Google Scholar]
- 31. Broskey NT, Greggio C, Boss A et al. . Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99:1852–1861. doi:10.1210/jc.2013-3983 [DOI] [PubMed] [Google Scholar]
- 32. Larsen S, Hey-Mogensen M, Rabøl R, Stride N, Helge JW, Dela F. The influence of age and aerobic fitness: effects on mitochondrial respiration in skeletal muscle. Acta Physiol (Oxf). 2012;205:423–432. doi:10.1111/j.1748-1716.2012.02408.x [DOI] [PubMed] [Google Scholar]
- 33. Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986;9:585–591. doi:10.1002/mus.880090702 [DOI] [PubMed] [Google Scholar]
- 34. Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi:10.1038/nrm3412 [DOI] [PubMed] [Google Scholar]
- 35. Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc Res. 2012;96:32–37. doi:10.1093/cvr/cvs163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mailloux RJ, McBride SL, Harper ME. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem Sci. 2013;38:592–602. doi:10.1016/j.tibs.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 37. Capel F, Rimbert V, Lioger D et al. . Due to reverse electron transfer, mitochondrial H2O2 release increases with age in human vastus lateralis muscle although oxidative capacity is preserved. Mech Ageing Dev. 2005;126:505–511. doi:10.1016/j.mad.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 38. Bailey DM, McEneny J, Mathieu-Costello O et al. . Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol (1985). 2010;109:449–456. doi:10.1152/japplphysiol.00354.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi:10.1186/2046-2395-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–555. doi:10.1016/j.freeradbiomed.2011.10.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siegel MP, Kruse SE, Percival JM et al. . Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell. 2013;12:763–771. doi:10.1111/acel.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–1400. doi:10.1016/j.exger.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 43. Mecocci P, Fanó G, Fulle S et al. . Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–308. [DOI] [PubMed] [Google Scholar]
- 44. Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q. 1994;10:43–63. [PubMed] [Google Scholar]
- 45. Wray DW, Nishiyama SK, Donato AJ et al. . The paradox of oxidative stress and exercise with advancing age. Exerc Sport Sci Rev. 2011;39:68–76. doi:10.1097/JES.0b013e31820d7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nyberg M, Hellsten Y. Reduced blood flow to contracting skeletal muscle in ageing humans: is it all an effect of sand through the hourglass?J Physiol. 2016;594:2297–2305. doi:10.1113/JP270594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Behringer EJ, Segal SS. Impact of Aging on calcium signaling and membrane potential in endothelium of resistance arteries: a role for Mitochondria. J Gerontol A Biol Sci Med Sci. 2017;72:1627–1637. doi:10.1093/gerona/glx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muller-Delp JM, Gurovich AN, Christou DD, Leeuwenburgh C. Redox balance in the aging microcirculation: new friends, new foes, and new clinical directions. Microcirculation. 2012;19:19–28. doi:10.1111/j.1549-8719.2011.00139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi:10.1113/jphysiol.2009.172221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. In: Proceedings from the National Academy of Sciences of the United States of America 2007;104:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frontera WR, Reid KF, Phillips EM et al. . Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985). 2008;105:637–642. doi:10.1152/japplphysiol.90332.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grosicki GJ, Standley RA, Murach KA, Raue U, Minchev K, Coen PM et al. . Improved single muscle fiber quality in the oldest-old. J Appl Physiol (1985). 2016;121:878–884. doi:10.1152/japplphysiol.00479.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dalton BH, Harwood B, Davidson AW, Rice CL. Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol (1985). 2009;107:1781–1788. doi:10.1152/japplphysiol.00464.2009 [DOI] [PubMed] [Google Scholar]
- 54. Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008;586:71–81. doi:10.1113/jphysiol.2007.139105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ochala J, Lambertz D, Pousson M, Goubel F, Hoecke JV. Changes in mechanical properties of human plantar flexor muscles in ageing. Exp Gerontol. 2004;39:349–358. doi:10.1016/j.exger.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 56. Christie AD, Tonson A, Larsen RG, DeBlois JP, Kent JA. Human skeletal muscle metabolic economy in vivo: effects of contraction intensity, age, and mobility impairment. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1124–R1135. doi:10.1152/ajpregu.00083.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barclay CJ, Woledge RC, Curtin NA. Energy turnover for Ca2+ cycling in skeletal muscle. J Muscle Res Cell Motil. 2007;28:259–274. doi:10.1007/s10974-007-9116-7 [DOI] [PubMed] [Google Scholar]
- 58. Hunter SK, Thompson MW, Ruell PA et al. . Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol (1985). 1999;86:1858–1865. doi:10.1152/jappl.1999.86.6.1858 [DOI] [PubMed] [Google Scholar]
- 59. Klitgaard H, Ausoni S, Damiani E. Sarcoplasmic reticulum of human skeletal muscle: age-related changes and effect of training. Acta Physiol Scand. 1989;137:23–31. doi:10.1111/j.1748-1716.1989.tb08717.x [DOI] [PubMed] [Google Scholar]
- 60. Ferrington DA, Krainev AG, Bigelow DJ. Altered turnover of calcium regulatory proteins of the sarcoplasmic reticulum in aged skeletal muscle. J Biol Chem. 1998;273:5885–5891. [DOI] [PubMed] [Google Scholar]
- 61. Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schöneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J. 1999;340:657–669. [PMC free article] [PubMed] [Google Scholar]
- 62. Thompson BJ, Ryan ED, Herda TJ, Costa PB, Herda AA, Cramer JT. Age-related changes in the rate of muscle activation and rapid force characteristics. Age (Dordrecht, Netherlands). 2014;36:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dalton BH, McNeil CJ, Doherty TJ, Rice CL. Age-related reductions in the estimated numbers of motor units are minimal in the human soleus. Muscle Nerve. 2008;38:1108–1115. doi:10.1002/mus.20984 [DOI] [PubMed] [Google Scholar]
- 64. McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467. doi:10.1002/mus.20276 [DOI] [PubMed] [Google Scholar]
- 65. Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7:e29082. doi:10.1371/journal.pone.0029082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mittal KR, Logmani FH. Age-related reduction in 8th cervical ventral nerve root myelinated fiber diameters and numbers in man. J Gerontol. 1987;42:8–10. [DOI] [PubMed] [Google Scholar]
- 67. Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190:W8–12. doi:10.2214/AJR.07.2732 [DOI] [PubMed] [Google Scholar]
- 68. Miljkovic I, Kuipers AL, Cauley JA et al. ; Osteoporotic Fractures in Men Study Group Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. J Gerontol A Biol Sci Med Sci. 2015;70:1133–1140. doi:10.1093/gerona/glv027 [DOI] [PMC free article] [PubMed] [Google Scholar]