Abstract
TP53 is the most frequently mutated gene in human cancer and thus an attractive target for novel cancer therapy. Several compounds that can reactive mutant p53 protein have been identified. APR-246 is currently being tested in a phase II clinical trial in high-grade serous ovarian cancer. We have used RNA-seq analysis to study the effects of APR-246 on gene expression in human breast cancer cell lines. Although the effect of APR-246 on gene expression was largely cell line dependent, six genes were upregulated across all three cell lines studied, i.e., TRIM16, SLC7A11, TXNRD1, SRXN1, LOC344887, and SLC7A11-AS1. We did not detect upregulation of canonical p53 target genes such as CDKN1A (p21), 14-3-3σ, BBC3 (PUMA), and PMAIP1 (NOXA) by RNA-seq, but these genes were induced according to analysis by qPCR. Gene ontology analysis showed that APR-246 induced changes in pathways such as response to oxidative stress, gene expression, cell proliferation, response to nitrosative stress, and the glutathione biosynthesis process. Our results are consistent with the dual action of APR-246, i.e., reactivation of mutant p53 and modulation of redox activity. SLC7A11, TRIM16, TXNRD1, and SRXN1 are potential new pharmacodynamic biomarkers for assessing the response to APR-246 in both preclinical and clinical studies.
Introduction
The TP53 gene which encodes the p53 tumor suppressor protein is the most frequently mutated gene in human cancer. Thus, in a study of 12 different cancer types, TP53 was the most frequently mutated genes in 10 of the tumor types investigated [1]. Indeed, in some of the most difficult to treat cancers such as squamous esophageal cancer, squamous cell lung cancer, small cell lung cancer, high-grade serous ovarian cancer, and triple-negative breast cancer, TP53 is mutated in approximately 80% of cases [2], [3], [4], [5], [6], [7]. This high prevalence makes mutant p53 protein an attractive therapeutic target for treating multiple types of aggressive cancers.
Mutant p53, along with oncoproteins like Ras and Myc, have been regarded as “undruggable.” However, in recent years, a number of low–molecular weight compounds have been reported to promote refolding and reactivation of mutant p53 to a conformation possessing wild-type properties [8], [9], [10], [11]. Of the mutant p53-reactivating compounds described to date, the most thoroughly investigated and most clinically advanced is APR-246 (also known as PRIMA-1MET) [8], [9], [10], [11]. APR-246 has previously been investigated in two phase I clinical trials [12], [13] and is currently undergoing further clinical trials in patients with high-grade serous ovarian [14], [15] and esophageal cancers (NCT02999893) as well as in patients with myeloid neoplasms (NCT03072043).
APR-246 is converted nonenzymatically to the Michael acceptor methylene quinuclidinone (MQ) that binds covalently to thiol groups in mutant p53, leading to reactivation of the mutant protein and induction of apoptosis [16]. Consistent with its ability to bind to and reactivate mutant p53, several preclinical studies have shown that APR-246 preferentially targets mutant TP53-carrying cells [17], [18], [19], [20], [21]. Other studies, however, have shown that APR-246 can mediate anticancer activity independently of the TP53 mutational status [22], [23], [24].
In addition to binding to p53, APR-246/MQ can also deplete intracellular GSH and induce reactive oxygen species (ROS) [22], [23], [24], [25]. Furthermore, APR-246/MQ has been shown to inhibit the redox enzyme thioredoxin reductase (TrxR1) and convert the enzyme to an active oxidase, which also increases levels of ROS [26]. These effects on the cellular redox system are thought to contribute to the anticancer activity of APR-246. Malignant cells are believed to be particularly sensitive to ROS induction, as these cells already possess high levels of ROS and thus should reach the apoptotic threshold faster than normal cells [27], [28], [29]. The ability of APR-246 to induce ROS may explain why the compound exerts anticancer activity in some wild-type TP53-carrying cells [22], [23], [24].
Thus, APR-246 can inhibit cancer cell growth and trigger cell death through at least two different mechanisms: reactivation of mutant p53 and generation of ROS. To gain further insights into the mechanism of action of APR-246, we investigated the effects of the compound on global gene expression using RNA-seq analysis in three different breast cancer cell lines.
Materials and Methods
Cell Culture
The following panel of breast cell lines was used: BT549, MDA-MD-468, MDA-MB-231, HCC1143, MDA-MD-453, SKBR3 (all p53 mutated), UACC-812, MCF7, and MCF10A (all p53 WT). Both the molecular subtype and the specific p53 mutation of these cell lines are summarized in Table 1. All cell lines were purchased from the American Type Culture Collection and maintained as previously described [21]. Cell line identity was confirmed by analysis of short-term repeat loci. Cells were routinely tested for mycoplasma infection.
Table 1.
Breast cancer cell lines used in this investigation, together with their molecular subtype, p53 mutational status, response to APR-246 measured at IC50 value, and the fold-change of SLC7A11, TRIM16, SRXN1, and TXNRD1 following treatment with 50 μM APR-246 for 12 Hours*
| Cell Line | Molecular Subtype | p53 Mutational Status⁎ | APR-246 IC50 (μM) |
SLC7A11 |
TRIM16 |
SRXN1 |
TXNRD1 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FC | P Value | FC | P Value | FC | P Value | FC | P Value | ||||
| BT549 | TN | p.Arg249Ser | 3.1 ± 0.4 | 21.42 ± 3.6 | .0307 | 2.39 ± 0.2 | .0272 | 4.6 ± 0.8 | .0488 | 1.83 ± 0.1 | .0075 |
| MDA-MB-468 | TN | p.Arg280Lys | 1.9 ± 0.3 | 5.0 ± 0.7 | .0093 | 2.79 ± 0.7 | .05 | 1.72 ± 0.1 | .0267 | 1.72 ± 0.3 | .0442 |
| MDA-MB-231 | TN | p.Arg273His | 4.1 ± 1.6 | 5.47 ± 0.9 | .0057 | 9.6 ± 2.8 | .0409 | 1.28 ± 0.1 | .1452 | 2.26 ± 0.4 | .1067 |
| HCC1143 | TN | p.Arg248Gln | 6.8 ± 1.1 | 6.06 ± 0.9 | .0027 | 3.38 ± 0.4 | .01 | 2.5 ± 0.3 | .02 | 3.33 ± 0.64 | .0357 |
| MDA-MB-453 | Her2+ | p.His368delinsGln | 0.9 ± 0.2 | 11.53 ± 1.9 | .003 | 7.8 ± 1.16 | .0042 | 3.71 ± 0.4 | .006 | 3.36 ± 0.5 | .0135 |
| SKBR3 | Her2+ | p.Arg175His | 5.1 ± 0.6 | 2.73 ± 0.5 | .0237 | 1.51 ± 0.2 | .086 | 2.17 ± 0.9 | .272 | 2.96 ± 0.9 | .119 |
| UACC812 | Her2+ | Wild type | 11.3 ± 1.8 | 2.4 ± 0.6 | .0927 | 1.29 ± 0.1 | .0747 | 0.77 ± 0.1 | .1328 | 0.77 ± 0.3 | .4989 |
| MCF7 | ER+ | Wild type | 31.1 ± 24.6 | 2.17 ± 0.3 | .0223 | 1.65 ± 0.15 | .0248 | 1.45 ± 0.1 | .0115 | 1.74 ± 0.2 | .0654 |
| MCF10A | Epithelial | Wild type | 5.2 ± 1.3 | 2.16 ± 0.5 | .0923 | 0.88 ± 0.17 | .5203 | 0.99 ± 0.2 | .0717 | 0.59 ± 0.11 | .0674 |
Gene expression was based on qPCR. P values were calculated using paired t test. Cell lines shown in bold were also analyzed by RNA-seq. FC = fold change.
Source; UMD TP53 Mutation database.
RNA Sequencing
Cells were treated with 50 μM of APR-246 or DMSO control in a 10-cm dish for 12 hours. A short treatment time was used in order to detect early response genes. Total RNA from the three biological replicates of each of the cell lines investigated was extracted using the miRNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. RNA concentration and integrity were determined using RNA 6000 Nano Kit on an Agilent Bioanalyzer 2100 (Agilent Systems). RNA-seq cDNA libraries were prepared using the Illumina TruSeq Stranded Total RNA Library PrepKit with Ribo-Zero according to the manufacturer's instructions (Illumina). Clustering was carried out by the cBot system. Pair-end sequencing was carried out on an Illumina HiSeq2500 (HiSeq Control Software 2.2.58/RTA 1.18.64) using HiSeq sequencing by synthesis (SBS) v4 kit. The Bcl to FastQ conversion was performed using the CASAVA software suite. The quality scale used was Sanger/phred33/Illumina 1.8+. Library preparation and sequencing were carried out by the Science for Life Laboratory (Stockholm, Sweden).
Biostatistical Analysis
Analysis was performed as previously described [30]. An adjusted P value of less than .05 and a fold-change greater than 1.4-fold were considered significant.
RNA Isolation and Real-Time PCR
RNA from cell lines was isolated using the RNeasy Mini kit (Qiagen). One microgram of RNA was reverse transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen). All primers were purchased from Qiagen. GADPH and 18s RNA were used as loading controls. The amplification process was carried out as recommended by Qiagen for the Roche LightCycler480.
Detection of Confirmation Specific Antibodies PAb1620 and PAb240
SKBR3 cells (which contain the p53 structural mutation, Arg175His) were seeded to confluency in an eight-well chamber slide. The following day, cells were treated with 50 μM APR-246 or DMSO control for 3 hours. Cells were fixed and stained as previously described [30]. Stained cells were visualized using a Leitz DM40 microscope (Leica Microsystems), and images were captured using the AxioCam system and AxioVision 3.0.6. In addition, FACS analysis was performed using a BD FACSCanto. Data were analyzed using FlowJo software v10.1.3.
Results
Effect of APR-246 on Global Gene Expression
To investigate effects of APR-246 on global gene expression, we treated BT549, MDA-MB-468, and MCF7 cells with 50 μM APR-246 for 12 hours and analyzed the differentially expressed genes using RNA-seq. We used 50 μM as this concentration of APR-246 induced significant cell death in the mutant TP53-carrying cells after 24 hours of treatment (Supplementary Figure 1). Furthermore, in this study, we deliberatively used a relatively short treatment schedule (12 hours) to detect the early response genes to APR-246 treatment.
With the BT549 cells, 99 genes were significantly upregulated and 63 genes significantly downregulated following treatment with APR-246 (Supplementary Table 1). For the MDA-MB-468 cells, 24 genes were significantly upregulated and 2 genes downregulated following treatment with APR-246 (Supplementary Table 2). For the MCF7 cells, 25 genes were significantly upregulated and 1 gene downregulated following treatment with APR-246 (Supplementary Table 3). Six genes were differentially expressed in all three cell lines investigated following treatment with APR-246. Of these genes, four were protein coding, i.e., TRIM16, SLC7A11, TXNRD1, and SRXN1, and two noncoding, i.e., LOC344887 and SLC7A11-AS1.
Effect of APR-246 on Cellular Signaling Pathways
To investigate how the genes differentially regulated by APR-246 impacted on cellular signaling pathways, we applied the modulated gene lists to the gene ontology (GO), R package GoSeq program. The top 20 GO terms significantly enriched in BT549, MDA-MB-468, or MCF7 cell lines are listed in Supplementary Tables 4, 5, and 6, respectively. As might be expected from targeting p53, the GO pathways modified in the BT549 cells, which had the greatest number of differentially regulated genes, include pathways implicated in positive regulation of gene expression and cell death. In addition, the GO pathways differentially regulated in two or more of the cell lines investigated included response to oxidative stress, gene expression, cell proliferation, response to nitrosative stress, and the glutathione biosynthesis process.
Effects of APR-246 on p53 Canonical Gene Expression
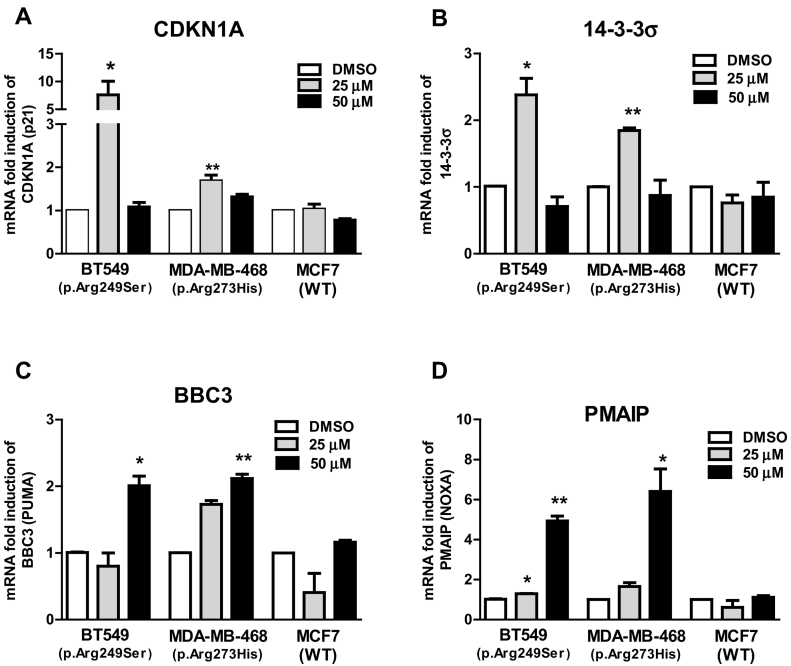
Since we did not detect any upregulation of canonical p53 target genes by RNA-seq, we used qPCR to investigate the effects of APR-246 on established p53 target genes associated with cell cycle arrest and apoptosis. Twelve hours of treatment with 25 μM APR-246 resulted in a significant induction of the cell cycle arrest-associated genes CDKN1A and 14-3-3σ in the two mutant TP53-carrying cell lines investigated (Figure 1, A and B). Similarly, treatment with 50 μM APR-246 resulted in a significant upregulation of the proapoptotic genes BBC3 (PUMA) and PMAIP1 (NOXA) (Figure 1, C and D). We did not detect any significant changes in expression of classical p53 target genes in the wild-type-TP53–carrying MCF7 cells at the APR-246 concentrations used.
Figure 1.
Fold-change in mRNA expression of CDKN1A (A), 14-3-3σ (B), BBC3 (C), or PMAIP1 (D) in three breast cancer cell lines. Cells were treated with 25 or 50 μM APR-246 or DMSO for 12 hours. Data were analyzed using paired t test. All experiments were carried out in triplicate. Values are means ± SEM, *P > .01, **P > .001, ***P > .0001.
Validation of RNAseq Results by qPCR
To validate the results from the RNA-seq experiments, we investigated the effects of APR-246 on the expression of TRIM16, SLC7A11, SRXN1, and TXNRD1 using qPCR. Using this standard method for assessing gene expression, we confirmed the increased expression of TRIM16, SLC7A11, and SRXN1 in all the three cell lines (Table 1). Similarly, we observed an increase in expression of TXNRD1 in the two mutant TP53 cell lines, i.e., in BT549 and MDA-MB-468 cells, using both RNA-seq and qPCR. However, the increased expression of TXNRD1 observed in the MCF7 cells according to RNA-seq was not confirmed by qPCR.
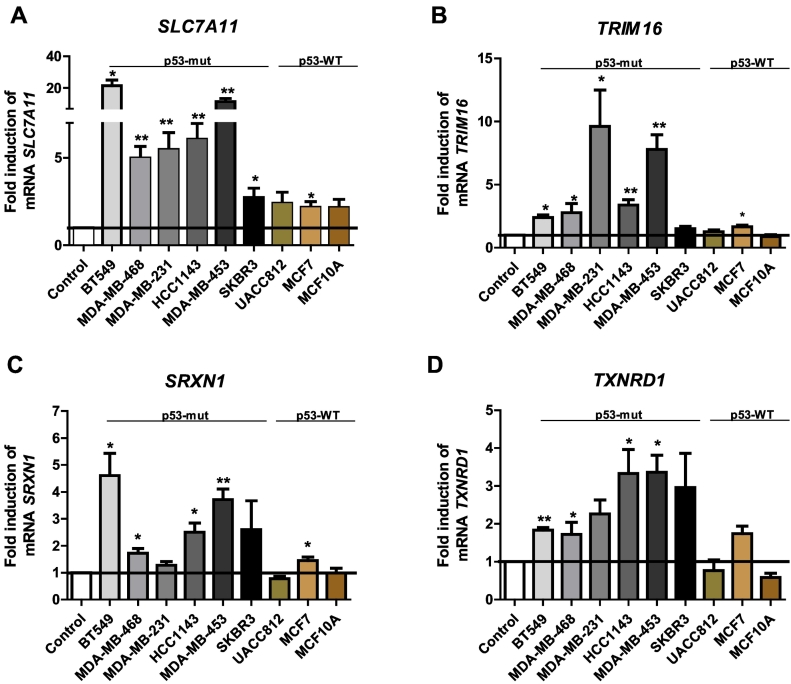
In order to address the generality of the increased expression of TRIM16, SLC7A11, SRXN1, and TXNRD1 by APR-246, we carried out qPCR in another six breast cancer cell lines, i.e., in a total of nine cell lines (Table 1, Figure 2). All mutant TP53-carrying cell lines showed a significantly increased expression of SLC7A11 following treatment with APR-246 (Figure 2A). Furthermore, six of nine cell lines showed a significant increase in the expression of TRIM16, five of nine showed increased expression of SRXN1, and four of nine showed increased expression of TXNRD1.
Figure 2.
Fold-change in mRNA expression of SLC7A11 (A), TRIM16 (B), SRXN1 (C), or TXNRD1 (D) in a panel of nine breast cell lines. Cells were treated with 50 μM APR-246 or DMSO for 12 hours. Data were analyzed using paired t test. All experiments were carried out in triplicate. Values are means ± SEM, *P > .01, **P > .001, ***P > .0001; p53-mut, p53 mutated; P53-WT; p53 wild-type.
Effects of APR-246 on Mutant p53 Refolding
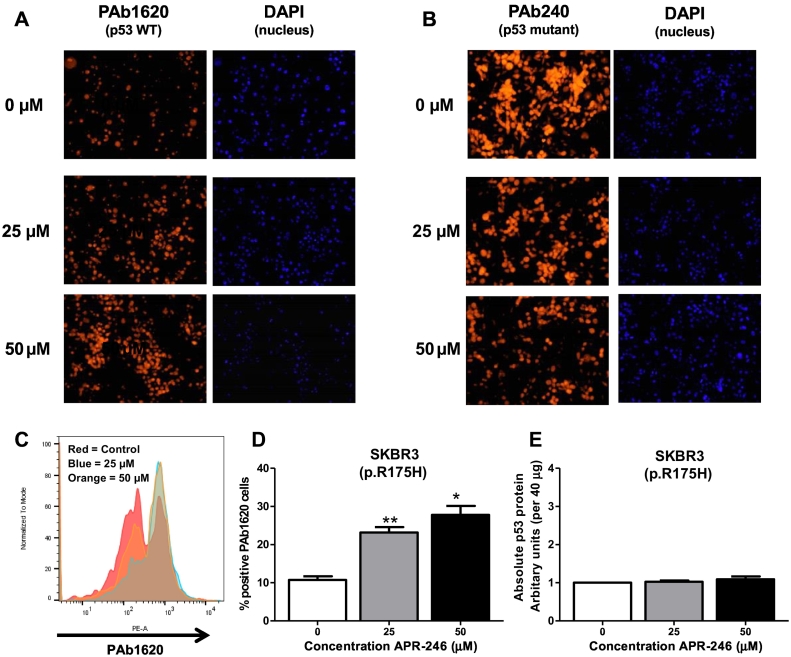
Unfolding of the p53 protein is one of the main consequences of structural p53 mutations. To establish if the addition of APR-246 reversed this unfolding, we used the p53 conformation-specific antibodies PAb240 and PAb1620. As seen in Figure 3A, treatment with APR-246 resulted in a dose-dependent increase in staining with the wild-type associated p53 antibody PAb1620. Simultaneously, there was a dose-dependent decrease in fluorescence using the mutant specific p53 antibody PAb240 (Figure 3B). To confirm these results, we quantified the fluorescent staining of PAb1620 by flow cytometry. A dose-dependent increase in PAb1620 staining was seen in the p53 mutated cell line SKBR3 (Figure 3, C and D). To ensure that the changes in fluorescent staining, i.e., p53 folding, were not due to changes in the total p53 protein levels, we quantified the absolute p53 protein by ELISA following the same treatment conditions (Figure 3E). No significant change in p53 protein levels was seen after APR-246 treatment.
Figure 3.
Image representative of SKBR3 cells treated with APR-246 and stained with PAb1620 (A) to detect wild-type-p53 or PAb240 (B) to detect mutant p53. DAPI nuclear stain was used as a control. All experiments were carried out in triplicate. (C) Histogram representatives of SKBR3 cells treated with APR-246 and stained with PAb1620 to detect WT-p53. (D) Bar chart representation of Pab1620 staining measured by flow cytometry and analyzed using FlowJo v.10 software. Data were analyzed using paired t test. (E) Bar chart representation of absolute p53 protein levels quantified using the PathScan p53 ELISA kit.
Discussion
To our knowledge, this is the first study to investigate the effects of the mutant p53-targeting compound APR-246 on global gene expression using RNA-seq analysis, although this has been studied by microarrays in several reports (see below). Our results showed that the effect of APR-246 on gene expression was largely breast cancer cell line dependent. This is consistent with studies indicating that the effects of p53 on gene expression are also largely cell type specific [31]. The number of genes whose expression was modulated by APR-246 also varied between the three cell lines. APR-246 induced changes in expression of 162 genes in the BT549 cells, whereas only 26 genes showed altered expression in APR-246–treated MDA-MB-468 and MCF7 cells. The BT549 cells express the structural p53 mutant R249S. MDA-MB-468 cells express the contact p53 mutant R273H which can also be reactivated by APR-246, while MCF7 cells carry wild-type TP53. Previously, the p53 R273H mutation was shown to be reactivated by PRIMA-1 in the Saos-2-His273 cell line [17], while the R175H mutation was shown to be reactivated by APR-246/MQ [32].
Although the effect of APR-246 on gene regulation was largely cell line specific, the expression of six genes, i.e., TRIM16, SLC7A11, TXNRD1, SRXN1, LOC344887, and SLC7A11-AS1, was altered in all three cell lines investigated. Four of these genes are protein encoding, namely, TRIM16, SLC7A11, TXNRD1, and SRXN1, while LOC344887 and SLC7A11-AS1 do not code for proteins. Indeed, increased expression of all four protein-encoding genes by APR-246 was confirmed by qPCR in the two mutant p53-expressing cell lines. We observed upregulation of TRIM16, SLC7A11, and SRXN1 but not TXDRD1 in wild-type TP53-carrying MCF7 cells. Furthermore, APR-246–mediated increased expression of these genes was observed in additional breast cancer cell lines. The increased expression of the above four genes in multiple breast cancer cell lines suggests that they play an important role in the mode of action of APR-246.
Consistent with the dual action of APR-246, i.e., reactivation of mutant p53 and generation of ROS, all four consistently regulated protein-coding genes have previously been associated with p53 function and/or redox regulation. Thus, using esophageal cell lines, Liu et al. [25] showed that binding of mutant p53 to the transcription factor NRF2 suppressed expression of SLC7A11. If mutant p53 suppresses expression of SLC7A11 via NRF2, reactivation of mutant p53 by APR-246 would be expected to upregulate SLC7A11, which is what we observed. Consistent with our findings in breast cancer cells, increased expression of SLC7A11 was previously found in leukemia cell lines following treatment with APR-246 [23]. Similarly, SLC7A11 was shown to be upregulated by another mutant p53-reactivating compound, PK11007, in HCC1143 breast cancer cells [30].
Upregulation of SLC7A11 by APR-246 may modulate ROS levels [25]. SLC7A11 encodes a component of the cystine/glutamate antiporter system xC−, which functions to import cystine into cells. As this is the rate-limiting step for the formation of the antioxidant tripeptide GSH, the upregulation of SLC7A11 might be interpreted as a cellular response to protect against excessive oxidative stress [23].
To our knowledge, there are no published studies showing that expression of TXNRD1 encoding thioredoxin reductase (TrxR) or SRXN1 which encodes sulfiredoxin is directly regulated by p53. However, both TrxR and sulfiredoxin are important redox regulators [26], [33], [34], [35], [36]. TrxR is a selenoprotein involved in scavenging ROS and protecting cells against oxidative damage. Thus, the upregulation of TXNRD1, like that of SLC7A11, might also be interpreted as a response to increased ROS. The observed upregulation of TXNRD1 by APR-246 is particularly interesting given the previous finding that APR-246 and MQ are efficient inhibitors of TrxR activity [26].
Although TRIM16 has not, to our knowledge, been previously reported to be p53-regulated or implicated in controlling redox balance, other members of the TRIM family, especially TRIM19 (also known as PML), were shown to be direct targets of p53 [37]. Indeed, TRIM19/PML has been found to contribute to p53-mediated cell cycle arrest, apoptosis, and senescence [37]. Other members of the TRIM family linked to p53 action include TRIM8 [38] and TRIM29 [39]. Interestingly, TRIM16 is upregulated by the mutant p53-reactivating compound PK11007 in HCC1143 breast cancer cells [30].
Two previous studies have investigated the effects of APR-246 on gene expression in mutant TP53-carrying cells using microarray analysis [23], [40]. Lambert et al. found that 185 genes were regulated by APR-246 in Saos-2 cells expressing R273H mutant p53 [40]. However, only six of these genes overlap with the identified APR-246–regulated genes in the present study, i.e., SESN2, SLC1A4, SLC7A1, TFE3, KIAA0226, and XBP1. Using the acute myeloid leukemia cell line KMB3, Ali et al. [23] reported that APR-246 upregulates expression of 10 genes, including SLC7A11, which is in agreement with our results.
Surprisingly, we did not detect upregulation of canonical p53 target genes such as CDKN1A (p21) PMAIP1 (NOXA), and BBC3 (PUMA) by RNA-seq following treatment with APR-246. However, our qPCR analysis showed that expression of CDKN1A, PMAIP1, BBC3, and 14-3-3σ was increased by APR-246 in the two mutant p53-expressing cell lines. The ability to induce expression of these canonical p53-regulated genes is consistent with reactivation of mutant p53 by APR-246. Consistent with our findings, Lambert et al. [40] also failed to detect upregulation of some classical p53-regulated genes such as CDKN1A, BAX, and MDM2 after treatment of Saos-His273 cells with APR-246, whereas increased expression of BAX was detected by RT-PCR. These results suggest that global methods for detecting gene expression changes such microarray and RNA-seq may have inadequate sensitivity for detecting the expression of specific genes but rather provide identification of stress-response patterns of gene expression.
In addition to investigating the effects of APR-246 on global gene expression, we carried out GO analysis to identify specific pathways that were altered by the compound. Following analysis of BT549 cells which had the greatest number of genes altered by APR-246, we found that several of the significantly enriched terms identified might be expected from the reactivation of mutant p53. These included regulation of cell death, regulation of apoptosis, protein refolding, programmed cell death, cellular response to stimuli, and signal transduction. Previously, using microarrays, Lambert et al. [40] reported that pathways involving cell-cycle arrest, apoptosis, and endoplasmic reticulum stress were altered following treatment with APR-246.
Although this is one of the most comprehensive studies to date on gene modulation by the p53 reactivating compound APR-246, our study has some limitations. One of these relates to the use of a single time point, i.e., 12 hours, for investigating the effects of APR-246. However, as stated in the Methods section above, we deliberatively used a short treatment time to focus on the early/relatively early response genes. A further limitation is that we did not investigate whether different extents of glutathione depletion or ROS formation contributed to the cell line–specific effects observed.
In conclusion, this work confirms and extends our knowledge on the mode of action of the first mutant p53-reactivating compound to enter clinical trials. We show that the effects of APR-246 on modulating gene expression are largely but not totally cell line specific. Indeed, genes such as SLC7A11, TRIM16, TXNRD1, and SRXN1 were upregulated in all the three cell lines investigated. The known ability of three of these genes to modulate ROS levels is further evidence that APR-246 exerts its anticancer activity at least partly by inducing ROS. Finally, genes such as SLC7A11, TRIM16, TXNRD1, and SRXN1 which appear to be widely regulated by APR-246 are potential new pharmacodynamic biomarkers for assessing the response to APR-246 in the clinic.
Acknowledgments
Acknowledgements
The authors wish to thank the Irish Cancer Society Collaborative Cancer Research Centre BREAST-PREDICT program (CCRC13GAL) and the Cancer Clinical Research Trust for funding this work. K. G. W. is supported by the Swedish Cancer Society (Cancerfonden), the Swedish Research Council (VR), Radiumhemmets Forskningsfonder, an ERC Advanced grant (TRANSREAD 694825), and Karolinska Institutet.
Conflict of Interest
K. G. W. and V. J. N. B. are cofounders and shareholders of Aprea Therapeutics AB, a company that develops p53-based cancer therapy including APR-246. K. G. W. is a member of its Clinical Advisory Board. Research in the K. G. W. laboratory has received financial support from Aprea Therapeutics AB. K. G. W. has received a salary from Aprea Therapeutics AB. N. S., S. M., J. C., and M. J. D. have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2018.08.009.
Appendix A. Supplementary data
Supplementary material
References
- 1.Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet. 2013;14:703–718. doi: 10.1038/nrg3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [Erratum in: Nature 2012 Nov 8;491:288. Rogers, Kristen [corrected to Rodgers, Kristen]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, Plenker D, Leenders F, Sun R, Zander T. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MJ, Synnott NC, Crown J. Mutant p53 as a target for cancer treatment. Eur J Cancer. 2017;83:258–265. doi: 10.1016/j.ejca.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Sabapathy K, Lane DP. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat Rev Clin Oncol. 2018;15:13–30. doi: 10.1038/nrclinonc.2017.151. [DOI] [PubMed] [Google Scholar]
- 11.Blandino G, Di Agostino S. New therapeutic strategies to treat human cancers expressing mutant p53 proteins. J Exp Clin Cancer Res. 2018;37(1):30–43. doi: 10.1186/s13046-018-0705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann S, Bykov VJ, Ali D, Andrén O, Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30:3633–3639. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 13.Deneberg S, Cherif H, Lazarevic V, Andersson PO, von Euler M, Juliusson G, Lehmann S. An open-label phase I dose-finding study of APR-246 in hematological malignancies. Blood Cancer J. 2016;6:e447. doi: 10.1038/bcj.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gourley C, Green J, Gabra H, Vergote I, Basu B, Brenton JD, Björklund U, Smith A, Von Euler M. PISARRO: A EUTROC phase Ib study of APR-246 in combination with carboplatin (C) and pegylated liposomal doxorubicin (PLD) in platinum sensitive relapsed high grade serous ovarian cancer (HGSOC) J Clin Oncol. 2016;34:5571. [suppl; abstr 5571] [Google Scholar]
- 15.Gourley C, Gabra H, Vergote I, Basu B, Brenton J, Von Euler M, Björklund U, Smith AM, Green J. EUTROC PiSARRO: A phase Ib study combining APR-246 with standard chemotherapy in platinum sensitive relapsed high grade serous ovarian carcinoma (HGSOC) J Clin Oncol. 2015;33:5605. [suppl; abstr TPS5605] [Google Scholar]
- 16.Lambert JM, Gorzov P, Veprintsev DB, Söderqvist M, Segerbäck D, Bergman J, Fersht AR, Hainaut P, Wiman KG, Bykov VJ. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15:376–388. doi: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 18.Bykov VJ, Zache N, Stridh H, Westman J, Bergman J, Selivanova G, Wiman KG. PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene. 2005;24:3484–3491. doi: 10.1038/sj.onc.1208419. [DOI] [PubMed] [Google Scholar]
- 19.Bykov VJ, Issaeva N, Selivanova G, Wiman KG. Mutant p53-dependent growth suppression distinguishes PRIMA-1 from known anticancer drugs: a statistical analysis of information in the National Cancer Institute database. Carcinogenesis. 2002;23:2011–2018. doi: 10.1093/carcin/23.12.2011. [DOI] [PubMed] [Google Scholar]
- 20.Shi H, Lambert JM, Hautefeuille A, Bykov VJ, Wiman KG, Hainaut P, Caron de Fromentel C. In vitro and in vivo cytotoxic effects of PRIMA-1 on hepatocellular carcinoma cells expressing mutant p53ser249. Carcinogenesis. 2008;29:1428–1434. doi: 10.1093/carcin/bgm266. [DOI] [PubMed] [Google Scholar]
- 21.Synnott NC, Murray A, McGowan PM, Kiely M, Kiely PA, O'Donovan N, O'Connor DP, Gallagher WM, Crown J, Duffy MJ. Mutant p53: a novel target for the treatment of patients with triple-negative breast cancer? Int J Cancer. 2017;140:234–246. doi: 10.1002/ijc.30425. [DOI] [PubMed] [Google Scholar]
- 22.Tessoulin B, Descamps G, Moreau P, Maïga S, Lodé L, Godon C, Marionneau-Lambot S, Oullier T, Le Gouill S, Amiot M. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood. 2014;124:1626–1636. doi: 10.1182/blood-2014-01-548800. [DOI] [PubMed] [Google Scholar]
- 23.Ali D, Mohammad DK, Mujahed H, Jonson-Videsäter K, Nore B, Paul C, Lehmann S. Anti-leukaemic effects induced by APR-246 are dependent on induction of oxidative stress and the NFE2L2/HMOX1 axis that can be targeted by PI3K and mTOR inhibitors in acute myeloid leukaemia cells. Br J Haematol. 2016;174:117–126. doi: 10.1111/bjh.14036. [DOI] [PubMed] [Google Scholar]
- 24.Mohell N, Alfredsson J, Fransson Å, Uustalu M, Byström S, Gullbo J, Hallberg A, Bykov VJ, Björklund U, Wiman KG. PR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu DS, Duong CP, Haupt S, Montgomery KG, House CM, Azar WJ, Pearson HB, Fisher OM, Read M, Guerra GR. Inhibiting the system xC−/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun. 2017;8 doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng X, Zhang MQ, Conserva F, Hosny G, Selivanova G, Bykov VJ, Arnér ES, Wiman KG. APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis. 2013;4:e881. doi: 10.1038/cddis.2013.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 28.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 29.Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochem Pharmacol. 2014;92:90–101. doi: 10.1016/j.bcp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Synnott NC, Bauer MR, Madden S, Murray A, Klinger R, O'Donovan N, O'Connor D, Gallagher WM, Crown J, Fersht AR. Mutant p53 as a therapeutic target for the treatment of triple-negative breast cancer: preclinical investigation with the anti-p53 drug, PK11007. Cancer Lett. 2018;414:99–106. doi: 10.1016/j.canlet.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 31.Allen MA, Andrysik Z, Dengler VL, Mellert HS, Guarnieri A, Freeman JA, Sullivan KD, Galbraith MD, Luo X, Kraus WL. Elife. 2014;3 doi: 10.7554/eLife.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Bykov VJN, Wiman KG, Zawacka-Pankau J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 2018;9:439. doi: 10.1038/s41419-018-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W, Zou P, Zhao Z, Weng Q, Chen X, Ying S, Ye Q, Wang Z, Ji J, Liang G. Selective killing of gastric cancer cells by a small molecule via targeting TrxR1 and ROS-mediated ER stress activation. Oncotarget. 2016;7:16593–16609. doi: 10.18632/oncotarget.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 35.Cunniff B, Snider GW, Fredette N, Stumpff J, Hondal RJ, Heintz NH. Resolution of oxidative stress by thioredoxin reductase: cysteine versus selenocysteine. Redox Biol. 2014;2:475–484. doi: 10.1016/j.redox.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae SH, Sung SH, Lee HE, Kang HT, Lee SK, Oh SY, Woo HA, Kil IS, Rhee SG. Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury. Antioxid Redox Signal. 2012;17:1351–1361. doi: 10.1089/ars.2011.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Stanchina E, Querido E, Narita M, Davuluri RV, Pandolfi PP, Ferbeyre G, Lowe SW. PML is a direct p53 target that modulates p53 effector functions. Mol Cell. 2004;13:523–535. doi: 10.1016/s1097-2765(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 38.Caratozzolo MF, Marzano F, Mastropasqua F, Sbisà E, Tullo A. TRIM8: Making the right decision between the oncogene and tumour suppressor role. Genes (Basel) 2017;8(12):354–368. doi: 10.3390/genes8120354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sho T, Tsukiyama T, Sato T, Kondo T, Cheng J, Saku T, Asaka M, Hatakeyama S. TRIM29 negatively regulates p53 via inhibition of Tip60. Biochim Biophys Acta. 2011;1813:1245–1253. doi: 10.1016/j.bbamcr.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Lambert JM, Moshfegh A, Hainaut P, Wiman KG, Bykov VJ. Mutant p53 reactivation by PRIMA-1MET induces multiple signaling pathways converging on apoptosis. Oncogene. 2010;29:1329–1338. doi: 10.1038/onc.2009.425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material