Abstract
Objective
To determine the association between serum lipid measurements and the occurrence of out-of-hospital sudden unexpected death (OHSUD).
Patients and Methods
We compared 139 OHSUD cases (43 female patients [30.9%]) and 968 controls (539 female patients [55.7%]) from Wake County, North Carolina, from March 1, 2013, through February 28, 2015. Individuals were included if they were aged 18 to 64 years and had lipid measurements in the 5 years before their death (cases) or the most recent health care encounter (controls). Covariates were abstracted from medical records for all subjects, and those with triglyceride (TG) levels greater than 400 mg/dL (to convert to mmol/L, multiply by 0.0259) were excluded for low-density lipoprotein (LDL)–related analyses.
Results
By linear regression using age- and sex-adjusted models, cases of OHSUD had lower adjusted mean total cholesterol (170.3±52.2 mg/dL vs 188.9±39.7 mg/dL; P<.001), LDL cholesterol (90.9±39.6 mg/dL vs 109.6±35.2 mg/dL; P<.001), and non–high-density lipoprotein (HDL) (121.6±49.8 mg/dL vs 134.3±39.6 mg/dL; P<.001) levels and a higher adjusted TG/HDL-C ratio (4.7±7 vs 3±2.7; P<.001) than did controls. By logistic regression using age- and sex-adjusted models, the odds of OHSUD were elevated per unit increase in TG/HDL-C ratio (1.08; 95% CI, 1.03-1.12).
Conclusion
Out-of-hospital sudden unexpected death cases had more favorable levels of total cholesterol, LDL cholesterol, and non-HDL, possibly indicating a lack of association between traditional lipid cardiovascular risk factors and sudden unexpected death. A comparatively elevated TG/HDL-C ratio in cases may corroborate an evolving hypothesis of how vasoactive and prothrombotic remnant-like lipoprotein particles contribute to sudden unexpected death.
Abbreviations and Acronyms: BMI, body mass index; CDW, Carolina Data Warehouse for Health; EMS, emergency medical services; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NHANES, National Health and Nutrition Examination Survey; OHSUD, out-of-hospital sudden unexpected death; OR, odds ratio; RLP, remnant lipoprotein particle; SUDDEN, Sudden Unexpected Death in North Carolina; TC, total cholesterol; TG, triglyceride
Sudden death remains a public health challenge, despite substantial progress in identifying associated clinical, structural, and genetic factors,1, 2 with an annual incidence ranging from 150,000 to 400,000 in the United States. Attempts to further elucidate characteristics predictive of sudden death are therefore crucial for identifying populations at risk and instituting preventive measures.
The association between high total cholesterol (TC) levels,3 high low-density lipoprotein cholesterol (LDL-C) levels,4 high triglyceride (TG) levels,5 low high-density lipoprotein cholesterol (HDL-C) levels, and cardiovascular risk is well known. However, few studies have examined the relationship between lipid measurements and sudden death, with varied conclusions. Large prospective cohort studies have found both an elevated6, 7 and a similar8 risk of sudden death associated with higher baseline TC measurements. Postmortem studies have reported higher levels of remnant lipoprotein particles (RLPs) in cases of sudden death.9
There is a lack of comprehensive data on lipid measurements in nonelderly adults temporally closer to death. We aimed to fill this gap by conducting a case-control study comparing lipid profiles of out-of-hospital sudden unexpected death (OHSUD) cases with available lipid profiles with those who did not experience sudden unexpected death within a population-based case-control study from Wake County, a socioeconomically and racially diverse region in North Carolina. We hypothesized that sudden death cases would have a similar or less favorable lipid profile relative to control subjects.
Patients and Methods
The Sudden Unexpected Death in North Carolina (SUDDEN) study is an ongoing population-based registry of adjudicated premature OHSUD cases in North Carolina. The SUDDEN study aims to capture all natural OHSUDs and includes sudden death cases regardless of whether the fatal event was witnessed and/or resuscitation attempted by using criteria similar to those of the Resuscitation Outcomes Consortium for out-of-hospital cardiac arrest.10 The pilot study was conducted from March 1, 2013, through February 28, 2015, in Wake County, NC, a diverse and predominantly urban region (2015 population estimate: 1,024,198; 21.3% blacks11).
Out-of-Hospital Sudden Unexpected Death Cases
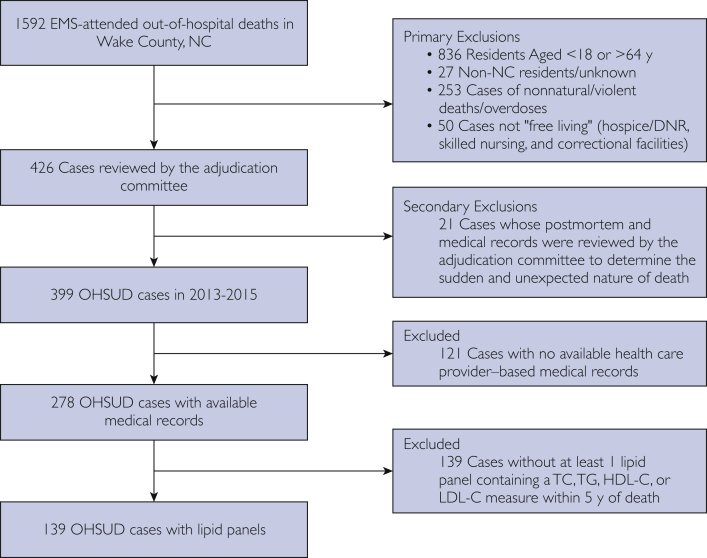
Cases of OHSUD were adjudicated according the pilot criteria for the SUDDEN study described elsewhere.12 We briefly present them here. All emergency medical services (EMS)–attended out-of-hospital deaths occurring from March 1, 2013, through February 28, 2015, in North Carolina residents aged 18 to 64 years were queried from Wake County EMS patient care reporting software. We then excluded those not “free living” such as inmates of prisons/correctional institutions, residents of skilled nursing facilities/hospice, and individuals with “do not resuscitate” orders. Those who had nonnatural/violent deaths were also excluded using information from EMS narratives, medical examiner, and/or autopsy reports (Figure 1). For all included cases, we requested medical records of 5 years preceding death from area health care providers. A committee of cardiologists reviewed all records acquired to adjudicate the sudden and unexpected nature of death for a case. For the present study, we included only adjudicated OHSUD cases with 1 or more lipid panel within their medical records.
Figure 1.
Ascertainment of OHSUD cases. DNR = do not resuscitate; EMS = emergency medical services; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; OHSUD = out-of-hospital sudden unexpected death; TC = total cholesterol; TG = triglyceride.
Control Group
A control group was formed by randomly sampling 15,000 patients from the Carolina Data Warehouse for Health (CDW), a data repository containing electronic health records of 18 hospitals and practices within the University of North Carolina Healthcare Systems.13 This sample size was chosen by estimating the number of patients required for sufficient Wake County residents to be randomly included in the sample and serve as controls in an approximately 3:1 control-case ratio for various analyses. Patients were eligible for selection if they had visited University of North Carolina Healthcare Systems’ hospital systems or affiliate providers from March 1, 2013, through February 28, 2015 (index visit), and were aged 18 to 64 years during the index visit. We obtained 5 years of CDW electronic medical records before the index visit of selected patients. Patients were excluded if their home address was not in Wake County, NC (n=9710), or if they did not have any medical history recorded within the previous 5 years in CDW (n=590). We excluded patients with documented discharge to a correctional institution, law enforcement, skilled nursing facility, assisted care facility, long-term acute care facility, or hospice (n=592). Finally, we included in our analysis only patients with 1 or more lipid measurement in 5 years preceding their index visit (n=968).
Data Ascertainment
For OHSUD cases and controls, we obtained age at death or index visit, sex, and race from death certificates and electronic health care records, respectively.
Dates of lipid measurements and values of TC, TG, HDL-C, and LDL-C were abstracted from the most recent laboratory reports or health care provider notes for cases; for controls, the most recent lipid measurements within the electronic record preceding the index visit were used. If, for any subject, a single lipid panel had only 3 of 4 lipid measures available, we used the Friedewald equation (LDL=(TC–HDL–TG)/5)14 to estimate the missing value, with the LDL-C value calculated only if a TG value was 400 mg/dL or less (to convert to mmol/L, multiply by 38.67) and the TG value calculated only if a calculated LDL value was available. For all subjects, we calculated non-HDL values using the following equation: non-HDL=TC–HDL-C. We also derived the TG/HDL-C ratio, TC/HDL-C ratio, and LDL-C/HDL-C ratio.
We obtained heart rate, systolic blood pressure, diastolic blood pressure, and body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) from the most recent record available. Body mass index was either directly abstracted or calculated from abstracted height and weight.
For cases, comorbid medical conditions such as diabetes mellitus, dyslipidemia, hypertension, and coronary artery disease were abstracted from medical records according to the presence of diagnostic terms, whereas for controls, International Classification of Diseases, Ninth Revision/International Statistical Classification of Diseases, 10th Revision codes were used (Supplemental Table 1, available online at http://www.mcpiqojournal.org). We also recorded the use of lipid-lowering medication by searching the medical record for generic and brand medication names.
Reliability of the abstraction process for OHSUD cases was ensured via blind reabstraction of a 10% sample by a quality officer. Electronic record abstraction queries for controls were developed by B.M.B. and verified by N.H. and G.J.
Statistical Analyses
Differences were tested using a 2-sided t test for continuous variables and Pearson chi-square test for categorical variables. We used multiple linear regression to assess the unadjusted (model 1) and age- and sex-adjusted (model 2) mean differences in lipid measures between cases and controls. We performed logistic regression to calculate the unadjusted (model 1) and age- and sex-adjusted (model 2) odds of OHSUD associated with each lipid measurement. We additionally included another model (model 3), which adjusts for age, sex, BMI, diabetes mellitus, and interaction between each lipid measure and lipid-lowering medication use. For multivariate analysis, we partitioned TC, TG, HDL-C, LDL-C, and non-HDL values into quartiles (lowest quartile as reference). For all analysis pertaining to LDL-C, subjects with TG levels greater than 400 mg/dL were excluded if the direct LDL-C value was missing. For all analyses, we used a significance level of .05 and SAS 9.4 (SAS Institute Inc).
To test the robustness of our conclusions, we defined a second control group using 18- to 64-year-old participants from the 2009 to 2010 National Health and Nutrition Examination Survey (NHANES) who were confirmed alive 1 year after the survey and had a TC, TG, HDL-C, or LDL-C measurement (n=4571).15 All information was obtained from publicly available databases.15 National Health and Nutrition Examination Survey methods are detailed elsewhere.16 Briefly, lipid measurements and BMI of participating subjects were recorded during clinical examinations; comorbid medical conditions were self-reported.
Results
From March 1, 2013, through February 28, 2015, we identified 139 OHSUD cases with at least 1 lipid measurement available in clinical records. After exclusions, we identified 968 controls. As presented in Table 1, OHSUD cases were older (54.8±7.6 years vs 47.2±8.7 years; P<.001), less likely to be female (30.9% [n= 43] vs 55.7% [n=539]; P<.01), and more likely to be black (36.7% [n=51] vs 20.4% [n=197]; P<.05).
Table 1.
Population Characteristics of OHSUD Cases, Local Controls, and 2009-2010 NHANES Participantsa,b,c,d,e
| Characteristic | OHSUD cases (n=139) | Local controls (n=968) | NHANES participants (n=4571) |
|---|---|---|---|
| Age (y) | 54.8±7.6 | 47.2±8.7 | 40.2±13.7f |
| Sex: female | 43 (30.9) | 539 (55.7) | 2374 (51.9)f |
| Racef | |||
| White | 86 (61.9) | 628 (64.9)f | 2003 (43.8) |
| Black | 51 (36.7) | 197 (20.4)f | 834 (18.2) |
| Asian | 2 (1.4) | 32 (3.31) | – |
| Other | 1 (0.7) | 20 (5.7) | 275 (6) |
| Hispanic | – | – | 1459 (31.9) |
| American Indian or Alaska Native | – | 3 (0.3) | – |
| Refused | – | 7 (0.7) | – |
| Unknown | – | 51 (5.3) | – |
| Heart rate (beats/min) | 78±11.4 | 76±13.5 | 77.2±14.5 |
| Systolic blood pressure (mm Hg) | 132.6±21.5 | 125.7±16.8f | 130.4±25.6 |
| Diastolic blood pressure (mm Hg) | 80.9±13.8 | 78±10.8g | 79.4±15.2 |
| BMI (kg/m2) | 31.3±9.7 | 30±7.1 | 29±7f |
| Dyslipidemia | 103 (74.1) | 348 (36)f | 1034 (38)f |
| Dyslipidemia diagnosis or LL medication use | 104 (74.8) | 371 (40.1)f | 1034 (37.8)f |
| Use of LL medication | 60 (43.2) | 265 (27.4)f | 493 (78.6)f |
| Diabetes mellitus | 61 (43.9) | 164 (16.9)f | 335 (7.3)f |
| Hypertension | 110 (79.1) | 360 (37.2)f | 1074 (23.5)f |
| Coronary artery disease | 40 (28.8) | 42 (4.3)f | 161 (3.7)f |
| Annual health care encounters | 5.8±6 | 5.0±6.1 | 3.8±4f |
BMI = body mass index; LL = lipid-lowering; NHANES = National Health and Nutrition Examination Survey; OHSUD = out-of-hospital sudden unexpected death.
Data are presented as mean ± SD or No. (percentage).
P values reported for comparisons between OHSUD cases and each control group.
Missing data: BMI missing for 2 OHSUD cases (1.4%) and 75 local controls (7.7%); dyslipidemia status missing for 1849 NHANES participants (40.5%); use of LL medication missing for 3944 NHANES participants (86.3%); diabetes mellitus status missing for 2 NHANES participants (0.04%); hypertension status missing for 9 NHANES participants (0.2%); coronary artery disease status missing for 277 NHANES participants (6.1%).
P values for race are reported for only black and white race groups. Dash indicates absence of that particular race classification within the data for that group.
P<.001.
P<.05.
Although the mean BMI was similar between cases and controls, the prevalence of comorbidities was higher in OHSUD cases, including dyslipidemia (74.1% [n=103] vs 36% [n=348]; P<.001), diabetes mellitus (43.9% [n=61] vs 16.9% [n=164]; P<.001), hypertension (79.1% [n=110] vs 37.2% [n=360]; P<.001), coronary artery disease (28.8% [n=40]vs 4.3% [n=42]; P<.001), and use of lipid-lowering medication (43.2% [n=60] vs 27.4% [n=265]; P<.001). Notably, both cases and controls had a similar average number of health care encounters in the preceding year (Table 1).
The unadjusted mean TC (170.3±52.2 mg/dL vs 188.9±39.7 mg/dL; P<.001), LDL-C (90.9±39.6 mg/dL vs 109.6±35.2 mg/dL; P<.001), and non-HDL (121.6±49.8 mg/dL vs 134.3±39.6 mg/dL; P<.001) levels were all significantly lower in OHSUD cases than in controls (Table 2). Although the unadjusted mean HDL-C level was significantly lower in cases than in controls (48.3±20.2 mg/dL vs 54.6±17.8 mg/dL; P<.001), there was no significant difference in unadjusted mean TG level. The TG/HDL-C ratio was the only ratio examined that was significantly higher in OHSUD cases (4.7±7 vs 3±2.7; P<.001). Differences between TC, LDL-C, and non-HDL levels remained statistically significant after adjustment for age and sex (Table 2).
Table 2.
| Variable | OHSUD cases (n=139) | Local controls (n=968) | NHANES participants (n=4571) |
|---|---|---|---|
| TC level (mg/dL) | 170.3±52.2 | 188.9±39.7f | 194.9±41.5f |
| TG level (mg/dL) | 170.9±210.6 | 135.8±90g | 129.4±120.7f |
| HDL-C level (mg/dL) | 48.3±20.2 | 54.6±17.8 | 51.9±16.1f |
| LDL-C (mg/dL) | 90.9±39.6 | 109.6±35.2f | 116.1±34.8f |
| Non–HDL-C level (mg/dL) | 121.6±49.8 | 134.3±39.6f | 143±42.5f |
| TG/HDL-C ratio | 4.7±7 | 3±2.7f | 3.1±5.1f |
| TC/HDL-C ratio | 4±1.8 | 3.8±1.3 | 4.1±1.6 |
| LDL-C/HDL-C ratio | 2.1±1.2 | 2.2±1g | 2.4±1f |
HDL = high-density lipoprotein cholesterol; LDL = low-density lipoprotein cholesterol; NHANES = National Health and Nutrition Examination Survey; OHSUD = out-of-hospital sudden unexpected death; TC = total cholesterol; TG = triglyceride.
SI conversion factor: To convert mg/dL values to mmol/L, multiply by 0.0259.
Data are presented as mean ± SD.
P values reported are adjusted for age- and sex-adjusted mean differences in each lipid measure as compared to OHSUD cases.
Missing data: TC level missing for 383 NHANES participants (7.7%) and 5 local controls (50%); TG level missing for 2738 NHANES participants (55.3%), 70 local controls (7.2%), and 1 OHSUD case (0.7%); HDL-C level missing for 383 NHANES participants (7.7%), 14 local controls (1.4%), and 1 OHSUD case (0.7%); LDL-C level missing for 2780 NHANES participants (56.1%), 54 local controls (5.6%), and 4 OHSUD cases (2.9%).
P<.001.
P<.05.
The odds of OHSUD were elevated per unit increase in TG/HDL-C ratio in models that were unadjusted (model 1: odds ratio [OR], 1.10; 95% CI, 1.05-1.15) and age and sex adjusted (model 2: OR, 1.08; 95% CI, 1.03-1.12) (Table 3).
Table 3.
Odds Ratios (95% CIs) for OHSUD Associated With Various Lipid Measurements by Univariate and Multivariate Analysesa,b
| Variable | Model 1c | Model 2 | Model 3 |
|---|---|---|---|
| TC level (highest vs lowest quartile) | 0.4 (0.25-0.65) | 0.43 (0.25-0.74) | 0.45 (0.22-0.95) |
| TG level (highest vs lowest quartile) | 1.00 (1.00-1.00) | 1.00 (1-1.00) | 0.73 (0.34-1.59) |
| HDL-C level (highest vs lowest quartile) | 0.43 (0.27-0.70) | 0.64 (0.37-1.13) | 0.77 (0.37-1.62) |
| LDL-C (highest vs lowest quartile) | 0.30 (0.18-0.50) | 0.32 (0.18-0.56) | 0.36 (0.17-0.77) |
| Non–HDL-C (highest vs lowest quartile) | 0.46 (0.28-0.73) | 0.45 (0.27-0.75) | 0.44 (0.21-0.93) |
| TG/HDL-C ratio (per unit increase) | 1.10 (1.05-1.15) | 1.08 (1.03-1.12) | 1.05 (1.01-1.10) |
| TC/HDL-C ratio (per unit increase) | 1.12 (0.99-1.26) | 1.04 (0.90-1.19) | 1.01 (0.83-1.23) |
| LDL-C/HDL-C ratio (per unit increase) | 0.94 (0.78-1.13) | 0.88 (0.72-1.07) | 0.85 (0.65-1.12) |
HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; OHSUD = out-of-hospital sudden unexpected death; NHANES = National Health and Nutrition Examination Survey; TC = total cholesterol; TG = triglyceride.
Local controls are the reference group.
Model 1: unadjusted; model 2: adjusted for age and sex; model 3: adjusted for age, sex, body mass index, diabetes mellitus, and interaction between respective lipid measure and lipid-lowering medication use. For the TG/HDL-C ratio, model 3 adjusts for age, sex, body mass index, and diabetes mellitus. For the LDL-C level and LDL-C/HDL-C ratio, model 3 additionally adjusts for TG level (as continuous variable).
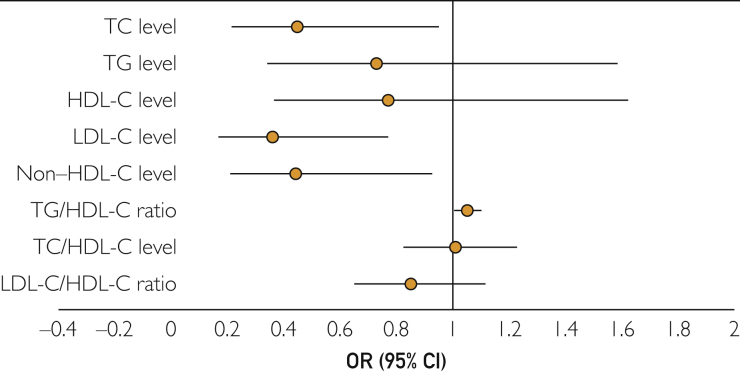
The odds of OHSUD were lower for the highest quartile of TC values in both model 1 (OR, 0.4; 95% CI, 0.25-0.65) and model 2 (OR, 0.43; 95% CI, 0.25-0.74). The highest quartile of LDL-C values was also associated with lower odds of OHSUD (model 1: OR, 0.30; 95% CI, 0.18-0.50; model 2: OR, 0.32; 95% CI, 0.18-0.56). Similarly, the highest quartile of non-HDL values was associated with lower odds of OHSUD (model 1: OR, 0.46; 95% CI, 0.28-0.73; model 2: OR, 0.45; 95% CI, 0.24-0.75). Similar trends were observed even after additional adjustment for plausible confounders (model 3) (Figure 2).
Figure 2.
Odds ratios (95% CIs) of out-of-hospital sudden unexpected death associated with various lipid measures, using local controls as the reference group. Odds ratios (95% CIs) depicted are obtained from model 3, which is adjusted for age, sex, body mass index, diabetes mellitus, and interaction between respective lipid measure and lipid-lowering medication use. For the TG/HDL-C ratio, model 3 adjusts for age, sex, body mass index, and diabetes mellitus. For the LDL-C level and LDL-C/HDL-C ratio, model 3 additionally adjusts for TG level (as continuous variable). HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; OR = odds ratio; TC = total cholesterol; TG = triglyceride.
We compared OHSUD cases with NHANES participants (n=4571) to serve as sensitivity analyses. As seen in Tables 1 and 2, comparison trends between OHSUD cases and NHANES participants were similar to those seen in Wake County (local) controls.
By finding a lower TC level, an LDL-C level in OHSUD cases was unanticipated. We therefore performed an array of sensitivity analyses. Excluding direct LDL-C values, which are typically numerically lower than measured LDL values, did not yield results markedly different from our main results (Supplemental Table 2, available online at http://www.mcpiqojournal.org).
Although race was excluded from the analytical models given the variable race distribution of subjects with few nonblack, nonwhite, or unknown subjects among OHSUD cases (n=3) and many among controls (n=113), adjusting for race in a sensitivity analysis did not markedly alter our results (Supplemental Table 3, available online at http://www.mcpiqojournal.org).
When stratified by age, although the odds of OHSUD associated with TC, HDL-C, LDL-C and non-HDL levels in those younger than 45 years differed significantly as compared with the overall results, the same odds in those 45 years or older were similar to the overall results (Supplemental Table 4, available online at http://www.mcpiqojournal.org). The larger number of older OHSUD cases (≥45 years: n=123 vs <45 years: n=16) may therefore be responsible for the overall lower levels of lipid measures. Notably, however, higher unadjusted odds of OHSUD associated with the TG/HDL-C ratio were seen in both age groups. When stratified by sex, the LDL-C level was associated with lower odds and the TG/HDL-C ratio with higher odds of OHSUD in both men and women (Supplemental Table 5, available online at http://www.mcpiqojournal.org). Stratifying by a previous diagnosis of coronary artery disease also did not alter our findings (Supplemental Table 6, available online at http://www.mcpiqojournal.org).
Discussion
We characterized standard lipid profile measures in OHSUD cases and a geographically based control group of living individuals. We found that OHSUD cases had significantly lower mean TC, non–HDL-C, and, most notably, LDL-C levels than did controls at approximately 1 year from either death (cases) or index visit (controls). Lower levels of these components were unanticipatedly associated with higher odds of OHSUD even after multivariate adjustment. Although no meaningful trend could be delineated between OHSUD and TG or HDL-C levels individually, the mean TG/HDL-C ratio was found to be associated with higher odds of OHSUD.
To our knowledge we are the first study to have categorically studied lipid measurements within a few years of sudden death. Most prospective studies have reported baseline lipid measurements of sudden death cases, often decades preceding death. A 20-year follow-up of male Framingham Heart Study17 participants aged 45 to 74 years reported no association between cholesterol levels and sudden death, but a 38-year follow-up of 30- to 62-year-old female Framingham Heart Study participants18 reported an elevated risk of sudden death in women who had higher cholesterol levels. In contrast, the Honolulu Heart Study,6 the Paris Prospective Study,19 and the British Regional Heart Study7 all reported a significant association of cholesterol levels with the risk of sudden death in middle-aged men. Few studies have examined LDL-C levels in relationship to sudden death, but the Physicians’ Health Study20 and Kuopio Ischemic Heart Disease Risk Factor Study21 both reported similar LDL-C levels in sudden death cases and participants who did not die suddenly during follow-up.
It is worth noting that despite the established association between lower LDL-C levels and reduced atherosclerosis, some studies have found inverse, U-shaped, and no association between TC or LDL-C level reduction and cardiovascular events22, 23, 24 in individuals older than 60 years. Similar findings in middle-aged individuals have been reported. The Atherosclerosis Risk in Communities Study reported a J-shaped relationship between all-cause mortality and LDL-C levels in participants with a mean age of 54 years at baseline during 10 years of follow-up.25 A U-shaped relationship between TC levels and all-cause mortality and a linearly increasing relationship between TC levels and coronary disease mortality were observed in 35- to 69-year-old Framingham Heart Study participants.26 Similarly, the Tecumseh Community Health Study found a low risk of all-cause mortality in 45- to 64-year-old subjects in the middle tertile of serum cholesterol levels but found cholesterol to be a significant predictor of fatal coronary heart disease.27
The lower odds of OHSUD observed with higher quartiles of TC, LDL-C, and non-HDL values may indicate a lack of association between the traditional lipid cardiovascular risk factors and OHSUD. It may also be indicative of a residual sudden death risk in the community despite adequate control of TC and/or LDL-C levels with medication, as previously suggested.28
High TG29 and low HDL30 levels, the hallmarks of combined dyslipidemia, are established predictors of adverse cardiovascular events, but their relationship to sudden death is largely inconclusive.19, 21 However, studies have found a correlation between high levels of circulating free fatty acids and sudden death.31, 32 As free fatty acids contribute to circulating TGs,33 this is a plausible biological pathway suggesting an association between elevated TG levels and sudden death. In this study, although OHSUD cases had higher unadjusted mean TG levels and lower unadjusted mean HDL-C levels, the difference was rendered insignificant on multivariate adjustment. However, the TG/HDL-C ratio was significantly higher in sudden death cases and this difference persisted even after adjustment for age, sex, and comorbid medical conditions. The TG/HDL-C ratio is increasingly recognized as an indicator of insulin resistance,34 a measure of atherogenicity representative of small dense LDL,35 an independent predictor of poor cardiac fitness36 and adverse cardiovascular events.37 The TG/HDL-C ratio serves as a marker of the complex interaction between circulating TG and HDL levels that results in increased circulating levels of RLPs, which are dense apolipoprotein E–rich catabolites of very low-density lipoproteins and chylomicrons. Remnant cholesterol, the cholesterol content of RLPs, has been identified as an independent indicator of cardiovascular risk in several studies38 including Framingham Heart Study39 and Honolulu Heart Study.40 It was recently found by Quispe et al41 that the TG/HDL-C ratio strongly correlated with plasma remnant cholesterol. With regard to sudden death, several postmortem studies reported that RLP levels were significantly elevated42 and were the strongest predictor of sudden death in cases both with and without evidence of coronary atherosclerosis at autopsy.31 Given the vasoactive43 and prothrombotic44 properties of RLPs, it has been hypothesized that these particles may play a key role in inciting the coronary event that results in sudden death. Some evidence suggests that the proposed residual risk of cardiovascular mortality that persists after achieving recommended targets of LDL-C or TC may be predicted by remnant lipoproteins.45 In this context, the significantly higher odds of sudden death associated with unit increases in TG/HDL-C ratio (measured at ∼1 year before death) in both univariate and multivariate models lend credence to this evolving hypothesis of how vasoactive remnant particles could act as triggers for fatal cardiac events.
We must note our study’s limitations. Of the 399 subjects originally adjudicated as OHSUD cases, only 67.7% (n=270) had medical records. Furthermore, for this study, subject inclusion was limited by the availability of lipid profiles, although cases with and without lipid profiles had similar age, sex, race distributions, and socioeconomic characteristics (data not shown). Reliable electrocardiographic and echocardiographic data were unavailable for most cases and therefore, though relevant to sudden death, were not described.
Although control group subjects were residents of the same county as of cases, they came from a health care system and not a community-based source. The mean age of the control group was also younger than that of controls, which is a potential source of bias. However, we would expect a younger age to bias controls toward even lower LDL-C and TC levels. Comorbid medical conditions were abstracted differently for cases (exact terms abstracted from medical records) and controls (International Classification of Diseases, Ninth Revision/International Statistical Classification of Diseases, 10th Revision codes). Despite these limitations, we believe that the consistency observed in the differences in mean lipid measurements when compared between the 2 control group populations (Wake county and NHANES participants) increases the reliability of our results.
Conclusion
In this community-based case-control study of nonelderly adults, lower serum TC, LDL-C, and non–HDL-C levels but a higher TG/HDL-C ratio were associated with an increased risk of OHSUD. These findings indicate the need for prospective studies to elucidate the role of lipids in sudden death.
Acknowledgments
We thank the emergency medical services (EMS) of Wake County, NC, for providing the EMS transport records necessary to conduct the Sudden Unexpected Death in North Carolina study. The Wake County EMS Data System supports, maintains, and monitors EMS service delivery, patient care, and disaster preparedness for the Wake County community at large. This manuscript has been reviewed by Wake County EMS Data System investigators for scientific content and consistency of data interpretation with previous Wake County EMS Data System publications. We thank Jefferson G. Williams, MD, MPH, Deputy Medical Director of Wake County Department of Emergency Medical Services and Clinical Assistant Professor (Adjunct), University of North Carolina Department of Emergency Medicine. We also thank the SUDDEN team abstractors and adjudicators as well as the Carolina Data Warehouse/North Carolina Translational and Clinical Sciences Institute, University of North Carolina at Chapel Hill for providing electronic health records for the local control subjects.
Footnotes
Grant Support: The Sudden Unexpected Death in North Carolina project is funded by individual private donations; the Heart and Vascular Division of the University of North Carolina at Chapel Hill; and the McAllister Heart Institute, Chapel Hill, NC. The project described was supported by grant 1UL1TR001111 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Potential Competing Interests: Dr Mendys is an employee of Pfizer. Dr Simpson consults with Merck, Pfizer, and Amgen.
Supplemental Online Material
Supplemental material can be found online at: http://mcpiqojournal.org Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Myerburg R.J. Scientific gaps in the prediction and prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2002;13(7):709–723. doi: 10.1046/j.1540-8167.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 2.Kong M.H., Fonarow G.C., Peterson E.D., et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57(7):794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel W.B., Castelli W.P., Gordon T. Cholesterol in the prediction of atherosclerotic disease: new perspectives based on the Framingham study. Ann Intern Med. 1979;90(1):85–91. doi: 10.7326/0003-4819-90-1-85. [DOI] [PubMed] [Google Scholar]
- 4.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 5.Austin M.A., Hokanson J.E., Edwards K.L. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 6.Kagan A., Yano K., Reed D.M., MacLean C.J. Predictors of sudden cardiac death among Hawaiian-Japanese men. Am J Epidemiol. 1989;130(2):268–277. doi: 10.1093/oxfordjournals.aje.a115333. [DOI] [PubMed] [Google Scholar]
- 7.Wannamethee G., Shaper A.G., Macfarlane P.W., Walker M. Risk factors for sudden cardiac death in middle-aged British men. Circulation. 1995;91(6):1749–1756. doi: 10.1161/01.cir.91.6.1749. [DOI] [PubMed] [Google Scholar]
- 8.Doyle J.T., Kannel W.B., McNamara P.M., Quickenton P., Gordon T. Factors related to suddenness of death from coronary disease: combined Albany Framingham studies. Am J Cardiol. 1976;37(7):1073–1078. doi: 10.1016/0002-9149(76)90427-6. [DOI] [PubMed] [Google Scholar]
- 9.Oi K., Shimokawa H., Hiroki J., et al. Remnant lipoproteins from patients with sudden cardiac death enhance coronary vasospastic activity through upregulation of Rho-kinase. Arterioscler Thromb Vasc Biol. 2004;24(5):918–922. doi: 10.1161/01.ATV.0000126678.93747.80. [DOI] [PubMed] [Google Scholar]
- 10.Morrison L.J., Nichol G., Rea T.D., et al. ROC Investigators Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Resuscitation. 2008;78(2):161–169. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.QuickFacts: Wake County, North Carolina. United States Census Bureau website. https://www.census.gov/quickfacts/table/PST045215/37183,37,00 Available at: Accessed May 4, 2017.
- 12.Nanavati P.P., Mounsey J.P., Pursell I.W., et al. Sudden Unexpected Death in North Carolina (SUDDEN): methodology review and screening results. Open Heart. 2014;1(1):e000150. doi: 10.1136/openhrt-2014-000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carolina Data Warehouse for Health website. https://tracs.unc.edu/index.php/services/biomedical-informatics/cdw-h Available at: Accessed May 4, 2017.
- 14.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data. Hyattsville, MD: Centers for Disease Control and Prevention, US Dept of Health and Human Services. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2009 Accessed May 4, 2017.
- 16.Zipf G., Chiappa M., Porter K.S., et al. National Health and Nutrition Examination Survey: Plan and Operations, 1999-2010. Hyattsville, MD: National Center for Health Statistics; 2013. Vital and Health Statistics; series 1, no. 56. https://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf Available at: [PubMed]
- 17.Kannel W.B., Thomas H.E., Jr. Sudden coronary death: the Framingham Study. Ann N Y Acad Sci. 1982;382(1):3–21. doi: 10.1111/j.1749-6632.1982.tb55203.x. [DOI] [PubMed] [Google Scholar]
- 18.Kannel W.B., Wilson P.W.F., D’Agostino R.B., Cobb J. Sudden coronary death in women. Am Heart J. 1998;136(2):205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 19.Jouven X., Desnos M., Guerot C., Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 20.Albert C.M., Ma J., Rifai N., Stampfer M.J., Ridker P.M. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 21.Kurl S., Laaksonen D.E., Jae S.Y., et al. Metabolic syndrome and the risk of sudden cardiac death in middle-aged men. Int J Cardiol. 2016;203:792–797. doi: 10.1016/j.ijcard.2015.10.218. [DOI] [PubMed] [Google Scholar]
- 22.Larson M.G. Assessment of cardiovascular risk factors in the elderly: the Framingham Heart Study. Stat Med. 1995;14(16):1745–1756. doi: 10.1002/sim.4780141604. [DOI] [PubMed] [Google Scholar]
- 23.Psaty B.M., Anderson M., Kronmal R.A., et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(10):1639–1647. doi: 10.1111/j.1532-5415.2004.52455.x. [DOI] [PubMed] [Google Scholar]
- 24.Ravnskov U., Diamond D.M., Hama R., et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open. 2016;6(6):e010401. doi: 10.1136/bmjopen-2015-010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilling K., Sterne J.A., Szklo M. Estimating the effect of cardiovascular risk factors on all-cause mortality and incidence of coronary heart disease using G-estimation: the atherosclerosis risk in communities study. Am J Epidemiol. 2002;155(8):710–718. doi: 10.1093/aje/155.8.710. [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino R.B., Belanger A.J., Kannel W.B., Higgins M. Role of smoking in the U-shaped relation of cholesterol to mortality in men: the Framingham Study. Am J Epidemiol. 1995;141(9):822–827. doi: 10.1093/oxfordjournals.aje.a117517. [DOI] [PubMed] [Google Scholar]
- 27.Higgins M., Keller J.B. Cholesterol, coronary heart disease, and total mortality in middle-aged and elderly men and women in Tecumseh. Ann Epidemiol. 1992;2(1-2):69–76. doi: 10.1016/1047-2797(92)90039-s. [DOI] [PubMed] [Google Scholar]
- 28.Sampson U.K., Fazio S., Linton M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14(1):1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller M., Stone N.J., Ballantyne C., et al. American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 30.Tariq S.M., Sidhu M.S., Toth P.P., Boden W.E. HDL hypothesis: where do we stand now? Curr Atheroscler Rep. 2014;16(4):398. doi: 10.1007/s11883-014-0398-0. [DOI] [PubMed] [Google Scholar]
- 31.Jouven X., Charles M.A., Desnos M., Ducimetière P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation. 2001;104(7):756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- 32.Havmoeller R., Reinier K., Teodorescu C., et al. Elevated plasma free fatty acids are associated with sudden death: a prospective community-based evaluation at the time of cardiac arrest. Hear Rhythm. 2014;11(4):691–696. doi: 10.1016/j.hrthm.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vedala A., Wang W., Neese R.A., Christiansen M.P., Hellerstein M.K. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res. 2006;47(11):2562–2574. doi: 10.1194/jlr.M600200-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Murguía-Romero M., Jiménez-Flores J.R., Sigrist-Flores S.C., et al. Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J Lipid Res. 2013;54(10):2795–2799. doi: 10.1194/jlr.M040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruyama C., Imamura K., Teramoto T. Assessment of LDL particle size by triglyceride/HDL-cholesterol ratio in non-diabetic, healthy subjects without prominent hyperlipidemia. J Atheroscler Thromb. 2003;10(3):186–191. doi: 10.5551/jat.10.186. [DOI] [PubMed] [Google Scholar]
- 36.Vega G.L., Grundy S.M., Barlow C.E., et al. Association of triglyceride-to-high density lipoprotein cholesterol ratio to cardiorespiratory fitness in men. J Clin Lipidol. 2016;10(6):1414–1422.e1. doi: 10.1016/j.jacl.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Jeppesen J., Hein H.O., Suadicani P., Gyntelberg F. Relation of high TG-low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease: an 8-year follow-up in the Copenhagen Male Study. Arterioscler Thromb Vasc Biol. 1997;17(6):1114–1120. doi: 10.1161/01.atv.17.6.1114. [DOI] [PubMed] [Google Scholar]
- 38.Goliasch G., Wiesbauer F., Blessberger H., et al. Premature myocardial infarction is strongly associated with increased levels of remnant cholesterol. J Clin Lipidol. 2015;9(6):801–806.e1. doi: 10.1016/j.jacl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Joshi P.H., Khokhar A.A., Massaro J.M., et al. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson Heart and Framingham Offspring Cohort Studies. J Am Heart Assoc. 2016;5(5) doi: 10.1161/JAHA.115.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imke C., Rodriguez B.L., Grove J.S., et al. Are remnant-like particles independent predictors of coronary heart disease incidence? The Honolulu Heart Study. Arterioscler Thromb Vasc Biol. 2005;25(8):1718–1722. doi: 10.1161/01.ATV.0000173310.85845.7b. [DOI] [PubMed] [Google Scholar]
- 41.Quispe R., Manalac R.J., Faridi K.F., et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242(1):243–250. doi: 10.1016/j.atherosclerosis.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima K., Nakajima Y., Takeichi S., Fujita M.Q. Plasma remnant-like lipoprotein particles or LDL-C as major pathologic factors in sudden cardiac death cases. Atherosclerosis. 2008;198(1):237–246. doi: 10.1016/j.atherosclerosis.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Doi H., Kugiyama K., Ohgushi M., et al. Remnants of chylomicron and very low density lipoprotein impair endothelium-dependent vasorelaxation. Atherosclerosis. 1998;137(2):341–349. doi: 10.1016/s0021-9150(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 44.Knöfler R., Nakano T., Nakajima K., Takada Y., Takada A. Remnant-like lipoproteins stimulate whole blood platelet aggregation in vitro. Thromb Res. 1995;78(2):161–171. doi: 10.1016/0049-3848(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T., Obata J.E., Hirano M., et al. Predictive value of remnant lipoprotein for cardiovascular events in patients with coronary artery disease after achievement of LDL-cholesterol goals. Atherosclerosis. 2011;218(1):163–167. doi: 10.1016/j.atherosclerosis.2011.04.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.