Abstract
Erdheim-Chester disease (ECD) is a rare form of non–Langerhans cell histiocytosis characterized by infiltration of organs by CD68+ and CD1a− lipid-laden histiocytes, including the central nervous system in more than a third of patients. Molecular analysis of ECD samples has demonstrated the prevalence of BRAF V600E mutations as high as 54%. Recently, vemurafenib became the only Food and Drug Administration–approved treatment for patients with ECD who carry the BRAF V600E mutation. However, dabrafenib has been suggested to have greater brain distribution. We describe a 44-year-old female patient treated from August of 2015 through November 2017. She presented with a 2-year history of light-headedness, fatigue, and vertigo. She was moderately dysmetric, diffusely hyperreflexic, and dysarthric in the bilateral upper and lower extremities. Her gait was wide-based. She had dysarthria and nystagmus on horizontal gaze bilaterally. Magnetic resonance imaging showed an extensive area of increased T2/fluid-attenuated inversion recovery signal in the brain stem, enhancement in the pons and midbrain, and thickening of the pituitary stalk. Positron emission tomography/computed tomography (PET/CT) and whole-body technetium Tc99m bone scintigraphy showed intense symmetrical radiotracer uptake in the distal femur and tibia bilaterally, which was biopsied. Immunohistochemistry was negative for BRAF V600E, but genomic sequencing revealed the mutation. The patient received combination therapy with dabrafenib and trametinib. Her nystagmus, dysarthria, dysmetria, and gait improved remarkably. Subsequent PET/CT and magnetic resonance imaging showed complete resolution of all radiographic evidence of disease. In this case report, we demonstrate the success of a combination therapy with dabrafenib and trametinib.
Abbreviations and Acronyms: CNS, central nervous system; CSF, cerebrospinal fluid; ECD, Erdheim-Chester disease; FDA, Food and Drug Administration; IFN-α, interferon alfa; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography
Erdheim-Chester disease (ECD) is a rare form of non–Langerhans cell histiocytosis characterized by xanthogranulomatous infiltration of multiple organs by lipid-laden histiocytes. It is classified within the histiocytic neoplasms in the revised 2016 World Health Organization Classification. Histiocytes in this disease are characteristically CD68+ and CD1a−. Erdheim-Chester disease has a wide-ranging, nonspecific clinical spectrum and multiorgan involvement. The central nervous system (CNS) is commonly involved, with more than one-third of patients presenting with CNS lesions.1, 2 Conventionally, experience-based management of ECD involves the use of tumor necrosis factor–blocking agents, interleukin 1 antagonists, interferon alfa (IFN-α), corticosteroids, surgery, chemotherapy, and radiation.2
Molecular analysis of ECD samples has demonstrated a high prevalence of somatic BRAF V600E mutations in ECD, as high as 54% in one study.3 Currently, 2 United States Food and Drug Administration (FDA)–approved inhibitors of the mutant BRAF V600E kinase have been developed through structure-guided drug discovery approach: vemurafenib and dabrafenib.4 Accordingly, recent studies have demonstrated efficacy of vemurafenib and dabrafenib in the treatment of BRAF V600E–positive multisystem ECD as well as other BRAF V600E–positive malignancies.1, 5, 6, 7, 8, 9, 10, 11, 12, 13 This has led to recent FDA approval of vemurafenib for the treatment of ECD.14
Both these inhibitors have been shown through in vitro melanoma and in vivo murine models to have limited blood-brain barrier penetration due to active efflux. However, both BRAF V600E kinase inhibitors have been shown to have efficacy in melanoma metastatic to the brain.5, 15 In addition, there is growing evidence that both vemurafenib and dabrafenib have activity against BRAF V600E–positive mutated ECD with CNS involvement.9, 16
Both BRAF V600E kinase inhibitors are known to cause considerable cutaneous adverse effects, as well as headaches and arthralgias, but only vemurafenib is known to frequently cause photosensitivity.17 Between the 2 drugs, dabrafenib has been shown to have greater brain distribution in comparison to vemurafenib.18, 19 In combination with the mitogen-activated protein kinase inhibitor trametinib, dabrafenib has demonstrated a substantial increased overall survival and lower rate of cutaneous neoplasms in comparison to vemurafenib alone in the treatment of metastatic melanoma.16
Report of Case
We describe a 44-year-old female patient who has been under our care from August of 2015 through November 2017. The patient presented with a 2-year history of light-headedness and fatigue, ataxia, and dysarthria who was responsive to systemic treatment with a BRAF inhibitor.
The patient's medical history was notable for anxiety and depression. In addition, the patient had been evaluated by magnetic resonance imaging (MRI) for an episode of vertigo 3 years before presentation, which showed a focal T2 signal abnormality and enhancement in the pons and thickening of the pituitary stalk (Supplemental Figure 1, available online at http://mcpiqojournal.org/). However, the patient did not receive initial treatment or follow-up and the etiology of the lesion was unclear.
The patient was an unemployed librarian, with both parents living in good health. Her personal family history was unremarkable. She did not smoke, drink alcohol, or use illicit drugs.
On examination, the patient was oriented, with cranial nerves intact and symmetric. Sensation was intact in upper and lower extremities, but she was diffusely hyperreflexic in the bilateral upper and lower extremities. The patient had dysmetria of her upper and lower extremities with preserved motor strength. The patient had moderate dysmetria on finger-to-nose testing and heel-to-shin testing bilaterally. She had a wide-based gait but was able to maintain balance. She also had gross dysarthria and nystagmus on horizontal gaze bilaterally.
A lumbar puncture was performed to analyze the patient's cerebrospinal fluid (CSF). Flow cytometry of CSF revealed cells that were mostly CD56+CD3−. Her glucose level was 66 mg/dL (to convert to mmol/L, multiply by 0.0555), red blood cell count was 4 cells/μL, and total protein level was 26 mg/dL (to convert to g/L, multiply by 10.0). Pathologic analysis of the CSF was negative for malignant cells but showed few small mature lymphocytes. Venereal Disease Research Laboratory test results were negative and no cryptococcal antibodies were detected. The CSF cultures were negative for any fungi or viruses.
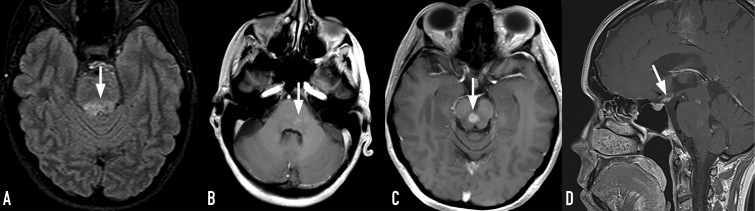
The MRI showed a more extensive area of increased T2/fluid-attenuated inversion recovery signal in the brain stem, persistent enhancement in the pons, thickening of the pituitary stalk, and a new enhancing lesion in the midbrain (Figure 1). Positron emission tomography/computed tomography (PET/CT) showed intense symmetrically increased radiotracer uptake involving the distal femur and tibia bilaterally (Supplemental Figure 2, available online at http://mcpiqojournal.org/).
Figure 1.
The MRI at presentation. A, Axial FLAIR image demonstrates progression of the brain-stem lesion (arrow). B and D, Axial and sagittal T1-weighted postcontrast images show irregular heterogeneous enhancement in the pons and thickening of the pituitary stalk (arrows). C, Axial T1-weighted postcontrast image shows new enhancing lesion in the midbrain. FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging.
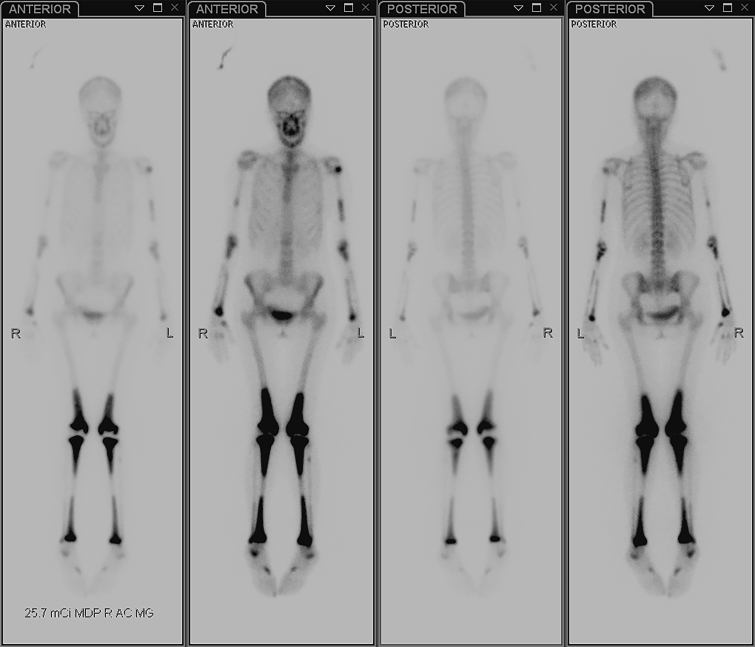
On December 28, 2015, a right tibial bone biopsy was performed (Supplemental Figure 3, available online at http://mcpiqojournal.org/). Immunohistochemical staining demonstrated an abundant population of histiocytes among the CD45-positive chronic inflammatory cells. Immunohistochemical staining for S100, Pax-8, keratin, and the BRAF V600E (VE-1 antibody) mutation was negative. However, next-generation sequencing analysis of the same bone tissue in January 2016 demonstrated the presence of a BRAF V600E mutation. Shortly afterward, the patient underwent whole-body technetium Tc99m bone scintigraphy, which showed bilateral symmetric increased activity involving the long bones, predominantly of the lower extremities (Figure 2).
Figure 2.
Whole-body technetium Tc99m bone scintigraphy performed before treatment shows bilateral symmetric increased activity involving the long bones, predominantly of the lower extremities.
In April 2016 (2 years after initial presentation), the patient was formally diagnosed with ECD and placed on combination therapy with dabrafenib and trametinib. Trametinib was added to prevent the development of squamous cell carcinoma of the skin.20 The patient was started on 2 mg of trametinib daily by mouth and her dabrafenib dose was gradually increased from 75 mg twice daily to 150 mg twice daily. She developed a rash on the torso and extremities, requiring a short course of corticosteroids and multiple dose adjustments of dabrafenib and trametinib. Her final regimen was dabrafenib at 75 mg twice daily and trametinib at 1 mg every evening.
In August 2016, her dysarthria and gait had remarkably improved. The PET/CT at 4 months and 9 months after the initiation of treatment demonstrated remarkable interval improvement in the hypermetabolic skeletal lesions and resolution of the increased metabolic activity in the right hepatic lobe. No new hypermetabolic lesions were seen.
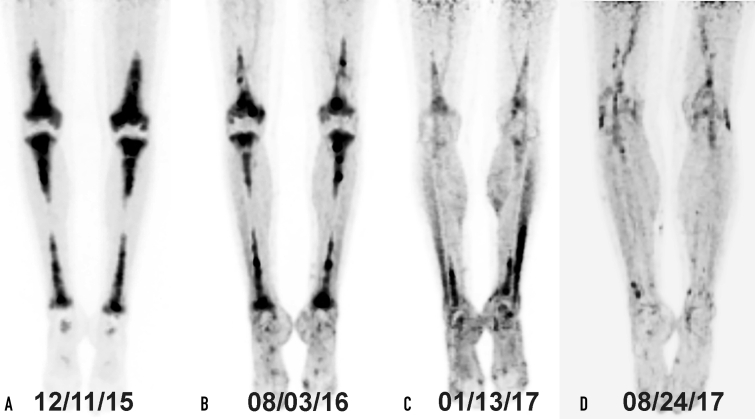
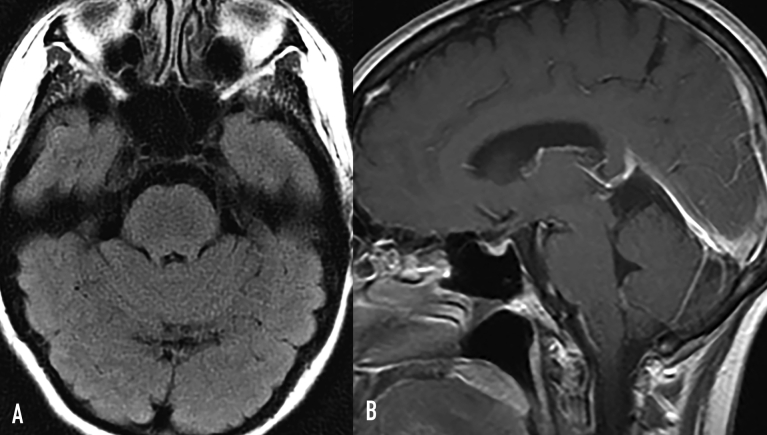
The PET/CT (Figure 3) and MRI of the brain (Figure 4) showed response to treatment, with resolved signal abnormality and enhancement in the brain and decreased bone uptake.
Figure 3.
The FDG-PET scan demonstrates a symmetrical radiotracer uptake in the diaphysis and metaphysis of the long bones of legs (A). Response to treatment in the long bones assessed by FDG-PET scan performed after 4 (B), 10 (C), and 16 months (D) of treatment shows a marked reduction in tracer uptake especially in long bones of the tibias and the femurs. FDG-PET = fluorodeoxyglucose-positron emission tomography.
Figure 4.
Response to treatment. A, FLAIR axial image shows complete resolution of brain-stem signal abnormality. B, Postcontrast T1 sagittal image shows resolved brain-stem enhancement and normal pituitary stalk. FLAIR = fluid-attenuated inversion recovery.
She was evaluated 10 months after continuous treatment. Although she had minor neurological deficits, her dysarthria had improved considerably. Finger-to-nose and heel-to-shin test results remained mildly abnormal on the left side but much improved. Her gait was steady and she was able to pivot without difficulty. Performance on the Romberg test was improved from previous examinations and demonstrated only minor instability. Her speech pattern had normalized, with minimal residual slurring. She continued to have minimal nystagmus on horizontal gaze bilaterally. Muscle tone and sensory examinations were normal and symmetric.
Discussion
Erdheim-Chester disease is marked by bilaterally symmetric bone infiltration composed of foamy histiocytes causing granulomatous and fibrotic changes. Neurologic involvement in ECD is not infrequent, as this patient exemplified through her mild cerebellar findings and pronounced dysarthria. Oftentimes, ECD is associated with soft-tissue sheathing of the thoracic or abdominal aorta, as seen on the PET/CT for this patient.1, 2
In a retrospective multicenter analysis of 53 patients with ECD, Arnaud et al21 demonstrated that CNS involvement is an independent factor for increased mortality while treatment with IFN-α was identified as an independent predictor of survival. In a phase 2 trial, Hyman et al10 investigated the efficacy of vemurafenib in nonmelanoma cancers with the BRAF V600E mutations in a study that included a cohort of 14 patients with ECD or Langerhans'-cell histiocytosis. In this study, 6 patients responded including 1 complete response and 5 patients who responded partially. The extensive CNS disease and presence of the BRAF V600E mutation in this patient's ECD steered the treatment of choice from IFN-α toward BRAF inhibitors.8, 9
Although there are more reports in the literature demonstrating the efficacy of BRAF inhibitor vemurafenib in treating BRAF V600E–positive ECD, we chose the alternative BRAF inhibitor dabrafenib on the basis of improved overall survival without increased toxicity demonstrated in clinical trials of melanoma treatment, as well as improved CNS penetrance relative to vemurafenib.5, 6, 7, 9, 10, 13 A recent study following 51 patients with ECD treated with BRAF inhibitors demonstrated disease regression in all patients, with 1 patient treated with dabrafenib monotherapy from the beginning and 3 patients switched to dabrafenib from vemurafenib because of adverse effects.22
Conclusion
Recently, the FDA approved the use of vemurafenib in patients with ECD who carry the BRAF V600E mutation.14 There is currently no standard accepted regimen to treat ECD. Of the various treatments for this disease, IFN-α is the most often tried but there are Serious adverse effects and the response rate is unclear. In this case report, we demonstrate a progressive and sustained response of a combination therapy with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor. In a patient presenting with brain-stem disease, the presence of BRAF V600E mutation presents unique opportunities for targeted treatment. We believe that a clinical trial comparing the efficacy of dabrafenib to vemurafenib is warranted, possibly leading the way for the FDA approval for another treatment option for this orphan disease. Currently, there is a phase 2 clinical trial sponsored by the Cancer Therapy Evaluation Program that is enrolling new patients with ECD with BRAF V600E mutations and treating with dabrafenib and trametinib (www.clinicaltrials.gov #NCT02281760).
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org/. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Estrada-Veras J.I., O'Brien K.J., Boyd L.C., et al. The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv. 2017;1(6):357–366. doi: 10.1182/bloodadvances.2016001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz J., Janku F., Cohen P.R., Kurzrock R. Erdheim-Chester disease: characteristics and management. Mayo Clin Proc. 2014;89(7):985–996. doi: 10.1016/j.mayocp.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Haroche J., Charlotte F., Arnaud L., et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 4.Menzies A.M., Long G.V., Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther. 2012;6:391–405. doi: 10.2147/DDDT.S38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies M.A., Saiag P., Robert C., et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. doi: 10.1016/S1470-2045(17)30429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falchook G.S., Long G.V., Kurzrock R., et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grob J.J., Amonkar M.M., Karaszewska B., et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015;16(13):1389–1398. doi: 10.1016/S1470-2045(15)00087-X. [DOI] [PubMed] [Google Scholar]
- 8.Haroche J., Cohen-Aubart F., Emile J.F., et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121(9):1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]
- 9.Haroche J., Cohen-Aubart F., Emile J.F., et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411–418. doi: 10.1200/JCO.2014.57.1950. [DOI] [PubMed] [Google Scholar]
- 10.Hyman D.M., Puzanov I., Subbiah V., et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long G.V., Trefzer U., Davies M.A., et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 12.Nordmann T.M., Juengling F.D., Recher M., et al. Trametinib after disease reactivation under dabrafenib in Erdheim-Chester disease with both BRAF and KRAS mutations. Blood. 2017;129(7):879–882. doi: 10.1182/blood-2016-09-740217. [DOI] [PubMed] [Google Scholar]
- 13.Robert C., Karaszewska B., Schachter J., et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 14.Drug and device news. P T. 2018;43(1):13–30. [PMC free article] [PubMed] [Google Scholar]
- 15.McArthur G.A., Maio M., Arance A., et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol. 2017;28(3):634–641. doi: 10.1093/annonc/mdw641. [DOI] [PubMed] [Google Scholar]
- 16.Tzoulis C., Schwarzlmuller T., Gjerde I.O., et al. Excellent response of intramedullary Erdheim-Chester disease to vemurafenib: a case report. BMC Res Notes. 2015;8:171. doi: 10.1186/s13104-015-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh S.J., Corrie P.G. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7(2):122–136. doi: 10.1177/1758834014566428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittapalli R.K., Vaidhyanathan S., Dudek A.Z., Elmquist W.F. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344(3):655–664. doi: 10.1124/jpet.112.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittapalli R.K., Vaidhyanathan S., Sane R., Elmquist W.F. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032) J Pharmacol Exp Ther. 2012;342(1):33–40. doi: 10.1124/jpet.112.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King A.J., Arnone M.R., Bleam M.R., et al. Dabrafenib: preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One. 2013;8(7):e67583. doi: 10.1371/journal.pone.0067583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnaud L., Hervier B., Néel A., et al. CNS involvement and treatment with interferon-alpha are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117(10):2778–2782. doi: 10.1182/blood-2010-06-294108. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Aubart F., Emile J.F., Carrat F., et al. Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (the LOVE study) Blood. 2017;130(11):1377–1380. doi: 10.1182/blood-2017-03-771873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.