Abstract
Purpose
Orofacial myofunctional therapy (OMT) is a modality of treatment for children and adults with obstructive sleep apnea (OSA) to promote changes in the musculature of the upper airways. This review summarizes and discusses the effects of OMT on OSA, the therapeutic programs employed, and their possible mechanisms of action.
Methods
We conducted an online literature search using the databases MEDLINE/PubMed, EMBASE, and Web of Science. Search terms were “obstructive sleep apnea” in combination with “myofunctional therapy” OR “oropharyngeal exercises” OR “speech therapy”. We considered original articles in English and Portuguese containing a diagnosis of OSA based on polysomnography (PSG). The primary outcomes of interest for this review were objective measurement derived from PSG and subjective sleep symptoms. The secondary outcome was the evaluation of orofacial myofunctional status.
Results
Eleven studies were included in this review. The studies reviewed reveal that several benefits of OMT were demonstrated in adults, which include significant decrease of apnea–hypopnea index (AHI), reduced arousal index, improvement in subjective symptoms of daytime sleepiness, sleep quality, and life quality. In children with residual apnea, OMT promoted a decrease of AHI, increase in oxygen saturation, and improvement of orofacial myofunctional status. Few of the studies reviewed reported the effects of OMT on the musculature.
Conclusion
The present review showed that OMT is effective for the treatment of adults in reducing the severity of OSA and snoring, and improving the quality of life. OMT is also successful for the treatment of children with residual apnea. In addition, OMT favors the adherence to continuous positive airway pressure. However, randomized and high-quality studies are still rare, and the effects of treatment should also be analyzed on a long-term basis, including measures showing if changes occurred in the musculature.
Keywords: sleep-disordered breathing, myofunctional therapy, oropharyngeal exercises, speech therapy, oral motor exercises
Introduction
Health depends on various factors, with breathing, eating, hydration, and sleep being the primordial among them. However, each of them is susceptible to changes caused by a large number of variables with different degrees of physical and mental consequences. Sleep-disordered breathing (SDB) is a relatively common problem, with obstructive sleep apnea (OSA) being its most severe manifestation.1
OSA is a relevant health problem that involves repeated upper airway collapse during sleep, causing a reduction (hypopnea) or cessation (apnea) of airflow, oxygen desaturation, and fragmented sleep, accompanied by respiratory effort.2,3 Snoring is a characteristic signal, but not when it occurs in an isolated manner. The disease must be diagnosed based on laboratory full-night polysomnography (PSG), considering that criteria are different for children younger than 18 years and adults.4
The pathophysiology of OSA during childhood is poorly known, although adenotonsillar hypertrophy and the installation of oral breathing are the major factors contributing to its occurrence.5–9 In general, studies report that the problem affects 1%–5% of the child population,2 with a peak of incidence among preschoolers, that is, in the age range when tonsil hypertrophy is more common.10 The consequences of OSA in children are low school performance, attention deficit and hyperactivity,11,12 low weight–height development,13 and cardiovascular dysfunction.2
During adulthood, anatomical and nonanatomical factors interact and contribute to the manifestation of OSA, such as narrow pharynx, increased upper airway length, specific pharyngeal lumen shapes, and collapsible upper airway. The factors that may play a role in OSA pathogenesis are changes in the activity of oropharyngeal muscles occurring during sleep, a poor genioglossus muscle responsiveness to negative pharyngeal pressure, a low respiratory arousal threshold, and an oversensitive ventilatory control system.14–18
The disease is more common among men than women and its prevalence increases with age and in obese persons.17,19 However, menopause is a risk factor for women regardless of age or body mass index (BMI),20 a factor that tends to reduce the difference from men, especially when no hormone replacement therapy is used.21 The symptoms of OSA include loud snoring, sleep disruption, excessive daytime sleepiness, nocturia,3,22,23 fatigue, morning headache, irritability, decreased concentration, and memory loss.24
Considered to be a progressive and incapacitating chronic disease,4 OSA impairs the quality of life and results in comorbidities such as arterial hypertension, cardiovascular diseases, and diabetes.25–27 However, the clinical manifestations are heterogeneous, and if the patients do not present characteristics such as a high BMI and subjective sleepiness, the symptoms may also be attributed to comorbid diseases and OSA will not be investigated and diagnosed.18
The first-line treatment during childhood is adenotonsillectomy, with reported cure or improvement of the disorder in most cases.9 Orthodontic treatment for correction of mandibular or maxillomandibular anomalies has been shown to improve OSA.28 Continuous positive airway pressure (CPAP) is the treatment of choice for adults with OSA,20 especially in severe cases, to relieve symptoms and to reduce the sequelae.18,29 However, mandibular advancement device (MAD), an intraoral dental splint used to protrude the mandible in a forward position during sleep and thus enlarge the upper airway,30,31 is mainly indicated as the first-stage treatment of adults with mild-to-moderate OSA and in severe cases in which attempts with CPAP treatment fail.30–32 MAD has been considered as a treatment option for children,33 although it is still under study, and it requires accurate indication and follow-up due to the craniofacial growth and development.
Still, surgical interventions are recommended for the correction of anatomical and morphological problems or as a second option for adult patients who fail to adhere or respond to noninvasive treatments. Surgical procedures include uvulopalatopharyngoplasty,34 surgically assisted rapid maxillary expansion,35 and maxillomandibular advancement.31,36,37
Despite the success rate of these interventions in reducing apnea–hypopnea index (AHI) and symptoms related to OSA,20 the need for new or complementary therapeutic modalities for OSA has been pointed out. The main reasons for this are the percentage of patients who do not respond satisfactorily to available treatments, the reduced adherence to CPAP, especially when the severity of the disease is moderate,2,18,29 and the possible complications of surgical procedures, even when limited.2,28,34
The orofacial myofunctional therapy (OMT), or oropharyngeal exercises, with one of the focal points in the promotion of changes in dysfunctional upper airway muscles, was proposed with success for reducing OSA severity and associated symptoms in adults.38 Since then, the potential of OMT has also been investigated to promote reduction of snoring,39 improvement of quality of life,40 and adherence to the use of CPAP,29 as well to treat residual OSA in children.41 A retrospective study summarized the clinical data of children with OSA and concluded that the OMT after surgery improved the outcome of treatment.42 Previously, systematic reviews,43 meta-analysis,44,45 and narrative reviews46,47 analyzed the myofunctional therapy for patients with OSA.
The difference in the current review from the previous ones is the emphasis on the strategies of therapeutics used by the various authors and their possible implications regarding the pathophysiology of OSA, in addition to suggestions for future investigations.
The objective of the present review is to summarize and discuss the available literature about OMT for patients with OSA, the benefits described, the strategies employed, and the way they can act on the functionality of the upper airway.
Methods
We conducted a bibliographic search up to December 3, 2017, of the following electronic databases: MEDLINE/PubMed, EMBASE, and Web of Science, limited to humans. A manual search of the references of selected studies was conducted as well. Articles in English and Portuguese were accepted. Search terms were “obstructive sleep apnea” in combination with “myofunctional therapy” OR “oropharyngeal exercises” OR “speech therapy”.
Inclusion criteria
Participants
This review was restricted to studies with participants meeting the following criteria: (1) diagnosis of OSA according to the measurement derived from PSG; (2) clinical symptoms of OSA; and (3) no other comorbid conditions (eg, trauma in the head and neck region, cancer, or neurological disease).
Studies
This review included clinical investigations published in journals with peer-review policy, which analyzed the effects of OMT (or oropharyngeal exercises) intervention alone for OSA patients. Randomized studies comparing OMT with a placebo intervention or a controlled intervention and prospective case–control studies were prioritized and mentioned in more detail. However, prospective case series were also included to explore the interventional models, due to the scarcity in relevant publications.
Outcomes of interest
The primary outcomes of interest for this review were objective measurements derived from PSG and subjective sleep symptoms including snoring, daytime sleepiness, and sleep quality. The secondary outcome of interest was the evaluation of orofacial myofunctional status.
Data screening and extraction
The initial screening of the detected documents was conducted blindly in triplicate. The titles were analyzed, as well as the abstract of the publication when available. Articles were selected if at least one reviewer identified them as relevant. A detailed full-text analysis was conducted and two reviewers extracted data from each paper such as study purpose, design, participants, interventions, and main and secondary outcomes. Disagreements regarding data screening and extraction were resolved by consensus among the three reviewers. Twelve questions were elaborated to guide the analyses (Supplementary material) based on a previous study.48
Results
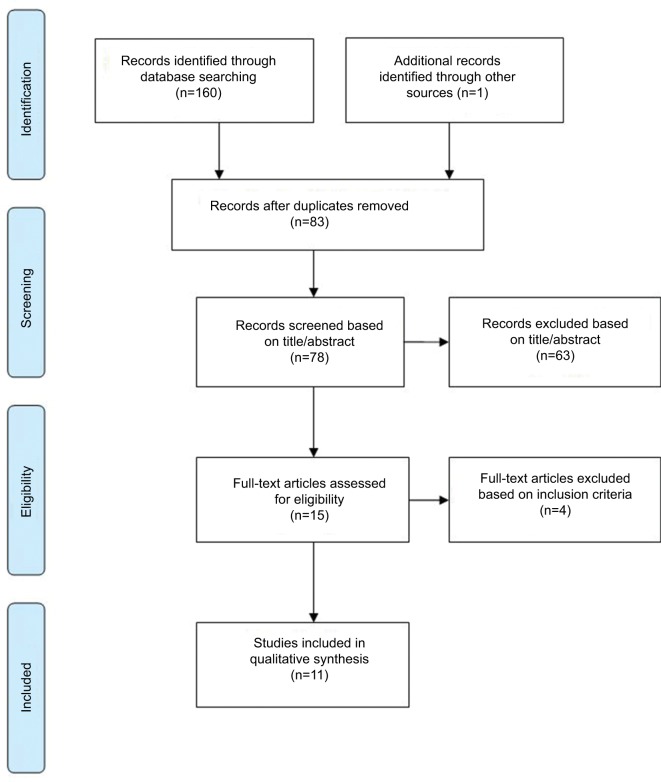
A total of 161 published articles were retrieved from the initial search. After exclusion of duplicates (83) and ineligible studies (67), 11 studies were included. Figure 1 shows a flowchart of the search process used in this review, which is based on PRISMA guidelines for systematic review49 with the exception that other types of studies were also included here along with randomized controlled trial (RCT) studies.
Figure 1.
Study search flowchart.
The types of study included were as follows: RCTs (n=4),29–40 prospective randomized (n=1),41 prospective case–control (n=1),1 and prospective case series (n=5).50–54 Table 1 provides a summary of the reviewed studies.
Table 1.
Summary of studies included
| Study, design, country | Objectives/participants | Intervention | Outcomes of interest | Results |
|---|---|---|---|---|
| Guimarães et al38 RCT Brazil |
To verify the effects of oropharyngeal exercises in patients with moderate OSAS on objective measurement of severity derived from PSG OE group – N: 16 Age: 51.5±6.8 Male: 63% BMI: 29.6±3.8 Control group – N: 15 Age: 47.7±9.8 Male: 73% BMI: 31.0±2.8 |
Three months for all groups, with weekly visits OE group Nasal lavage and OE HT: Three times a day Control group Nasal lavage Deep breathing exercises through the nose while sitting HT: Once a day, 30 min |
Full-night PSG AHI Lowest oxygen saturation SaO2 Sleep-related questionnaires Anthropometry |
Patients in OE group had significant improvement of PSG measures, snoring symptoms, subjective sleepiness, and sleep quality scores, and decreased NC Before vs after AHI: 22.4±4.8 vs 13.7±8.5 AHI REM: 29.8±12.7 vs 7.4±15.9 AI: 6.6±4.7 vs 3.3±3.2 Lowest SaO2 (%): 83±6 vs 85±7 NC: 39.6±3.6 vs 38.5±4.0 Control group showed no significant changes AHI: 22.4±5.4 vs 25.9±8.5 AHI REM: 29.9±11.6 vs 22.4±5.4 AI: 9.1±6.6 vs 9.6±6.0 Lowest SaO2 (%): 82±4 vs 80±4 NC: 40.9±3.5 vs 40.7±3.7 |
| Baz et al50 PCS Egypt |
To evaluate the effect of OMT as a simple method for treatment of patients with mild- to-moderate OSA N: 30 OMT Age: 44.07±7.54 Male: 73.3% BMI: 33.59±1.98 |
Three months, with two sessions weekly OMT HT: 3–5 times per day with minimum 10 min for each time |
Full PSG History Anthropometry ESS |
Patients showed significant improvement of PSG measures, snoring symptoms, subjective sleepiness, and decreased NC AHI: 22.27±4.51 vs 11.53±5.38 Minimum SaO2%: 84±4 vs 87±5 Arousal index: 28.87±8.41 vs 15.33±6.11 % TSTS: 14.05±4.89 vs 3.87±4.12 ESS: 16.40±1.96 vs 9.27±2.89 NC (cm): 42.77±1.67 vs 42.01±1.96 Subjective snoring (n): 30 vs 16 Excessive daytime sleepiness (n): 30 vs 12 Morning headache (n): 18 vs 6 |
| Diaféria et al40 RCT Brazil |
To assess the effect of ST alone and combined with CPAP on the QoL of patients with OSA Placebo group – N: 24 Age: 42.9±10.5 Male: 100% AHI: 27.8±20.3, BMI: 28.6±4.0 ESS: 12.8±3.1 ST group – N: 27 Age: 45.2±13.0 Male: 100% AHI: 28.0±22.7, BMI: 25.0±7.4 ESS: 13.7±3.2 CPAP group – N: 27 Age: 46.4±9.1 Male: 100% AHI: 34.4±22.4, BMI: 28.7±3.3 ESS: 12.0±2.1 Combination group – N: 22 Age: 47.5±10.9 Male: 100% AHI: 30.4±19.8, BMI: 27.9±2.4 ESS: 12.0±2.6 |
The patients were treated for three months Placebo group: head movements without any therapeutic function HT: Three times a day, with 20 min each ST group: OE HT: Three times a day, with 20 min each CPAP group: device with nasal mask, without humidifier, which was set to the optimal pressure according to the PSG to each patient Combination group (ST+CPAP): both protocols |
ESS, FOSQ, WHOQoL-Bref, and SF-36 PSG All outcomes were measured before and after therapy and after 3-week washout |
Significant improvement was observed in the physical domain of the WHOQoL-Bref in the ST and combination groups after treatment and washout compared to the pretreatment assessment. The functional capacity domain of the SF-36 improved in the ST group After ST vs washout Placebo group AHI: 30.6±21.8 vs 27.8±15.0 BMI: 28.3±3.9 vs 29.0±4.0 ESS: 12.2±5.2 vs 10.5±5.1 ST group AHI: 13.9±18.5 vs 21.3±21.4 BMI: 26.7±2.9 vs 26.9±2.9 ESS: 7.5±3.7 vs 10.4±4.3 CPAP group AHI: 4.3±4.0 vs 29.7±25.4 BMI: 29.5±3.2 vs 27.4±6.9 ESS: 7.2±3.6 vs 8.8±4.4 Combination group AHI: 3.4±2.7 vs 29.6±25.1 BMI: 28.3±2.6 vs 28.2±2.8 ESS: 7.3±5.7 vs 9.5±6.3 |
| Suzuki et al51 PCS Japan |
To assess the OMT for improving AHI and SpO2 during sleep N: 6 – Age: 22±0.5 Male: 50% BMI: 23.8±1.8 |
Two months of LCF training at the clinic with lip trainer | LCF measurement SpO2 and AHI obtained during sleep at home |
Patients had significant improvement of LCF, AHI, and SpO LCF: 8.8±1.6 vs 12.9±0.6 AHI: 15.1±3.4 vs 9.2±1.5 SpO2 (%): 90.0±2.9 vs 96.8±0.8 |
| Matsumura et al52 PCS Brazil |
To assess perceptions of the bed partner and the self-evaluation of snoring, myofunctional evaluation, AC and NC of individual with snoring or mild–severe OSA, before and after therapy N: 9 (7 OSA, 2 primary snoring) Age: 55.1 Male: 44.44% |
Twelve sessions lasting 40 min each OE | Snoring intensity and frequency indexes ESS Myofunctional evaluation Anthropometry |
The group had significant improvement of snoring intensity, snoring frequency, and ESS score (11.67 vs 4.67) Positive changes were observed in myofunctional evaluation NC: 40.3 vs 39.4 (p>0.05) AC: 96.8 vs 97.1 (p>0.05) |
| Ieto et al39 RCT Brazil |
To determine the effects of OE on snoring patients with a primary complaint of snoring and diagnosis of primary snoring or mild-to-moderate OSA OE group – N: 19 Age: 48±14 Male: 57.9% BMI: 28.3±2.7, AHI: 15.6±9.3 Control group – N: 20 Age: 45±13 Male: 55% BMI: 28.3±2.5, AHI: 15.1±9.5 |
Three months for all groups, with weekly visits OE group Nasal lavage and OE Control group Nasal lavage, deep breathing exercises, nasal dilator strips during sleep |
Objective snore index and the total snore index obtained after snore recording during PSG plus anthropometry questionnaires | OE group had significant lower snore index, total snore index, NC, intensity and frequency of snoring as reported by the bed partner Only a subgroup of patients with moderate OSA had a significantly decreased AHI (n=8, AHI: 25.4 [22.1–28.7] vs 18.1 [15.4–24.1], p=0.017) Control group showed only decrease on the subjective frequency of snoring reported by the patient |
| Villa et al41 PR Italy |
To evaluate the efficacy of OE as a means of reducing residual OSA in children after AT Children with residual OSA after AT (AHI>1) and persistence of respiratory symptoms after AT Group 1 – N: 14 Age: 6.01±1.55, AHI: 4.87±2.96 BMI (centile): 81.85±29.94 Group 2 – N: 13 Age: 5.76±0.82, AHI: 4.56±3.22 BMI (centile): 68.22±28.68 |
Two months, three meeting with the therapist Group 1: nasal washing and exercises HT: three times a day, with 10–20 repetitions each time Group 2: nasal washing HT: two times a day, in the morning and evening |
Full-night PSG before AT, 6 months after AT and after 2 months of exercises The improvement in OSA was defined by ΔAHI: (AHI at T1−AHI at T2)/AHI at T1×100 Morphofunctional evaluation |
Group 1 had significantly decreased AHI, reduction in oral breathing, a positive Glatzel test, a positive Rosenthal test, as well as increased labial seal and lip tone Group 2 had no significant difference after two months of nasal washing |
| Verma et al53 PCS India |
To evaluate the effect of oropharyngeal exercises in graded level of difficulty for mild-to-moderate OSA Patients with OSAS, N: 20 Age: 41.1±10.6 Male: 75% AHI: 20.1±9.1, BMI: 25.6±3.1 |
Three months, with weekly visits Three phases of OE in graded level of difficulty HT: 5 sets per day, with 10 repetitions of each exercise |
ESS PSG |
Patients showed significant improvement of ESS and PSG parameters and reduced NC ESS: 15.4±2.3 vs 13.6±3.1 AHI:19.7±9.4 Arousal index: 15.6±9.5 vs 12.8±7.1 Minimum SaO2 (%): 87.6±1.1 vs 88.5±1.6 Time duration for SaO2<90%: 6.7±6.6 vs 5.1±6.1 |
| Diaféria et al40 RCT Brazil |
To evaluate the effect of myofunctional therapy on CPAP adherence N=100 Male: 100% Placebo group – N: 24 Age: 42.9±10.5, AHI: 27.8±20.3 BMI: 28.6 ± 4.0 MT group – N: 27, Age: 45.2±13 AHI: 28±22.7, BMI: 25.0±7.4 CPAP group – N: 27 Age: 46.4±9.1, AHI: 34.4±22.4 BMI: 28.7±3.3 CPAP+OMT group – N: 22 Age: 47.5±10.9, AHI: 30.4±19.8 BMI: 27.9±2.4 |
Three months for all groups. Weekly visits for placebo, MT, and MT+CPAP groups Three visits for CPAP Placebo: relaxation and stretching of the neck muscles HT: Three times a day, 20 min each MT: OE HT: Three times a day, 20 min each CPAP device with nasal mask, without humidifier MT+CPAP: both protocols |
Adherence evaluation PSG, ESS, scale of snoring intensity and snoring frequency, myofunctional evaluation, Malampatti index All outcomes measured before and after three months of treatment, and after a 3-week washout period |
The average adherence to treatment was placebo (55%), MT (63%), CPAP (30%), MT+CPAP (65%) Time using the device: MT+CPAP>CPAP after one week and after three months of treatment The AHI decreased in the MT (50%), CPAP (87%), and MT+CPAP (89%). All groups were significantly different from the placebo ESS: Only the OMT group maintained the improvement in the snoring intensity and frequency after the washout period The Mallampati index improved in the OMT and CPAP+OMT groups and it was correlated with the increased strength of the tongue and soft palate |
| Villa et al1 PC-C Italy |
To evaluate the efficacy of MT to reduce oral breathing in children with SDB and to evaluate the increase in tongue tone 54 children with SDB (14 primary snoring [PSG, AHI 0.35±0.3] and 40 mild–moderate OSA [AHI 2.2±2.0]) MT group: (n: 36) Age: 6.7±2.3 Male: 38.8% Non-MT group: (n: 18) Age: 6.7±2.8 8 Male: 44.4% Healthy group: (n: 38) Age: 7.8±2.2 Male: 65.8% |
Two months, with two monthly meetings with a therapist MT group: MT plus nasal washing HT: three times a day, with 10–20 repetitions each time Non-MT group: nasal washing, two times a day, in the morning and evening. |
All the patients were evaluated before (T0) and after (T1) two months of treatment: tongue strength, tongue peak pressure, and endurance using the IOPI, myofunctional evaluation, nocturnal pulse oximetry The Healthy group underwent only IOPI measurements |
T0: MT vs non-MT (p>0.05) Compared to healthy group, MT and non-MT groups had lower tongue strength, tongue peak, and longer endurance (s) T1: MT group showed significant increase of tongue strength, tongue peak, endurance, mean and minimum SaO2, decrease of oxygen desaturation index, as well as decrease of the number of children with oral breathing habit (15 vs 3) and lip hypotonia (14 vs 6) and abnormal tongue resting position (17 vs 12) No differences were observed in the non-MT |
| Mohamed et al54 PCS Egypt |
To evaluate the effect of upper airway muscle exercise and rehabilitation as a new and simple technique to treat OSAS Group I moderate OSAS N: 15 Age: 46.39±2.04 Male: 80% BMI: 28.62±1.86 Group II: severe OSAS N: 15 Age: 47.5±9 Male: 86.7% BMI: 27.2±2.03 |
Three months, with weekly visits OE HT: 3–5 times per day with minimum 10 min for each time |
Full-night PSG AHI Lowest SaO2 Snoring ESS Neck circumference |
Daytime sleepiness (ESS), AHI, SaO2, and snoring index improved significantly in Group I (all p≤0.003), but not in Group II Group I: ESS: 14±6 vs 9.5±4.9 AHI: 22.51±5.03 vs 12.4±5.12 Snoring index: 312±8.8 vs 237.8±27.4 SaO2 (%): 83±4 vs 86±5 NC: 39.65±3.52 vs 38.92±2.92 Group II: ESS: 20.9±6.2 vs 18.91±5.1 AHI: 46.1±21.1 vs 42.8±15.65 SaO2 (%): 75±5.8 vs 78±4.9 Snoring index: 615±96.8 vs 554.6±81.43 NC: 43.02±2.06 vs 42.86±1.87 |
Note: Study type: RCT, PR, PC-C, and PCS; diagnosis: OSAS, OSA, and SDB; intervention: OE, MT, OMT, HT, ST, CPAP, AT, and LCF; age and anthropometric measures: age (mean age in years), BMI, AC, NC, and PSG and its variables such as AI, AHI, AHI REM, SaO2, SpO2, and TSTS; subjective scale: ESS, QoL, FOSQ, WHOQoL-Bref, SF-36, and IOPI.
Abbreviations: RCT, randomized controlled trial; PR, prospective randomized; PC-C, prospective case–control; PCS, prospective case series; OSAS, obstructive sleep apnea syndrome; OSA, obstructive sleep apnea; SDB, sleep-disordered breathing; OE, oropharyngeal exercises; MT, myofunctional therapy; OMT, orofacial myofunctional therapy or oral myofunctional therapy; HT, home training; ST, speech therapy; CPAP, continuous positive airway pressure; AT, adenotonsillectomy; LCF, labial closure force; Age, mean age in years; BMI, body mass index (kg/m2); AC, abdominal circumference; NC, neck circumference; PSG, polysomnography; AI, apnea index (events/hour); AHI, apnea–hypopnea index (events/hour); AHI REM, apnea–hypopnea index during rapid eye movement (events/hour); SaO2, oxygen saturation; SpO2, saturation of peripheral oxygen; TSTS, total sleep time snoring; ESS, Epworth Sleepiness Scale; QoL, quality of life; FOSQ, Functional Outcomes of Sleep Questionnaire; WHOQoL-Bref, World Health Organization Quality of Life Assessment; SF-36, Medical Outcomes Study 36-Item Short-Form Health Survey; IOPI, Iowa Oral Performance Instrument.
All RCT studies, with clinical trials registered, analyzed the effect of OMT in young subjects and adults with mild and/or moderate OSA, and the authors were Brazilians.29–40 Two of them included the same sample to answer distinct research questions.29,40 The prospective randomized and prospective case–control studies analyzed the benefits of OMT for children with SDB residual apnea1,41 and the authors were Italians.
OMT
OMT is a treatment modality applied to subjects with orofacial myofunctional disorders (OMDs), that is, with changes in the orofacial structure, in the cervical musculature, or both, which may interfere with the development or functioning of orofacial structures and functions.55 OMT is based on exercises and other strategies that favor sensitivity, proprioception, mobility, coordination, and strength of orofacial structures, as well as promote an appropriate performance of respiration, mastication, deglutition, and speech.
In 1990, the American Speech and Hearing Association first recognized the role of the speech-language pathologist in providing services to persons with OMD, and the knowledge and skills required to evaluate and treat OMD were later described.56 However, the starting point in the development of OMT was the recognition that the correction of malocclusion requires equilibrium of orofacial muscles.57–59 The mutual interest of orthodontists and speech pathologists in oropharyngeal mechanisms led them to cooperate in the study of some associated problems.59 Since then, the range of health problems, treatment strategies,60 and scientific evidence has expanded.
An example of this is that the patients with sequelae of chronic mouth breathing have been long treated by speech-language pathologists61 due to the orofacial myofunctional impairments.62,63 However, the use of PSG for the diagnosis of OSA was not widespread, and therefore, only the symptoms and clinical condition of the patients were considered. Since the last decade, due to the dissemination of knowledge, OSA has attracted the attention of these professionals. The reason for this interest lies in the fact that orofacial and pharyngeal muscles dysfunction64 and impaired oropharyngeal control are possible contributing factors to airway collapse,65 mainly when associated with an anatomical predisposition.
Therapeutic procedures in this area can be selected based on scientific evidence of benefits of a particular treatment or theoretical solidity when clinical efficacy has not been previously documented.66 Thus, when proposing the first exercise protocol for the reduction of obstructive sleep apnea syndrome (OSAS) severity, Guimarães et al38 used the scientific reports available about the pathogenesis of OSA and the empirical foundations of speech therapy. According to the authors,38 the oropharyngeal exercises target soft palate elevation that recruits several upper airway muscles such as the tensor and levator veli palatini, as well as muscle fibers of the palatopharyngeal and palatoglossus muscles, tongue repositioning, and training of mandibular elevation to avoid mouth opening. The results obtained in an RCT showed that, after three months of therapy, patients with moderate OSA had a significant reduction of AHI (22.4±4.8 vs 13.7±8.5), an increase of lowest oxygen saturation (83±6 vs 85±7), as well as a reduction of associated symptoms. These changes did not occur in the control (placebo) group.
This protocol was applied in other studies in full52 or with some modifications in the exercises.29,40 An author added 12 other exercises,53 although giving no reason for this addition. In another study39 whose objective was to treat snoring, the original protocol was simplified to facilitate the incorporation of the training into the daily activities. The number of exercises was reduced to 50% and the duration of each training was set to 8 min. This model was later used for the treatment of OSA.54 Although Baz et al50 have maintained the goals of increasing the tone and endurance of the same muscles for the treatment of OSA, they employed different exercises.
In most of the studies reviewed, the therapeutic program for adults lasted three months, with a weekly visit and training at home for three29,38–40,52 to five times a day.53,54 The authors adopted systems of control of adherence to treatment considering the frequency of supervised sessions and a diary indicating home practice. Only one pilot study51 lasted two months, with training at the clinic for 5 min twice a day and four times per week. The strategies and exercises used during therapy are listed in Table 2.
Table 2.
Strategies and exercises for young and adult patients with OSA recommended in the reviewed studies
| Frequency (times a day)/dose | |
|---|---|
| Nasal lavage with an application of saline in each nostril | Three times/10 mL38,39 |
| Soft palate | |
| Pronounce an oral vowel intermittently (isotonic exercise)/elevate the soft palate and uvula while intermittently saying the vowel “A” | Three times/3 min29,38,52 |
| Five times/10 repetitions53 | |
| Three times/20 repetitions39,54 | |
| Pronounce an oral vowel continuously (isometric exercise) | Three times/3 min29,38 |
| Five times/10 repetitions53 | |
| Elevate the soft palate and uvula without vocalization, after gaining control and coordination of movement (~after 3–5 weeks) | Three times/5 s39,54 |
| Elevate the soft palate with and without a yawn | Three to five times per day50 |
| Five times/10 repetitions53 | |
| Produce lingua-velar sounds by contacting the dorsum of the tongue and the velum several times each | Three to five times per day50 |
| Produce uvular sounds by contraction of the uvula several times each | Three to five times per day50 |
| Tongue | |
| Brush the superior surfaces of the tongue while it is positioned in the floor of the mouth. Also, brush the lateral surfaces of the tongue | Three times/5 repetitions29,38 |
| Five times/10 repetitions53 | |
| Place the tip of the tongue against the front of the palate and slide the tongue backward | A total of 3 min throughout the day |
| Three times/20 repetitions29,39,54 | |
| Five times/10 repetitions53 | |
| Place the tongue tip as far as possible on the palate | Three to five times per day50 |
| Press the entire tongue upward against the palate | A total of 3 min throughout the day38 |
| Three to five times per day50 | |
| Three times/20 repetitions29,39,54 | |
| Five times/10 repetitions50 | |
| Press the tongue against the palate and apply a counterresistance on both cheeks using the hands | Three to five times per day50 |
| Place the tongue tip in contact with the inferior incisive teeth and force its posterior region of the tongue downward | A total of 3 min throughout the day38 |
| Three times/20 repetitions29,39,54 | |
| Protrude the tongue tip forward just in front of the lips, without touching the teeth or lips,50 and without deviation. Hold, relax, and repeat53 | Three to five times per day/30 s50 |
| Five times/10 repetitions53 | |
| Repeatedly stick the tongue in and out as fast as possible | Five times/10 repetitions53 |
| Spread center of the tongue, so the sides of the tongue touch the bottom of the upper teeth | Three to five times per day/30 s50 |
| Protrude the tongue outside the mouth and move the tip (lift and down) | Three to five times per day50 |
| Move the tongue to the right/left corner of the mouth and keep it pointed | Three to five times per day50 |
| Flick the tongue from corner-to-corner as quickly as possible. Move the tongue all around the lips in a circle quickly | Five times/10 repetitions53 |
| Stick out the tongue to reach the chin with the tip. Hold at the farthest extension | Five times/10 repetitions53 |
| Stick out the tongue. Hold a spoon upright against the tip of your extended tongue and try to push it away while your hand holds the spoon in place | Five times/10 repetitions53 |
| Rotate the tongue in the oral vestibule | Three times/10 repetitions starting in the right side and 10 left side29 |
| Facial | |
| Pressure the lips (orbicularis oris muscle) with the mouth closed (isometric exercise) | Three times/30 s38 |
| Five times/10 repetitions53 | |
| Open and close the jaw slowly and widely, keeping the lips in contact (orbicularis oris muscle) | Five times/10 repetitions53 |
| Pucker the lips (as if about to kiss). Hold for a count of 10 and relax | Five times/10 repetitions53 |
| Spread the lips into a big, exaggerated smile. Hold and relax | Five times/10 repetitions53 |
| Pucker lips–hold–smile–hold | Five times/10 repetitions53 |
| Pucker the lips with the mouth wide open, without closing the jaws. Hold and relax | Five times/10 repetitions53 |
| Close the lips firmly, and then make a “slurping” noise, as if sipping a drink | Five times/10 repetitions53 |
| Perform suction movements contracting only the buccinators. These exercises are performed with repetitions (isotonic) and holding position (isometric) | Unclear38 |
| Five times/10 repetitions53 | |
| Suck air from a syringe of 20 mL | Three times a day/5 repetitions29 |
| Recruitment of the buccinator muscle against the finger that is introduced into the oral cavity | Unclear38 |
| Three times a day/10 repetitions each side29,39,54 | |
| Five times/10 repetitions53 | |
| Alternated elevation of the mouth angle muscle (isometric exercise), with repetitions (isotonic exercise) | Three times a day/10 intermittent elevations three times38 |
| Lateral jaw movements with alternating elevation of the mouth angle muscle | Unclear38 |
| Five times/10 repetitions53 | |
| Open and close mouth as quickly as you can, making sure your lips close each time | Five times/10 repetitions53 |
| Say the syllable “Ma” quickly and repeatedly. Do the same with “La” and “Kala” | Five times/10 repetitions53 |
| Sing “A–E–I–O–U” as loud as possible | Five times/10 repetitions53 |
| Stomatognathic functions | |
| Suction | |
| Suck the yogurt with a narrow straw | Unclear38 |
| Breathing and speech | |
| Forced nasal inspiration and oral expiration in conjunction with phonation of open vowels, while sitting | Unclear29,38 |
| Five times/10 repetitions53 | |
| Balloon inflation with prolonged nasal inspiration and then forced blowing | Repeated five times without taking the balloon out of the mouth29,38,53 |
| Swallowing and chewing | |
| Alternate bilateral chewing | The patients were instructed to incorporate this pattern whenever they were eating29,38,39,53,54 |
| Deglutition: swallow with the tongue positioned on the palate, occluded teeth, and without perioral muscle contraction | The patients were instructed to incorporate this pattern whenever they were eating29,38,39,53,54 |
| Holding the tongue tip between teeth anteriorly while trying to swallow | Three to five times per day50 |
Abbreviation: OSA, obstructive sleep apnea.
The program applied to children lasted two months, and the exercises included differed from those applied to adults. One study41 involved three meetings with the therapist, the first for evaluation and explanation of the exercises, the second for supervision, and the third for reevaluation, while the other1 involved two monthly meetings. Villa et al41 described the exercises.
OMT and sleep-breathing variables
Among the randomized studies that analyzed the effect of OMT on sleep breathing based on full-PSG data, the investigators detected evidence of AHI reduction in adults with moderate OSA,29,38–40 an increased percentage of lowest oxygen saturation (SaO2),38 and a reduction of arousal index (AI).38 In children, the authors observed a decrease of AHI41 and a higher SaO21 after OMT.
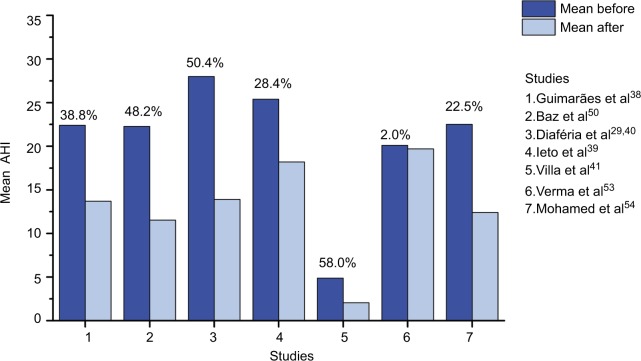
Authors of non-randomized studies also reported that, after OMT, patients with mild or moderate OSA showed a significant reduction of AHI50,51,53,54 and AI,50,53,54 and an increase of lowest SaO2 (%)50,51,53,54 compared to baseline. They also observed a decrease in the percentage of the duration of the SaO2<90.50,53,54 Mohamed et al54 analyzed in parallel the effects of OMT in a group of patients with severe OSA, which showed a decrease in mean AHI, but was not statistically significant. The AHI before and after OMT reported by reviewed authors, as well as the ΔAHI%: (AHI before −AHI after)/AHI before × 100) are summarized in Figure 2.
Figure 2.
Apnea–hypopnea index (AHI): mean (or median for Ieto et al39) before and after OMT and percentage of AHI decrease.
Abbreviations: AHI, apnea–hypopnea index; OMT, orofacial myofunctional therapy.
OMT and snoring
A study39 reported a primary efficacy end point of OMT for the objective measurement of snoring in patients with a complaint of snoring and a diagnosis of primary snoring or mild-to-moderate OSA. For this purpose, based on recordings obtained during PSG, the authors established a methodology for the calculation of snore index (total number of snores/total sleep time) and total snore index (sound intensity power generated by all snoring episodes/total sleep time, expressed in arbitrary unit/107). The results showed that OMT promoted a reduction of snoring index (99.5 vs 48.2, p=0.041) and total snore index (60.4 vs 31.0, p=0.041).39 Other authors also reported a reduction of snoring index and episodes of loud snoring.40,54 Additionally, the intensity and frequency of snoring as reported by the bed partner39,52 or as perceived by the patient decreased after the therapy.38,39,53
OMT and daytime sleepiness, sleep quality, and life quality
A large number of studies, as noted from the literature as well as observed in the present review, had employed the Epworth Sleepiness Scale (ESS) proposed by Johns67 that evaluates the propensity to sleep in eight different situations, with a total score ranging from zero (absence) to 24 (intense). Although the ESS has been criticized for its sensitivity and specificity and low predictive value, limiting its value in the screening of patients with sleep apnea,68 in almost all papers analyzed, it was used as a measure of the results of treatment regarding daytime sleepiness.
Patients with a mean baseline ESS score ranging from 12±2.6 to 15.4±2.3 showed a significant improvement after OMT, with a mean reduction of six points.29,38,40,50,52,54 However, no change occurred in a group of patients with a median ESS score of 7.0 (3–11) who were, on average, not sleepy39 or in a group with severe OSA and an ESS score of 20.9±6.2.54
Considering the damage to sleep caused by OSA and its consequences in the daytime and for health in general, other relevant findings were an improved quality of sleep evaluated with the Pittsburg questionnaire38,39 and quality of life.40 The quality of life similarly improved after treatment with OMT alone or combined with CPAP, while no effect was observed in the CPAP-alone group.40 The morning headache symptom was investigated in two studies,50,53 only one of which detected a reduction from 60% to 20% in the number of patients with this complaint.50
All of these reported data concern the effect of therapy measured immediately after the conclusion of OMT. Only Diaféria et al29,40 followed up their patients for three weeks after the end of the intervention and observed that, among the variables that improved after the OMT, the frequency and intensity of snoring were maintained. Another positive contribution of OMT is that it favors adherence to the use of CPAP.29 The educational process and the support received on a weekly basis20 during the sessions probably favored greater adherence to CPAP when combined with OMT (65%) than treatment with CPAP alone (30%).29 Verma et al53 reported differences in response to therapy between men and women. However, the limited number of female participants (n=5) hinders the interpretation.
OMT and orofacial myofunctional status
The efficacy of the OMT for patients with mild–moderate OSA is well characterized by PSG data and questionnaires in the studies analyzed. However, reports about the myofunctional evaluation are limited and not based on a standardized assessment tool that hampers the interpretation and comparison of findings. Only some studies reported the effects promoted by OMT in the oropharyngeal and facial muscles and functions.1,41,51,52
According to Matsumura et al,52 the percentage of patients with an appropriate pattern of structure and functions, such as lowering of the back of the tongue, high soft palate, suprahyoid muscles tonus, nasal breathing, bilateral chewing, and speech, increased in response to the OMT program. An increase in labial closure force resulted from the training specifically applied for this purpose, aiming to promote nasal breathing.51 Diaféria et al29 reported that the Mallampati index improved in the OMT and CPAP+OMT groups and was correlated with the increased strength of the tongue and soft palate, but results of the myofunctional evaluation were not presented.
Villa et al1,41 used a set of exercises divided into three categories: (1) nasal breathing rehabilitation, (2) labial seal and lip tone exercises, and (3) tongue posture exercises. In a group of children with residual apnea, OMT increased the number of patients with a proper labial seal, lip tone, and nasal breathing.41 In another study conducted on subjects with primary snoring or mild–moderate OSA, there was a significant increase in the objective measures of tongue strength, tongue peak, and endurance. Also, the number of children with oral breathing, abnormal tongue rest position, and lip hypotonia decreased after therapy.1
Future studies would determine whether the effects of OMT in patients with SDB are, in fact, related to improved muscle and orofacial functions.65 For this, validated tools that enable identifying, classifying, and grading changes in muscles and functions status would be used, rather than using dichotomous judgment (yes/no), which only inform the frequency of individuals with normal or altered conditions.
Currently, this is possible with the Orofacial Myofunctional Evaluation with Scores (OMES)-expanded protocol validated for OSA patients.65 The OMES protocol, which is the original version, has also shown to be useful for the characterization of the orofacial myofunctional conditions of children64 and adults with OSA.34,69 Moreover, objective measures of strength/force1,51,64 and electromyography64 can contribute to the myofunctional diagnosis and the assessment of the results of the intervention. Therefore, the parameters for the indication of OMT, as well as the benefits of the therapeutic strategies, could be defined appropriately in the future.
Next, based on the literature, we will consider the possible influences of OMT on the oropharyngeal structures. Because many assumptions still need confirmation, this discussion may suggest new ways for further investigations.
Therapeutic strategies and fundamentals
Most of the authors of the papers reviewed adopted a set of exercises for OMT to cover the various oropharyngeal structures,1,38,39,41,50,52–54 that is, tongue, palate, lateral pharyngeal walls, or epiglottis which, separately or in combination, are involved in the collapse of the pharyngeal airway.16,17
Because the increased soft palate length has been associated with higher rates of obstructive apnea, AHI, and respiratory disturbance index,70 to increase the tone of elongated and floppy soft palate and uvula is one of the goals of training.29,38–40,52–54
Exercises focused on the tongue are widely applied, except for a pilot study,51 and are fully justified by the findings related to the pathogenesis of OSA. The dimensions of the tongue (area and volume) are significantly associated with upper airway collapsibility, which in turn is associated with AHI.17 Tongue fat (in mm3) is the main factor explaining the increased tongue volume in patients with OSA compared to controls.71 The possible implications of this finding are the compromised ability of extrinsic muscles to position the tongue and the reduction of the air space in the retroglossal region, where there is a larger percentage of fat in patients with OSA, increasing the risk of sleep apnea.71 Moreover, the genioglossus muscle activity is significantly reduced during rapid eye movement (REM) sleep, especially phasic REM, coincident with the onset of REM hypopnea/obstructive apnea.15
Thus, we should point out the significant reduction of AHI during REM sleep observed after OMT.38 The exercises, as performed during OMT, with a large number of repetitions at a low level of resistance, may promote adaptation of Type I fatigue-resistant muscles, which represent more than half of the muscle fibers in the posterior region of the tongue.66–72 Thus, the training during wakefulness seems to have contributed to the minimization of hypotonia of the genioglos-sus muscle, the major upper airway dilator muscle, during the most critical phase of sleep when the severity of OSA worsens. According to a recent review, REM sleep apnea may be more important in mediating insulin resistance and the cardiovascular consequences of OSA.18
The suprahyoid muscles, whose adequacy is also mentioned in some studies as a result of OMT,52–54 include the geniohyoid, stylohyoid, mylohyoid, anterior digastric, and posterior digastric muscles.73 These muscles, together with the styloglossus and genioglossus muscles, participate in specific tasks. For example, “placing the tip of the tongue against the front of the palate and sliding the tongue backward” and “forced tongue sucking upward against the palate, pressing the entire tongue against the palate”.38 These tasks are potentially favorable to an increase in resistance and fatigue threshold of the muscles involved and consequently to reach the goal of appropriate tongue positioning during rest and deglutition.
During the oral phase of deglutition, the surface of the tongue rises, gradually expanding the area of tongue–palate contact from the anterior to the posterior region and compressing the bolus in the direction of the pharynx.74 Elevation of the tongue is promoted by the suprahyoid muscles,75 which also displace the hyoid bone in an anterior direction (geniohyoid) and upper direction (mylohyoid).73,76 Therefore, another result of OMT may be a higher positioning of the hyoid bone, which should also be analyzed in future studies because a lower positioned hyoid bone is a common finding in patients with OSA.17
Mouth breathing during sleep lengthens and narrows the upper airway, which in turn may aggravate OSA severity.71 Presumably, for this reason, Suzuki et al51 decided to train labial closure force to the patients. However, this training alone does not seem to be sufficient to modify the signs and symptoms of apnea since the muscles involved in the collapse of the airways are not directly exercised.
Jaw muscles exercises38,51,53 and training of alternate bilateral mastication and deglutition with teeth in occlusion, focusing on the equilibrium of these functions, have been reported.29,38,39,52–54 However, no statement was made regarding the fact that not all patients have an occlusal condition to chew alternately on the right and left sides. Moreover, patients with OSA may have associated temporomandibular disorders (TMDs), and a prospective cohort study demonstrated that OSA symptoms preceded first-onset TMD.77
Thus, the goals of rehabilitation of mastication and swallowing should take into account the dynamics relationship between occlusions, muscles, and temporomandibular joint.78 In this sense, there should be bilateral occlusal guides without occlusal interference, especially on the balancing side, as well as muscular coordination to grind the food, to transfer it from one side of the oral cavity to the other, and into the oropharynx. Also, during or after mastication, the patients should not have either joint noise or pain.78
Exercise for buccinator muscles has also been included in various studies.29,38,39,52–54 It has been recently suggested that the appearance of the cheeks (volume and flaccidity) may be an additional predictor of OSA risk and a visible signal of fat deposition also affecting the tongue and pharynx.69 However, it would be necessary to demonstrate how the type of cheek exercise can favor the muscles related to OSA since their remote location renders improbable a meaningful contribution to remodeling of the oropharyngeal airway.79
The neck circumference, abdominal circumference measures, and BMI are anthropometric predictors of OSA severity.80 Obese patients with a large neck circumference show worse collapsibility of the upper airway.17 Thus, a significant decrease in neck circumference38,39,50,53 correlated with changes in AHI after OMT38,54 indicates that the exercises induced upper airway remodeling.38 Although weight loss is recommended for overweight or obese patients, it is essential to control any variation in BMI when the intention is to analyze the effect of interventions on the severity of OSA, a fact that was not reported in two previous studies.51,54 It should also be pointed out that Verma et al53 emphasized that their experimental design had an advantage over a previous study that did not permit to conclude which set of exercises resulted in maximum benefit for the patients. However, they failed to consider that the effects of training are progressive. Therefore, the highest percentage of improvement of symptoms achieved by patients at the last phase of treatment compared to the previous two does not indicate which set of exercises was most effective.
In summary, the principle of OMT (oropharyngeal exercises) is repetitive muscle training, with specific gains in the coordination, tonicity, and endurance of the muscles, considering the specificity of the exercises adopted (isotonic and isometric). The exercises may improve the condition of muscle fatigue in subjects with OSA81 and perhaps act on the equilibrium of contraction between the different muscles that involve the velopharyngeal, oropharyngeal, and hypopharyngeal segments.82 Further, they can decrease the volume of specific structures and fat in the pharynx-dilating muscles, thus also reducing the potential upper airway collapse in apneic subjects. However, these hypotheses have not been verified so far.
As pointed out previously, it needs to be determined whether there is a relationship between tongue exercises and changes in the tongue itself and the palate regarding muscle tone, strength, size, and upper airway volume.44 It would also be relevant to compare different OMT programs in randomized controlled studies. In addition, whether the therapy results are stable over time remains to be determined; however, this is a hard task, given the difficulty of keeping the participants tied to the research team and of convincing them to submit again to the complex exams.
Conclusion
In recent years, OMT for the treatment of patients with OSA has represented a new path in the fight aiming at the minimization or cure of a disease with serious consequences for human beings. The results of randomized studies, although of a limited number, have shown that OMT is effective for the treatment of adult patients with mild and moderate OSA and with primary snoring, and of children with residual apnea. In addition, it provides benefits such as an improved quality of life and increased adherence to CPAP. In general, the goal of the therapy is to induce changes in weak and dysfunctional upper airway muscles, although there is a lack of demonstration based on standardized measures showing whether such changes do occur. New RCTs, including analyses of the effects on the muscles and motor control of the upper airway, could contribute to the dissemination of the indication of OMT for the treatment of OSA.
Supplementary material
Table S1.
Questions for analysis of complete texts
| Number | Question |
|---|---|
| 1 | Is the document an original research published in a journal with peer-review policies? |
| 2 | Does the article include patients with OSA diagnosis based on PSG? |
| 3 | Does the article describe the OMT program (oral, facial, or oropharyngeal exercises) or quoted some program? |
| 4 | What was the research objective? |
| 5 | What were the outcomes measures? |
| 6 | Does the article describe the measurement used in a way that can be replicated, or provide quantitative measures of PSG and validated or recognized questionnaires/scales for OSA symptoms? |
| 7 | Does the article describe the measurements used in a way that can be replicated, or provide specific quantitative measures of orofacial myofunctional evaluation? |
| 8 | Does the article describe participant eligibility criteria such as age, sex, BMI, and AHI? |
| 9 | Does the article describe the participant’s characteristics? |
| 10 | Were the participants randomized for groups? |
| 11 | Were the results express as quantitative measures? |
| 12 | What were the main and secondary findings of the study? |
Abbreviations: OSA, obstructive sleep apnea; PSG, polysomnography; OMT, orofacial myofunctional therapy; BMI, body mass index; AHI, apnea–hypopnea index.
Acknowledgments
This study was supported by the Provost’s Office for Research of the University of São Paulo (Process No. 11.1.21626.01.7).
Footnotes
Author contributions
CMF defined the study design with support from FVSD and LVVT and drafted the article. All authors conducted the literature review, contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Villa MP, Evangelisti M, Martella S, Barreto M, Del Pozzo M. Can myofunctional therapy increase tongue tone and reduce symptoms in children with sleep-disordered breathing? Sleep Breath. 2017;21:1025–1032. doi: 10.1007/s11325-017-1489-2. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 3.Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9:687–701. doi: 10.1007/s13311-012-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine (AASM) International Classification of Sleep Disorders. 3rd ed. Darien: AASM; 2014. [Google Scholar]
- 5.Woodside DG, Linder-Aronson S, Lundstrom A, McWilliam J. Mandibular and maxillary growth after change mode of breathing. Am J Orthod Dentofacial Orthop. 1991;100:1–18. doi: 10.1016/0889-5406(91)70044-W. [DOI] [PubMed] [Google Scholar]
- 6.Katz ES, D’Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:253–262. doi: 10.1513/pats.200707-111MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilleminault C, Riley R, Powell N. Obstructive sleep apnea and abnormal cephalometric measurements. Chest. 1984;86:793–794. doi: 10.1378/chest.86.5.793. [DOI] [PubMed] [Google Scholar]
- 8.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Moore RH, Rosen CL, et al. Childhood Adenotonsillectomy Trial (CHAT) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164:16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 11.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ, et al. Symptoms of sleep disorders, inattention and hyperactivity in children. Sleep. 1997;20:1185–1192. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 12.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 13.Schechter MS. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 14.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McSharry DG, Saboisky JP, Deyoung P, et al. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep. 2014;37:561–569. doi: 10.5665/sleep.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genta PR, Sands SA, Butler JP, et al. Airflow shape is associated with the pharyngeal structure causing OSA. Chest. 2017;152:537–546. doi: 10.1016/j.chest.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata RP, Schorr F, Kayamori F, et al. Upper airway collapsibility assessed by negative expiratory pressure while awake is associated with upper airway anatomy. J Clin Sleep Med. 2016;12:1339–1346. doi: 10.5664/jcsm.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osman AM, Carter SG, Carberry JC, Eckert DJ. Obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:21–34. doi: 10.2147/NSS.S124657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the São Paulo epidemiologic sleep study. Sleep Med. 2010;11:441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 22.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero E, Krakow B, Haynes P, Ulibarri V. Nocturia and snoring: predictive symptoms for obstructive sleep apnea. Sleep Breath. 2010;14:337–343. doi: 10.1007/s11325-009-0310-2. [DOI] [PubMed] [Google Scholar]
- 24.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 25.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 26.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 27.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 28.Villa MP, Rizzoli A, Rabasco J, et al. Rapid maxillary expansion outcomes in treatment of obstructive sleep apnea in children. Sleep Med. 2015;16:709–716. doi: 10.1016/j.sleep.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Diaféria G, Santos-Silva R, Truksinas E, et al. Myofunctional therapy improves adherence to continuous positive airway pressure treatment. Sleep Breath. 2017;21:387–395. doi: 10.1007/s11325-016-1429-6. [DOI] [PubMed] [Google Scholar]
- 30.Isacsson G, Fodor C, Sturebrand M. Obstructive sleep apnea treated with custom-made bibloc and monobloc oral appliances: a retrospective comparative study. Sleep Breath. 2017;21:93–100. doi: 10.1007/s11325-016-1377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knappe SW, Sonnesen L. Mandibular positioning techniques to improve sleep quality in patients with obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:65–72. doi: 10.2147/NSS.S135760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health Quality Ontario Oral appliances for obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9(5):1–51. [PMC free article] [PubMed] [Google Scholar]
- 33.Machado-Júnior AJ, Signorelli LG, Zancanella E, Crespo AN. Randomized controlled study of a mandibular advancement appliance for the treatment of obstructive sleep apnea in children: a pilot study. Med Oral Patol Oral Cir Bucal. 2016;21:e403–e407. doi: 10.4317/medoral.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braga A, Grechi TH, Eckeli A, et al. Predictors of uvulopalatopharyngoplasty success in the treatment of obstructive sleep apnea syndrome. Sleep Med. 2013;14:1266–1271. doi: 10.1016/j.sleep.2013.08.777. [DOI] [PubMed] [Google Scholar]
- 35.Vinha PP, Eckeli AL, Faria AC, Xavier SP, de Mello-Filho FV. Effects of surgically assisted rapid maxillary expansion on obstructive sleep apnea and daytime sleepiness. Sleep Breath. 2016;20:501–508. doi: 10.1007/s11325-015-1214-y. [DOI] [PubMed] [Google Scholar]
- 36.Faria AC, da Silva-Junior SN, Garcia LV, dos Santos AC, Fernandes MR, de Mello-Filho FV. Volumetric analysis of the pharynx in patients with obstructive sleep apnea (OSA) treated with maxillomandibular advancement (MMA) Sleep Breath. 2013;17:395–401. doi: 10.1007/s11325-012-0707-1. [DOI] [PubMed] [Google Scholar]
- 37.Faria AC, Garcia LV, Santos AC, Eckeli AL, Garcia DM, Mello-Filho FV. Dynamic comparison of pharyngeal stability during sleep in patients with obstructive sleep apnea syndrome treated with maxillomandibular advancement. Sleep Breath. 2017;21:25–30. doi: 10.1007/s11325-016-1362-8. [DOI] [PubMed] [Google Scholar]
- 38.Guimarães KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179:962–966. doi: 10.1164/rccm.200806-981OC. [DOI] [PubMed] [Google Scholar]
- 39.Ieto V, Kayamori F, Montes MI, et al. Effects of oropharyngeal exercises on snoring: a randomized trial. Chest. 2015;148:683–691. doi: 10.1378/chest.14-2953. [DOI] [PubMed] [Google Scholar]
- 40.Diaféria G, Badke L, Santos-Silva R, Bommarito S, Tufik S, Bittencourt L. Effect of speech therapy as adjunct treatment to continuous positive airway pressure on the quality of life of patients with obstructive sleep apnea. Sleep Med. 2013;14:628–635. doi: 10.1016/j.sleep.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Villa MP, Brasili L, Ferretti A, et al. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath. 2015;19:281–289. doi: 10.1007/s11325-014-1011-z. [DOI] [PubMed] [Google Scholar]
- 42.Guilleminault C, Huang YS, Monteyrol PJ, Sato R, Quo S, Lin CH. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Med. 2013;14:518–525. doi: 10.1016/j.sleep.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Valbuza JS, de Oliveira MM, Conti CF, Prado LB, de Carvalho LB, do Prado GF. Methods for increasing upper airway muscle tonus in treating obstructive sleep apnea: systematic review. Sleep Breath. 2010;14:299–305. doi: 10.1007/s11325-010-0377-9. [DOI] [PubMed] [Google Scholar]
- 44.Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38:669–675. doi: 10.5665/sleep.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camacho M, Guilleminault C, Wei JM, et al. Oropharyngeal and tongue exercises (myofunctional therapy) for snoring: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2017;275:849–855. doi: 10.1007/s00405-017-4848-5. [DOI] [PubMed] [Google Scholar]
- 46.Soares EB, Pires JB, Menezes MA, Santana SKS, Fraga J. Speech therapy and snore and sleep apnea. Rev CEFAC. 2010;12:317–325. [Google Scholar]
- 47.Moeller JL, Paskay LC, Gelb ML. Myofunctional therapy: a novel treatment of pediatrics sleep-disordered breathing. Sleep Med Clin. 2014;9:235–243. [Google Scholar]
- 48.Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30:2–26. doi: 10.1007/s00455-014-9578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baz H, Elshafey M, Elmorsy S, Abu-Samra M. The role of oral myofunctional therapy in managing patients with mild to moderate obstructive sleep apnea. PAN Arab J Rhinol. 2012;2:17–22. [Google Scholar]
- 51.Suzuki H, Watanabe A, Akihiro Y, et al. Pilot study to assess the potential of oral myofunctional therapy for improving respiration during sleep. J Prosthodont Res. 2013;57:195–199. doi: 10.1016/j.jpor.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Matsumura E, Tonisi GABR, Vecina ALC, Inocêncio LB, Guimarães KCC, Nemr NK. A percepção do acompanhante e do indivíduo com ronco/saos antes e após fonoterapia [Perception of the bed partner and the individual suffering from SNORING/OSAS before and after speech therapy] Rev CEFAC. 2014;16:907–916. Portuguese [with English abstract] [Google Scholar]
- 53.Verma RK, Johnson J, Jr, Goyal M, Banumathy N, Goswami U, Panda NK. Oropharyngeal exercises in the treatment of obstructive sleep apnoea: our experience. Sleep Breath. 2016;20:1193–1201. doi: 10.1007/s11325-016-1332-1. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed AS, Sharshar RS, Elkolaly RM, Serageldin M. Upper airway muscle exercises outcome in patients with obstructive sleep apnea syndrome. Chest. 2017;66:121–125. [Google Scholar]
- 55.Sociedade Brasileira de Fonoaudiologia Documento do Comitê de Motricidade Orofacial [Document of the Orofacial Motricity Committee] 2007. [Accessed August 6, 2018]. Available from: http://www.sbfa.org.br/portal/pdf/dicionario_mfo.pdf. Spanish.
- 56.American Speech-Language-Hearing Association Orofacial myofunctional disorders: knowledge and skills [guidelines, knowledge and skills] 2004. [Accessed August 6, 2018]. Available from: http://www.asha.org/policy. [PubMed]
- 57.Rogers AP. Muscle training and its relation of orthodontia. Int J Orthod. 1918;4:555–577. [Google Scholar]
- 58.Rogers AP. A restatement of the myofunctional concept in orthodontics. Am J Orthod. 1950;36:845–855. doi: 10.1016/0002-9416(50)90039-x. [DOI] [PubMed] [Google Scholar]
- 59.Subtelny JD, Subtelny JD. Malocclusion, speech, and deglutition. Am J Orthod. 1962;48:685–697. [Google Scholar]
- 60.Mason RM. A retrospective and prospective view of orofacial myology. Int J Orofacial Myology. 2008;34:5–14. [PubMed] [Google Scholar]
- 61.Schievano D, Puppin-Rontani RM, Bérzin F. Influence of myofunctional therapy on the perioral muscles – clinical and electromyographic evaluations. J Oral Rehabil. 1999;26:564–569. doi: 10.1046/j.1365-2842.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 62.Marchesan IQ, Krakauer LR. The importance of respiratory activity in myofunctional therapy. Int J Orofacial Myology. 1996;22:23–27. [PubMed] [Google Scholar]
- 63.Valera FCP, Trawitzki LVV, Mattar SEM, Matsumoto MA, Elias AM, Anselmo-Lima WT. Muscular, functional and orthodontic changes in pre school children with enlarged adenoids and tonsils. Int J Pediatr Otorhinolaryngol. 2003;67:761–770. doi: 10.1016/s0165-5876(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 64.de Felício CM, Silva Dias FV, Folha GA, et al. Orofacial motor functions in pediatric obstructive sleep apnea and implications for myofunctional therapy. Int J Pediatr Otorhinolaryngol. 2016;90:5–11. doi: 10.1016/j.ijporl.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 65.Folha GA, Valera FC, de Felício CM. Validity and reliability of a protocol of orofacial myofunctional evaluation for patients with obstructive sleep apnea. Eur J Oral Sci. 2015;123:165–172. doi: 10.1111/eos.12180. [DOI] [PubMed] [Google Scholar]
- 66.Clark HM. Neuromuscular treatments for speech and swallowing: a tutorial. Am J Speech Lang Pathol. 2003;12:400–415. doi: 10.1044/1058-0360(2003/086). [DOI] [PubMed] [Google Scholar]
- 67.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 68.Sil A, Barr G. Assessment of predictive ability of Epworth scoring in screening of patients with sleep apnoea. J Laryngol Otol. 2012;126:372–379. doi: 10.1017/S0022215111003082. [DOI] [PubMed] [Google Scholar]
- 69.Prikladnicki A, Martinez D, Brunetto MG, Fiori CZ, Lenz MDCS, Gomes E. Diagnostic performance of cheeks appearance in sleep apnea. Cranio. 2018;21:1–8. doi: 10.1080/08869634.2017.1376426. [DOI] [PubMed] [Google Scholar]
- 70.Lim JS, Lee JW, Han C, Kwon JW. Correlation of soft palate length with velum obstruction and severity of obstructive sleep apnea syndrome. Auris Nasus Larynx. 2018;45:499–503. doi: 10.1016/j.anl.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 71.Kim EJ, Choi JH, Kim KW, et al. The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur Arch Otorhinolaryngol. 2011;268:533–539. doi: 10.1007/s00405-010-1397-6. [DOI] [PubMed] [Google Scholar]
- 72.Clark HM. Specificity of training in the lingual musculature. J Speech Lang Hear Res. 2012;55:657–667. doi: 10.1044/1092-4388(2011/11-0045). [DOI] [PubMed] [Google Scholar]
- 73.Pearson WG, Jr, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–351. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114:2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 76.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14:77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 77.Sanders AE, Essick GK, Fillingim R, et al. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res. 2013;92:70S–77S. doi: 10.1177/0022034513488140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Felício CM. Desordens temporomandibulares: terapia fonoaudiológica [Temporomandibular disorders: speech-language therapy] In: Felício CM, Trawitzki LVV, editors. Interfaces da Medicina - Odontologia e Fonoaudiologia no Complexo Cérvico-craniofacial [Interfaces of Medicine, Dentistry and Speech-Language Therapy in the Cervico-Craniofacial Complex] 1st ed. Barueri: Pró-Fono; 2009. pp. 145–198. Portuguese. [Google Scholar]
- 79.Steele CM. On the plausibility of upper airway remodeling as an outcome of orofacial exercise. Am J Respir Crit Care Med. 2009;179:858–859. doi: 10.1164/rccm.200901-0016ED. [DOI] [PubMed] [Google Scholar]
- 80.Pinto JA, Godoy LB, Marquis VW, Sonego TB, Leal Cde F, Artico MS. Anthropometric data as predictors of obstructive sleep apnea severity. Braz J Otorhinolaryngol. 2011;77:516–521. doi: 10.1590/S1808-86942011000400017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eckert DJ, Lo YL, Saboisky JP, Jordan AS, White DP, Malhotra A. Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol. 2011;111:1644–1653. doi: 10.1152/japplphysiol.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saboisky JP, Butler JE, Luu BL, Gandevia SC. Neurogenic changes in the upper airway of obstructive sleep apnoea. Curr Neurol Neurosci Rep. 2015;15:12. doi: 10.1007/s11910-015-0537-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Questions for analysis of complete texts
| Number | Question |
|---|---|
| 1 | Is the document an original research published in a journal with peer-review policies? |
| 2 | Does the article include patients with OSA diagnosis based on PSG? |
| 3 | Does the article describe the OMT program (oral, facial, or oropharyngeal exercises) or quoted some program? |
| 4 | What was the research objective? |
| 5 | What were the outcomes measures? |
| 6 | Does the article describe the measurement used in a way that can be replicated, or provide quantitative measures of PSG and validated or recognized questionnaires/scales for OSA symptoms? |
| 7 | Does the article describe the measurements used in a way that can be replicated, or provide specific quantitative measures of orofacial myofunctional evaluation? |
| 8 | Does the article describe participant eligibility criteria such as age, sex, BMI, and AHI? |
| 9 | Does the article describe the participant’s characteristics? |
| 10 | Were the participants randomized for groups? |
| 11 | Were the results express as quantitative measures? |
| 12 | What were the main and secondary findings of the study? |
Abbreviations: OSA, obstructive sleep apnea; PSG, polysomnography; OMT, orofacial myofunctional therapy; BMI, body mass index; AHI, apnea–hypopnea index.