Key Clinical Message
Sjögren's syndrome concurrent with protein‐losing gastroenteropathy can develop into secondary systemic capillary leak syndrome. Thus, it is important to diagnose the condition as soon as possible and simultaneously administer treatment for Sjögren's syndrome, protein‐losing gastroenteropathy, and systemic capillary leak syndrome.
Keywords: gamma globulin therapy, protein‐losing gastroenteropathy, Sjögren's syndrome, steroid therapy, systemic capillary leak syndrome
1. INTRODUCTION
Protein‐losing gastroenteropathy is a rare condition. In some cases, it has been found to coexist with connective tissue diseases, such as Sjögren's syndrome.1 We experienced the case of a patient with Sjögren's syndrome concurrent with protein‐losing gastroenteropathy who subsequently developed secondary systemic capillary leak syndrome, making their condition temporarily critical. However, the combination of various treatments resulted in an improvement. Here, we report the first case of Sjögren's syndrome concurrent with protein‐losing gastroenteropathy in which secondary systemic capillary leak syndrome developed, together with a review of the literature.
2. CASE HISTORY
The patient was an 88‐year‐old man with a history of dyslipidemia, right corneal transplantation, and cataract surgery. One month prior to hospitalization, he experienced respiratory distress on exertion and pedal edema, for which he consulted his local physician. Upon receiving a diuretic, Chinese herbal medicine, and albumin drip infusion, his symptoms improved. One week prior to hospitalization, the patient experienced abdominal bloating and was referred to our hospital after being diagnosed with pleural effusion with ascites; he was subsequently hospitalized.
On admission, the patient's height was 155 cm and weight was 62.2 kg, and he presented with mild edema of the fingers and marked fast pitting edema in both legs. Laboratory tests on admission revealed hypoalbuminemia, with 2.8 g/dL Albumin (Table 1). However, as the urinary protein/creatinine ratio was 0.926 g/g Creatinine, nephrotic syndrome was ruled out.
Table 1.
Blood tests results
| Initial | |
|---|---|
| Hemoglobin (g/dL) | 11.3 |
| Hematocrit (%) | 34.0 |
| Mean corpuscular volume (fL) | 85.9 |
| Platelets (×109/L) | 254 |
| Leukocytes (×109/L) | 6.7 |
| Neutrophils (×109/L) | 3.5 |
| Lymphocytes (×109/L) | 2.4 |
| Prothrombin time ratio (INR) | 1.03 |
| D‐dimers (μg/mL) | 3.8 |
| Uric acid (mg/dL) | 4.4 |
| Blood urea nitrogen (mg/dL) | 22 |
| Creatinine (mg/dL) | 0.75 |
| Total protein (g/dL) | 5.8 |
| Albumin (g/dL) | 2.8 |
| Total bilirubin (mg/dL) | 0.4 |
| AST (IU/L) | 28 |
| ALT (IU/L) | 11 |
| Alkaline phosphatase (IU/L) | 227 |
| LDH (IU/L) | 210 |
| Total cholesterol (mg/dL) | 211 |
| Sodium (mEq/L) | 133 |
| Potassium (mEq/L) | 3.9 |
| Chloride (mEq/L) | 102 |
| Calcium (mg/dL) | 8.2 |
| Creatine phosphokinase (IU/L) | 63 |
| CRP (mg/dL) | 2.3 |
| TSH (μU/mL) | 3.4 |
| BNP (pg/mL) | 88.2 |
| Interleukin‐6 (pg/mL) | 25.2 |
| Vascular endothelial growth factor (pg/mL) | 179 |
| Gamma globulin (g/dL) | 0.91 |
| IgG4 (mg/dL) | 48 |
| Anti‐SS‐A antibody | Positive |
| Anti‐SS‐B antibody | Positive |
As platelet count was normal and abdominal ultrasonography revealed no sign of liver cirrhosis, liver failure was also ruled out. Heart failure and hypothyroidism were also ruled out. Pleural effusin test revealed exudative, with 83 IU/L LDH and 3.5 g/dL protein, but it was modified according to infusing albumin by physician who treated him before the admission. So, actually pleural effusion seemed to be transudative.
Contrast‐enhanced computed tomography of the chest and abdomen revealed lymph node edema of <1 cm in the bilateral axilla and mediastinum.
A right axillary lymph node biopsy was normal and laboratory tests revealed mildly elevated levels of interleukin‐6 at 25.2 pg/mL, vascular endothelial growth factor (VEGF) at 179 pg/mL, gamma globulin at 0.91 g/dL, CRP at 2.3 mg/dL, and IgG4 at 48.0 mg/dL (Table 1); thus, Castleman disease was ruled out. Serum positivity for anti‐SS‐A and anti‐SS‐B antibodies (Table 1), as well as an ocular staining score of >4 points in both eyes, indicated that the subject satisfied 2 out of 3 items of the 2012 classification for Sjögren's syndrome established by the American College of Rheumatology and The Sjögren's International Collaborative Clinical Alliance; thus, the patient was diagnosed with Sjögren's syndrome.2 Furthermore, gum test revealed dry mouth, with a saliva flow rate of 0.5 mL/10 min.
Biopsies of the stomach, duodenum, rectum, skin, abdominal wall fat, and bone marrow were performed; however, since amyloid deposition was not observed, amyloidosis was ruled out. Biopsies of the stomach and duodenum showed only mild lymphangiectasis. Immunologic studies to assess complements were not performed.
Fecal fat staining was negative. However, technetium‐99 m‐labeled human serum albumin scintigraphy revealed early radioisotope accumulation in the small bowel (Figure 1). Because of this typical finding of protein‐losing gastroenteropathy and absence of gastrointestinal bleeding, we diagnosed protein‐losing gastroenteropathy.
Figure 1.
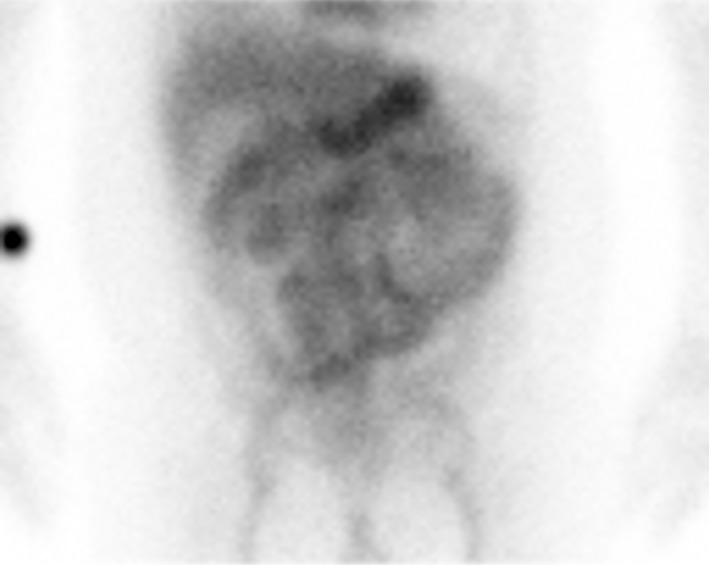
Technetium‐99 m‐labelled (99mTc‐labelled) human serum albumin (HSA) scintigraphy. 2 minutes after intravaenous injection of 99mTc‐labelled HSA, there was mild accumulation in the small bowel, and it became clear. 3 hours and 30 minutes after injection, there was movement of accumulation in the anus side. Typical features of protein losing gastroenteropathy were documented
Fecal alpha‐1‐antitrypsin test was useful but unavailable at our hospital.
3. OUTCOME
On day 18 of hospitalization, a dose of prednisolone at 30 mg/d (0.5 mg/kg/d) was initiated. On day 24, a sudden drop in blood pressure, reduced level of consciousness, elevated level of hematocrit (Ht) at 48.3%, pleural effusion with ascites, pericardial effusion, systemic edema and decreased level of serum albumin at 1.7 g/dL were observed. This led to a suspicion of protein leakage from a location other than the gastrointestinal tract, and upon diagnosis of secondary systemic capillary leak syndrome,3 human gamma globulin at a dose of 0.4 g/kg/d was administered for 5 days. Because the improvement in edema was poor, the dosage of prednisolone was increased to 50 mg/d starting on day 16; however, the patient showed little response to this increase in dosage of prednisolone. From day 28 to day 30, steroid pulse therapy was administered with methylprednisolone at 1 g/d and theophylline therapy was simultaneously initiated and maintained at a serum concentration of 10‐20 μg/mL. On day 31, his body weight reached its maximum value of 71.2 kg (9.0 kg increase since admission), and prednisolone was recommenced at 50 mg/day. From day 32 to day 42, 20% albumin at 100 mL/d and furosemide at 20 mg/d were administered. As a result, his body weight gradually decreased and the edema also improved. On day 45, theophylline was discontinued, and the dosage of prednisolone was reduced on days 46, 61, and 75 to 40 mg/d, 30 mg/d, and 25 mg/d, respectively. On day 80, he was discharged from the hospital, and he subsequently continues to receive treatment on an outpatient basis.
4. DISCUSSION
Autoimmume diseases that cause protein‐losing gastroenteropathy include scleroderma, systemic lupus erythematosus, Sjögren's syndrome, rheumatoid arthritis, mixed connective tissue disease, and dermatomyositis, and the cause of protein‐losing gastroenteropathy associated with autoimmune disease is thought to be related to capillary hyperpermeability.1 Among autoimmune diseases, there have been 21 published reports from 1988 to 2017 in English and Japanese of cases presenting with Sjögren's syndrome concurrent with protein‐losing gastroenteropathy, as in the present case (Table 2).1, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Among such publications, there are no reports of secondary systemic capillary leak syndrome, thereby making our case the first case to be reported.
Table 2.
The previous reports of PLGE patients associated with SS
| Author | Publish year | Age | Sex | Nationality | Alb (g/dL) | SS‐A | SS‐B | ANA | Complication | Steroid | Other therapy | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugiyama T | 1988 | 47 | F | Japanese | 1.6 | + | ‐ | 64 | Chronic thyroiditis | PSL 60 mg (p.o.) | Improve | 1, 4 | |
| Yamada H | 1994 | 38 | F | Japanese | 2.3 | ND | ND | ND | SLE | PSL | Improve | 5 | |
| Iizuka H | 1996 | 28 | F | Japanese | 1.4 | ND | ND | ND | Chronic thyroiditis | PSL 40 mg | Improve | 6 | |
| Inoue R | 1996 | 49 | F | Japanese | 2.4 | ND | ND | ND | n.p. | PSL 40 mg | Improve | 7 | |
| Mok MY | 1997 | 54 | F | ‐ | 1.4 | ND | ND | ND | ‐ | PSL 60 mg (p.o.) | CPA 100 mg | Improve | 8 |
| Imai K | 2002 | 64 | F | Japanese | 2.7 | + | + | ND | RA | ‐ | Ubai‐en (Kampo medicine) | Improve | 9 |
| Hsieh TY (case1) | 2002 | 37 | F | Taiwanese | 1.4 | + | ND | 320 | n.p. | m‐PSL 750 mg × 3 d 2course (i.v.) + PSL 30 mg (p.o.) | HCQ 200 mg | Improve | 10 |
| Hsieh TY (case2) | 2002 | 50 | F | Taiwanese | 1.1 | + | ND | 640 | n.p. | m‐PSL 750 mg × 3 d 3course (i.v.) + PSL 30 mg (p.o.) | HCQ 200 mg | Improve | 10 |
| Choi HJ | 2004 | 50 | F | Korean | ND | ND | ND | ND | ND | PSL 60 mg (p.o.) | Improve | 11 | |
| Ushiyama A | 2004 | 61 | F | Japanese | 1.8 | + | ‐ | 320 | Chronic thyroiditis | PSL 40 mg (i.v.) | Improve | 12 | |
| Nagashima T | 2009 | 41 | M | Japanese | 1.3 | + | + | 1280 | n.p. | PSL 70 mg (i.v.) + m‐PSL 1 g × 3 d (i.v.) | Improve | 13 | |
| Nasu T | 2011 | 59 | F | Japanese | 2.8 | + | ‐ | ND | RA, Chronic thyroiditis | PSL 50 mg (p.o.) + m‐PSL1 g × 3 d 2course (i.v.) | CPA pulse + MZR 150 mg | Improve | 14 |
| Uraoka Y | 2012 | 42 | F | Japanese | 1.5 | + | ND | ND | n.p. | m‐PSL 1000 mg×3 d (i.v.) + PSL 20 mg (p.o.) | CPA pulse + rituximab | Improve | 15 |
| Kakigao K | 2012 | 58 | F | Japanese | 1.5 | + | ‐ | ND | MCTD, hypothyroidism | PSL 45 mg (p.o.) | Improve | 16 | |
| Chen HY | 2013 | 69 | F | Chinese | ND | + | + | ND | ND | PSL (p.o.) + m‐PSL (i.v.) | Improve | 17 | |
| Yamashita H | 2014 | 51 | F | Japanese | 1.5 | + | + | 2560 | Interstitial pneumonia | PSL 60 mg (p.o.) | Improve | 18 | |
| Liao CY | 2015 | 30 | F | Taiwanese | 1.8 | + | ND | 5120 | n.p. | PSL 30 mg (p.o.) | HCQ 400 mg | Improve | 19 |
| Gupta A | 2015 | 58 | F | White | 2.6 | + | + | 1280 | Type 1 RTA | PSL 60 mg (p.o.) | CPA 800 mg/mo | 20 | |
| Izumi Y | 2016 | 64 | F | Japanese | 3.0 | + | ‐ | ‐ | n.p. | PSL 50 mg (p.o.) + m‐PSL 500 mg × 3 d (i.v.) | MZR 200 mg | Improve | 21 |
| Ofuji K | 2016 | 73 | M | Japanese | 2.7 | + | ‐ | 80 | Dermatomyositis | PSL 45 mg (p.o.) | Improve | 22 | |
| Hadigal S | 2017 | 67 | M | United States | 2.5 | + | ND | 640 | Pleural effusion | PSL | HCQ | Improve | 23 |
| This case | 2018 | 88 | M | Japanese | 2.8 | + | + | 40 | n.p. | PSL 30 mg (p.o.) + m‐PSL 1000 mg (i.v.) | IVIG 20 g + theophylline | Improve |
PLGE, protein‐losing gastroenteropathy; SS, Sjögren's syndrome; SS‐A, Anti SS‐A antibody; SS‐B, Anti SS‐B antibody; ANA, Anti nuclear antibody; PSL, prednisolone, p.o., per os.; SLE, systemic lupus erythematosus; n.p., not particular; CPA, cyclophosphamide; ND, no data; RA, rheumatoid arthritis; m‐PSL, methylprednisolone; HCQ, hydroxychloroquine; MZR, mizoribine; IVIG, intravenous immunoglobulin; i.v., intravenous; MCTD, mixed connective tissue disease; RTA, renal tubular acidosis.
Among the 21 reported cases, 18 were females and 19 were reported from East Asia. The present case is also considered a rare case, as the patient is elderly and male. Among the 21 patients reported, 20 received prednisolone, and in the event of a poor response to steroids, additional treatment was administered (eg, cyclophosphamide, hydroxychloroquine, mizoribine, and rituximab), resulting in the alleviation of symptoms in all patients.
In the present case, a sudden drop in blood pressure and hemoconcentration were observed on day 24. Moreover, pleural effusion with ascites, pericardial effusion, and systemic edema were observed. Thus, protein leak other than that from the gastrointestinal tract was suspected, leading to the diagnosis of secondary systemic capillary leak syndrome.
Systemic capillary leak syndrome is a rare disease characterized by 3 features, comprising hypotension, hypoalbuminemia, and hemoconcentration, and is said to cause disruption of vascular endothelial cells, resulting in the leakage of plasma proteins into the interstitial compartment. Because of normal blood pressure and no sign of hemoconcentration on admission, systemic capillary leak syndrome did not present on admission.
The involvement of VEGF has been suggested in the extravascular leakage of protein associated with systemic capillary leak syndrome. Moreover, because our patient also presented with elevated levels of VEGF, VEGF appears to be involved. There has been a reported case of systemic capillary leak syndrome treated with high‐dose intravenous immunoglobulin therapy (IVIG), theophylline therapy (blood concentration at 15‐25 μg/mL), terbutaline (beta‐2 receptor agonist), anti‐human‐TNF‐alpha monoclonal antibodies, and anti‐VEGF antibodies.3
Although our patient was first treated with prednisolone at a dose of 30 mg/d for protein‐losing gastroenteropathy, he later developed secondary systemic capillary leak syndrome, and thus IVIG was administered at a dose of 0.4 g/kg for 5 days, a regimen that is also considered to be a valid treatment for Sjögren's syndrome. Upon completion of IVIG therapy, there was no clear therapeutic effect, and additional treatment with steroid pule therapy and theophylline therapy were administered, which successfully alleviated the symptoms. However, it is unclear whether the steroid, IVIG, or theophylline therapy was effective.
In conclusion, we report the case of a patient who presented with Sjögren's syndrome concurrent with protein‐losing gastroenteropathy and whose condition became severe upon developing secondary systemic capillary leak syndrome. However, the patient's condition was immediately identified, and he recovered with the simultaneous administration of treatment for Sjögren's syndrome and systemic capillary leak syndrome.
CONSENT
While ensuring the anonymity of the patient, we obtained the patient's written consent to report his case.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare with regard to this report.
AUTHORSHIP
KW: played a key role in the patient's treatment and wrote the draft of the manuscript. SM: played a key role in the patient's treatment. All authors were involved in the treatment, preparation of the original manuscript, and revision of the original manuscript. They have agreed to hold accountability for the translation of this report.
ACKNOWLEDGMENT
We would like to express our gratitude to Dr. Yasuhiro Kato for his clinical advice, Dr. Seika Higuchi‐Uemura for her assistance on revision and Mr. Tomoyuki Uto for his technical advice on revision. We also thank Climson Interactive Pvt. Ltd. (Ulatus) ‐ http://www.ulatus.jp ‐ for its assistance in manuscript translation.
Watanabe K, Murakami S, Misago M, et al. Sjögren's syndrome concurrent with protein‐losing gastroenteropathy with secondary systemic capillary leak syndrome : A case report. Clin Case Rep. 2018;6:1829–1833. 10.1002/ccr3.1675
REFERENCES
- 1. Tsutsumi A, Sugiyama T, Matsumura R, et al. Protein losing enteropathy associated with collagen diseases. Ann Rheum Dis. 1991;50:178‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shiboski SC, Shiboski CH, Criswell LA, et al. American college of rheumatology classification criteria for Sjögren's syndrome: a data‐driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 2012;64:475‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Druey KM, Greipp PR. Narrative Review: Clarkson disease‐systemic capillary leak syndrome. Ann Intern Med. 2010;153:90‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sugiyama T, Koike T, Imaizumi T, et al. A case of Sjögren's syndrome associated with protein‐losing enteropathy. Jpn J Clin Immunol. 1988;11:80‐85. [Japanese]. [Google Scholar]
- 5. Yamada H, Utahashi K, Arai M, et al. A case of erosive gastritis with protein‐losing enteropathy. Shokakika. 1994;18:574‐582. [Japanese]. [Google Scholar]
- 6. Iizuka H, Momoi Y, Suenaga M, et al. A case of protein losing gastroenteropathy with latent Sjögren's syndrome. Kanto Riumachi. 1996;30:33‐40. [Japanese]. [Google Scholar]
- 7. Inoue R, Hotta S, Kurokawa M, et al. A case of protein losing gastroenteropathy with Sjögren's syndrome. Kyushu Riumachi. 1996;15:151‐155. [Japanese]. [Google Scholar]
- 8. Mok MY, Lau CS. Protein losing enteropathy and primary Sjögren's syndrome. Clin Exp Rheumatol. 1997;15:705. [PubMed] [Google Scholar]
- 9. Imai K, Kainuma M, Kohta K, Mitsuma T. The potential effect of Ubai‐en on protein−losing enteropathy. Jpn J Orient Med. 2002;53:229‐234. [Japanese]. [Google Scholar]
- 10. Hsieh TY, Lan JL, Chen DY. Primary Sjögren's syndrome with protein‐losing gastroenteropathy: report of two cases. J Formos Med Assoc. 2002;101:519‐522. [PubMed] [Google Scholar]
- 11. Choi HJ, Shin K, Bae YD, et al. A case of primary Sjögren's syndrome with protein‐losing enteropathy. J Korean Rheumat Assoc. 2004;11:61‐65. [Google Scholar]
- 12. Ushiyama A, Teraoka H, Shiba T, et al. Protein‐losing gastroenteropathy associated with Sjögren's syndrome‐case report and review of the Japanese literature. Nihon Shokakibyo Gakkai Zasshi. 2004;101:1314‐1319. [Japanese]. [PubMed] [Google Scholar]
- 13. Nagashima T, Hoshino M, Shimoji S, et al. Protein‐losing gastroenteropathy associated with primary Sjögren's syndrome: a characteristic oriental variant. Rhumatol Int. 2009;29:817‐820. [DOI] [PubMed] [Google Scholar]
- 14. Nasu T, Miyata K, Uno A, et al. Successful treatment of protein‐losing gastroenteropathy with steroid pulse and immunosuppressive therapies in a patient with Sjögren syndrome. Case Rep Gastroenterol. 2011;5:372‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uraoka Y, Tanigawa T, Watanabe K, et al. Complete remission of protein‐losing gastroenteropathy associated with Sjögren's syndrome by B cell‐targeted therapy with rituximab. Am J Gastroenterol. 2012;107:1266‐1268. [DOI] [PubMed] [Google Scholar]
- 16. Kakigao K, Fukushima N, Mizutani T, et al. A case of protein‐losing gastroenteropathy accompanied by Sjögren syndrome and mixed connective tissue disease. Nihon Shokakibyo Gakkai Zasshi. 2012;109:1770‐1775. [Japanese]. [PubMed] [Google Scholar]
- 17. Chen HY, Hsu CY, Huang WC, et al. An uncommon cause of generalized edema: protein‐losing gastroenteropathy with primary Sjögren's syndrome. Acta Nephrologica. 2013;27:52‐56. [Google Scholar]
- 18. Yamashita H, Muto G, Hachiya R, et al. A case of Sjögren's syndrome complicated by protein‐losing gastroenteropathy with unprecedented pulmonary interstitial lesions. Mod Rhuematol. 2014;24:877‐879. [DOI] [PubMed] [Google Scholar]
- 19. Liao CY, Chien ST, Wang CC, et al. Sjögren's syndrome associated with protein losing gastroenteropathy manifested by intestinal lymphangiectasia successfully treated with prednisolone and hydroxychloroquine. Lupus. 2015;24:1552‐1556. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, Cohen NL, McCarthy S, McHugh JB, Kwon R. Protein‐losing gastroenteropathy associated with Sjögren's syndrome: first known case reported outside of Asia. ACG Case Rep J. 2015;2:184‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izumi Y, Nakaoka K, Kamata M, et al. Steroid‐resistant protein‐losing gastroenteropathy complicated with Sjögren's syndrome successfully treated with mizoribine. Mod Rheumatol. 2016; published online:04 Apr 2016. https://www.tandfonline.com/doi/full/10.3109/14397595.2016.1145570. Accessed June 29, 2018. [DOI] [PubMed] [Google Scholar]
- 22. Ofuji K, Otani M, Matsuda H, et al. A case of protein‐losing gastroenteropathy associated with dermatomyositis and secondory Sjögren's Syndrome. Gastroenerol Endosc. 2016;58:2405‐2411. [Japanese]. [Google Scholar]
- 23. Hadigal S, Lowther G, Prasad A. Primary Sjögrens syndrome causing pleural effusions. Am J Respir Crit Care Med. 2017;195:A1484. [Google Scholar]


