Abstract
Treatments for hepatitis C virus (HCV) have advanced greatly, becoming more efficacious with fewer adverse events, due to the availability of direct-acting antiviral agents, which target specific steps in the HCV life cycle. Recently, a combination regimen consisting of the HCV nonstructural protein 5A inhibitor elbasvir (EBR) and the HCV NS3/4A protease inhibitor grazoprevir (GZR) was approved for the treatment of patients with chronic HCV and genotypes (Gts) 1 and 4 in various countries. In Phase III trials, the combination of EBR/GZR (fixed-dose combination table or single agent) for 12 or 16 weeks of treatment with or without ribavirin resulted in a high sustained virological response at 12 weeks in treatment-naïve and treatment-experienced patients with HCV Gt 1a, 1b, 4, or 6, including special populations, such as individuals with advanced chronic kidney disease, HCV-HIV coinfection, and compensated cirrhosis. In this review, we focus on the mode of action, pharmacokinetics, clinical applications, efficacy, and safety profile of EBR/GZR, including special populations who have been considered refractory from the extensive evidence of clinical trials.
Keywords: HCV, DAAs, compensated LC, HCV/HIV
Introduction
Chronic hepatitis C virus (HCV) occurs in association with chronic inflammatory cell infiltration and hepatocellular necrosis because of HCV infection of the liver. Liver cirrhosis and the progression of hepatic fibrosis are critical stages of HCV-related liver disease, and can develop into hepatocellular carcinoma (HCC) in a multicentric manner. Therefore, the primary therapeutic goal of eradicating HCV by treatment with an antiviral drug is to terminate its progression to liver cirrhosis and HCC.1,2 Currently, the estimated number of patients with HCV is 71 million globally.3 HCV is classified into six major genotypes (Gts), with Gt 1 being the most prevalent globally (49.1%), followed by Gt 3 (17.9%), 4 (16.8%), 2 (11.0%), and 5 or 6 (,5%).4 Antiviral treatment for HCV infection has significantly improved since the advent of direct-acting antiviral (DAA) agents, such as boceprevir and telaprevir. First-generation DAAs with concomitant use of Pegylated interferon (IFN) and ribavirin (RBV) is associated with a high rate of adverse events (AEs). However, the combined use of second-generation DAAs without IFN, such as IFN-free regimens, improves treatment efficacy and decreases the incidence of AEs, yet a number of issues remain. As HCV patients get older, it becomes necessary to administer safer drugs. In addition, there are very limited treatment options for patients with chronic kidney disease (CKD), patients coinfected with HCV and HIV, patients with resistance-associated substitutions (RASs) as a result of prior treatment including DAAs, and patients with coadministered drugs due to complicated diseases. As such, novel agents with enhanced efficacy, tolerability, and convenience are required for unmet medical needs.
Recently, combination treatment with both the HCV nonstructural (NS) protein 5A inhibitor elbasvir (EBR; Figure 1A)5 and the HCV NS3/4A protease inhibitor grazoprevir (GZR; Figure 1B)6 was approved in many countries for the treatment of patients with chronic HCV and Gt 1 and 4. This review covers the mode of action, pharmacokinetics, clinical applications, efficacy, and safety profile of EBR/GZR, including special populations, from evidence to date.
Figure 1.

Chemical structural formulae of elbasvir (A) and grazoprevir (B).
Properties of elbasvir/grazoprevir
EBR inhibits HCV NS5A, which is necessary for assembly of the viral replication complex, RNA synthesis, and virion assembly (Figure 2).7–10 EBR has median effective concentration (EC50) values against chimeric replicons containing NS5A from clinical isolates of Gt 1a, 1b, 2a, 2b, 3a, 4a, 5a, and 6 of 4, 3, 3, 3,400, 140, 0.3, 1, and 9 pM, respectively (Table 1).11 GZR inhibits HCV NS3/4A protease, which has serine protease and NTPase/RNA helicase activities and is essential for viral polyprotein processing, RNA replication, and assembly (Figure 2).12–14 GZR has median EC50 values against chimeric replicons containing NS3/4A from clinical isolates of Gt 1a, 1b, 2a, 2b, 3a, 4a, 5a, and 6 of 400, 500, 2,300, 3,700, 35,000, 300, 6,600, and 200 pM, respectively (Table 2).11 In the HCV-replicon assay, the antiviral activity of EBR is reduced one- to 929-fold by single NS5A substitutions (known as RASs) at amino-acid positions M28T/V/A, Q30D/H/E/R, L31M/V/F, H58D, and Y93H/N/C in the HCV Gt 1a replicon, one- to 17-fold by single NS5A substitutions at amino-acid positions L28M, L31M/F/V, Y93H, and V121I in the HCV Gt 1b replicon, and one- to 7.5-fold by single NS5A substitutions at amino-acid positions L30F/P/S, M31V, N69K, and Y93H in the HCV Gt four replicon (Table 3).11,15,16 In the same way, the antiviral activity of GZR is reduced 1.1- to 114-fold by single NS3 substitutions at amino-acid positions V36A, T54S, Y56H, Q80K, R155K, A156S, and D168A/Y/T in the HCV Gt 1a replicon, 0.6- to 262-fold by single NS3 substitutions at amino-acid positions Q41R, F43S, R155K, A156T, and D168Y in the HCV genotype 1b replicon, and 47.1- to 137.1-fold by double-NS3 substitutions at amino-acid positions G162R with D168A, and G162R with D168V in the HCV Gt 4 replicon (Table 4).11,15,16
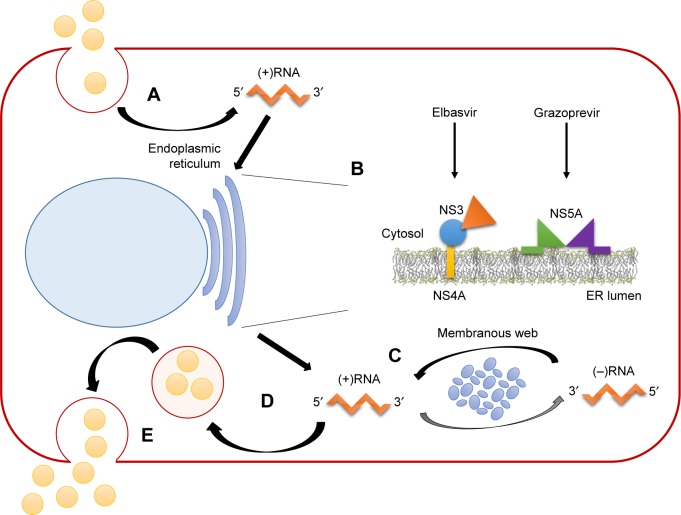
Figure 2.
Life cycle of hepatitis C virus (HCV) and targeting points of elbasvir and grazoprevir.
Notes: Major steps of the HCV life cycle are summarized: virus entry and uncoating of viral genome (A); translation and polyprotein processing (B); RNA replication (C); packaging and assembly (D); and virion release (E). Upon cleavage of the polyprotein, nonstructural HCV proteins form the replication complex in association with cellular factors, which leads to the formation of double-membrane vesicles, also called the membranous web, where replication takes place. Grazoprevir targets HCV NS3/4A protease, which has serine protease and NTPase/RNA helicase activities and is essential for viral polyprotein processing, RNA replication, and assembly. Elbasvir targets HCV NS5A, which is necessary for assembly of the viral replication complex, RNA synthesis, and virion assembly. Adapted by permission from Springer Nature. Nature, Nat Rev Microbiol. Replication of hepatitis C virus. Moradpour D, Penin F, Rice CM. Copyright 2007;5(6):453–463.12
Table 1.
Antiviral activity of elbasvir in HCV replicons of genotypes 1–6
| Genotype (Gt) of HCV replicona | EC50 (nM) | EC50 (nM) |
|---|---|---|
| Gt 1a_H77_NC004102 | 0.004±0.002 | 0.006±0.002 |
| Gt 1b_con1_AJ238799 | 0.003±0.001 | 0.006±0.004 |
| Gt 2a_JFH1_AB047639 | 0.003±0.001 | 0.019±0.01 |
| Gt 2b_AB030907/JFH1b | 3.4±2.6 | 11±4.8 |
| Gt 3a_S52_GU814263 | 0.14±0.09 | 0.49±0.19 |
| Gt 4a_ED43_GU814265 | 0.0003±0.0001 | 0.0005±0.0001 |
| Gt 5a_SA13_AF064490/JFH1b | 0.001±0.001 | 0.002±0.002 |
| Gt 6_DQ278892/JFH1b | 0.009±0.006 | 0.017±0.009 |
Notes:
Names and numbers are strain designations and GenBank accession numbers;
JFH1-based chimeric replicon harboring NS5A sequences of Gt 2b, 5a, and 6 were used. Values are mean ± SD (n≥3). Adapted with permission from Lahser FC, Bystol K, Curry S, et al. The combination of grazoprevir, a hepatitis C virus (HCV) NS3/4A protease inhibitor, and elbasvir, an HCV NS5A inhibitor, demonstrates a high genetic barrier to resistance in HCV genotype 1a replicons. Antimicrob Agents Chemother. 2016;60(5):2954–2964.11
Abbreviations: EC, effective concentration; HCV, hepatitis C virus.
Table 2.
Antiviral activity of grazoprevir in HCV replicons of genotypes 1–6
| Genotype (Gt) of HCV replicona | EC50 (nM) | EC50 (nM) |
|---|---|---|
| Gt 1a_H77_NC004102 | 0.4±0.2 | 0.9±0.5 |
| Gt 1b_con1_AJ238799 | 0.5±0.3 | 1.1±0.6 |
| Gt 2a_JFH1_AB047639 | 2.3±1.2 | 7.1±3.1 |
| Gt 2b_AY232740/JFH1b | 3.7±1.1 | 7.8±2.1 |
| Gt 3a_S52_GU814263 | 35±15 | 153±35 |
| Gt 4a_ED43_GU814265 | 0.3±0.2 | 0.8±0.4 |
| Gt 5a_SA13_AF064490/JFH1b | 6.6±0.6 | 12.8±2.2 |
| Gt 6_DQ278892/JFH1b | 0.2±0.04 | 0.3±0.1 |
Notes:
Names and numbers are strain designations and GenBank accession numbers;
JFH1-based chimeric replicon harboring NS3-4A sequences of Gt 2b, 5a, and 6 were used. Values are mean ± SD (n≥3). Adapted with permission from Lahser FC, Bystol K, Curry S, et al. The combination of grazoprevir, a hepatitis C virus (HCV) NS3/4A protease inhibitor, and elbasvir, an HCV NS5A inhibitor, demonstrates a high genetic barrier to resistance in HCV genotype 1a replicons. Antimicrob Agents Chemother. 2016;60(5):2954–2964.11
Abbreviations: EC, effective concentration; HCV, hepatitis C virus.
Table 3.
Inhibitory effects of elbasvir against NS5A-variant replicons
| HCV replicon | Fold shift relative to WTa |
|---|---|
| 1a H77 (WT) | 1 |
| 1a M28T | 15 |
| 1a M28V | 1 |
| 1a M28A | 61 |
| 1a Q30D | 925 |
| 1a Q30H | 6 |
| 1a Q30E | 56 |
| 1a Q30R | 16 |
| 1a L31M | 10 |
| 1a L31F | 96 |
| 1a L31V | 61 |
| 1a H58D | 6 |
| 1a Y93H | 220 |
| 1a Y93N | 929 |
| 1a Y93C | 11 |
| 1b con1 (WT) | 1 |
| 1b L28M | 2 |
| 1b L31M | 1 |
| 1b L31F | 15 |
| 1b L31V | 4 |
| 1b Y93H | 17 |
| 1b V121I | 0.2 |
| 4a ED43 (WT)b | 1 |
| 4a L30Fb | 15 |
| 4a L30Pb | 1 |
| 4a L30Sb | 4 |
| 4a M31Vb | 2.5 |
| 4a N69Kb | 1.5 |
| Y93Hb | 7.5 |
Notes:
The fold shift calculated using EC50 concentration;
chimeric replicons bearing resistant-associated substitutions generated in a Gt 2a (JFH1) backbone. Modified from Lahser et al,11 Asante-Appiah et al,15 and Liu et al.16
Abbreviations: EC, effective concentration; HCV, hepatitis C virus; Gt, genotype; WT, wild type.
Table 4.
Inhibitory effects of grazoprevir against NS3A-variant replicons
| HCV replicon | Fold shift relative to WTa |
|---|---|
| 1a H77 (WT) | 1 |
| 1a V36A | 1.2 |
| 1a T54S | 1.1 |
| 1a Y56H | 46 |
| 1a Q80K | 1.1 |
| 1a R155K | 3 |
| 1a A156S | 2.5 |
| 1a D168A | 114 |
| 1a D168Y | 27 |
| 1a V170T | 2 |
| 1b con1 (WT) | 1 |
| 1b Q41R | 3.6 |
| 1b F43S | 2.6 |
| 1b R155K | 0.6 |
| 1b A156T | 262 |
| 1b D168Y | 8 |
| 4a ED43 (WT)b | 1 |
| 4a G162Rb | 1 |
| 4a G162R D168Ab | 137.1 |
| 4a G162R D168Vb | 47.1 |
Notes:
Fold shift calculated using EC90 concentration in Gt 1a and EC50 concentration in Gt 1b and 4a;
chimeric replicons bearing resistant-associated substitutions generated in a Gt 2a (JFH1) backbone. Modified from Lahser et al,11 Asante-Appiah et al,15 and Liu et al.16
Abbreviations: EC, effective concentration; HCV, hepatitis C virus; Gt, genotype; WT, wild type.
Concentrations of 50 mg EBR and 100 mg GZR can be administered as a fixed-dose combination of a single once-daily tablet. Peak plasma concentrations of EBR and GZR are reached at a median of 3 and 2 hours, respectively, after oral administration,5,6 and steady-state pharmacokinetics reached within 6 days. EBR/GZR can be administered without regard to food, as changes in drug exposure are not clinically relevant after a high-calorie, high-fat meal in healthy subjects. EBR and GZR are highly bound to 99.9% and 98.8% of plasma proteins, respectively. Both EBR and GZR are mainly metabolized by CYP3A, after which both agents are predominantly excreted in feces. The approximate half-lives of EBR and GZR are 24 and 31 hours, respectively. EBR/GZR dose does not need to be adjusted in patients with renal impairment, including those on hemodialysis. Both EBR and GZR are CYP3A substrates, and thus coadministration of EBR/GZR with strong CYP3A inducers is contraindicated. GZR is a substrate of organic anion-transporting polypeptides 1B1/3 (OATP1B1/3), and thus coadministration of EBR/GZR with OATP1B1/3 inhibitors is also contraindicated. Plasma concentrations of tacrolimus and statins have the potential to increase after combination use of EBR/GZR, so clinical monitoring after administration of those drugs is recommended. However, EBR/GZR does not have any specific prohibition and caution as concomitant medications relative to the same class of drugs. It is not necessary either to take special consideration in combination use of EBR/GZR with calcium blockers, proton-pump inhibitors, or oral contraceptive drugs.
Clinical outcomes of elbasvir–grazoprevir
In the C-EDGE TN trial, in treatment-naïve (TN) patients infected with Gt 1a or 1b and receiving EBR/GZR for 12 weeks without RBV, sustained virological response at 12 weeks (SVR12) was 92% in patients infected with Gt 1a and 99% in those infected with Gt 1b (Table 5).17 The presence of compensated cirrhosis in 23% of patients had no effect on SVR12. In the open-label C-EDGE COINFECTION trial, TN patients coinfected with HIV with or without compensated cirrhosis were treated with EBR/GZR for 12 weeks. SVR12 rates were 97% in patients infected with Gt 1a and 95% in those infected with Gt 1b.18 In DAA-naïve HCV Gt 1a-infected patients who received a 12-week regimen of EBR/GZR, the presence of baseline NS5A polymorphisms at amino-acid positions M28, Q30, L31, and Y93 was associated with reduced efficacy.
Table 5.
Efficacy and safety of elbasvir/grazoprevir (EBR/GZR) ± ribavirin (RBV) in key clinical trials
| Clinical trial | Population | Regimen | Duration (weeks) | Gt | Overall SVR (%) | Gt 1a SVR (%) | Gt 1b SVR (%) | Gt 4 SVR (%) | Severe AEs (%) |
|---|---|---|---|---|---|---|---|---|---|
| C-SURFER30 | Advanced CKD | EBR/GZR | 12 | 1 | 94 | 97 | 92 | – | 15.6 |
| C-EDGE TN17 | TN ± cirrhosis | EBR/GZR | 12 | 1, 4, 6 | 95 | 92 | 99 | 100 | 3.0 |
| C-EDGE COINFECTION18 | HIV, TN ± cirrhosis | EBR/GZR | 12 | 1, 4, 6 | 96 | 97 | 96 | 96 | 0.9 |
| C-EDGE TE24 | P/R ± cirrhosis ± HIV | EBR/GZR ± RBV | 12 or 16 | 1, 4, 6 | 95 | 95 | 99 | 89 | 3.3 |
| C-WORTHY33 | ± HIV | EBR/GZR ± RBV | 8 or 12 | 1 | 97 | 94 | 93 | – | 1.4 |
| C-SALVAGE22 | PI + P/R ± cirrhosis | EBR/GZR + RBV | 12 | 1 | 96 | 93 | 98 | – | 5.1 |
| Japanese20 | No cirrhosis | EBR/GZR | 12 | 1 | 97 | 100 | 96 | – | 0 |
Abbreviations: AEs, adverse events; CKD, chronic kidney disease; Gt, genotype; PI, protease inhibitor; P/R, PEGylated interferon and ribavirin; SVR, sustained virological response; TE, treatment-experienced; TN, treatment-naïve.
Patients with NS5A polymorphisms at amino-acid positions Q30H/L/R or L31M had particularly low SVR12 rates (<50%).19 In HCV Gt 1b-infected patients who received a 12-week regimen of EBR/GZR, the presence of baseline NS5A polymorphisms was associated with slightly reduced SVR12 rates.19,20 Moreover, in HCV Gt 4-infected patients who received a 12-week regimen of EBR/GZR, the presence of baseline NS5A polymorphisms had little influence on SVR12 rates.19 Despite the fact that baseline NS3 RASs were commonly observed, a 12-week regimen of EBR/GZR demonstrated high SVR12 rates among patients infected with Gt 1a, 1b, or 4.17,19–24 In treatment-experienced (TE) patients in the C-EDGE TE Phase III trial, including 34% of patients with compensated cirrhosis, SVR12 rates in patients infected with Gt 1a and 1b were 92% and 100%, respectively, after 12 weeks of EBR/GZR without RBV, 93% and 97%, respectively, after 12 weeks with RBV, 94% and 98%, respectively, after 16 weeks without RBV, and 100% and 100%, respectively, after 16 weeks with RBV.24 Treatment-emergent NS5A substitutions were observed at amino-acid positions M28A/G/T, Q30H/K/R/Y, L31F/M/V, H58D, and Y93H/N/S in HCV Gt 1a, L28M, L31F/V, and Y93H in HCV Gt 1b, and L28S/T, M31I/V, P58D and Y93H in HCV Gt 4. Treatment-emergent NS3 substitutions were also observed at amino-acid positions V36L/M, Y56H, V107I, R155I/K, A156G/T/V, V158A, and D168A/G/N/V/Y in HCV Gt 1a, Y56F, V107I and A156T in HCV Gt 1b, and A156M/T/V, D168A/G, and V170I in HCV Gt 4 among patients receiving EBR/GZR with and without RBV and who experienced virologic failure in Phase II or III clinical trials.19 The safety of EBR/GZR has been based on Phase II and III clinical studies, and the most commonly reported AEs are fatigue and headache. Rare cases (0.8%) of substantial elevations in alanine aminotransferase levels have been reported. Less than 1% of subjects treated with EBR/GZR with or without RBV discontinued treatment due to AEs.
Advanced CKD
The prevalence of HCV in patients with CKD is higher than that in the general population, with an incidence of 5%–10% in Europe and the USA.25,26 The prevalence of HCV is also closely associated with the length of time on hemodialysis.27 Moreover, dialysis patients with HCV have a higher mortality rate than those without HCV.28 In the long term, the treatment of HCV-infected patients with CKD is limited by low efficacy and high AEs with IFN and RBV treatment.29 The development of DAAs has dramatically changed the prognosis for patients with HCV receiving dialysis. Until recently, all clinical trials excluded patients with CKD stage 4–5, due to a lack of safety data in patients with advanced CKD. However, this situation changed with the contribution of the C-SURFER study,30 which was the first randomized trial to include patients with CKD stage 4 to evaluate the efficacy and safety of EBR/GZR for HCV treatment.30 In this trial, 224 patients, 76% of whom were hemodialysis patients, were enrolled. This study included equal proportions of patients infected with HCV Gt 1a and 1b. SVR12 was achieved in 94% of patients. Six patients discontinued treatment for reasons other than virologic failure, and one noncirrhotic patient with HCV Gt 1b and NS5A-L31M RASs experienced a virological relapse. AEs were observed in 15.6% of patients and were generally mild. The most frequent AEs were headache, nausea, and fatigue. Serious AEs did not lead to drug discontinuation. There was no significant renal or hepatic impairment. The well-tolerated oral regimens for HCV have expanded the treatment strategies available for patients with CKD stage 4–5 and hemodialysis.
HIV coinfection
The overall global prevalence of HIV-HCV coinfection is about 6.2%.31 HIV accelerates liver fibrosis in patients coinfected with HCV through several different mechanisms,32 and treatment for chronic HCV infection is urgently needed in this patient population. Sulkowski et al33 reported Phase II results of the C-WORTHY trial, which evaluated the efficacy of 8 or 12 weeks of EBR/GZR with or without RBV in HCV Gt 1 monoinfected and HIV-HCV coinfected patients. Rockstroh et al18 also reported Phase III results of the C-EDGE COINFECTION trial, which tested the efficacy of 12 weeks of EBR/GZR without RBV in patients with HIV and HCV Gt 1, 4, or 6 coinfection. This EBR/GZR regimen can be used in combination with HIV-integrase inhibitors and a number of nucleoside reverse-transcriptase inhibitors without any dosage adjustment. Both clinical trials showed high SVR rates and a good safety profile for EBR/GZR regimens.
Compensated cirrhosis
Rockstroh et al18 reported the effects of EBR/GZR in patients with HCV infection and compensated cirrhosis, combining data from six clinical trials (C-SURFER,30 C-EDGE COINFECTION, C-EDGE TN,17 and C-EDGE TE24 [Phase III], and C-WORTHY33 and C-SALVAGE22 [Phase II]). The authors performed integrated analysis of 402 patients with HCV genotype 1, 4, or 6 infection and Child–Pugh A compensated cirrhosis enrolled in six clinical trials. All patients received EBR/GZR with or without RBV for 12–18 weeks. Among TN and TE patients receiving EBR/GZR for 12 weeks, 97.8% and 88.9% achieved SVR12, respectively. Among patients receiving EBR/GZR for 12 weeks with RBV, the SVR12 rate did not increase in TN or TE patients. TE patients receiving EBR/GZR with or without RBV for 16 or 18 weeks achieved SVR12 of 100% and 93.9%, respectively. Virologic failure was observed more frequently in Gt 1a-infected patients than in patients infected with Gt 1b or 4. HCV Gt 1a-infected patients with baseline RASs in NS5A receiving EBR/GZR for 12 weeks achieved SVR12 of 73%. Serious AEs were reported in 3% of patients, and no patient had a hepatic failure-related event.
Decompensated cirrhosis
Pharmacokinetic data from hepatic-impairment studies in non-HCV-infected subjects have demonstrated a decrease in EBR area under the curve in Child–Pugh A (39%), Child–Pugh B (28%), and Child–Pugh C (12%).34 In contrast, GZR exposure is increased in Child–Pugh A (double), Child–Pugh B (fivefold), and Child–Pugh C (12-fold).35 EBR/GZR use is contraindicated in patients with moderate (Child–Pugh B) or severe (Child–Pugh C) liver cirrhosis, as they may have significantly increased GZR exposure that might lead to an increased risk of elevated transaminase levels.
Pangenotypic use
The Phase II C-SCAPE study evaluated EBR/GZR with or without RBV in participants with HCV genotype 2, 4, 5, or 6 infection.36 Among participants with Gt 2 infection, SVR12 was achieved by 80% (24 of 30) of those receiving EBR/GZR + RBV. The addition of RBV to EBR/GZR appeared to increase SVR12 rates in participants with Gt 5 infection from 25% (one of four) to 100% (four of four). These results indicated that EBR/GZR ± RBV was unsatisfactory in patients with HCV Gt 2 or 5 infection. SVR12 rates for HCV Gt 4 and 6 were similar to those found in other studies.
Conclusion
The ultimate goal of HCV therapy is not only to eradicate HCV infection but also to prevent HCV-related deaths, including liver-related disease, HCC, and extrahepatic complications. Current recommendations from the American Association for the Study of Liver Disease, European Association for the Study of the Liver, and Infectious Diseases Society of America guidelines on EBR/GZR are shown in Table 6. Limitations of EBR/GZR are the occurrence of potential concomitant drug–drug interactions, the absence of pangenotypic efficacy, the need for baseline evaluation of RASs in Gt 1a-infected patients, and the putative hepatotoxicity of protease inhibitors in Child–Pugh B and C cirrhosis in a minority of clinical situations. However, the fixed-dose once-daily oral combination regimen of EBR/GZR has beneficial effects in antiviral treatment for HCV Gt 1- and 4-infected TN and TE patients, with or without compensated cirrhosis, HIV coinfection, or advanced CKD.
Table 6.
Treatment recommendations with elbasvir–grazoprevir (EBR/GZR) for HCV genotypes 1 and 4
| Guideline | Patients | Regimen | Duration (weeks) |
|---|---|---|---|
| AASLD/IDSA | Gt 1a: TN or TE (P/R), without NS5A RASs | EBR/GZR | 12 |
| Gt 1a: TN or TE (P/R), with NS5A RASs | EBR/GZR + RBV | 16 | |
| Gt 1b: TN or TE (P/R) | EBR/GZR | 12 | |
| Gt 1a or 1b: TE (P/R/PI) | EBR/GZR + RBV | 12 | |
| Gt 4: TN | EBR/GZR | 12 | |
| Gt 4: TE (P/R) | EBR/GZR + RBV | 16 | |
| EASL | Gt 1a: TN or TE (P/R), no NS5A resistance testing, low HCV RNAa | EBR/GZR | 12 |
| Gt 1a: TN or TE (P/R), no NS5A resistance testing, high HCV RNAb | EBR/GZR + RBV | 16 | |
| Gt 1a: TN or TE (P/R), without NS5A RASs, low HCV RNAa | EBR/GZR | 12 | |
| Gt 1a: TN or TE (P/R), without NS5A RASs, high HCV RNAb | EBR/GZR | 12 | |
| Gt 1a: TN or TE, with NS5A RASs, low HCV RNAa | EBR/GZR | 12 | |
| Gt 1a: TN or TE (P/R), with NS5A RASs, high HCV RNAb | EBR/GZR + RBV or alternative treatment | 16 | |
| Gt 1b: TN or TE (P/R) | EBR/GZR | 12 | |
| Gt 4: TN | EBR/GZR | 12 | |
| Gt 4: TE (P/R), low HCV RNAa | EBR/GZR | 12 | |
| Gt 4: TE (P/R), high HCV RNAb | EBR/GZR + RBV | 16 |
Notes:
Low HCV RNA defined as ≤800,000 (5.9 log) IU/mL;
high HCV RNA defined as >800,000 (5.9 log) IU/mL.
Abbreviations: AASLD, American Association for the Study of Liver Disease; EASL, European Association for the Study of the Liver; Gt, genotype; HCV, hepatitis C virus; IDSA, Infectious Diseases Society of America; PI, protease inhibitor; P/R, PEGylated interferon and ribavirin; RASs, resistance-associated substitutions; RBV, ribavirin; TE, treatment-experienced; TN, treatment-naïve.
Acknowledgments
The authors gratefully acknowledge Koji Ogawa, Goki Suda, Takuya Sho, Masato Nakai, Takaaki Izumi, Machiko Umemura, Naoki Kawagishi, Masatsugu Ohara, and Kazuharu Suzuki from the Hepatology Department of Hokkaido University Hospital for helpful discussions. This review was not supported by external funding.
Abbreviations
- AASLD
American Association for the Study of Liver Disease
- AE
adverse event
- CKD
chronic kidney disease
- DAA
direct-acting antiviral
- EASL
European Association for the Study of the Liver
- EBR
elbasvir
- Gt
genotype
- GZR
grazoprevir
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IDSA
Infectious Diseases Society of America
- LC
liver cirrhosis
- NS
nonstructural
- RAS
resistance-associated substitution
- RBV
ribavirin
- SVR12
sustained virological response at 12 weeks
- TE
treatment-experienced
- TN
treatment-naïve
Footnotes
Author contributions
KM, AN, and TS cowrote the manuscript. KM and NS discussed and edited the paper. NS supervised the writing of the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
NS has received lecture fees from Bristol-Myers Squibb and Janssen Pharmaceutical, grants and endowments from MSD and Chugai Pharmaceutical, and a research grant from Gilead Sciences. KM has received research grants from Gilead Sciences, Bristol-Myers Squibb, Otsuka Pharmaceutical, and Takeda Pharmaceutical. The authors report no other conflicts of interest in this work.
References
- 1.Alkhouri N, Lawitz E, Poordad F. Novel treatments for chronic hepatitis C: closing the remaining gaps. Curr Opin Pharmacol. 2017;37:107–111. doi: 10.1016/j.coph.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Bukh J. The history of hepatitis C virus (HCV): basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65(1 Suppl):S2–S21. doi: 10.1016/j.jhep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Global Hepatitis Report. Geneva: WHO; 2017. [Google Scholar]
- 4.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–7840. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn CA, Meinke PT, Chang W, et al. Discovery of MK-8742: an HCV NS5A inhibitor with broad genotype activity. ChemMedChem. 2013;8(12):1930–1940. doi: 10.1002/cmdc.201300343. [DOI] [PubMed] [Google Scholar]
- 6.Summa V, Ludmerer SW, Mccauley JA, et al. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob Agents Chemother. 2012;56(8):4161–4167. doi: 10.1128/AAC.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macdonald A, Harris M. Hepatitis C virus NS5A: tales of a promiscuous protein. J Gen Virol. 2004;85(Pt 9):2485–2502. doi: 10.1099/vir.0.80204-0. [DOI] [PubMed] [Google Scholar]
- 8.Masaki T, Matsunaga S, Takahashi H, et al. Involvement of hepatitis C virus NS5A hyperphosphorylation mediated by casein kinase I-α in infectious virus production. J Virol. 2014;88(13):7541–7555. doi: 10.1128/JVI.03170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGivern DR, Masaki T, Williford S, et al. Kinetic analyses reveal potent and early blockade of hepatitis C virus assembly by NS5A inhibitors. Gastroenterology. 2014;147(2):453.e7–462.e7. doi: 10.1053/j.gastro.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross-Thriepland D, Harris M. Hepatitis C virus NS5A: enigmatic but still promiscuous 10 years on! J Gen Virol. 2015;96(Pt 4):727–738. doi: 10.1099/jgv.0.000009. [DOI] [PubMed] [Google Scholar]
- 11.Lahser FC, Bystol K, Curry S, et al. The combination of grazoprevir, a hepatitis C virus (HCV) NS3/4A protease inhibitor, and elbasvir, an HCV NS5A inhibitor, demonstrates a high genetic barrier to resistance in HCV genotype 1a replicons. Antimicrob Agents Chemother. 2016;60(5):2954–2964. doi: 10.1128/AAC.00051-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5(6):453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 13.Morikawa K, Gouttenoire J, Hernandez C, et al. Quantitative proteomics identifies the membrane-associated peroxidase GPx8 as a cellular substrate of the hepatitis C virus NS3-4A protease. Hepatology. 2014;59(2):423–433. doi: 10.1002/hep.26671. [DOI] [PubMed] [Google Scholar]
- 14.Morikawa K, Lange CM, Gouttenoire J, et al. Nonstructural protein 3-4A: the Swiss army knife of hepatitis C virus. J Viral Hepat. 2011;18(5):305–315. doi: 10.1111/j.1365-2893.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 15.Asante-Appiah E, Curry S, Mcmonagle P, et al. Antiviral activity and resistance analysis of NS3/4A protease inhibitor grazoprevir and NS5A inhibitor elbasvir in hepatitis C virus GT4 replicons. Antimicrob Agents Chemother. 2017;61(7):e00363–17. doi: 10.1128/AAC.00363-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Curry S, Mcmonagle P, et al. Susceptibilities of genotype 1a, 1b, and 3 hepatitis C virus variants to the NS5A inhibitor elbasvir. Antimicrob Agents Chemother. 2015;59(11):6922–6929. doi: 10.1128/AAC.01390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevirelbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 18.Rockstroh JK, Nelson M, Katlama C, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2(8):e319–e327. doi: 10.1016/S2352-3018(15)00114-9. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu TE, Boyd S, Sherwat A, et al. Regulatory analysis of effects of hepatitis C virus NS5A polymorphisms on efficacy of elbasvir and grazoprevir. Gastroenterology. 2017;152(3):586–597. doi: 10.1053/j.gastro.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Kumada H, Suzuki Y, Karino Y, et al. The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients: a randomized phase II/III study. J Gastroenterol. 2017;52(4):520–533. doi: 10.1007/s00535-016-1285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dore GJ, Altice F, Litwin AH, et al. Elbasvirgrazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625–634. doi: 10.7326/M16-0816. [DOI] [PubMed] [Google Scholar]
- 22.Forns X, Gordon SC, Zuckerman E, et al. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol. 2015;63(3):564–572. doi: 10.1016/j.jhep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Hézode C, Colombo M, Bourlière M, et al. Elbasvir/grazoprevir for patients with hepatitis C virus infection and inherited blood disorders: a phase III study. Hepatology. 2017;66(3):736–745. doi: 10.1002/hep.29139. [DOI] [PubMed] [Google Scholar]
- 24.Kwo P, Gane EJ, Peng CY, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. 2017;152(1):164.e4–175.e4. doi: 10.1053/j.gastro.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Baid-Agrawal S, Pascual M, Moradpour D, Frei U, Tolkoff-Rubin N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol. 2008;18(2):97–115. doi: 10.1002/rmv.565. [DOI] [PubMed] [Google Scholar]
- 26.Marinaki S, Boletis JN, Sakellariou S, Delladetsima IK. Hepatitis C in hemodialysis patients. World J Hepatol. 2015;7(3):548–558. doi: 10.4254/wjh.v7.i3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabrizi F. Hepatitis C virus infection and dialysis: 2012 update. ISRN Nephrol. 2013;2013:159760. doi: 10.5402/2013/159760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone M, Mutimer D, Neuberger J. Hepatitis C virus and nonliver solid organ transplantation. Transplantation. 2013;95(6):779–786. doi: 10.1097/TP.0b013e318273fec4. [DOI] [PubMed] [Google Scholar]
- 29.McHutchison JG, Lawitz EJ, Shiffman ML, et al. PEGinterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361(6):580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 30.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 31.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11(6):362–371. doi: 10.1038/nrgastro.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1087–1097. doi: 10.1016/S0140-6736(14)61793-1. [DOI] [PubMed] [Google Scholar]
- 34.Marshall WL, Feng HP, Wenning L, et al. Pharmacokinetics, safety, and tolerability of single-dose elbasvir in participants with hepatic impairment. Eur J Drug Metab Pharmacokinet. 2018;43(3):321–329. doi: 10.1007/s13318-017-0451-9. [DOI] [PubMed] [Google Scholar]
- 35.Caro L, Wenning L, Guo Z, et al. Effect of hepatic impairment on the pharmacokinetics of grazoprevir, a hepatitis C virus protease inhibitor. Antimicrob Agents Chemother. 2017;61(12):e00813–17. doi: 10.1128/AAC.00813-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown A, Hézode C, Zuckerman E, et al. Efficacy and safety of 12 weeks of elbasvir ± grazoprevir ± ribavirin in participants with HCV genotype 2, 4, 5, or 6 infection: the C-SCAPE study. J Viral Hepat. 2018;25(5):457–464. doi: 10.1111/jvh.12801. [DOI] [PubMed] [Google Scholar]