Abstract
Aims/hypothesis
Insulin secretion is widely studied because it plays a central role in glucose homeostasis and diabetes. Processes from insulin granule fusion in beta cells to in vivo insulin secretion have been elucidated, but data at the cellular level do not fully account for several aspects of the macroscopic secretory pattern. Here we investigated how individual secretory events are coordinated spatially and temporally within intact human islets.
Methods
We used the fluorescent probe neuropeptide Y (NPY)–pHluorin to visualise insulin granule secretion in isolated intact human islets.
Results
We found that individual beta cells respond to increases in glucose concentration by releasing insulin granules in very discrete bursts with periods consistent with in vivo pulsatile insulin secretion. In successive secretory bursts during prolonged exposure to high glucose levels, secretory events progressively localised to preferential release sites, coinciding with the transition to second phase insulin secretion. Granule secretion was very synchronised in neighbouring beta cells, forming discrete regional clusters of activity.
Conclusions/interpretation
These results reveal how individual secretory events are coordinated to produce pulsatile insulin secretion from human islets.
Keywords: Exocytosis, Human islets, Insulin granule, Pulsatile secretion
Introduction
The beta cell of the pancreatic islet is the only cell in the body that secretes insulin, which in turn is the only hormone able to lower plasma glucose levels. Death or dysfunction of beta cells thus disturbs glucose homeostasis and leads to diabetes. Not surprisingly, beta cell function has been studied so exhaustively that the signalling pathways coupling glucose detection to insulin granule release are part of the physiological canon. Different aspects of insulin secretion, such as its dose–response relationship for glucose and its dynamic temporal profile, have been examined in vitro and in vivo in many species including humans. A major feature of insulin secretion is its pulsatile pattern, in which most insulin is released in discrete bursts with periods ranging from 4 to 10 min [1, 2]. At the molecular level, insulin is packed in granules that are released in a Ca2+-dependent process similar to that in neurons, which is Ca2+-dependent and requires soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins [3].
Granule exocytosis in beta cells has been studied using electrophysiological approaches, such as membrane capacitance measurements and amperometry [4–6], and microscopic approaches such as two-photon or total internal reflection (TIRF) microscopy in combination with fluorescent probes and vesicle cargo proteins [7–11]. Data have mainly been obtained from clonal beta cell lines or dispersed beta cells, which do not allow the study of how insulin release is coordinated in the islet. Attempts at measuring granule exocytosis in beta cells within intact islets have been made only with rodent islets [9, 12, 13], which differ structurally and functionally from human islets [14, 15].
For these reasons, it is still unclear how individual granule secretory events are coordinated in individual beta cells within an islet to produce a concerted secretory response that is pulsatile. Indeed, most studies show that insulin granules are released at a steady rate. Only two studies report a pulsatile pattern of granule fusion, though at a frequency not related to that of in vivo secretion [11, 13]. We therefore investigated insulin granule exocytosis by using the fluorescent reporter neuropeptide Y (NPY)–pHluorin and confocal microscopy to visualise granule secretion in real time in beta cells within intact human islets. This technique allowed us to study the spatial and temporal patterns of granule secretion in individual beta cells as well as in beta cell populations throughout the human islet.
Methods
Islets
We obtained human pancreatic islets from the Integrated Islet Distribution Program (NIDDK, NIH (Bethesda, MD, USA)). We received eleven healthy human islet preparations for this study but we observed responses to KCl and high glucose in only seven islet preparations that we used for the experiments included in the manuscript (male and female donors, 44.6 ± 6.2 years old, BMI 26.0 ± 3.1 kg/m2). Experiments have been approved by the ethics committee of the University of Miami.
Adenoviral production and islet infection
Human islets were infected with adenoviruses containing NPY fused to the pH-dependent green fluorescent protein (GFP) pHluorin for 48 h and cultured at 37°C for 5–7 days in CMRL 1066 (Cellgro, Herndon, Virginia, USA), 10% (vol./vol.) FBS and 2 mmol/l L-glutamine [16]. Approximately 20–40% of the islet cells were infected. Most of the infected cells showed no background fluorescence, with few granules visible at basal glucose concentration (3 mmol/l) in the absence of stimulation. Some cells had increased background fluorescence in the cytoplasm but did not show secretory events upon stimulation and were therefore not included in the analyses.
Confocal imaging and data quantification
Human islets were placed on a coverslip in an imaging chamber (Warner instruments, Hamden, CT, USA) for imaging on a Leica TCS SP5 upright laser-scanning confocal microscope (Leica Microsystems, Wetzlar, Germany). Islets were continuously perfused with extracellular solution (in mmol/l: 125 NaCl, 5.9 KCl, 2.56 CaCl2, 1 MgCl2, 25 HEPES, 0.1% BSA, 3 mmol/l glucose, pH 7.4, 37°C) and confocal images were acquired with LAS AF software (Leica Microsystems) using a 63× water immersion objective (HCX APO L 63×/0.9 NA). We used a resonance scanner for fast image acquisition to produce time-lapse recordings spanning 50 µm of the islet (z-step: 5 µm, stack of 10 confocal images with a size of 512 × 512 pixels) at 1.5 s resolution (XYZT imaging). NPY-pHluorin fluorescence was excited at 488 nm and emission detected at 535–550 nm. Insulin granule exocytosis was stimulated by increasing glucose concentration from 3 mmol/l to 16 mmol/l, in the presence cAMP-raising agents 3-isobutyl-1-methylxanthine (IBMX) (100 µmol/l) and forskolin (10 µmol/l) [5] or by KCl depolarisation (30 mmol/l). cAMP-raising agents increased the consistency of secretory responses but did not change temporal patterns of granule secretion so that secretory events appeared in discrete bursts synchronised with the average cell response (see below) and with similar burst periods (range 1.4–6.6 min with IBMX/forskolin vs 1.5–10 min without). Intracellular pH was increased by using 50 mmol/l NH4Cl (to replace NaCl on an equimolar basis) and the plasma membrane was labelled with di-8-ANEPP (2 µmol/l for 1 h at 37°C; Invitrogen), excited at 488 nm and detected at 620 nm.
ImageJ (http://imagej.nih.gov/ij/) was used to determine changes in fluorescence intensity over time in regions of interest (ROI) placed around single secretory events in confocal planes (see electronic supplementary material [ESM] Methods for complete description of imaging data analysis). To identify sites of preferential secretion, we plotted fluorescence intensity along cell membranes and determined the number of secretory events that reappeared at the same site in two secretory bursts (adaptation from the birthday problem). Temporal stacks of images acquired during 30 s (~ 20 images) were used to calculate the CV of the fluorescence signal in the cell [17], according to the formula: CV = SD / mean. Higher CV values indicate higher concentration of fluorescence signals. Pearson’s correlation coefficient was used to determine co-localisation of secretory events in 30 s temporal stacks from two bursts.
Perifusion experiments
Insulin secretion from non-infected or NPY–pHluorin-infected islets was measured 5 days after infection with adenoviruses as described previously [18].
Immunohistochemistry
Human islets infected for 5 days with NPY-pHluorin were fixed with 4% (vol./vol.) paraformaldehyde for 30–60 min on a microscope slide and immunostained for insulin, glucagon or somatostatin and GFP (Invitrogen, Carlsbad, CA, USA) using protocols previously described [15]. To assess co-localisation of NPY–pHluorin with islet hormones, we used ImageJ co-localisation analysis plugin ‘Intensity Correlation Analysis’ and calculated Mander’s overlap coefficients (R). R values over 0.6 denote co-localisation [19].
Statistical analyses
We used Prism 5.0 software (GraphPad Software, version 5, San Diego, CA, USA) to perform one-way analysis of variance (ANOVA) followed by a Tukey’s multiple comparison test and considered statistical significance when p values were lower than 0.05. Bar graphs show means ± SEM.
Results
NPY-pHluorin works as a reporter for insulin secretory events
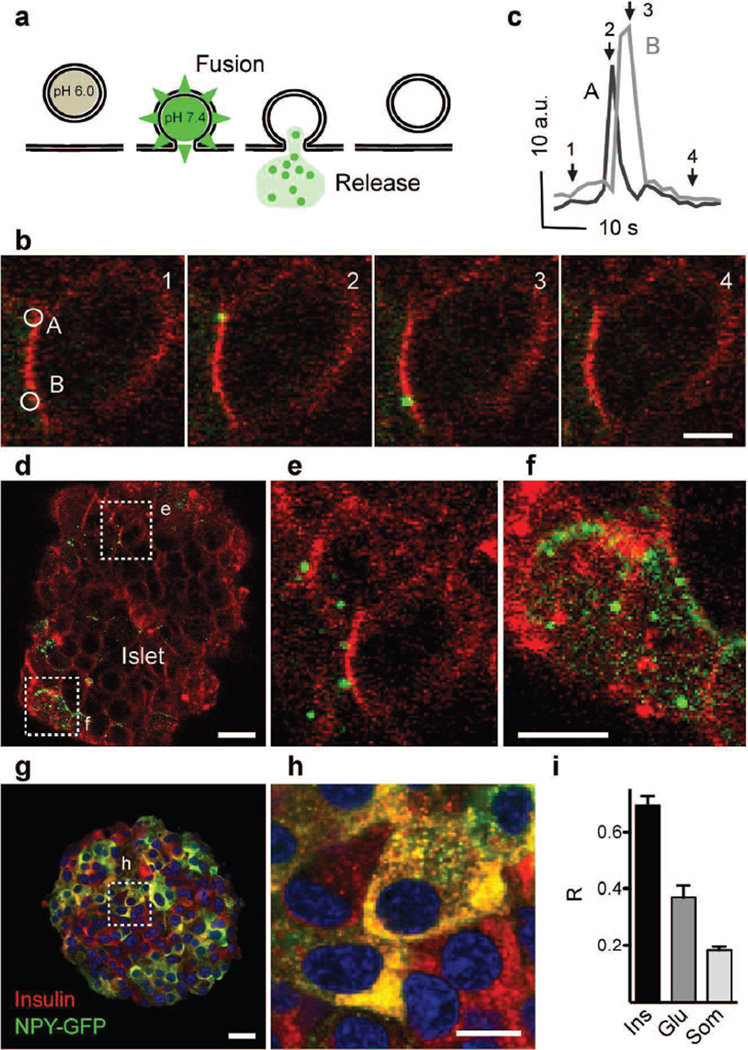
We infected human islets with adenoviruses containing NPY–pHluorin, a fluorescent probe in which the vesicle cargo neuropeptide Y (NPY) is fused to the pH-sensitive GFP (pHluorin) (Fig. 1a) [20]. By performing confocal time-lapse imaging in three dimensions (XYZT imaging), we visualised single secretory events in beta cells within the intact human islet (Fig. 1 b–f). In response to stimulation with high glucose (16 mmol/l), granules containing NPY–pHluorin became visible upon fusing with the plasma membrane (Fig. 1 b, d–f). These transient increases in NPY–pHluorin fluorescence could be readily recorded (Fig. 1c and ESM Video 1). KCl depolarisation also triggered an abrupt appearance of bright fluorescent structures at the cell surface (ESM Fig. 1). These secretory events were a minor fraction (~ 5%) of the total cellular pool of NPY–pHluorin-containing granules, as visualised by application of NH4Cl (ESM Fig. 1).
Fig. 1.
Imaging insulin granule secretion in intact human islets. (a) The vesicle cargo NPY fused to pHluorin is used to follow insulin granule dynamics in real time. (b) Confocal images showing vesicle secretion (green) at the plasma membrane (red, labelled with di-8-ANEPP) of a cell within an intact human islet, triggered by high glucose, at particular times indicated in (c). (c) Traces of the changes in fluorescence intensity in ROIs A and B shown in (b); AU, arbitrary units. Arrows indicate time points of images in (b). (d–f) Temporal stack of 20 images (30 s) of a single confocal plane showing high glucose-triggered vesicle secretion in two cells (d) cut transversally by the confocal plane (e) and a cell cut tangentially and exposing its surface (f). (g, h) Confocal image (g) and higher magnification (h) of an isolated islet immunostained for insulin (red) and GFP (green). (i) Quantification of data in (g) showing Mander’s overlap coefficients (R), n=5 islets. Ins, insulin; Glu, glucagon; Som, somatostatin. Scale bars, 5 µm (b, h), 10 µm (f) or 20 µm (d, g)
Most NPY–pHluorin-expressing cells were beta cells (92 ± 3%) and only a small proportion of glucagon-containing alpha and somatostatin-containing delta cells were GFP-labelled (11 ± 3% and 13 ± 2% for alpha and delta cells, respectively, n = 5 islets). Insulin co-localised with NPY–pHluorin, confirming that NPY fusion protein was stored in granules containing endogenous insulin (Fig. 1 g–i). Although a subset of alpha cells was also infected with NPY–pHluorin (~ 10%), lowering the glucose concentration from 16 mmol/l to 1 mmol/l, a specific stimulus for alpha cells, did not elicit detectable granule fusion. Given their small number in the islet (~ 10%), the contribution of delta cells is negligible. While part of the exogenous NPY–pHluorin could segregate to granules other than those containing insulin [21], their glucose dependency and co-localisation with insulin indicate that most secretory events under high glucose conditions represent cargo release from insulin granules.
Although NPY has been shown to decrease insulin secretion from rodent islets [22, 23] and to be present in human islets [24], the total amount of glucose-stimulated insulin released from NPY–pHluorin-infected human islets was not different from that released from non-infected islets (AUC during 15 min stimulation with 16 mmol/l glucose was 0.14 ± 0.01 ng/µl × min for non-infected islets and 0.11 ± 0.005 ng/µl × min for NPY–pHluorin-infected islets, p = 0.11, n = 3 perifusions).
Secretory events are highly synchronised and generate discrete secretory bursts
In healthy people insulin is secreted in pulses. Pulsatile secretion from the islet is crucial for the action of hormones in target tissues as it maintains a higher expression of insulin receptors at the membrane and facilitates post-insulin receptor signalling [2, 25]. Insulin secretion oscillates in periods of 4–10 min in portal blood [26, 27]; a similar range has been observed in vitro with perfused pancreases or isolated islets from dogs (10 min), rhesus monkeys (~ 6 min) and humans (4–7 min [28–34]).
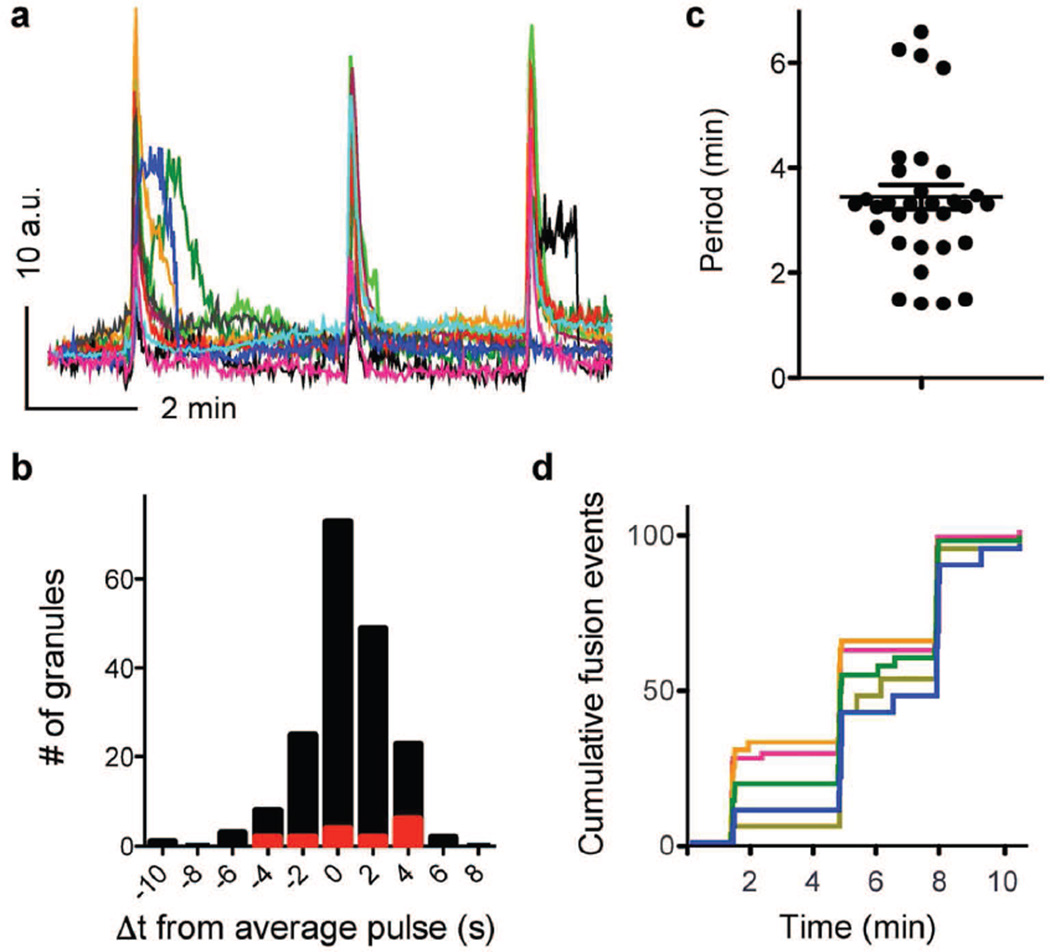
To determine how this regular pattern of insulin secretion is generated from multiple single secretory events, we stimulated NPY–pHluorin-infected islets with an elevated glucose concentration (16 mmol/l) for prolonged periods (20 min). Secretory events appeared in discrete bursts every 3.4 ± 0.23 min (range 1.4–6.6 min; Fig. 2 a, c and ESM Video 2). During each secretory burst, granules were very synchronised (Fig. 2 b, d), with most granules (~ 90%) being secreted within a time difference of 4 s from the average cell response (Fig. 2b). Secretory events between bursts were rare (8.6 ± 1.0%, n = 5 cells, Fig. 2d). Our data show that individual secretory events are highly coordinated in beta cells within intact human islets.
Fig. 2.
Secretory events are highly synchronised and generate discrete pulses of secretion in individual beta cells. (a) Traces of fluorescence intensity in ROIs placed around individual secretory sites in a beta cell within an intact islet during sustained stimulation with 16 mmol/l glucose; AU, arbitrary units. Each colour represents an individual site. (b) Histogram showing the difference in time (Δt) from the start of each secretory event to that of the cell response. Black bars, with IBMX/forskolin; red bars, without IBMX/forskolin. (c) Time between bursts of vesicle secretion (period), n=9 cells, n=3 islet preparations. Periods varied within a cell, from cell to cell, and from preparation to preparation. Line represents the average period ± SEM. (d) Running sum of the number of secreted vesicles (each coloured trace represents secretory events from a single cell; n=5 cells, normalised to the total number of secretion events). Three bursts of vesicle secretion can be distinguished
Spatial pattern of granule secretion
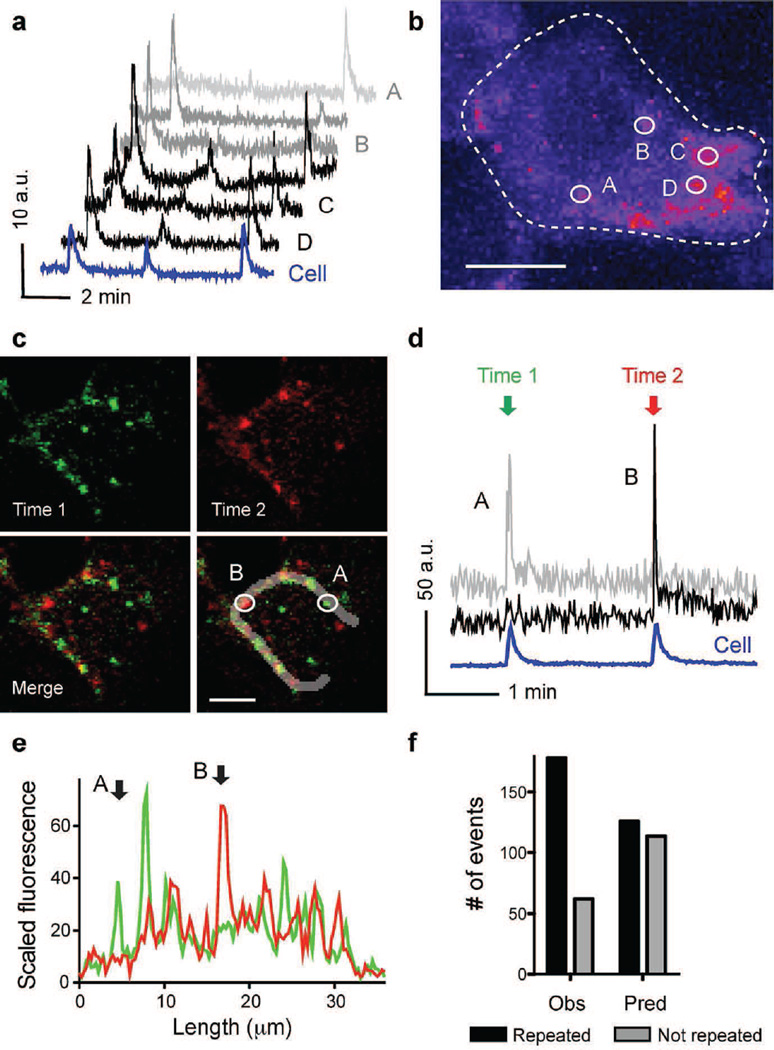
To ensure fast insulin release upon increases in glucose concentration, pools of insulin granules are pre-docked at the plasma membrane at specific sites (e.g. where syntaxin1A/munc18 and other SNARE complexes are pre-assembled, or presynaptic structural proteins are enriched [35–37]). To determine whether granule secretion occurred randomly over the cell surface or preferentially at specific release sites, we compared the localisation of secretory events in successive secretory bursts. We found that some regions were used only once as release sites and others were used as exocytotic sites in two or three consecutive bursts (Fig. 3 a, b). In the latter regions, several secretory events occurred in very close proximity, while the events that occurred in singly used release sites were usually isolated (Fig. 3 b–d). To discover whether granule secretion preferentially occurred at specific membrane regions and not randomly over the cell surface, we used line plots of fluorescence intensity along the cell membrane (Fig. 3e) and calculated the probability of two secretory events in different bursts occurring at the same release site (see Methods). We found that the proportion of secretory events reappearing in a subsequent burst was greater than that predicted to occur by chance (Fig. 3f), reflecting the contribution of repeated secretion at sites of preferred secretion.
Fig. 3.
Secretory events show distinct spatial patterns within a beta cell. (a) Traces of fluorescence intensity in ROIs placed around individual secretory sites during high glucose condition. At some sites secretion occurred repeatedly (black traces), while at others it only occurred once (grey traces); AU, arbitrary units. (b) Temporal stack (13 min 20 s) of confocal images (n=536) of a cell showing the sum of granule secretion during sustained stimulation with high glucose. Fluorescence intensity is highest where more secretory events occurred (pseudocolour scale). Circles denote ROIs for the traces shown in (a). (c) Temporal stacks (30 s) of confocal images of a cell showing secretory events during two secretory bursts (first in green [Time 1], second in red [Time 2]). Scale bar, 5 µm (b, c). (d) Traces of fluorescence intensity in ROIs placed around the individual secretory sites shown in (c). (e) Line scans along the cell membrane outline in (c) showing the profile of fluorescence intensity during the first (green) and second secretory burst (red). Secretory events did not recur at sites A and B (ROIs shown in [c]). (f) Quantification of recurrence of secretory events using line scans as those in (e). Bars represent values from a contingency table (black and grey bars for repeated and not repeated events, respectively). The observed number (Obs) of recurring secretory events (black bars) was higher than the predicted number (Pred) (Fisher exact test, p<0.01, n=13 cells, n=4 islets)
Release sites become more localised with each subsequent secretory burst
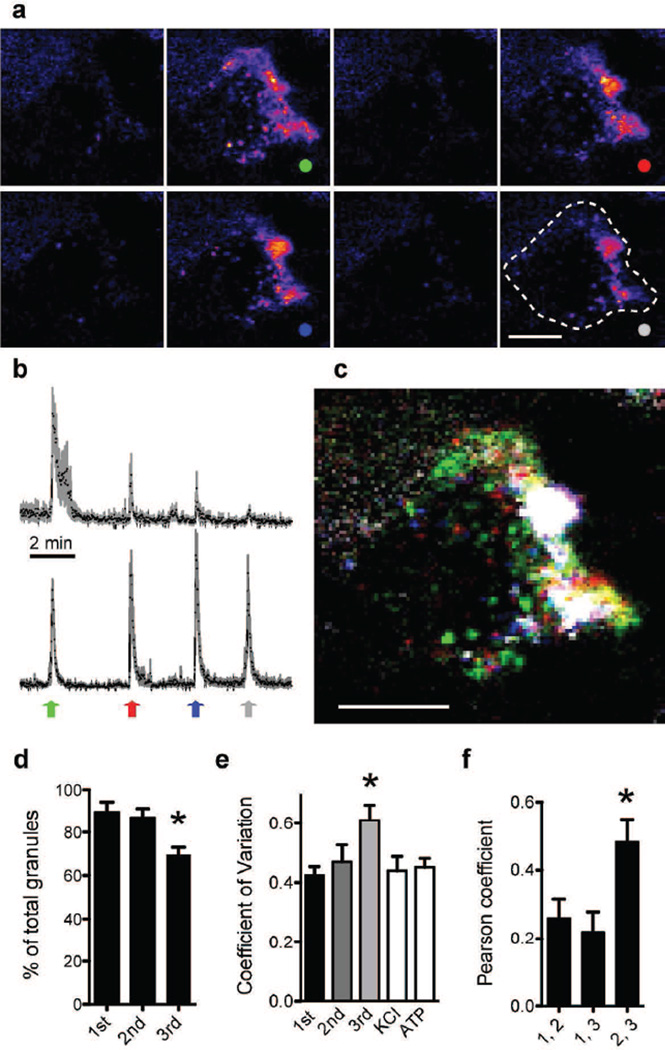
Prolonged stimulation with high glucose triggered discrete secretory bursts in individual beta cells. We observed that in early bursts, granules were secreted in isolated and cluster-like release sites (Fig. 3), but with increasing burst number and duration of the high glucose stimulus, secretory sites progressively localised to sites of preferred secretion (Fig. 4 a–d). To quantify this change in localisation of exocytotic sites, we calculated the CV of the fluorescence signals in temporal stacks of each burst. Greater CV values indicated that secretory events in later secretory bursts became more compartmentalised (Fig. 4e). In addition to a higher CV value, spatial co-localisation of secretory events between later bursts was also greater, as indicated by an increase in the Pearson coefficient (Fig. 4f). Stimulation with KCl after glucose or with ATP elicited granule secretion that was as broadly distributed as that of the first burst in high glucose, with CV values as low as those of the first burst (Fig. 4e). In conclusion, protracted stimulation with high glucose induced a progressive compartmentalisation of granule secretion that was specific to sustained glucose stimulation and was reversed after glucose concentration returned to basal levels.
Fig. 4.
Secretory events become more localised in subsequent bursts. (a) Sequence of eight temporal stacks (20 s) of confocal images of a cell within an intact islet showing the sum of secretory events before, between and during four secretory bursts (dots) in sustained 16 mmol/l glucose. Scale bar, 5 µm. (b) Average traces of fluorescence intensity in ROIs outside (top) and inside sites of preferential secretion (bottom) (n=7 ROIs in cell shown in (a). Peaks of top trace decrease, indicating fewer secretory events. Arrows indicate when images in (a) were taken. (c) Merged image of the temporal stacks of secretory bursts shown in (a). The sums of secretory events per burst are shown in different colours (colours as in a and b). Sites of preferential secretion appear white. Scale bar, 5 µm. (d) The percentage of ROIs with a secretory event (based on recordings as in b) decreases with burst number, reflecting a reduction in secretory events outside regions of preferential secretion (n=7 cells, *p<0.05 vs 1st burst). (e) CV of cell fluorescence in secretory bursts (30 s temporal stacks) during high glucose, or upon KCl (30 mmol/l) or ATP (100 µmol/l) stimulation (n≥4 cells, * p<0.05 for comparison between 1st and 3rd bursts during high glucose). (f) Higher Pearson’s correlation coefficient shows greater co-localisation of secretory events between pulses 2 and 3 than between pulses 1 and 2 (*p< 0.05, n=5 cells)
Secretory patterns reveal clusters of synchronous cells
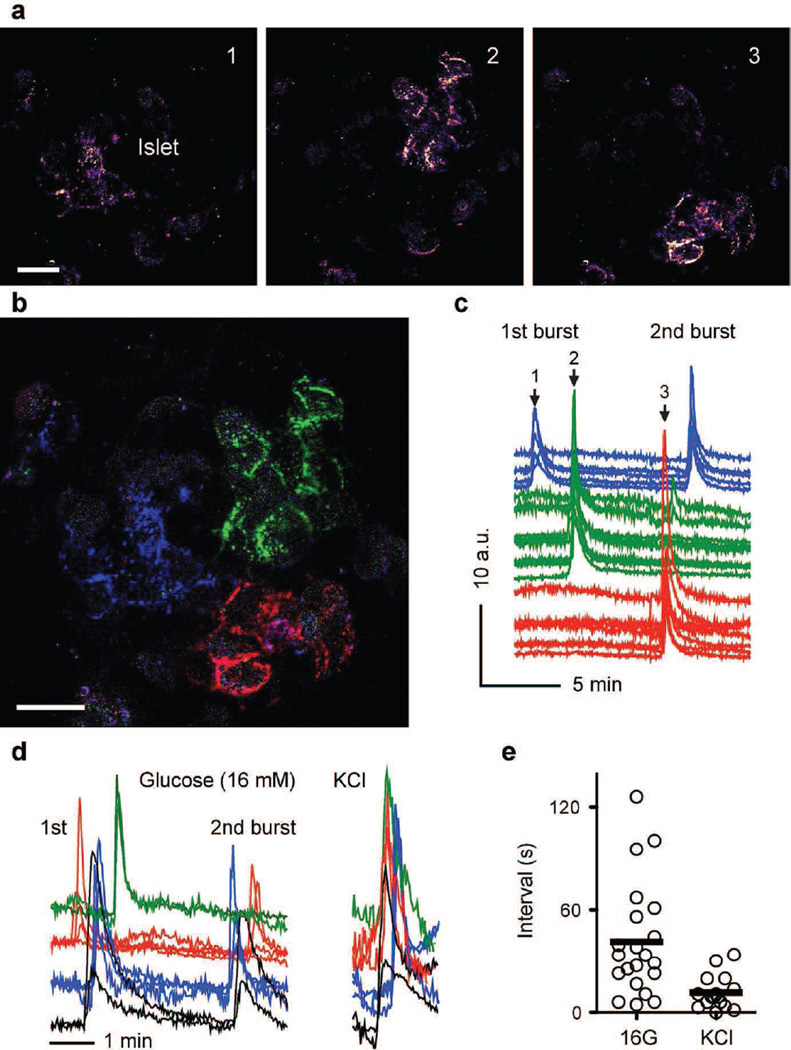
Beta cells must respond in a coordinated way to generate a pulsatile pattern in insulin secretion from the islet. We therefore monitored secretion from multiple cells within the same islet and observed that secretory events in high glucose concentrations occurred within 10 s in beta cells localised in close proximity (Figs 2d, 5 a–d and 6). Beta cells formed distinct regional clusters of synchronised activity. In contrast, beta cell clusters in different islet regions showed delays in their responses within a secretory burst (~ 40 s; Fig. 5). The sequence in the activation of the different clusters varied from burst to burst (Fig. 5 c, d), suggesting that the response did not originate from a leading cluster. When KCl depolarisation was used as stimulus, the delay between clusters was absent and beta cells from different islet regions responded simultaneously (Fig. 5 d, e).
Fig. 5.
Islet cells form discrete clusters of synchronised activity. (a) Sequence of temporal stacks (30 s) of confocal images showing secretory events stimulated by high glucose in an intact human islet. (b) Merged image of the temporal stacks shown in (a), with secretory events colour-coded: blue at time 1; green at time 2 and red at time 3. Three non-overlapping clusters comprising ~10 cells become visible. Scale bars, 20 µm. (c) Traces of secretory responses from cells shown in (b), with corresponding colours; AU, arbitrary units. Arrows indicate when images in (a) were acquired. (d) Traces of secretory responses from cells in response to high glucose and KCl stimulation (neighbouring cells in a cluster in same colour). The sequence in the activation of cell clusters changes from burst to burst. (e) Quantification of delays in the secretory responses between different clusters in the same islet stimulated with high glucose (16 G) or KCl. Lines represent average values (n=3–6 regions per islet, n=3 islets)
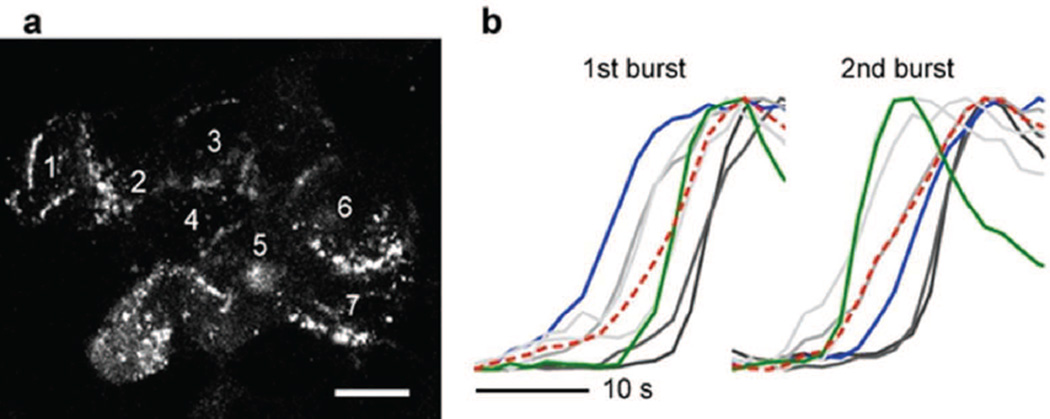
Fig. 6.
The sequence of activation of cells within a cluster is not fixed. (a) Temporal stack of confocal images acquired during 30 s showing secretory events in a cell cluster within an intact islet. Numbers indicate different cells within the region. Scale bar, 10 µm. (b) Secretory responses of the cells shown in (a) in two distinct secretory bursts show different activation sequences (see blue and green traces). Red lines show the response of the whole region. Responses are scaled to peak fluorescence increase
The order in which beta cells showed exocytotic responses changed from burst to burst, indicating that there was no dominant cell serving as a pacemaker within a cluster (Fig. 6b).
Discussion
Our results show that imaging of NPY–pHluorin can be used to visualise insulin granule exocytosis in beta cells within intact human islets. We determined that in response to glucose stimulation insulin granules in individual beta cells are secreted in distinct bursts with exquisite synchronisation. These bursts appear in periods similar to those of pulsatile in vivo insulin secretion. With prolonged glucose stimulation and increasing pulse number, secretory events within a beta cell become localised to regions of preferred secretion. We further demonstrated that neighbouring human beta cells form distinct clusters comprised of ~ 5–10 cells in which activity is synchronised. These clusters of synchronised activity are preserved from burst to burst and differ from surrounding clusters in their timing within the global secretory pulse of the islet. Most imaging studies on insulin exocytosis have been performed on either cell lines or isolated beta cells, mainly using TIRF microscopy (e.g. [8, 11, 35, 38–41]). Islet dissociation and cell culture induce cellular rearrangements that not only disrupt cell-to-cell communication, but may also affect granule pool distribution. Dispersed beta cells show marked variability in their responses to glucose due to metabolic heterogeneity and different activation thresholds. Because TIRF microscopy does not allow imaging of cells within intact islets, other imaging modalities have been used to measure insulin secretion in situ in the intact islet [9, 12, 13, 37]. These experiments, however, were conducted on rodent islets, which are structurally different from human islets [14, 15]. Not surprisingly, our results with intact human islets have revealed features in insulin exocytosis not predicted by previous studies (see below).
Our findings show that multiple secretory events in individual beta cells occur almost simultaneously, within a narrow temporal window of 8 s, and in discrete bursts. Few secretory events (< 9%) occurred in the periods between bursts. This pattern is in contrast to that found in other studies in which insulin granules underwent exocytosis at a steady rate, most likely as a result of averaging the behaviour of asynchronous dispersed cells. Our results are in line with those of a study on small clusters of human beta cells showing that insulin granules are secreted in recurrent discrete bursts [11]. However, bursts appeared with a periodicity of 15–45 s, which is different from the periods we determined and from those of insulin secretion in vitro and in vivo [26, 27, 29, 31, 33, 34, 42].
Our findings further indicate that prolonged stimulation with high glucose changes the spatial pattern of granule secretion. With each subsequent secretory burst, granule secretion becomes more localised to preferred sites of exocytosis. We currently do not have a morphological or molecular correlate for the observed sites of preferred secretion, but they could have a high density of P/Q-type Ca2+ channels [43], be areas where SNARE proteins cluster [44] or be plasma membrane hubs for the delivery of components of the exocytotic machinery orientated towards the vasculature [36]. Interestingly, granule secretion becomes more confined at a time that coincides with the transition from first phase to the sustained second phase of insulin secretion (~ 5–10 min into glucose stimulation) [45, 46]. The transition to the second phase of insulin secretion can be explained by the depletion of primed granules and the recruitment of different functional pools of insulin granules with different release competence [3, 4]. Our results now suggest that this transition is also associated with changes in the spatial pattern of insulin granule secretion.
We also found that neighbouring beta cells form highly synchronised functional clusters of up to ten cells, consistent with the six to seven cells that are coupled through gap junctions in human islets [47]. There was no preferential sequence in which cells within a cluster were activated, which is consistent with the notion that electrical coupling lessens the effects of physiological cell-to-cell variations [48, 49]. In contrast to the fast coupling seen within clusters, longer time delays in activation and their spatial segregation within the islet suggest that coordination between clusters requires paracrine signalling. The looser coupling between cell clusters may explain why the overall secretory pulse of the islet is less discrete than that of granule secretion from individual cells or clusters [33, 34].
Characterising insulin exocytosis in vitro is a first step towards fully understanding the cellular mechanisms that orchestrate individual secretory events to achieve the complex oscillatory pattern of insulin release. The ability to measure insulin granule secretion in real time from multiple cells in intact human islets provides an exceptional tool that can be combined with mouse models of in vivo imaging of vascularised and innervated islets [50]. It is important to determine whether the subcellular spatial pattern of insulin granule secretion we report here is associated with vascular arrangements [36].
Supplementary Material
Acknowledgments
Funding
This work was funded as follows: NIH grants R56DK084321 (AC) and R01DK084321 (AC); the Institute for Basic Science IBS-R013-D1 (HGN); the Juvenile Diabetes Research Foundation (P-OB); the Swedish Research Council; the Novo Nordisk Foundation; the Swedish Diabetes Association; the Family Erling-Persson Foundation; the Skandia Insurance Company Ltd; Strategic Research Program in Diabetes at Karolinska Institutet; the Berth von Kantzow’s Foundation; VIBRANT Grant FP7-2288933; the Knut and Alice Wallenberg Foundation and Lee Kong Chien School of Medicine, Nanyang Technical University, Singapore and Imperial College, London, UK ERC-2013-AdG 338936-BetaImage. JA is a recipient of a postdoctoral fellowship from the American Heart Association (14POST20380499).
Abbreviations
- GFP
Green fluorescent protein
- IBMX
3-Isobutyl-1-methylxanthine
- NPY
Neuropeptide Y
- ROI
Regions of interest
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TIRF
Two-photon or total internal reflection
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
JA and AC conceived and designed the study and wrote the manuscript. P-OB, HGN and AC raised funding. JA acquired the data, JA and AC analysed the data and all authors interpreted the data. All authors revised the article and approved its final version. JA is the guarantor of this work.
References
- 1.Tengholm A, Gylfe E. Oscillatory control of insulin secretion. Molecular and cellular endocrinology. 2009;297:58–72. doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Satin LS, Butler PC, Ha J, Sherman AS. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Molecular aspects of medicine. 2015;42:61–77. doi: 10.1016/j.mam.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaisano HY. Here come the newcomer granules, better late than never. Trends in endocrinology and metabolism: TEM. 2014;25:381–388. doi: 10.1016/j.tem.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 5.Hanna ST, Pigeau GM, Galvanovskis J, Clark A, Rorsman P, MacDonald PE. Kiss-and-run exocytosis and fusion pores of secretory vesicles in human beta-cells. Pflugers Arch. 2009;457:1343–1350. doi: 10.1007/s00424-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa RM, Silva AM, Tome AR, Stamford JA, Santos RM, Rosario LM. Control of pulsatile 5-HT/insulin secretion from single mouse pancreatic islets by intracellular calcium dynamics. The Journal of physiology. 1998;510:135–143. doi: 10.1111/j.1469-7793.1998.135bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron A, Pucci L, Bezzi P, Regazzi R. Analysis of synaptic-like microvesicle exocytosis of B cells using a live imaging technique. PloS one. 2014;9:e87758. doi: 10.1371/journal.pone.0087758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravier MA, Tsuboi T, Rutter GA. Imaging a target of Ca2+ signalling: dense core granule exocytosis viewed by total internal reflection fluorescence microscopy. Methods. 2008;46:233–238. doi: 10.1016/j.ymeth.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D, Zhang Y, Lam PP, et al. Dual role of VAMP8 in regulating insulin exocytosis and islet beta cell growth. Cell metabolism. 2012;16:238–249. doi: 10.1016/j.cmet.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Michael DJ, Xiong W, Geng X, Drain P, Chow RH. Human insulin vesicle dynamics during pulsatile secretion. Diabetes. 2007;56:1277–1288. doi: 10.2337/db06-0367. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Chen S, Bellomo EA, et al. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR) Proceedings of the National Academy of Sciences of the United States of America. 2011;108:21063–21068. doi: 10.1073/pnas.1109773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low JT, Mitchell JM, Do OH, et al. Glucose principally regulates insulin secretion in mouse islets by controlling the numbers of granule fusion events per cell. Diabetologia. 2013;56:2629–2637. doi: 10.1007/s00125-013-3019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 15.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu D, Koo E, Kwan E, et al. Syntaxin-3 regulates newcomer insulin granule exocytosis and compound fusion in pancreatic beta cells. Diabetologia. 2013;56:359–369. doi: 10.1007/s00125-012-2757-0. [DOI] [PubMed] [Google Scholar]
- 17.Hoppa MB, Collins S, Ramracheya R, et al. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca2+ channels from secretory granules. Cell metabolism. 2009;10:455–465. doi: 10.1016/j.cmet.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera O, Jacques-Silva MC, Berman DM, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell transplantation. 2008;16:1039–1048. [PMC free article] [PubMed] [Google Scholar]
- 19.Zinchuk V, Grossenbacher-Zinchuk O. Recent advances in quantitative colocalization analysis: focus on neuroscience. Progress in histochemistry and cytochemistry. 2009;44:125–172. doi: 10.1016/j.proghi.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 21.Sobota JA, Ferraro F, Back N, Eipper BA, Mains RE. Not all secretory granules are created equal: partitioning of soluble content proteins. Molecular biology of the cell. 2006;17:5038–5052. doi: 10.1091/mbc.E06-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ZL, Bennet WM, Wang RM, Ghatei MA, Bloom SR. Evidence of a paracrine role of neuropeptide-Y in the regulation of insulin release from pancreatic islets of normal and dexamethasone-treated rats. Endocrinology. 1994;135:200–206. doi: 10.1210/endo.135.1.8013354. [DOI] [PubMed] [Google Scholar]
- 23.Morgan DG, Kulkarni RN, Hurley JD, et al. Inhibition of glucose stimulated insulin secretion by neuropeptide Y is mediated via the Y1 receptor and inhibition of adenylyl cyclase in RIN 5AH rat insulinoma cells. Diabetologia. 1998;41:1482–1491. doi: 10.1007/s001250051095. [DOI] [PubMed] [Google Scholar]
- 24.Bennet WM, Wang ZL, Jones PM, et al. Presence of neuropeptide Y and its messenger ribonucleic acid in human islets: evidence for a possible paracrine role. The Journal of clinical endocrinology and metabolism. 1996;81:2117–2120. doi: 10.1210/jcem.81.6.8964837. [DOI] [PubMed] [Google Scholar]
- 25.Goodner CJ, Sweet IR, Harrison HC., Jr Rapid reduction and return of surface insulin receptors after exposure to brief pulses of insulin in perifused rat hepatocytes. Diabetes. 1988;37:1316–1323. doi: 10.2337/diab.37.10.1316. [DOI] [PubMed] [Google Scholar]
- 26.Porksen N, Grofte T, Greisen J, et al. Human insulin release processes measured by intraportal sampling. American journal of physiology Endocrinology and metabolism. 2002;282:E695–E702. doi: 10.1152/ajpendo.00516.2000. [DOI] [PubMed] [Google Scholar]
- 27.Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. The Journal of clinical endocrinology and metabolism. 2000;85:4491–4499. doi: 10.1210/jcem.85.12.7043. [DOI] [PubMed] [Google Scholar]
- 28.Bertram R, Sherman A, Satin LS. Electrical bursting, calcium oscillations, and synchronization of pancreatic islets. Advances in experimental medicine and biology. 2010;654:261–279. doi: 10.1007/978-90-481-3271-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilon P, Ravier MA, Jonas JC, Henquin JC. Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes. 2002;51(Suppl. 1):S144–S151. doi: 10.2337/diabetes.51.2007.s144. [DOI] [PubMed] [Google Scholar]
- 30.Goodner CJ, Koerker DJ, Stagner JI, Samols E. In vitro pancreatic hormonal pulses are less regular and more frequent than in vivo. The American journal of physiology. 1991;260:E422–E429. doi: 10.1152/ajpendo.1991.260.3.E422. [DOI] [PubMed] [Google Scholar]
- 31.Hellman B, Salehi A, Gylfe E, Dansk H, Grapengiesser E. Glucose generates coincident insulin and somatostatin pulses and antisynchronous glucagon pulses from human pancreatic islets. Endocrinology. 2009;150:5334–5340. doi: 10.1210/en.2009-0600. [DOI] [PubMed] [Google Scholar]
- 32.Stagner JI, Samols E, Weir GC. Sustained oscillations of insulin, glucagon, and somatostatin from the isolated canine pancreas during exposure to a constant glucose concentration. The Journal of clinical investigation. 1980;65:939–942. doi: 10.1172/JCI109750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritzel RA, Veldhuis JD, Butler PC. The mass, but not the frequency, of insulin secretory bursts in isolated human islets is entrained by oscillatory glucose exposure. American journal of physiology Endocrinology and metabolism. 2006;290:E750–E756. doi: 10.1152/ajpendo.00381.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ritzel RA, Veldhuis JD, Butler PC. Glucose stimulates pulsatile insulin secretion from human pancreatic islets by increasing secretory burst mass: dose-response relationships. The Journal of clinical endocrinology and metabolism. 2003;88:742–747. doi: 10.1210/jc.2002-021250. [DOI] [PubMed] [Google Scholar]
- 35.Gandasi NR, Barg S. Contact-induced clustering of syntaxin and munc18 docks secretory granules at the exocytosis site. Nature communications. 2014;5:3914. doi: 10.1038/ncomms4914. [DOI] [PubMed] [Google Scholar]
- 36.Low JT, Zavortink M, Mitchell JM, et al. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia. 2014;57:1655–1663. doi: 10.1007/s00125-014-3252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi N, Hatakeyama H, Okado H, Noguchi J, Ohno M, Kasai H. SNARE conformational changes that prepare vesicles for exocytosis. Cell metabolism. 2010;12:19–29. doi: 10.1016/j.cmet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Shibasaki T, Takahashi H, Miki T, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuboi T, Ravier MA, Parton LE, Rutter GA. Sustained exposure to high glucose concentrations modifies glucose signaling and the mechanics of secretory vesicle fusion in primary rat pancreatic β-cells. Diabetes. 2006;55:1057–1065. doi: 10.2337/diabetes.55.04.06.db05-1577. [DOI] [PubMed] [Google Scholar]
- 40.Uenishi E, Shibasaki T, Takahashi H, et al. Actin dynamics regulated by the balance of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion. The Journal of biological chemistry. 2013;288:25851–25864. doi: 10.1074/jbc.M113.464420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krus U, King BC, Nagaraj V, et al. The complement inhibitor CD59 regulates insulin secretion by modulating exocytotic events. Cell metabolism. 2014;19:883–890. doi: 10.1016/j.cmet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Matveyenko AV, Liuwantara D, Gurlo T, et al. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes. 2012;61:2269–2279. doi: 10.2337/db11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annual review of physiology. 2013;75:155–179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 44.Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, et al. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. The Journal of cell biology. 2007;177:695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Haeften TW, Pimenta W, Mitrakou A, et al. Relative conributions of beta-cell function and tissue insulin sensitivity to fasting and postglucose-load glycemia. Metabolism: clinical and experimental. 2000;49:1318–1325. doi: 10.1053/meta.2000.9526. [DOI] [PubMed] [Google Scholar]
- 46.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. The Journal of clinical investigation. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serre-Beinier V, Bosco D, Zulianello L, et al. Cx36 makes channels coupling human pancreatic β-cells, and correlates with insulin expression. Human molecular genetics. 2009;18:428–439. doi: 10.1093/hmg/ddn370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocheleau JV, Remedi MS, Granada B, et al. Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS biology. 2006;4:e26. doi: 10.1371/journal.pbio.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces β-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes. 2007;56:1078–1086. doi: 10.2337/db06-0232. [DOI] [PubMed] [Google Scholar]
- 50.Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature medicine. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.