Abstract
Painful neuropathic injuries are accompanied by robust inflammatory and oxidative stress responses that contribute to the development and maintenance of pain. After neural trauma the inflammatory enzyme cyclooxygenase-2 (COX-2) increases concurrent with pain onset. Although pre-treatment with the COX-2 inhibitor, meloxicam, before a painful nerve root compression prevents the development of pain, the pathophysiological mechanisms are unknown. This study evaluated if pre-treatment with meloxicam prior to painful root injury prevents pain by reducing spinal inflammation and peripheral oxidative stress. Glial activation and expression of the inflammatory mediator secreted phospholipase A2 (sPLA2) in the spinal cord were assessed at day 7 using immunohistochemistry. The extent of oxidative damage was measured using the oxidative stress marker, 8-hydroxyguanosine (8-OHG) and localization of 8-OHG with neurons, microglia and astrocytes in the spinal cord and peripherally in the dorsal root ganglion (DRG) at day 7. In addition to reducing pain, meloxicam reduced both spinal microglial and astrocytic activation at day 7 after nerve root compression. Spinal sPLA2 was also reduced with meloxicam treatment, with decreased production in neurons, microglia and astrocytes. Oxidative damage following nerve root compression was found predominantly in neurons rather than glial cells. The expression of 8-OHG in DRG neurons at day 7 was reduced with meloxicam. These findings suggest that meloxicam may prevent the onset of pain following nerve root compression by suppressing inflammation and oxidative stress both centrally in the spinal cord and peripherally in the DRG.
Keywords: neuropathic pain, NSAID, phospholipase A2, inflammation, reactive oxygen species, radiculopathy
Introduction
Neuropathic pain has been estimated in 7–10% of the population, with chronic pain carrying societal costs of $560–635 billion annually (Institute of Medicine (US) Committee on Advancing Pain Research, 2011; van Hecke et al., 2014; Holmes, 2016). In the cervical spine, the dorsal nerve roots are a common source of painful neuropathic injury since they are susceptible to loading from compression by disc herniation, spondylosis or other forms of trauma (Côté et al., 2004; Carette and Fehlings, 2005; Abbed and Coumans, 2007). Even a transient compression of the nerve root can produce chronic radiculopathy, which often manifests as pain or numbness that can radiate down the arm or leg (Abbed and Coumans, 2007; Kuijper et al., 2009; Caridi et al., 2011). Despite the high prevalence of painful neuropathy, current treatments are not effective in providing pain relief, partially due to an incomplete understanding of the mechanisms involved in pain cascades (Institute of Medicine (US) Committee on Advancing Pain Research, 2011). Both inflammatory and oxidative stress pathways at the injury site and in the spinal cord where nociceptive processing occurs, contribute to pain through the release of inflammatory mediators and reactive oxygen species (Abbed and Coumans, 2007; Goupille et al., 1998; Klein, 2016; Nagashima et al., 2009, Salvemini 2011). By inhibiting cyclooxygenase2 (COX-2) mechanisms, non-steroidal anti-inflammatory drugs (NSAIDs) can reduce both inflammation and oxidative stress (Takahashi et al., 2005; Ma et al., 2002); because of this, they are a common analgesic treatment but have side effects (Han et al., 2015; Wong et al., 2016). The NSAID meloxicam is, a known selective COX-2 inhibitor (Furst, 1997; Hawkey, 1999; Kimura and Kontani, 2009) that has been shown to cross the blood brain barrier (Dehouck et al., 1992; Novakova et al., 2014). It provides effective pain relief in orthodontic pain and animal models of temporomandibular joint pain without the same risk of adverse side effects (Zarif Najafi et al., 2015; Montesinos et al., 2016; Zhang and Gan, 2017). Although meloxicam administration before a painful nerve root compression has been shown to prevent pain onset (Philips et al., 2017), the pathophysiologic mechanisms responsible its effectiveness after nerve root injury are unknown.
After painful neural trauma, even a transient injury, the central nervous system mounts a robust inflammatory response contributing to pain onset and maintenance (Hubbard and Winkelstein, 2005; Nicholson et al., 2012; Rothman et al., 2009, 2010; Smith et al., 2013; Winkelstein et al., 2002). As part of that immune response, resident spinal glia become activated over different time courses (Hubbard and Winkelstein, 2005; Nicholson et al., 2014; Rothman et al., 2010; Sun et al., 2012; Takahata et al., 2011). Upon activation, both microglia and astrocytes release a host of pro-inflammatory cytokines as early as 1 hour after painful nerve root compression (Rothman et al., 2009b; Smith et al., 2016) that further exacerbate neuroinflammation in the spinal cord (Ren and Dubner, 2008; Crown, 2012; Thomas et al., 2015; Johnson et al., 2017). Activated glia and neurons also increase production of phospholipase enzymes, including the subfamily phospholipase A2 (PLA2), (Lee et al., 1998; Svensson et al., 2005). PLA2 enzymes catalyze the hydrolysis of glycerophospholipids in the cellular membrane to produce free fatty acids, such as arachidonic acid (AA), which are used through the COX pathway to produce prostaglandins and other inflammatory mediators (Chacur et al., 2004; Svensson et al., 2005). sPLA2 increases in many painful conditions including discogenic pain (Ren et al., 2015), spinal cord injury (Titsworth et al., 2009), and inflammatory pain (Svensson et al., 2005) and its direct application to normal nerve tissue induces behavioral sensitivity (i.e. pain) and immune cell activation in the spinal cord (Chacur et al., 2004). The upregulation of sPLA2 during inflammation also increases activity of the COX enzymes, exacerbating inflammation (Chacur et al., 2004). Although COX-2 inhibition alleviates pain and suppresses neuroinflammation (Ripamonti et al., 1996.; Ma et al., 2002; Tzeng et al., 2005), it is not known if systemic COX-2 inhibition has effects on spinal sPLA2 production and associated glial activation.
In conjunction with inflammation, oxidative stress pathways also contribute to neuropathic pain through the production of reactive oxygen species (ROS) like hydroxyl radicals, superoxide and nitic oxide (Machelska and Celik, 2016; Geis et al., 2017; Kiasalari et al., 2017). ROS production early after neural trauma results from the dysregulation of many cellular metabolic processes including production by NADPH oxidase, mitochondrial respiratory chains and even from the COX-mediated metabolism of AA to produce prostaglandins (Simmons et al., 2004; Adibhatla and Hatcher, 2008). The accumulation of ROS after neural injury has been hypothesized to contribute to neuropathic pain by activating glia, through inflammatory mediator production (Mosley et al., 2006; Naziroğlu et al., 2012; Areti et al., 2014) and by mediating spinal dorsal horn sensitization (Chung, 2004). In the spinal cord, activated microglia are the main sources of free radicals, which in parallel to their production of inflammatory cytokines, further exacerbates inflammation, and leads to tissue damage (Mosley et al., 2006). ROS-dependent oxidative damage is observed in neuropathic pain and neurodegenerative disorders (Mosley et al., 2006; Kim et al., 2010; Hoffman et al., 2011). In particular, increases in the oxidative stress protein and a marker of DNA damage and oxidation, 8-hydroxyguanosine (8-OHG), has been reported in the spinal cord between 3–14 days after a lumbar spinal nerve transection (Kim et al., 2010). Although 8-OHG has been studied in the context of diabetes, Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS), its involvement in pain is not well-characterized (Nunomura et al., 2004; Aguirre et al., 2005; Mosley et al., 2006; Hoffman et al., 2011). Further, whether COX-2 inhibition prevents pain by reducing oxidative damage during neural injury is unknown.
Since systemic administration of meloxicam prior to a painful nerve root injury has been shown to prevent the onset of both evoked and spontaneous pain for up to 7 days (Philips et al., 2017), this study assessed whether selective COX-2 inhibition with meloxicam before a painful nerve root compression modulates aspects of the spinal inflammatory cascades and/or oxidative stress in the dorsal root ganglion (DRG) at a time when pain is evident (Rothman et al., 2010; Nicholson et al., 2012; Smith et al., 2013). In order to assess if meloxicam reduces spinal inflammation, both microglial and astrocytic activation and sPLA2 production were evaluated at day 7 using immunohistochemistry in spinal tissue from rats receiving meloxicam treatment prior to a painful nerve root compression (NRC). Spinal sPLA2 and its expression in spinal neurons, microglia and astrocytes were separately assessed at day 7. In addition, the extent of oxidative stress was also measured using immunohistochemistry by the DNA damage marker 8-OHG. The distribution of 8-OHG was first evaluated in spinal microglia, astrocytes and neurons. Based on those findings, 8-OHG expression was quantitatively assessed in the DRG neurons.
Experimental Procedures
Surgical Procedures
All experimental procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and carried out under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983). All surgical procedures were performed using male Holtzman rats (275–299g; Envigo; Indianapolis, IN) under inhalation isoflurane anesthesia (4% induction, 2–3% for maintenance). At the time of anesthetic induction, meloxicam (2mg/kg; Bimeda; Oakbrook Terrace, IL) was diluted in sterile saline to a volume of 1mL and administered subcutaneously immediately before applying the nerve root compression (NRC) (n=7, MxNRC). Additional rats underwent NRC only (n=5, NRC) or a sham surgical procedure (n=4, sham) to serve as controls as previously described (Weisshaar et al., 2011; Nicholson et al., 2014; Smith et al., 2016; Philips et al., 2017). Briefly, a midline incision was made to expose the cervical spine and a right dorsal hemilaminectomy at C6/C7 exposed the right C7 dorsal nerve root. A microvascular clip (10gf; World Precision Instruments; Sarasota, FL) was inserted through a small opening in the dura to compress the nerve root for 15 minutes. After 15 minutes, the clip was removed and the incision was closed using 3–0 polyester suture and surgical staples. Sham surgeries included all of the same procedures with no root compression in order to control for the effect of surgery. Rats were monitored during recovered in room air with a heating pad.
Behavioral Assessment
Sensitivity of the ipsilateral forepaw to mechanical stimuli was measured before surgery (baseline, day 0) and on postoperative days 1, 3, 5, and 7 as previously described (Crosby et al., 2015a; Kras et al., 2015; Smith and Winkelstein, 2017). The forepaw was stimulated using a series of von Frey filaments of increasing strengths ranging from 1.4g to 26g (Stoelting, Wood Dale, IL). The lowest strength filament to evoke a response was recorded as the response threshold if the next filament also elicited a positive response. If a rat was unresponsive to all filaments, the maximum filament strength (26g) was taken as the threshold. Each testing session consisted of 3 rounds with at least 10 minutes of rest between each round. The threshold for each rat on each day was determined by averaging the rounds and was normalized to each rat’s own baseline threshold. A repeated measures ANOVA with post-hoc Tukey test compared response thresholds over time and between groups.
Tissue Harvest & Immunohistochemical Labeling of Spinal Cord & DRG
After behavioral assessment on day 7, the C7 spinal cord and DRGs were harvested to evaluate effects of meloxicam treatment on inflammation and oxidative stress. Rats were deeply anesthetized with sodium pentobarbital (65mg/kg) and transcardially perfused with phosphate-buffered saline and 4% paraformaldehyde. Tissues were post-fixed overnight and stored in 30% sucrose for 6 days at 4ºC. Samples were axially sectioned (14¼m thick) onto slides for immunohistochemical labeling. Microglial and astrocytic activation in the C7 spinal cord at day 7 was assessed using markers of ionized calcium-binding adaptor molecule 1 (Iba1) and glial fibrillary acidic protein (GFAP), respectively. Tissue sections were blocked for 2 hours in goat serum (Vector Labs; Burlingame, CA) and incubated in primary antibody solutions containing rabbit anti-Iba1 (1:1000; Wako; Richmond, VA) and mouse anti-GFAP (1:500; Millipore; Billerca, MA) overnight at 4°C. The next day, sections were incubated in a secondary antibody solution containing goat anti-rabbit 568 (1:1000; Life Technologies; Carlsbad, CA) and goat anti-mouse 488 (1:1000; Life Technologies; Carlsbad, CA).
To evaluate spinal sPLA2 and its cell-specific expression in each of neurons, microglia and astrocytes at day 7, sections were incubated overnight in antibodies to goat anti-sPLA2-IIA (1:500; Santa Cruz; Dallas, TX), mouse anti-MAP2 (1:250; Covance; Cumberland, VA), rabbit anti-Iba1 (1:500; Wako; Richmond, VA), and mouse anti-GFAP (1:500, Millipore, Billerica, MA). After incubation with primary antibodies, sections were washed in PBS and incubated in secondary antibodies of donkey (all from Invitrogen; Carlsbad, CA) anti-goat Alexa Fluor 488 (1:1000), donkey anti-rabbit Alexa Fluor 546 (1:1000), and donkey anti-mouse Alexa Fluor 350 and 546 (1:1000).
At the same time point (day 7), spinal and DRG sections also were evaluated for the cellular source of 8-OHG expression and the extent of neuronal 8-OHG in DRG neurons. Tissue sections were labeled to determine those cell types associated with expression of 8-OHG. In separate runs, tissue sections were blocked in goat serum (Vector Labs; Burlingame, CA) and then incubated overnight at 4°C in a primary antibody solution of mouse anti-8-OHG (1:200; Abcam, Cambridge, MA) and either rabbit anti-Iba1 (1:1000; Wako; Richmond, VA), rabbit anti-GFAP (1:500; Millipore; Billerca, MA), or mouse anti-NeuN 555 conjugate (1:500; Millipore; Billerca, MA). The next day, samples were incubated in secondary antibody solutions containing goat anti-mouse 488 (1:1000; Life Technologies; Carlsbad, CA) and goat anti-rabbit 568 (1:1000; Life Technologies, Carlsbad, CA). Samples labeled for 8-OHG and NeuN were only exposed to goat anti-mouse 488 (1:1000; Life Technologies; Carlsbad, CA). The distribution of 8-OHG in DNA and RNA was separately assessed on a subset of DRG tissue sections at day 7 (n=3 each group) as previously described (Nunomura et al., 1999, 2004; Lovell et al., 2011). Sections were first digested with Proteinase K (10¼g/mL; Sigma; St. Louis, MO) for 40 minutes at 37°C and then separately pre-treated with either RNase free DNase-I (10U/¼L; Sigma) or DNase-I free RNase (0.5¼g/¼L; Sigma) for 2 hours at 37°C prior to incubation with 8-OHG antibodies.
Immunohistochemical Analyses
For all analyses, spinal cord and DRG samples were collected also from normal un-operated rats (n=2) in order to provide reference for expression levels in naïve control tissues; samples with no primary antibody were included in all runs and analyses as controls and to verify specificity of each antibody. Tissue sections were imaged at 20x using a digital camera and stereomicroscope with DP2-BSW software (Olympus; Center Valley, PA). Spinal cord images were cropped to include only the superficial dorsal horn (750×150 pixels) (Zhang et al., 2013; Nicholson et al., 2014b); densitometry was used to quantify the percentage of positive pixels as a measure of positive labeling (Zhang et al., 2013). For each label, the percentage of pixels above the threshold expression in normal samples was separately quantified in the dorsal horn on the side ipsilateral to the injury for each sample. The total percent positive pixels in the ipsilateral dorsal horn was normalized to that in the normal tissue sections; Spinal Iba1, GFAP and sPLA2 expression was separately averaged across each group and compared using separate ANOVAs with post-hoc Tukey tests to detect differences between groups.
To quantify colocalization of spinal sPLA2 in neurons, microglia and astrocytes, the total number of pixels positive for each of sPLA2 and MAP2, sPLA2 and Iba1, and sPLA2 and GFAP was separately quantified using a custom MATLAB script as previously described (Nicholson et al., 2012; Crosby et al., 2015b; Zeeman et al., 2016). Neuronal, microglial and astrocytic sPLA2 were each determined by dividing the total number pixels positive for sPLA2 and either MAP2, Iba1 and GFAP by the total number of positive pixels for sPLA2 (Zeeman et al., 2016) for each image and is presented as fold-change over normal. Each of neuronal, microglial and astrocytic sPLA2 expression was compared across groups using a two-way ANOVA with Tukey’s post hoc test (groupXday).
Merged images of 8-OHG and Iba1, GFAP, and NeuN were evaluated qualitatively to identify which cell type in the spinal cord exhibited the greatest extent of DNA and RNA damage from oxidative stress. Since that evaluation revealed expression almost exclusively in neurons, neuronal 8-OHG expression was evaluated in the ipsilateral DRG. Images of the DRG were cropped (450×450 pixels) to include 10–20 random neurons per image for intensity analysis. The mean signal intensity and size of each neuron were determined by manually outlining each neuron in ImageJ (Kras et al., 2013). Neurons with an average diameter in the range of 4–21¼m were classified as small diameter neurons, and those with an average diameter ranging from 21–40¼m were taken as medium diameter neurons (Weisshaar et al., 2010; Dong et al., 2012). Neurons greater than 40¼m were considered large diameter neurons. Accumulation of 8-OHG was analyzed in all neuron sizes with separate follow-up analyses of small- and medium-diameter neurons since those neurons contribute to nociception (Weisshaar et al., 2010; Dong et al., 2012). Intensity of 8-OHG in the DRG was compared between groups using a one-way ANOVA with post hoc Tukey test, with separate tests run for small- and medium-diameter neuron sizes.
Results
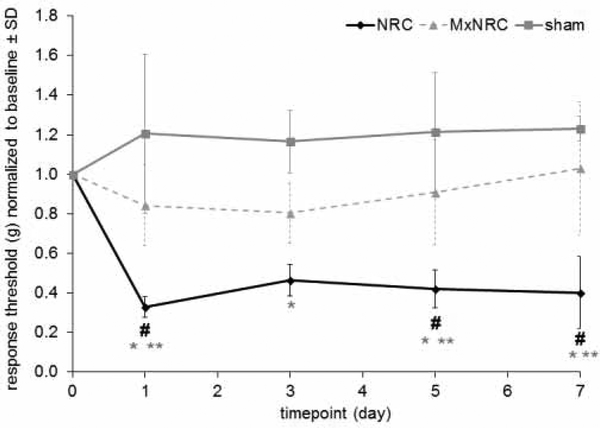
A single injection of meloxicam before a nerve root compression prevents the development of mechanical hyperalgesia in the ipsilateral forepaw that is typically produced by a nerve root compression (Fig. 1). Behavioral outcomes in the MxNRC and sham groups are not different and the withdrawal thresholds for those groups are unchanged from baseline. Meloxicam treatment (MxNRC) prevents the reduction in paw withdrawal thresholds that is seen after a NRC (p<0.0251), with thresholds for MxNRC significantly higher than those for NRC on days 1 (p<0.0056), 5 (p<0.0083), and 7 (p<0.0004) (Fig. 1). In contrast, responses in the NRC group significantly decrease from its baseline values on days 1 (p<0.0059), 5 (p<0.0366), and 7 (p<0.0384). In addition, although responses for the NRC group are significantly lower than sham overall (p<0.0012) and on each postoperative testing days (p<0.0030), there is no difference between MxNRC and sham on any day (Fig. 1).
Figure 1.
Pretreatment with meloxicam (MxNRC) prevents the reduction in normalized forepaw response thresholds (mean±SD) at days 1, 5, and 7 compared to NRC (**p<0.008). After NRC, responses decrease from baseline on days 1, 5, and 7 (#p<0.04). Normalized thresholds decrease significantly for the NRC group relative to sham starting at day 1 and lasting through day 7 (*p<0.003). Responses for MxNRC and sham groups are not different on any day.
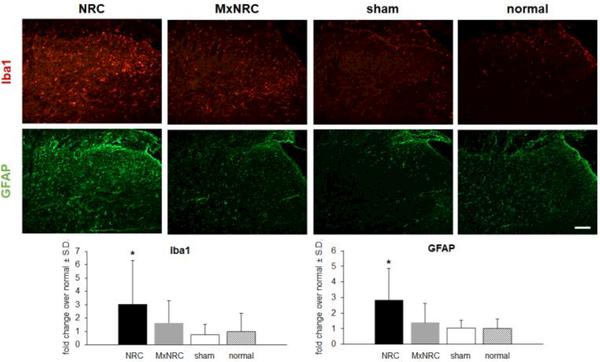
As with the behavioral responses at day 7, meloxicam also prevents increases in Iba1 and GFAP in the ipsilateral C7 superficial dorsal horn that occur after nerve root compression (Fig. 2). Treatment with meloxicam reduces spinal Iba1 to sham and normal levels, which is a significant reduction from levels after a painful NRC (p<0.0456) (Fig. 2). Likewise, Iba1 expression for sham (p<0.0016) and normal (p<0.0036) is significantly lower than for a NRC (Fig. 2). GFAP expression in the spinal cord is also significantly lower than levels after a NRC for the MxNRC (p<0.0010), sham (p<0.0002), and normal (p<0.0009) groups (Fig. 2).
Figure 2.
Meloxicam injection prevents the microglial and astrocytic activation that is typically observed after NRC in the superficial dorsal horn at day 7. Representative images show Iba1 (red) and GFAP (green) labeling in the ipsilateral dorsal horn of the C7 spinal cord. In the MxNRC group, Iba1 labeling remains at sham and normal levels and is significantly lower than levels in the NRC group (*p<0.0456). The increased GFAP in the superficial dorsal horn after NRC is prevented by meloxicam (*p<0.001), with GFAP expression similar to sham and normal levels. Scale bar is 100¼m and applies to all panels.
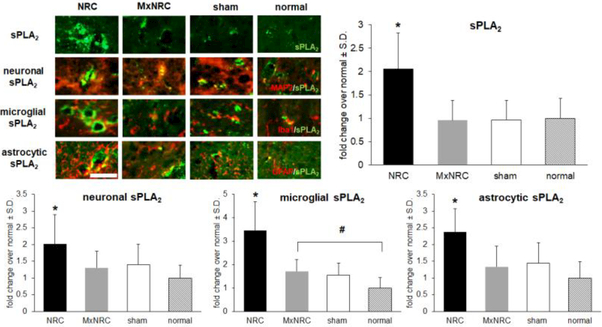
With meloxicam treatment, spinal sPLA2 is significantly decreased in the ipsilateral dorsal horn (p<0.0001) compared to that for NRC and has similar expression levels as those in spinal cords from rats receiving a sham procedure and from normal rats (Fig. 3). Spinal sPLA2 expression is significantly lower than that in the NRC group for both the sham (p<0.0001) and normal (p=0.0012) groups. Paralleling total sPLA2 expression, sPLA2 expression in each of neurons and astrocytes is also reduced to sham and normal levels and is significantly lower than expression after NRC for both neuronal sPLA2 (p=0.0007) and astrocytic sPLA2 (p= 0.002) respectively (Fig. 3). Spinal microglial sPLA2 expression is also significantly lower (p=0.0006) in the MxNRC group than in the NRC group, but meloxicam treatment does not reduce microglial sPLA2 levels to normal expression (p=0.0074) (Fig. 3).
Figure 3.
Pre-treatment with meloxicam before NRC prevents upregulation of spinal sPLA2. Representative images in the spinal dorsal horn show less expression of sPLA2 (green) with meloxicam treatment than NRC, and similar to sham and normal levels. Similarly, there is less colocalization (yellow) evident between sPLA2 (green) and each of MAP2, Iba1 and GFAP (red) in the meloxicam treated (MxNRC), sham and normal groups compared to the NRC group. Spinal sPLA2 expression in the NRC group is significantly higher (*p<0.0012) than levels in meloxicam-treated, sham and normal groups. The same is true of neuronal (*p=0.0007) microglial (*#p=0.0006) and astrocytic sPLA2 (*p=0.002) expression compared to NRC levels. Yet, microglial sPLA2 levels with meloxicam pre-treatment remain significantly higher (#p=0.0074) than normal levels. Scale bar is 200μm and applies to all panels.
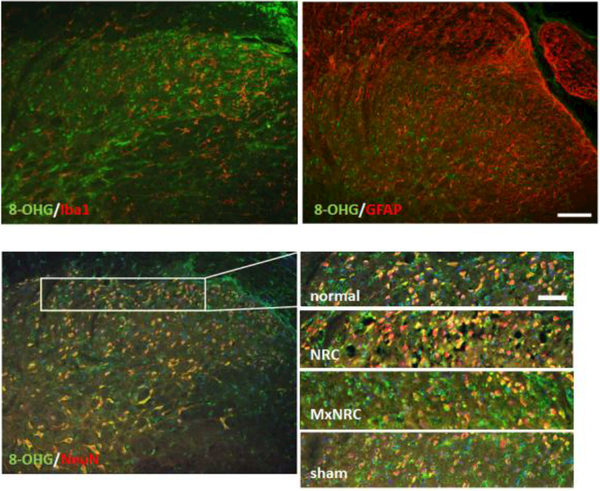
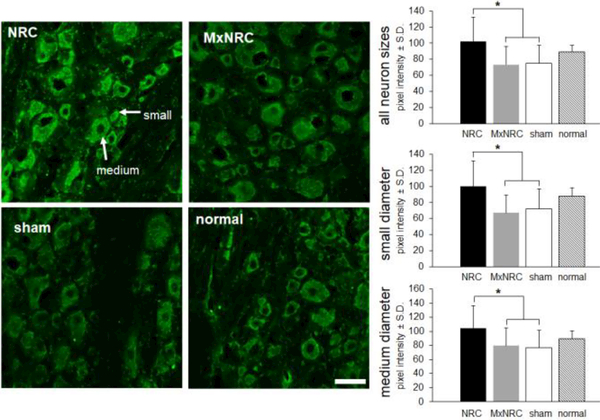
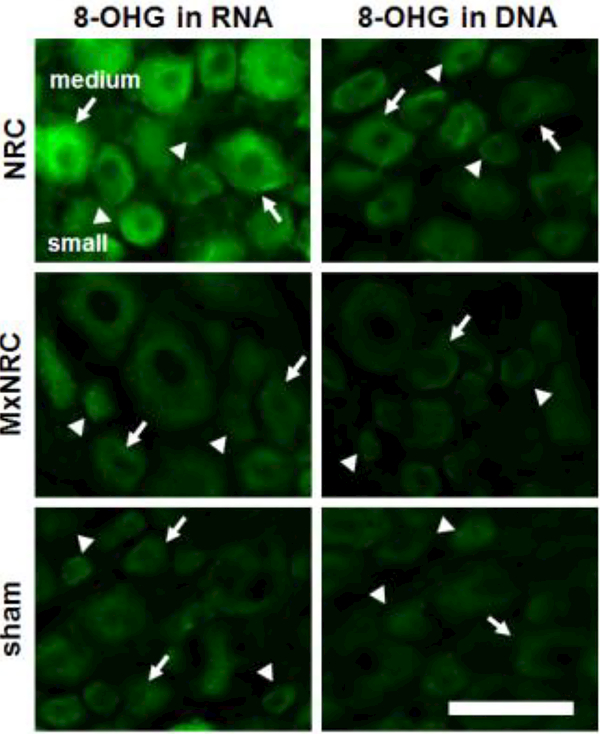
The marker of oxidative stress, 8-OHG, colocalizes predominantly with neurons in the spinal cord but not with microglia or astrocytes (Fig. 4). 8-OHG labeling in the spinal cord is not different from normal in any of the groups, regardless of injury or treatment. However, 8-OHG accumulation in the C7 DRG is modulated at day 7 following meloxicam treatment (Fig. 5). An average of 15±6 neurons was measured in each image. For neurons of all sizes, meloxicam treatment significantly reduces 8-OHG immunoreactivity compared to NRC alone (p<0.0004) (Fig. 5). This trend is also observed in small- and medium- diameter (p<0.0083) neurons (Fig. 5). Similarly, 8-OHG labeling is lower (p<0.0292) in sham tissue compared to NRC for all sizes of neurons, as well as explicitly in small-diameter and medium-diameter diameter neurons (Fig. 5). In neurons of all sizes, 8-OHG expression is elevated in both DNA and RNA following painful compression, with greater 8-OHG expression observed in RNA than DNA (Fig. 6). Meloxicam treatment reduces 8-OHG expression in both DNA and RNA to sham levels in neurons of all sizes, including small-diameter and medium-diameter neurons (Fig. 6)
Figure 4.
Representative images of the C7 spinal dorsal horn at day 7 showing that the oxidative stress marker 8-OHG does not colocalize with Iba1 or GFAP but does colocalize with the neuronal marker, NeuN. Enlarged images show colocalization of 8-OHG and NeuN in the superficial dorsal horn for each study group. Scale bar in the full size images is 100¼m and is 50μm in the insets of the superficial dorsal horn.
Figure 5.
Representative images and quantification of 8-OHG in the C7 DRG ipsilateral to the injury at day 7. Increases in 8-OHG is evident in the NRC group relative to MxNRC and sham for all neuron sizes (*p<0.0146). The same patterns of increases are observed in both the small-(*p<0.0160) and medium-diameter (*p<0.0292) neurons. Scale bar is 50¼m and arrows show small and medium neurons.
Figure 6.
Representative images of 8-OHG expression in RNA and DNA in the C7 ipsilateral DRG neurons of all sizes at day 7. 8-OHG in both RNA and DNA are elevated in the NRC group, with more 8-OHG observed in RNA than DNA. Meloxicam treatment reduces 8-OHG in both RNA and DNA to sham levels in neurons of all sizes, including small-diameter (arrowhead) and medium diameter (arrow) neurons. Scale bar is 150¼m and applies to all panels.
Discussion
This is the first study to show that in association with preventing pain onset (Fig. 1), systemic administration of meloxicam before an otherwise painful nerve root compression reduces spinal inflammation as well as peripheral oxidative damage in the DRG (Figs. 2–5). Meloxicam reduces the microglial and astrocytic activation in the spinal cord that is typical after painful nerve root injury (Hubbard and Winkelstein, 2005; Rothman et al., 2010; Nicholson et al., 2014) (Fig. 2). Paralleling the reduction in glial activation at day 7, spinal sPLA2 production is also suppressed, with reductions in both glia and neurons (Fig. 3). Interestingly, these reductions in spinal inflammation occur even though meloxicam is given systemically, suggesting that centrally-mediated responses may be involved in the pain attenuation. Unlike the inflammatory mediator sPLA2, the oxidative stress marker 8-OHG is almost exclusively in spinal neurons (Fig. 4) after painful nerve root injury, suggesting that accumulation of oxidative damage in neurons, rather than immune cells, may drive pain cascades in the periphery. In addition to pain and spinal inflammation, systemic meloxicam treatment immediately prior to neural trauma prevented the increase in 8-OHG in both DNA and RNA in peripheral DRG neurons (Figs. 5 & 6), further supporting the notion that meloxicam may attenuate pain by reducing peripheral oxidative stress. Taken together, these results suggest meloxicam may prevent the development of pain by reducing neuroinflammation and oxidative stress both centrally and peripherally.
Meloxicam’s reduction of spinal microglial and astrocytic activation a week after injury (Fig. 2) and which have been attributed to neuropathic pain maintenance (Winkelstein and DeLeo, 2002; Zhang and De Koninck, 2006; Rothman et al., 2010), suggests that its COX-2 inhibition may reduce the prolonged spinal neuroinflammation that is known to drive persistent neuropathic pain (Ripamonti et al., 1996; Ma et al., 2002; Tzeng et al., 2005). However, since meloxicam was given immediately before nerve root compression it is unclear if the associated analgesic effects were due to the prevention of glial activation at the later (day 7) time point or whether it mediated effects much earlier after the injury. Indeed, astrocytic activation in the spinal cord at day 7 or later has been associated with the maintenance of pain (Zhang and De Koninck, 2006; Rothman and Winkelstein, 2007; Nicholson et al., 2012; Smith et al., 2017). Of note, spinal microglia are activated robustly early (and before astrocytes) after neuropathic injury and so are associated with pain onset (Winkelstein et al., 2001; Scholz and Woolf, 2007; Rothman et al., 2010). Following spinal nerve ligation, which is a similar neuropathic injury to that used here, microglia and astrocyte activation occur sequentially with distinct roles in the temporal establishment of neuropathic pain (Zhuang et al., 2005). The absence of behavioral sensitivity with meloxicam treatment inhibiting COX-2 at day 7 when pain is typically evident after NRC may be due to the reduced spinal astrocytic activation at that time (Fig. 2). Similarly, microglial activation was also suppressed at that same time (Fig. 2); however, it is possible that the meloxicam pre-treatment inhibited earlier microglial activation that prevented both the pain and associated spinal inflammatory responses that were detected at the later time point (Figs. 1–3). Although early spinal microglial activation was not evaluated in this study, results do provide support for either a direct action by which meloxicam acts centrally to prevent pain or an indirect effect of reducing oxidative stress in peripheral afferents that drive spinal responses.
In conjunction with attenuating microglial activation, meloxicam also prevented the increase of overall and cell specific spinal sPLA2 expression after painful nerve root compression (Fig. 3), further supporting its effectiveness in suppressing spinal neuroinflammation. In addition, this is first study to show that spinal sPLA2 increases with a painful nerve root compression, implicating spinal sPLA2 in the maintenance of neuropathic pain after root injury. Although sPLA2 expression was probed using quantitative immunohistochemistry, previous work has shown that sPLA2 protein quantification from immunohistochemistry in the spinal cord follows similar trends to those found by alternate methods such as Western Blot (Titsworth et al., 2009). Application of sPLA2 alone onto a healthy sciatic nerve alone induces pain in the rat, as well as promotes both microglial and astrocytic activation in the spinal dorsal horn (Chacur et al., 2004). Further, expression of the neurotransmitter, substance P, is abolished for up to 24 hours after sPLA2 inhibition in cultured DRG neurons that are stimulated with inflammatory cytokines (Morioka et al., 2002). The robust increase in spinal sPLA2 after a painful nerve root compression injury is attributed to increases in neurons, microglia and astrocytes (Fig. 3), which could be from either increased trafficking of activated glia into the spinal cord or increased activation of resident spinal glia and neurons (Rothman and Winkelstein, 2007; Sheng et al., 2011). Since early after neural trauma, increased production of pro-inflammatory cytokines and ROS from activated glia and neurons promote the production of sPLA2 (Chacur et al., 2004; Svensson et al., 2005) and early glial activation and cytokine production occur after painful nerve root compression (Rothman et al., 2010; Smith et al., 2013), it may be possible that meloxicam pre-treatment may prevent the early inflammatory response associated with painful root injury, suppressing spinal glial activation (Fig. 2) and decreasing neuronal and glial sPLA2 production in the spinal cord at day 7 (Fig. 3). However, given the distinct differences in temporal activation between microglia and astrocytes (Zhuang et al., 2005), it is unknown if the analgesic effects of meloxicam are due to the prevention of overall or cell-specific sPLA2 production earlier than day 7. Nevertheless, these glial and sPLA2 findings (Figs. 2 & 3) suggest that meloxicam suppresses spinal neuroinflammation by inhibiting both the activation of spinal glia and the production of sPLA2, which likely also mediate other centrally-mediated mechanisms of pain.
Although both spinal neurons and glia produce ROS in neuropathic pain states (Mosley et al., 2006), DNA and RNA oxidative damage was evident predominantly in neurons in this study (Fig. 3), suggesting neuronal toxicity and dysfunction may play a role in radicular neuropathic pain. Neuronal oxidative damage has been reported to increase due to excess ROS production of several neuropathic pain states, including traumatic brain injury (Zhang et al., 2012) and peripheral nerve injury (Kim et al., 2010). Although glial cells are the major source of ROS (Mosley et al., 2006), extracellular ROS produced from microlgia has been shown to be directly toxic to neurons following neuropathic injury (Zhang et al., 2012; Manzanero et al., 2013). Further, intracellular ROS in microglia and macrophages can promote the production of neurotoxic cytokines (Block, 2008). After the same painful nerve root compression in this study, spinal 8-OHG is robustly elevated as early as 1 day lasting for up to 7 days (Kartha et al., 2018) which occurs concurrent with the timecouse of spinal glial activation (Rothman and Winkelstein, 2007; Rothman et al., 2010; Nicholson et al., 2012; Smith et al., 2016). As such, it may be possible that increased glial activation in the spinal cord contributes to the accumulation of spinal ROS and the subsequent increased spinal neuronal oxidative damage (Fig. 4). Spinal 8-OHG expression was not evaluated after meloxicam treatment in this study; so, that hypothesis remains only speculative.
In addition to spinal microglial activation, peripheral macrophages infiltrate the injured root after painful compression in this model (Chang and Winkelstein, 2011). Since those cells are a well-known source of extracellular ROS in neural and ischemic injuries (Hackel et al., 2013; Anwar et al., 2016), 8-OHG was also examined in peripheral DRG neurons. Systemic meloxicam that provided pain relief also reduced both the total 8-OHG and the 8-OHG that is specifically associated with DNA or RNA in the DRG at day 7 (Figs. 5 & 6), providing further evidence that peripheral oxidative stress, specifically oxidative DNA and RNA damage in neurons, is associated with nerve root compression as with neuropathic pain (Dirig et al., 1998; Takeda et al., 2005; Gmitterová et al., 2018). Interestingy, in addition to macrophage infiltration, painful compresson of the nerve root also induces production of sPLA2 by DRG neurons (Zhang et al., 2017). Because the use of metabolites (i.e. arachidonic acid) from sPLA2 cell membrane hydrolysis are well-established sources of reactive oxygen species in the production of COX-2 in neurons and immune cells (Adibhatla et al., 2006; Nanda et al., 2007), it is possible that ROS production and the resulting peripheral oxidative stress are also due to the elevated sPLA2 activity in the DRG after painful root injury. Given the role of COX-2 production in ROS generation and oxidative stress (Simmons et al., 2004; Adibhatla and Hatcher, 2008), the finding that 8-OHG in the DRG was reduced with meloxicam (Fig. 5) suggests that reducing peripheral oxidative stress by inhibiting COX-2 at the DRG, may be sufficient to prevent pain from nerve root compression. These findings begin to establish the contribution of oxidative stress in pain from neural trauma. However, since 8-OHG is only a marker of oxidative damage and ROS was not directly measured, further studies are needed to understand the spatiotemporal relationships between the peripherally- and centrally-derived oxidative stress responses and pain.
In addition to preventing neuropathic pain behaviors (Philips et al., 2017) (Fig. 1), systemic administration of meloxicam immediately prior to a painful nerve root compression prevents the spinal inflammation and peripheral oxidative stress that typically accompany that injury (Figs 2–5). Although additional studies are needed to further delineate the spatiotemporal mechanisms by which COX-2 inhibition alleviates pain following neuropathic injury, these findings suggest that a reduction in peripheral oxidative damage (Fig. 5) after selective COX-2 inhibition by meloxicam(Furst, 1997; Hawkey, 1999; Kimura and Kontani, 2009) may be due to a reduction in peripheral inflammation. In fact, decreased sPLA2 production and macrophage infiltration in the DRG has been found with this same meloxicam pre-treatment paradigm, along with prevention of the upregulation of spinal sPLA2 and activation of spinal glial cells that is typically observed after painful nerve root injury (Zhang et al., 2017). Together, these findings suggest that downregulation of sPLA2 in the spinal cord and DRG may contribute to the analgesic effects of meloxicam; further, the spinal changes may be due to regulation of neuroinflammation in the periphery. While COX-2 inhibitors have been recognized as alleviating pain by also reducing the robust neuroinflammatory response observed in neuropathic pain (Ma et al., 2002; Tzeng et al., 2005), this work also suggests COX-2 inhibition may alleviate pain by reducing inflammation-induced oxidative stress. Given that microglial activation (Winkelstein and DeLeo, 2002; Hubbard and Winkelstein, 2005; Rothman and Winkelstein, 2007; Rothman et al., 2009a) and oxidative stress tend to have more pronounced effects earlier after injury (Goecks et al., 2012; Nayernia et al., 2014; Geis et al., 2017), further studies probing immune cell-mediated ROS production at earlier times are needed to understand the interconnected contributions of inflammation and oxidative stress in the establishment of neuropathic pain. Nevertheless, this work begins to identify possible mechanisms by which meloxicam and agents that utilize COX-2 inhibition can be used as promising interventions in the treatment of neuropathic pain.
Highlights.
Pre-treatment with meloxicam reduces microglial and astrocytic activation in the spinal cord at day 7
Spinal sPLA2 upregulation is prevented by meloxicam, with reduced production in neurons, microglia and astrocytes
8-OHG colocalizes more in spinal neurons than glial cells and meloxicam reduces neuronal 8-OHG in the DRG
Acknowledgements
Support for this study was provided by the SK Foundation, the Catherine Sharpe Foundation, the Office of the Vice Provost for Research of the University of Pennsylvania, an NIH/NCCIH grant (#AT008291), and a grant (5R25OD010986) from the National Center for Advancing Translational Sciences, NIH, DHHS.
Abbreviations:
- 8-OHG
8-hydroxyguanosine
- AA
arachidonic acid
- COX-2
cyclooxygenase-2
- DNA
deoxyribonucleic acid
- DNase
deoxyribonuclease
- DRG
dorsal root ganglion
- GFAP
glial fibrillary acidic protein
- IBA1
ionized calcium binding adaptor molecule 1
- MAP2
microtubule associated protein 2
- NADPH
nicotinamide adenine dinucleotide phosphate
- NRC
nerve root compression
- NSAID
nonsteroidal anti-inflammatory drug
- RNA
ribonucleic acid
- RNase
ribonuclease
- ROS
reactive oxygen species
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbed KM, Coumans J-VCE (2007) Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery 60:S28–34. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF (2008) Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep 41:560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao FHC (2006) CDP-choline Significantly Restores Phosphatidylcholine Levels by Differentially Affecting Phospholipase A2 and CTP: Phosphocholine Cytidylyltransferase after Stroke. J Biol Chem 281:6718–6725. [DOI] [PubMed] [Google Scholar]
- Aguirre N, Beal MF, Matson WR, Bogdanov MB (2005) Increased oxidative damage to DNA in an animal model of amyotrophic lateral sclerosis. Free Radic Res 39:383–388. [DOI] [PubMed] [Google Scholar]
- Anwar MA, Al Shehabi TS, Eid AH (2016) Inflammogenesis of Secondary Spinal Cord Injury.Front Cell Neurosci 10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areti A, Yerra VG, Naidu V, Kumar A (2014) Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol 2:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiche F, Scheuerer S, Brune K, Geisslinger G, Goppelt-Struebe M (1996) Up-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett 390:165–169. [DOI] [PubMed] [Google Scholar]
- Block ML (2008) NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci 9 Suppl 2:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette S, Fehlings MG (2005) Clinical practice. Cervical radiculopathy. N Engl J Med 353:392–399. [DOI] [PubMed] [Google Scholar]
- Caridi JM, Pumberger M, Hughes AP (2011) Cervical radiculopathy: a review. HSS J 7:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Sloan EM, Wieseler-Frank J, Barrientos RM, Martin D, Poole S, Lomonte B, Gutiérrez JM, Maier SF, Cury Y, Watkins LR (2004) Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain 108:180–191. [DOI] [PubMed] [Google Scholar]
- Chang Y-W, Winkelstein BA (2011) Schwann cell proliferation and macrophage infiltration are evident at day 14 after painful cervical nerve root compression in the rat. J Neurotrauma 28:2429–2438. [DOI] [PubMed] [Google Scholar]
- Chung JM (2004) The role of reactive oxygen species (ROS) in persistent pain. Mol Interv 4:248–250. [DOI] [PubMed] [Google Scholar]
- Côté P, Cassidy DJ, Carroll LJ, Kristman V (2004) The annual incidence and course of neck pain in the general population: a population-based cohort study. Pain 112:267–273. [DOI] [PubMed] [Google Scholar]
- Crosby ND, Weisshaar CL, Smith JR, Zeeman ME, Goodman-Keiser MD, Winkelstein BA (2015a) Burst and tonic spinal cord stimulation differentially activate gabaergic mechanisms to attenuate pain in a rat model of cervical radiculopathy. IEEE Trans Biomed Eng 62:1604–1613. [DOI] [PubMed] [Google Scholar]
- Crosby ND, Zaucke F, Kras J V., Dong L, Luo ZD, Winkelstein BA (2015b) Thrombospondin-4 and excitatory synaptogenesis promote spinal sensitization after painful mechanical joint injury. Exp Neurol 264:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED (2012) The role of mitogen activated protein kinase signaling in microglia and neurons in the initiation and maintenance of chronic pain. Exp Neurol 234:330–339. [DOI] [PubMed] [Google Scholar]
- Dehouck MP, Jolliet-Riant P, Bree F, Fruchart JC, Cecchelli R, Tillement JP (1992) Drug transfer across the blood-brain barrier: correlation between in vitro and in vivo models. J Neurochem 58(5):1790–7. [DOI] [PubMed] [Google Scholar]
- Dirig DM, Isakson PC, Yaksh TL (1998) Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp Ther 285:1031–1038. [PubMed] [Google Scholar]
- Dong L, Quindlen JC, Lipschutz DE, Winkelstein BA (2012) Whiplash-like facet joint loading initiates glutamatergic responses in the DRG and spinal cord associated with behavioral hypersensitivity. Brain Res 1461:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst DE (1997) Meloxicam: Selective COX-2 inhibition in clinical practice. Semin Arthritis Rheum 26(6 Suppl 1):21–7. [DOI] [PubMed] [Google Scholar]
- Geis C, Geuss E, Sommer C, Schmidt HHHW, Kleinschnitz C (2017) NOX4 is an early initiator of neuropathic pain. Exp Neurol 288:94–103. [DOI] [PubMed] [Google Scholar]
- Gmitterová K, Gawinecka J, Heinemann U, Valkovič P, Zerr I (2018) DNA versus RNA oxidation in Parkinson’s disease: Which is more important? Neurosci Lett 662:22–8. [DOI] [PubMed] [Google Scholar]
- Goecks CSB, Horst A, Moraes MS, Scheid T, Kolberg C, Belló-Klein A, Partata WA (2012) Assessment of Oxidative Parameters in Rat Spinal Cord After Chronic Constriction of the Sciatic Nerve. Neurochem Res 37:1952–1958. [DOI] [PubMed] [Google Scholar]
- Goupille P, Jayson MIV, Valat J-P, Freemont AJ (1998) The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum 28:60–71. [DOI] [PubMed] [Google Scholar]
- Hackel D, Pflücke D, Neumann A, Viebahn J, Mousa S, Wischmeyer E, Roewer N, Brack A, Rittner HL (2013) The connection of monocytes and reactive oxygen species in pain. PLoS One 8:e63564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W-J, Chen L, Wang H-B, Liu X-Z, Hu S-J, Sun X-L, Luo C (2015) A Novel Nitronyl Nitroxide with Salicylic Acid Framework Attenuates Pain Hypersensitivity and Ectopic Neuronal Discharges in Radicular Low Back Pain. Neural Plast 2015:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey C (1999) COX-2 inhibitors. Lancet 353(9149):307–14. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Siedlak SL, Wang Y, Castellani RJ, Smith MA (2011) Oxidative damage is present in the fatal brain edema of diabetic ketoacidosis. Brain Res 1369:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D (2016) The pain drain. Nature 535:S2–S3. [DOI] [PubMed] [Google Scholar]
- Hubbard RD, Winkelstein BA (2005a) Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine (Phila Pa 1976) 30:1924–1932. [DOI] [PubMed] [Google Scholar]
- Hubbard RD, Winkelstein BA (2005b) Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine (Phila Pa 1976) 30:1924–1932. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Advancing Pain Research C and E (2011) Pain as a Public Health Challenge.
- Johnson MB, Young AD, Marriott I (2017) The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front Cell Neurosci 10:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiasalari Z, Rahmani T, Mahmoudi N, Baluchnejadmojarad T, Roghani M (2017) Diosgenin ameliorates development of neuropathic pain in diabetic rats: Involvement of oxidative stress and inflammation. Biomed Pharmacother 86:654–661. [DOI] [PubMed] [Google Scholar]
- Kim D, You B, Jo E-K, Han S-K, Simon MI, Lee SJ (2010) NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A 107:14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Kontani H (2009) Demonstration of Antiallodynic Effects of the Cyclooxygenase-2 Inhibitor Meloxicam on Established Diabetic Neuropathic Pain in Mice. J Pharmacol Sci 110(2):213–7. [DOI] [PubMed] [Google Scholar]
- Klein JP (2016) Imaging of progressive weakness or numbness of central or peripheral origin. In: Handbook of clinical neurology, pp 923–937. [DOI] [PubMed] [Google Scholar]
- Kras JV, Weisshaar CL, Pall PS, Winkelstein BA (2015) Pain from intra-articular NGF or joint injury in the rat requires contributions from peptidergic joint afferents. Neurosci Lett 604:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kras JV, Weisshaar CL, Quindlen J, Winkelstein BA (2013) Brain-derived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J Neurosci Res 91:1312–1321. [DOI] [PubMed] [Google Scholar]
- Kuijper B, Tans JTJ, Schimsheimer RJ, van der Kallen BFW, Beelen A, Nollet F, de Visser M (2009) Degenerative cervical radiculopathy: diagnosis and conservative treatment. A review. Eur J Neurol 16:15–20. [DOI] [PubMed] [Google Scholar]
- Lee HM, Weinstein JN, Meller ST, Hayashi N, Spratt KF, Gebhart GF (1998) The role of steroids and their effects on phospholipase A2. An animal model of radiculopathy. Spine (Phila Pa 1976) 23:1191–1196. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Soman S, Bradley MA (2011) Oxidatively modified nucleic acids in preclinical Alzheimer’s disease (PCAD) brain. Mech Ageing Dev.132(8–9):443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Du W, Eisenach JC (2002) Role for both spinal cord COX-1 and COX-2 in maintenance of mechanical hypersensitivity following peripheral nerve injury. Brain Res 937:94–99. [DOI] [PubMed] [Google Scholar]
- Machelska H, Celik MÖ (2016) Recent advances in understanding neuropathic pain: glia, sex differences, and epigenetics. F1000Research 5:2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Santro T, Arumugam T V. (2013) Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem Int 62:712–718. [DOI] [PubMed] [Google Scholar]
- Montesinos A, Ardiaca M, Gilabert JA, Bonvehí C, Oros J, Encinas T (2016) Pharmacokinetics of meloxicam after intravenous, intramuscular and oral administration of a single dose to African grey parrots ( Psittacus erithacus ). J Vet Pharmacol Ther 40(3):279–84. [DOI] [PubMed] [Google Scholar]
- Morioka N, Takeda K, Kumagai K, Hanada T, Ikoma K, Hide I, Inoue A, Nakata Y (2002) Interleukin-1beta-induced substance P release from rat cultured primary afferent neurons driven by two phospholipase A2 enzymes: secretory type IIA and cytosolic type IV. J Neurochem 80:989–997. [DOI] [PubMed] [Google Scholar]
- Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE (2006) Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin Neurosci Res 6:261–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima H, Morio Y, Yamane K, Nanjo Y, Teshima R (2009) Tumor necrosis factor-α, interleukin-1β, and interleukin-6 in the cerebrospinal fluid of patients with cervical myelopathy and lumbar radiculopathy. Eur Spine J 18:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS (2007) PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants--a new role as anti-inflammatory molecule. Curr Top Med Chem 7:765–777. [DOI] [PubMed] [Google Scholar]
- Nayernia Z, Jaquet V, Krause K-H (2014) New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal 20:2815–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroğlu M, Dikici DM, Dursun Ş (2012) Role of Oxidative Stress and Ca2+ Signaling on Molecular Pathways of Neuropathic Pain in Diabetes: Focus on TRP Channels. Neurochem Res 37:2065–2075. [DOI] [PubMed] [Google Scholar]
- Nicholson KJ, Gilliland TM, Winkelstein BA (2014a) Upregulation of GLT-1 by treatment with ceftriaxone alleviates radicular pain by reducing spinal astrocyte activation and neuronal hyperexcitability. J Neurosci Res 92:116–129. [DOI] [PubMed] [Google Scholar]
- Nicholson KJ, Guarino BB, Winkelstein BA (2012) Transient nerve root compression load and duration differentially mediate behavioral sensitivity and associated spinal astrocyte activation and mGLuR5 expression. Neuroscience 209:187–195. [DOI] [PubMed] [Google Scholar]
- Nicholson KJ, Zhang S, Gilliland TM, Winkelstein BA (2014b) Riluzole effects on behavioral sensitivity and the development of axonal damage and spinal modifications that occur after painful nerve root compression. J Neurosurg Spine 20:751–762. [DOI] [PubMed] [Google Scholar]
- Novakova I, Subileau EA, Toegel S, Gruber D, Lachmann B, Urban E, Chesne C, Noe CR, Neuhaus W (2014) Transport rankings of non-steroidal antiinflammatory drugs across blood-brain barrier in vitro models. PLoS One 9(1):e86806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Chiba S, Lippa CF, Cras P, Kalaria RN, Takeda A, Honda K, Smith MA, Perry G (2004) Neuronal RNA oxidation is a prominent feature of familial Alzheimer’s disease. Neurobiol Dis 17:108–113. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA (1999) RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 19(6):1959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips BH, Weisshaar CL, Winkelstein BA (2017) Use of the Rat Grimace Scale to Evaluate Neuropathic Pain in a Model of Cervical Radiculopathy. Comp Med 67:34–42. [PMC free article] [PubMed] [Google Scholar]
- Ren D, Zhang Z, Sun T, Li F (2015) Effect of percutaneous nucleoplasty with coblation on phospholipase A2 activity in the intervertebral disks of an animal model of intervertebral disk degeneration: a randomized controlled trial. J Orthop Surg Res 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R (2008) Neuron–glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol 21:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti C, Ticozzi C, Zecca E, Rodriguez CH, De Conno F (n.d.) Continuous subcutaneous infusion of ketorolac in cancer neuropathic pain unresponsive to opioid and adjuvant drugs. A case report. Tumori 82:413–415. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Guarino BB, Winkelstein BA (2009a) Spinal microglial proliferation is evident in a rat model of painful disc herniation both in the presence of behavioral hypersensitivity and following minocycline treatment sufficient to attenuate allodynia. J Neurosci Res 87:2709–2717. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Huang Z, Lee KE, Weisshaar CL, Winkelstein BA (2009b) Cytokine mRNA Expression in Painful Radiculopathy. J Pain 10:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Nicholson KJ, Winkelstein B a (2010) Time-dependent mechanics and measures of glial activation and behavioral sensitivity in a rodent model of radiculopathy. J Neurotrauma 27:803–814. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Winkelstein BA (2007) Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res 1181:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10:1361–1368. [DOI] [PubMed] [Google Scholar]
- Seybold VS, Jia Y-P, Abrahams LG (2003) Cyclo-oxygenase-2 contributes to central sensitization in rats with peripheral inflammation. Pain 105:47–55. [DOI] [PubMed] [Google Scholar]
- Sheng W, Zong Y, Mohammad A, Ajit D, Cui J, Han D, Hamilton JL, Simonyi A, Sun AY, Gu Z, Hong J-S, Weisman GA, Sun GY (2011) Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA2-IIA expression in astrocytes and microglia. J Neuroinflammation 8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T (2004) Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56:387–437. [DOI] [PubMed] [Google Scholar]
- Smith JR, Galie PA, Slochower DR, Weisshaar CL, Janmey PA, Winkelstein BA (2016) Salmon-derived thrombin inhibits development of chronic pain through an endothelial barrier protective mechanism dependent on APC. Biomaterials 80:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Lee J, Winkelstein BA (2017) Nerve Root Compression Increases Spinal Astrocytic Vimentin in Parallel with Sustained Pain and Endothelial Vimentin in Association with Spinal Vascular Reestablishment. Spine (Phila Pa 1976):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Syre PP, Oake SA, Nicholson KJ, Weisshaar CL, Cruz K, Bucki R, Baumann BC, Janmey PA, Winkelstein BA (2013) Salmon and human thrombin differentially regulate radicular pain, glial-induced inflammation and spinal neuronal excitability through protease-activated receptor-1. PLoS One 8:e80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Winkelstein BA (2017) The role of spinal thrombin through protease-activated receptor 1 in hyperalgesia after neural injury. J Neurosurg Spine 6:1–10. [DOI] [PubMed] [Google Scholar]
- Sun Y, Peng L, Sun X, Bo J, Yang D, Zheng Y, Liu C, Zhu B, Ma Z, Gu X (2012) Intrathecal Injection of Spironolactone Attenuates Radicular Pain by Inhibition of Spinal Microglia Activation in a Rat Model Minami M, ed. PLoS One 7:e39897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Lucas KK, Hua X-Y, Powell HC, Dennis EA, Yaksh TL (2005) Spinal phospholipase A2 in inflammatory hyperalgesia: Role of the small, secretory phospholipase A2. Neuroscience 133:543–553. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kawaguchi M, Shimada K, Nakashima T, Furuya H (n.d.) Systemic meloxicam reduces tactile allodynia development after L5 single spinal nerve injury in rats. Reg Anesth Pain Med 30:351–355. [DOI] [PubMed] [Google Scholar]
- Takahata S, Takebayashi T, Terasima Y, Tanimoto K, Wada T, Yamashita T, Sohma H, Kokai Y (2011) Activation of glial cells in the spinal cord of a model of lumbar radiculopathy. J Orthop Sci 16:313–320. [DOI] [PubMed] [Google Scholar]
- Takeda K, Sawamura S, Tamai H, Sekiyama H, Hanaoka K (2005) Role for cyclooxygenase 2 in the development and maintenance of neuropathic pain and spinal glial activation. Anesthesiology 103:837–844. [DOI] [PubMed] [Google Scholar]
- Thomas J, Mustafa S, Johnson J, Nicotra L, Hutchinson M (2015) The Relationship Between Opioids and Immune Signalling in the Spinal Cord. In: Handbook of experimental pharmacology, pp 207–238. [DOI] [PubMed] [Google Scholar]
- Titsworth WL, Cheng X, Ke Y, Deng L, Burckardt KA, Pendleton C, Liu N-K, Shao H, Cao Q-L, Xu X-M (2009) Differential expression of sPLA2 following spinal cord injury and a functional role for sPLA2-IIA in mediating oligodendrocyte death. Glia 57:1521–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S-F, Hsiao H-Y, Mak O-T (2005) Prostaglandins and cyclooxygenases in glial cells during brain inflammation. Curr Drug Targets Inflamm Allergy 4:335–340. [DOI] [PubMed] [Google Scholar]
- van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N (2014) Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155:654–662. [DOI] [PubMed] [Google Scholar]
- Weisshaar CL, Dong L, Bowman AS, Perez FM, Guarino BB, Sweitzer SM, Winkelstein BA (2010) Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J Neurotrauma 27:2261–2271. [DOI] [PubMed] [Google Scholar]
- Weisshaar CL, Winer JP, Guarino BB, Janmey PA, Winkelstein BA (2011) The potential for salmon fibrin and thrombin to mitigate pain subsequent to cervical nerve root injury. Biomaterials 32:9738–9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein BA, DeLeo JA (2002) Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: Considerations for persistent pain. Brain Res 956:294–301. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA (2001) Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol 439:127–139. [PubMed] [Google Scholar]
- Winkelstein BA, Weinstein JN, DeLeo JA (2002) The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine (Phila Pa 1976) 27:27–33. [DOI] [PubMed] [Google Scholar]
- Wong JJ, Côté P, Ameis A, Varatharajan S, Varatharajan T, Shearer HM, Brison RJ, Sutton D, Randhawa K, Yu H, Southerst D, Goldgrub R, Mior S, Stupar M, Carroll LJ, Taylor-Vaisey A (2016) Are non-steroidal anti-inflammatory drugs effective for the management of neck pain and associated disorders, whiplash-associated disorders, or non-specific low back pain? A systematic review of systematic reviews by the Ontario Protocol for Traffic Injur. Eur Spine J 25:34–61. [DOI] [PubMed] [Google Scholar]
- Zarif Najafi H, Oshagh M, Salehi P, Babanouri N, Torkan S (2015) Comparison of the effects of preemptive acetaminophen, ibuprofen, and meloxicam on pain after separator placement: a randomized clinical trial. Prog Orthod 16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman ME, Kartha S, Winkelstein BA (2016) Whole-body vibration induces pain and lumbar spinal inflammation responses in the rat that vary with the vibration profile. J Orthop Res 34:1439–1446. [DOI] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y (2006) Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem 97:772–783. [DOI] [PubMed] [Google Scholar]
- Zhang P, Gan Y-H (2017) Prostaglandin E2 Upregulated Trigeminal Ganglionic Sodium Channel 1.7 Involving Temporomandibular Joint Inflammatory Pain in Rats. Inflammation:1–8. [DOI] [PubMed] [Google Scholar]
- Zhang Q-G, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW (2012) Critical Role of NADPH Oxidase in Neuronal Oxidative Damage and Microglia Activation following Traumatic Brain Injury Combs C, ed. PLoS One 7:e34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kartha S, Lee J, Winkelstein BA (2017) Techniques for Multiscale Neuronal Regulation via Therapeutic Materials and Drug Design. ACS Biomater Sci Eng 3:2744–2760. [DOI] [PubMed] [Google Scholar]
- Zhang S, Nicholson KJ, Smith JR, Gilliland TM, Syré PP, Winkelstein BA (2013) The roles of mechanical compression and chemical irritation in regulating spinal neuronal signaling in painful cervical nerve root injury. Stapp Car Crash J 57:219–242. [DOI] [PubMed] [Google Scholar]
- Zhuang Z-Y, Gerner P, Woolf CJ, Ji R-R (2005) ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 114:149–159. [DOI] [PubMed] [Google Scholar]
- Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110. [DOI] [PubMed] [Google Scholar]