Abstract
Background
Antidepressant use is very common in the elderly, but the effects of antidepressants on cognition in the elderly are controversial with some studies suggesting harm and others protection. We aimed to investigate the association between different antidepressant use and change in cognition and risk of mild cognitive impairment (MCI) or dementia in very old women.
Methods
We examined 1,234 community-dwelling women (mean age 83.2 years) from the Study of Osteoporotic Fractures. Baseline antidepressant use was reported and verified by medication containers, and medications were coded with computerized dictionary. Cognitive status (normal, MCI, or dementia) was adjudicated by an expert clinical panel 5 years later. Change in a short-form Mini-Mental State Examination and Trails B were evaluated over 5 years.
Results
Eleven per cent of the women were taking antidepressants. Users of selective serotonin reuptake inhibitors (SSRIs) had the greatest cognitive decline over 5 years, after adjustment for demographics, medical comorbidities, benzodiazepine use, and baseline cognition. Multivariable logistic regression shows that the users of SSRIs were more than twice (OR = 2.69, 95% CI = 1.64–4.41) and trazodone users more than three times (3.48, 1.12–10.81) as likely to develop MCI or dementia compared with the nonusers. Further adjustment for baseline cognition or depressive symptoms did not appreciably alter the results, and the association remained after excluding women with high depressive symptoms. The use of tricyclic antidepressants or other antidepressants was not significantly associated with cognitive outcomes.
Conclusions
The use of antidepressants, especially SSRIs and trazodone, was associated with an increased risk of cognitive impairment 5 years later among the oldest old women.
Keywords: selective serotonin reuptake inhibitors, cognition, cognitive aging, dementia, epidemiology
Antidepressants are one of the most frequently prescribed medications, and the rate of antidepressant use worldwide has increased dramatically over the past two decades (1–3). Several mechanistic studies have suggested that antidepressants could be neuroprotective due to lower toxic amyloid-β formation, increasing neurotrophic factor, and promoting neurogenesis (4–6). Meanwhile, a growing number of population-based studies (7–10) have shown conflicting findings on the cognitive effects of antidepressants in older adults. Several studies reported either no significant association between antidepressant use and cognitive decline (9,11) or a reduced risk of cognitive decline or dementia associated with antidepressant use, especially among patients with depression or Alzheimer’s disease (10,12–14). However, several others suggested that antidepressant use was associated with an increased risk of developing mild cognitive impairment (MCI) or dementia (7,8,15). One recent meta-analysis summarized five population-based studies and concluded that those who developed cognitive impairment were twice as likely to have been using antidepressant drugs compared with the nonimpaired individuals (16). Notably, the majority of studies to date have included patients with depressive symptoms. It is critical to tease out the influence of depression, given that it could be depression or the reduction of depressive symptoms itself that is associated with changes in cognitive outcomes. Furthermore, few studies have examined the cognitive effects of different classes of antidepressant, despite some evidence showing a reduced risk of dementia associated with the use of tricyclic antidepressants (TCAs) rather than with other types of antidepressants such as selective serotonin reuptake inhibitors (SSRIs) (7,17).
Given the high burden of dementia in older adults, it is important to understand whether the use of different classes of antidepressants might reduce or increase the risk of cognitive impairment in older individuals. We prospectively investigated the relationship between the use of different antidepressants and change in cognitive function as well as risk of MCI or dementia in very old women who did not have cognitive impairment. We also examined whether the association is independent of baseline depression or cognitive status, and if it remains in individuals without depressive symptoms.
Methods
Participants
We studied participants enrolled in the Study of Osteoporotic Fractures (SOF) (18), a longitudinal cohort study of community-dwelling Caucasian women. From 1986 to 1988, women aged 65 years or older were recruited from population-based listings in four United States areas: Baltimore, Maryland; Minneapolis, Minnesota; Pittsburgh, Pennsylvania; and Portland, Oregon. At each site, the institutional review boards approved the study, and all participants provided written informed consent.
This study is part of an ancillary study of SOF, which was initiated at two (Minneapolis and Pittsburgh, n = 2,732) of the clinical sites during the eighth clinic visit (January 2002–April 2004). This visit is the “baseline” for this study, when eligible women provided medication information and completed a set of cognitive tests including Mini-Mental State Examination (MMSE), short-form MMSE, and Trail Making Test (Part B; Trails B). At the ninth visit (November 2006–September 2008), 1,300 women completed a battery of neuropsychological tests, and the cognitive status of these participants was adjudicated. After excluding 22 women who reported a history of Alzheimer’s disease or dementia and were taking dementia medications, and those with a MMSE score <24 at baseline, our primary analytic cohort included the 1,234 women who had complete data on antidepressant use at baseline, repeated cognitive measures, and cognitive status adjudication. Supplementary Figure 1 shows the progression of the study to our analytic cohort.
Antidepressant Use
At baseline, participants were asked to bring in all current (defined as daily or almost daily use in 30 days preceding the examination) prescription and over-the-counter medications. Interviewers then completed a medication history for each participant, including the name of the medication and frequency of use. A computerized coding dictionary was used to classify the type of medication from product brand and generic names or ingredients obtained from containers (19). In this study, “nonusers” were defined as women not taking any type of antidepressant. Users of antidepressants were further grouped into users of SSRIs alone, trazodone alone, TCAs alone, and users of any other or multiple antidepressants.
Cognitive Examination
Participants completed the short-form MMSE (20) and Trail Making Test (Part B; Trails B) (21) both at baseline and the follow-up. The short-form MMSE is a shortened version of MMSE with scores ranging from 0 to 26 and measures global cognition. The Trails B test is a timed test that measures attention, sequencing, visual scanning, and executive function with higher score representing worse cognitive functioning.
Baseline cognitive impairment was defined using a reported history of Alzheimer’s disease or dementia, dementia medication use, or a MMSE score of <24. At the follow-up visit 5 years later, women also completed an expanded neuropsychological battery, including the Modified MMSE (3MS) (22), a 100-point version of the MMSE that is particularly useful for dementia screening; the California Verbal Learning Test (Second Edition Short Form) (23), a test of verbal episodic memory with immediate and 20-minute delay scores; Digit Span (24), a test of attention with forward and backward scores; and Category and Verbal Fluency tests (25), a measure of sematic memory. Cognitive impairment was determined in a two-step process (26). First, women were screened for one or more of the following criteria: (i) score < 88 on the 3MS; (ii) score < 4 on the California Verbal Learning Test delayed recall; (iii) score ≥ 3.6 on the Informant Questionnaire on Cognitive Decline in the Elderly; (iv) previous dementia diagnosis or use of dementia medication; or (v) nursing home residence. The women who screen positive as well as a random sample of those who screen negative are adjudicated for clinical cognitive status determined by a panel of clinical experts who review all cognitive, self-reported medical history, and functional data. The remaining women who screen negative are considered cognitively normal. A diagnosis of dementia is made based on DSM-IV criteria (27). MCI is diagnosed using modified Petersen Criteria (28), which requires a cognitive impairment that is insufficient to meet criteria for dementia and generally intact function.
Other Measures
All participants completed questionnaires on demographics and medical history and underwent a physical examination at each clinical visit. Information on age, education, alcohol intake, history of stroke and myocardial infarction (MI), and benzodiazepine use was included in multivariable models as potential confounders. Body mass index was defined as weight in kilograms divided by height in meters squared. Depressive symptoms were determined using the Geriatric Depression Scale (GDS), a validated 15-item questionnaire commonly used for assessment of depressive symptoms in older adults, with a higher score representing more depressive symptoms (29). We defined high depressive symptoms as having a GDS score of 6 or greater.
Statistical Analysis
We first compared the characteristics of the participants by antidepressant use (yes/no). We used student’s t-tests for normally distributed continuous variables, Wilcoxon rank sum tests for skewed variables, and chi-square tests for categorical variables. We used multivariable linear regression to model log-transformed cognitive scores and change in cognitive scores. The adjusted means of short-form MMSE and Trails B scores by different types of antidepressant use were calculated for both the baseline and follow-up visit by exponentiation of the log-transformed scores. The multivariable model simultaneously included cognitive test scores at baseline and covariates that were associated with both antidepressant use and cognition at p < .10, including age, education, alcohol intake, history of stroke and MI, and benzodiazepine use. We then used logistic regression to examine the association between antidepressant use and risk of developing MCI or dementia, adjusting for the above covariates; additional models were adjusted for baseline cognitive test scores, and for high depressive symptoms. Finally, in order to address the influence of depression, we performed sensitivity analysis by (i) restricting the analysis only to women without evidence of high depressive symptoms; (ii) excluding women who had depressive symptoms but were not using antidepressants; and (iii) excluding women who have had history of high depressive symptoms (past history of GDS score ≥ 6) for up to 14 years prior to the baseline visit. Results are presented as odds ratios (OR) and 95% confidence intervals (CI). A two-sided p-value of <.05 was considered statistically significant. Analyses were conducted using Stata, version 14.1 (Stata Corp LP, College Station, Texas).
Results
After excluding participants who had significant cognitive impairment at baseline, the study sample included 1,234 women (mean age: 83.2 ± 2.9 years). One hundred forty (11 per cent) of these women were taking antidepressants, of which 77 were users of SSRIs alone, 15 trazodone alone, 32 TCA alone, and 16 other or multiple types of antidepressants. The baseline characteristics of the sample by use of antidepressants are summarized in Table 1. Compared with the nonusers, users of antidepressants had a higher body mass index and were about twice as likely to have a history of stroke or MI, to be taking benzodiazepines and to have high depressive symptoms (GDS score ≥ 6). Users of antidepressants and the nonusers did not differ significantly in age, education, or alcohol consumption. Of the 140 antidepressant users at baseline, 95 (67.9 per cent) were also using antidepressants at year 5; one hundred (9.2 per cent) of the nonusers at baseline became users of antidepressants at year 5.
Table 1.
Baseline Characteristics of 1,234 Older Women by Use of Antidepressants
| Characteristics, mean ± SD or N (%) | Nonusers | Users of antidepressant | p |
|---|---|---|---|
| (N = 1,094) | (N = 140) | ||
| Age | 83.2 ± 2.9 | 83.4 ± 2.8 | .32 |
| Education > high school | 427 (39.1) | 54 (38.6) | .90 |
| BMI (kg/m2) | 27.0 ± 4.5 | 28.0 ± 5.1 | .02 |
| Alcohol use (units/w) | 1.1 ± 2.9 | 0.8 ± 2.0 | .22 |
| Stroke | 101 (9.3) | 27 (19.3) | <.001 |
| Myocardial infarction | 102 (9.3) | 28 (20.0) | <.001 |
| Diabetes | 97 (8.9) | 11 (7.9) | .69 |
| Benzodiazepines use | 69 (6.3) | 21 (15.0) | <.001 |
| GDS ≥ 6 at baseline | 79 (7.3) | 22 (15.8) | .001 |
| History of depression (GDS ≥ 6) | 71 (6.5) | 18 (12.9) | .006 |
| GDS ≥ 6 at the follow-up visit | 118 (11.1) | 23 (17.0) | .04 |
| Baseline cognition | |||
| sMMSE | 25.0 ± 1.2 | 24.6 ± 1.5 | .15 |
| Trails B | 130.4 ± 54.9 | 147.4 ± 56.4 | .07 |
| Cognition after 5 y | |||
| sMMSE | 23.2 ± 3.5 | 21.9 ± 4.1 | <.001 |
| Trails B | 160.8 ± 73.8 | 195.5 ± 87.7 | <.001 |
Note: BMI = body mass index; GDS = Geriatric Depression Scale; sMMSE = Short-form Mini-Mental State Examination; Trails B = Trail Making Test (Part B).
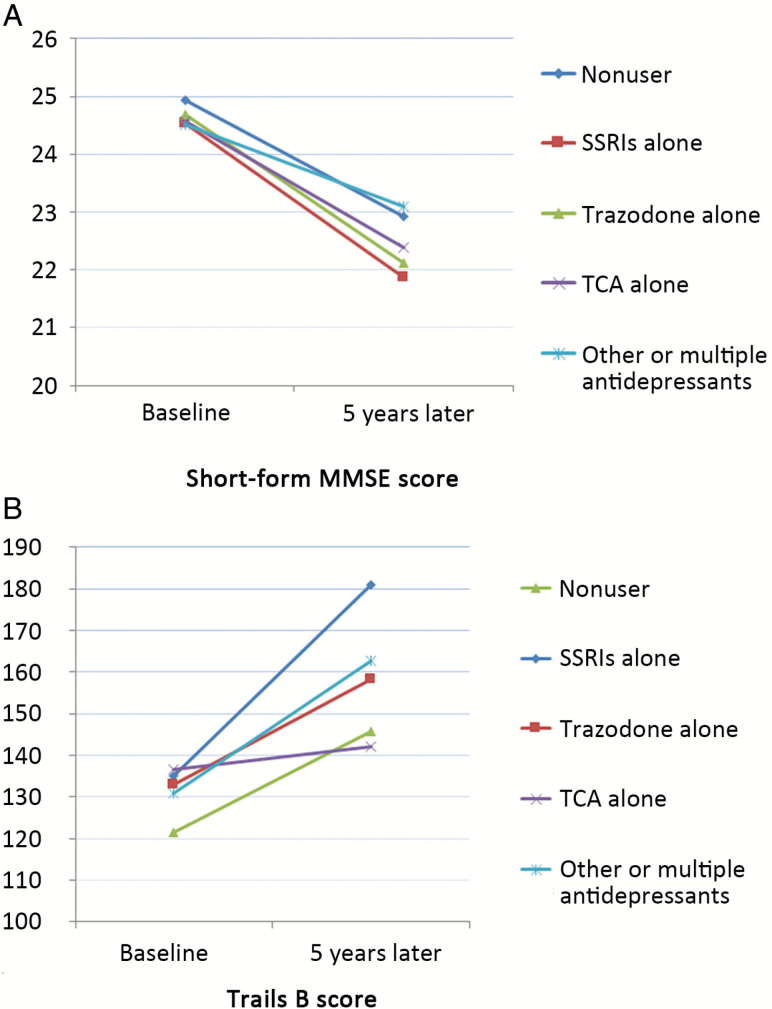
The baseline mean sMMSE scores were 24.6 ± 1.5 and 25.0 ± 1.2 points and baseline Trails B scores were 147.4 ± 56.4 and 130.4 ± 54.9 seconds for users and nonusers of antidepressants, respectively. Figure 1 shows the adjusted mean sMMSE and Trails B scores by antidepressant use, and suggests that SSRI users had the greatest decline from baseline to the follow-up visit on both cognitive tests. Linear regression reveals that the β associated with SSRI use was −1.09 (p = .007) and 42.59 (p < .001) for change in short-form MMSE and Trails B test scores, respectively, after adjusting for age, education, body mass index, history of stroke and MI, benzodiazepine use and baseline cognitive score. Use of other types of antidepressants was also associated with greater decline in cognitive tests compared with the nonusers, but the associations were not statistically significant.
Figure 1.
Adjusteda mean cognitive scores (A) Short-form MMSE and (B) Trails B at baseline and follow-up visits, by use of different antidepressants in 1,234 older women. aAdjusted for age, education, body mass index, history of stroke and myocardial infarction, benzodiazepine use; and for scores at follow-up visit additionally adjust for baseline cognitive scores.
Over a mean of 4.7 years of follow-up, 473 (38 per cent) women developed MCI or dementia, including 278 with MCI and 195 with dementia. Overall, the ORs of developing MCI or dementia were 2.17 (1.49–3.15) for antidepressant users at baseline, 1.61 (1.17–2.23) for antidepressant users at year 5, and 1.75 (1.13–2.73) for those who used antidepressants at both time points. Table 2 shows the adjusted ORs of developing MCI or dementia by usage of antidepressants. After adjustment for all covariates, users of SSRIs were more than twice (OR = 2.69, 95% CI = 1.64–4.41) and trazodone users more than three times (OR = 3.48, 95% CI = 1.12–10.81) as likely to develop MCI or dementia, compared with those not taking any antidepressants. Further adjustment for baseline cognitive scores or GDS scores did not appreciably alter the results. The use of TCAs and other or multiple antidepressants was not significantly associated with the risk of developing MCI or dementia. Sensitivity analysis shows that after excluding women with high depressive symptoms (n = 101), use of SSRIs or trazodone remained to be associated with the risk of developing MCI or dementia, with an OR of 2.87 (95% CI = 1.69–4.88) and 6.11 (1.16–32.21), respectively. Exclusion of women who did not use antidepressants but had high depressive symptoms (n = 79) showed similar findings. After excluding women with a past history of high depressive symptoms (n = 89), the ORs associated with the use of SSRIs and trazodone were 2.86 (1.68–4.88) and 5.05 (1.25–20.38), respectively.
Table 2.
Logistic Regression on the Risk of Mild Cognitive Impairment (MCI) or Dementia by Antidepressant Use in 1,234 Older Women
| Antidepressant use | Odds ratios (95% CI) | |||
|---|---|---|---|---|
| No. (% with MCI/ dementia) | Multivariable†- adjusted | Multivariable† + baseline cognitive scoreb-adjusted | Multivariable† + baseline cognitive scores‡ + GDS scores-adjusted | |
| Nonusers | 1,092 (36.7) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SSRIs alone | 77 (61.8) | 2.69 (1.64, 4.41)*** | 2.36 (1.39–4.01)** | 2.27 (1.33, 3.87)** |
| Trazodone alone | 15 (64.3) | 3.48 (1.12, 10.81)* | 3.46 (1.09–10.98)* | 3.20 (1.00, 10.22)* |
| Tricyclic antidepressants alone | 32 (45.2) | 1.24 (0.59, 2.61) | 1.09 (0.51, 2.36) | 1.10 (0.51, 2.36) |
| Other or multiple antidepressants | 16 (43.8) | 1.61 (0.58, 4.50) | 1.64 (0.53, 5.07) | 1.52 (0.48, 4.77) |
Notes: †Adjusted for age, education, body mass index, history of stroke and myocardial infarction, and benzodiazepine use
‡Short-form Mini-Mental State Examination and Trail Making Test B scores.
*<.05; **<.01; ***<.001. Bold values indicate statistical significance (p < 0.05).
Discussions
In a cohort of very old women, we found that those who were using SSRIs had the greatest decline in cognitive function over 5 years, compared with those not taking any antidepressant and those who were taking other types of antidepressants. Users of SSRIs or trazodone were more than twice or three times as likely to develop MCI or dementia 5 years later, compared with those not taking any antidepressant. The associations were independent of baseline cognition and depressive symptoms, and remained after excluding women with high depressive symptoms.
Previous studies have reported conflicting results on the relationship between antidepressant use and cognitive impairment (7,10,12,17). This could be partly due to the inclusion of patients with depression or cognitive impairment at baseline. Depression has been associated with an increased risk of dementia, both in the SOF cohort and in other populations (30–33). Growing evidence also suggested that late-life depressive symptoms could be prodromal features of dementia (34), and older adults who use antidepressants are more likely to have high depressive symptoms. Therefore, the influence of depression on the relationship between antidepressant use and cognitive impairment is of particular interest. It is unclear if the increased dementia risk associated with antidepressant use results from the depression itself, and if the reduced risk of cognitive impairment is because of the reduction in depressive symptoms rather than direct benefits of antidepressants. Besides, users of antidepressants are more likely to have remitted depression, but it is unknown whether the cognitive effects of antidepressants could be due to individuals’ past history of depression.
We investigated the independent effects of antidepressants on both cognitive decline and cognitive impairment, and found that those who were using antidepressants, especially SSRIs, had greater cognitive decline and an increased risk of MCI or dementia, irrespective of their depressive symptoms. The association remained even after excluding participants with high depressive symptoms at baseline or those with a history of high depressive symptoms; thus, it is unlikely that the observed relationship between antidepressant use and cognitive impairment is mainly through depression or past history of depression. Unlike our study, the Health and Retirement Study failed to find any significant association between antidepressant use and cognitive decline (9). This could be because they have used an unstandardized cognitive battery, which might not be particularly sensitive to the cognitive change related to antidepressant use. Moreover, their study sample was not restricted to cognitively nonimpaired people, and when stratified by baseline cognitive status, they did find significant cognitive decline among antidepressant users with normal cognition at baseline but nonsignificant decline for those with baseline cognitive impairment. It is possible that the use of antidepressants might help us to reduce cognitive decline in patients with cognitive impairment (12) but not in those with normal cognition. Given the complex role that baseline cognition might play in this relationship, we excluded participants with significant cognitive impairment at baseline and adjusted for baseline cognitive scores throughout the analysis.
Our results are consistent with prior findings from the Women’s Health Initiative Memory Study (WHIMS), which suggested that antidepressant use was associated with increased risk of incident MCI or probable dementia in both depressed and nondepressed postmenopausal women (8). Interestingly, the WHIMS found an increased risk in users of SSRIs or TCAs, whereas our study suggested an increased risk in users of SSRIs or trazodone but not in TCAs users. It should be noted that the WHIMS included 6,998 women and had higher number of users of specific types of antidepressants than our study. Meanwhile, two other studies (7,17) suggested that the use of TCAs was protective against dementia while use of other antidepressants, including the SSRIs, was associated with an increased risk or no risk of dementia. A review of 18 clinical studies concluded that trazodone might impair attention, whereas no detrimental cognitive effects were found for SSRIs (35). It is unclear whether and why certain types of antidepressants might influence cognition differently. Notably, the number of participants who were using specific classes of antidepressants, such as trazadone, was small in this study. Future studies should explore further the cognitive effects of different types of antidepressants in the elderly.
The cognitive effects of SSRIs and their potential mechanisms are controversial. Animal studies have shown that SSRIs play an important role in hippocampal neurogenesis and neuroplasticity, as well as suppressing τ pathology and production of amyloid-β (36,37). Treatment with SSRI fluoxetine might also help restore dendritic development and the expression of 5-hydroxytryptamine (5-HT) receptors and brain-derived neurotrophic factors, which in turn improves neurogenesis and cognitive performance (38,39). However, these benefits were found to lose with age in mice models (40), suggesting potentially different cognitive effects of antidepressants among the elderly. In addition, anticholinergic activity, which is often present in many antidepressants including SSRIs, is known to have adverse effects on cognitive function (41,42). It is possible that the greater cognitive decline and impairment found among the users of SSRIs could be due to greater anticholinergic properties, whereas the mechanism is less clear for trazodone, which has no anticholinergic activity. Moreover, epidemiologic evidence has suggested that the use of antidepressants, particularly the SSRIs, might be associated with an increased risk of stroke and MI due to their antiplatelet effects and serotonergic-related arterial vasoconstriction (43,44). The increased cardiovascular disease (CVD) risk could eventually contribute to an elevated risk of dementia, especially vascular dementia. Although women who were using antidepressants in this study were more likely to have had a history of stroke and MI, the observed relationship between antidepressant use and cognitive impairment remained after adjustment for a history of CVD.
Strengths and Limitations
This is the first study that examined the association between the use of different antidepressants and both cognitive decline and risk of cognitive impairment in very old women. Its prospective study design and focus on women without cognitive impairment at baseline have allowed for study of the direction of the relationship. We were also able to explore the impact of depression on this relationship by enrolling women both with and without depression, controlling for depressive symptoms, and by sensitivity analysis with exclusion of those with high depressive symptoms. Moreover, the assessment of antidepressant use was conducted and verified using a standardized medication classification system, and cognitive impairment was determined rigorously by a panel of clinical experts.
A few limitations need to be considered in interpreting these results. First, this cohort is comprised of older Caucasian women and thus our findings might not be generalizable to men, younger, or more ethnically diverse populations. Only a small proportion of women who were on antidepressants also had high depressive symptoms. Therefore, we could not examine the relationship specifically among depressed patients, or the interaction between antidepressants and depressive symptoms. Likewise, our findings on the cognitive effects of different classes of antidepressants should be interpreted with caution, given the small number of participants who were taking certain types of antidepressants. It should also be noted that prescriptions could change over time as participants’ cognitive status changes. We did not have information on the duration of antidepressant use, although our data did suggest change in the use of antidepressants from baseline to follow-up; we also did not have information regarding dose: trazodone and TCAs are often used for sleep-related indications at low doses. Finally, although we controlled for a number of potential confounders including baseline cognition and continuous scores of depressive symptoms, residual confounding remains a possibility. Those who were using antidepressants might already be experiencing subclinical cognitive impairment or had depression that was not captured by the GDS.
Conclusion
Among very old community-dwelling women with and without depression, the use of antidepressants, especially the SSRIs and trazodone, was associated with an increased risk of developing cognitive impairment 5 years later. These findings add to the heated debate over the risks and benefits of antidepressant use in the elderly, and highlight the complexities of the cognitive effects of different classes of antidepressants. Although causality cannot be established from this study and it is unclear whether antidepressant use is an independent predictor for cognitive impairment, the use of antidepressants does not seem to be protective against cognitive decline in the elderly. Further research is required to fully disentangle the effects and mechanisms of different types of antidepressants, and to explore options for optimal management of depression in older adults. Meanwhile, older adults who are taking antidepressants should be followed closely for cognitive function and risk of cognitive impairment.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720. This work is also in part supported by NIA grant number K24 AG031155, granted to Dr. Yaffe. Dr. Leng is funded through NIA grant number 1K99AG056598-01.
Conflict of interest statement
None declared.
References
- 1. Lockhart P, Guthrie B. Trends in primary care antidepressant prescribing 1995–2007: a longitudinal population database analysis. Br J Gen Pract. 2011;61:e565–572. doi: 10.3399/bjgp11X593848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 3. Patten SB, Beck C. Major depression and mental health care utilization in Canada: 1994 to 2000. Can J Psychiatry. 2004;49:303–309. doi: 10.1177/070674370404900505 [DOI] [PubMed] [Google Scholar]
- 4. Dixon O, Mead G. Selective serotonin reuptake inhibitors for mild cognitive impairment: A systematic review. J Neurol Disord Stroke. 2013;1:1022. [Google Scholar]
- 5. Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 6. Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee CW, Lin CL, Sung FC, Liang JA, Kao CH. Antidepressant treatment and risk of dementia: a population-based, retrospective case-control study. J Clin Psychiatry. 2016;77:117–22; quiz 122. doi: 10.4088/JCP.14m09580. [DOI] [PubMed] [Google Scholar]
- 8. Goveas JS, Hogan PE, Kotchen JM et al. . Depressive symptoms, antidepressant use, and future cognitive health in postmenopausal women: the Women’s Health Initiative Memory Study. Int Psychogeriatr. 2012;24:1252–1264. doi: 10.1017/S1041610211002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saczynski JS, Rosen AB, McCammon RJ et al. . Antidepressant use and cognitive decline: The Health and Retirement Study. Am J Med. 2015;128:739–746. doi: 10.1016/j.amjmed.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brodrick JE, Mathys ML. Antidepressant exposure and risk of dementia in older adults with major depressive disorder. J Am Geriatr Soc. 2016;64:2517–2521. doi: 10.1111/jgs.14378. [DOI] [PubMed] [Google Scholar]
- 11. Carrière I, Norton J, Farré A et al. . Antidepressant use and cognitive decline in community-dwelling elderly people – The Three-City Cohort. BMC Med. 2017;15:81. doi: 10.1186/s12916-017-0847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mossello E, Boncinelli M, Caleri V et al. . Is antidepressant treatment associated with reduced cognitive decline in Alzheimer’s disease?Dement Geriatr Cogn Disord. 2008;25:372–379. doi: 10.1159/000121334. [DOI] [PubMed] [Google Scholar]
- 13. Rozzini L, Chilovi BV, Conti M et al. . Efficacy of SSRIs on cognition of Alzheimer’s disease patients treated with cholinesterase inhibitors. Int Psychogeriatr. 2010;22:114–119. doi: 10.1017/S1041610209990184. [DOI] [PubMed] [Google Scholar]
- 14. Butters MA, Becker JT, Nebes RD et al. . Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Gao S, Hendrie HC et al. . Antidepressant use in the elderly is associated with an increased risk of dementia. Alzheimer Dis Assoc Disord. 2016;30:99–104. doi: 10.1097/WAD.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moraros J, Nwankwo C, Patten SB, Mousseau DD. The association of antidepressant drug usage with cognitive impairment or dementia, including Alzheimer disease: A systematic review and meta-analysis. Depress Anxiety. 2017;34:217–226. doi: 10.1002/da.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kessing LV, Forman JL, Andersen PK. Do continued antidepressants protect against dementia in patients with severe depressive disorder?Int Clin Psychopharmacol. 2011;26:316–322. doi: 10.1097/YIC.0b013e32834ace0f. [DOI] [PubMed] [Google Scholar]
- 18. Cummings SR, Nevitt MC, Browner WS et al. . Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 19. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/bf01719664. [DOI] [PubMed] [Google Scholar]
- 20. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1002/(sici)1099-1166 (199805)13:5<285::aid-gps753>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. doi: 10.2466/pms.8.7. 271-276. [Google Scholar]
- 22. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 23. Delis DC, Kramer JH, Kaplan E, Ober BA.. California Verbal Learning Test - Second Edition (CVLT-II). San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 24. Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 25. Spreen O, Strauss E.. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 26. Yaffe K, Middleton LE, Lui LY et al. . Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636. doi: 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28. Petersen RC, Doody R, Kurz A et al. . Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 29. Brink TL. Clinical gerontology: a guide to assessment and intervention. New York, NY: Howarth Press; 1986. doi: 10.1017/s0144686x00012733. [Google Scholar]
- 30. Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spira AP, Rebok GW, Stone KL, Kramer JH, Yaffe K. Depressive symptoms in oldest-old women: risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry. 2012;20:1006–1015. doi: 10.1097/JGP.0b013e318235b611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 33. Kaup AR, Byers AL, Falvey C et al. . Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry. 2016;73:525–531. doi: 10.1001/jamapsychiatry.2016.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh-Manoux A, Dugravot A, Fournier A et al. . Trajectories of depressive symptoms before diagnosis of dementia: A 28-year follow-up study. JAMA Psychiatry. 2017;74:712–718. doi: 10.1001/jamapsychiatry.2017.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knegtering H, Eijck M, Huijsman A. Effects of antidepressants on cognitive functioning of elderly patients. A review. Drugs Aging. 1994;5:192–199. doi: 10.2165/00002512-199405030-00005. [DOI] [PubMed] [Google Scholar]
- 36. Nelson RL, Guo Z, Halagappa VM et al. . Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007;205:166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Santarelli L, Saxe M, Gross C et al. . Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 38. Bianchi P, Ciani E, Guidi S et al. . Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci. 2010;30:8769–8779. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guidi S, Stagni F, Bianchi P et al. . Early pharmacotherapy with fluoxetine rescues dendritic pathology in the Ts65Dn mouse model of down syndrome. Brain Pathol. 2013;23:129–143. doi: 10.1111/j.1750-3639.2012.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heinen M, Hettich MM, Ryan DP, Schnell S, Paesler K, Ehninger D. Adult-onset fluoxetine treatment does not improve behavioral impairments and may have adverse effects on the Ts65Dn mouse model of Down syndrome. Neural Plast. 2012;2012:467251. doi: 10.1155/2012/467251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carrière I, Fourrier-Reglat A, Dartigues JF et al. . Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169:1317–1324. doi: 10.1001/archinternmed.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fox C, Richardson K, Maidment ID et al. . Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 43. Shin D, Oh YH, Eom CS, Park SM. Use of selective serotonin reuptake inhibitors and risk of stroke: a systematic review and meta-analysis. J Neurol. 2014;261:686–695. doi: 10.1007/s00415-014-7251-9. [DOI] [PubMed] [Google Scholar]
- 44. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.