Abstract
While vaccination remains the most cost effective strategy for disease prevention, communicable diseases persist as the second leading cause of death worldwide. There is a need to design safe, novel vaccine delivery methods to protect against unaddressed and emerging diseases. Development of vaccines administered orally is preferable to traditional injection-based formulations for numerous reasons including improved safety and compliance, and easier manufacturing and administration. Additionally, the oral route enables stimulation of humoral and cellular immune responses at both systemic and mucosal sites to establish broader and long-lasting protection. However, oral delivery is challenging, requiring formulations to overcome the harsh gastrointestinal (GI) environment and avoid tolerance induction to achieve effective protection. Here we address the rationale for oral vaccines, including key biological and physicochemical considerations for next-generation oral vaccine design.
Keywords: Oral vaccines, Adjuvants, Oral delivery, Protein deliver, Mucosal immunization
1. Introduction
Vaccines have substantially reduced the burden of infectious disease, second only to clean drinking water in reducing mortality worldwide [1]. Immunization is a cost effective strategy that protects not only the vaccinated individuals, but can indirectly protect the surrounding community through the generation of herd immunity [2].
Development of vaccines against a variety of diseases, including diphtheria, tetanus, polio, measles, mumps, rubella, hepatitis B, and meningitis, have reduced the associated mortality by 97–99% [3]. However, even with multiple successful vaccination campaigns, infectious diseases remain the second leading cause of death worldwide, disproportionately affecting children under the age of 5 and people in low income countries [4]. In fact, five of the top ten leading causes of death in low income countries are caused by infectious agents: lower respiratory infections (e.g. pneumonia), HIV/AIDS, diarrheal disease, malaria, and tuberculosis. While some of these pathogens currently lack a vaccine necessary for disease control, an estimated 20% of these deaths result from vaccine-preventable diseases, indicating the need for substantial improvement in vaccine technology and administration [4–6].
The majority of infections occur after crossing one of the body’s numerous protective mucosal barriers [5,7,8]. For example, potentially fatal diarrheal diseases are often caused by enteropathogens crossing the mucosal barrier of the GI tract after ingestion of contaminated water [9]. The formation of an immunologically strong mucosal barrier would be an effective strategy to prevent infection at the point of contact between microbes and the host. However, the current standards of vaccine technology typically only address pathogens that have already surpassed a mucosal barrier. The majority of licensed vaccines are administered either by subcutaneous or intramuscular injection. The resulting immune response is generally limited to systemic humoral immunity (e.g. antibody production) against the pathogen or toxin, with limited cellular immunity (e.g. T cell-mediated), and only weak protection generated at the mucosal surfaces [10,11]. In contrast, vaccination at mucosal surfaces successfully induces mucosal antibodies (IgA) and cell-mediated immune responses, while still producing a systemic antibody response (IgG) [12–15].
The largest mucosal surface, the GI tract, is readily accessible via oral administration. The oral delivery of therapeutic drugs represents the current gold standard of therapeutic drug administration due to the opportunity for self-administration, improved patient compliance, and the ease of distribution compared to injection-based therapies [16–19]. Vaccine efficacy is highly correlated to its regional coverage, which is affected by the accessibility, stability, and distribution of the formulation [2,20]. Consideration of these parameters is important in the development of next-generation vaccines.
Unfortunately, despite the numerous immunological and practical advantages associated with oral delivery, only a limited number of oral vaccines are available [21,22]. Herein, we present a systematic analysis of the barriers associated with the gastrointestinal delivery of vaccines, currently available oral vaccines, and design strategies for novel delivery vehicles and next-generation oral vaccine development.
2. Oral administration
Oral delivery is the most desirable and patient-accepted route of administration, with over 60% of commercialized small molecule drug products using the oral route [23,24]. Despite this, only a small fraction of currently licensed vaccines are oral formulations due to the inherent obstacles presented by the gastrointestinal system. The induction of a robust protective immune response by oral immunization requires: (i) successful delivery of the intact and active antigen to the intestine, (ii) transport across the mucosal barrier, and (iii) subsequent activation of antigen-presenting cells [14,23,25]. However, the GI tract poses difficulties to each step, including degradation of fragile antigens through the harsh environment in the stomach and requirement of adequate doses to generate immunity instead of tolerance [21,26]. Each challenge within the GI tract poses a unique engineering problem that requires careful consideration to achieve efficacious vaccine design.
2.1. Advantages of oral administration
Vaccine efficacy is dependent on both the degree of protection conferred to individuals as well as the total coverage, accessibility, and costs associated with administering the formulation [2]. Vaccine distribution represents one of the main limiting factors in the impact of these prophylactic systems, particularly in developing nations with limited resources [27,28]. Oral vaccines have the capacity to improve distribution compared to traditional injections due to their ease of administration, allowing for the self-administration of oral formulations. Self-administration is ideal for the widespread and rapid distribution of vaccines as it minimizes the need for trained healthcare personnel [16,29,30]. This could further reduce cost of vaccine programs, since training and mobilization of health care workers can account for up to 25% of the cost of introducing a new vaccine [31]. Additionally, needle-free administration would eliminate occupational needle-stick injuries, which occur in approximately 5% of health-care workers each year, exposing them to blood-borne infectious diseases such as HIV/AIDS and Hepatitis [32].
From a regulation standpoint, oral vaccines could enable more costeffective production since they do not require the extensive purification necessary for injected formulations. Parenteral injections require a) aseptic technique during synthesis and manufacturing, b) equipment and training of the healthcare personnel for optimal delivery, and c) appropriate use of sterile needles [33]. Moreover, use of these traditional techniques generates a huge amount of biohazardous waste [34], which the majority of developing countries simply do not have the infrastructure to handle properly. All of these factors increase the cost of immunizations, which can significantly affect their access in emergent regions.
Oral immunization has the potential to improve vaccine efficacy simply by increasing accessibility and coverage, however the oral route also provides the additional advantage of stimulating mucosal immunity. The mucosal epithelium covers the largest surface area in the body and constitutes the first line of defense against external pathogens [8,26,35]. These mucosal surfaces involve physicochemical and biological barriers working in unison to regulate entrance of nutrients and mount responses to foreign materials [36–38]. Eliciting prophylactic immunity in the infection entry site can help prevent infectious diseases. However, the same defense mechanisms designed to exclude pathogens must also be circumvented to develop efficacious oral vaccines.
2.2. Challenges of oral administration
In order to prompt a robust immune response, the oral delivery of antigens needs to overcome multiple physicochemical and biological barriers in the GI tract. Among them is the biological barrier of the intestinal epithelium and its mucus secreting layers which serve to digest consumed material for nutrient absorption and to protect the body from the invasion of pathogenic threats [37,38]. To accomplish these tasks, the GI tract includes a highly acidic environment in the stomach, a significant pH range along the length of the GI tract, and the presence of proteolytic enzymes responsible for protein degradation. These characteristics can interfere with the delivery of fragile biomolecules, such as antigenic proteins or peptides, which are highly susceptible to degradation and denaturation [23]. Furthermore, there is a temporal limitation for the absorption of these formulations due to the residence time in the small intestine (3–4 h), where the majority of absorption processes occur [39].
Another major hurdle in the development of oral vaccines is that a higher dose of antigen is needed to induce an immune response when compared to traditional parenteral immunizations [26]. This characteristic limits the possible formulations used as carriers as they must be able to successfully carry the required antigen dosage. Larger doses also increase the risk of inducing tolerance instead of stimulating a protective response [29,40,41]. The GI tract is constantly exposed to a variety of pathogens. If a vaccine does not induce the appropriate danger signals, the body can recognize it as non-pathogenic and avoid triggering an immune response, resulting in immune tolerance instead of protection [42,43]. Thus, it is critical in the design of oral vaccine carriers to include potent adjuvants in order to sufficiently stimulate the immune system.
3. The oral route: Physiology and immunology
The gastrointestinal tract is designed for the digestion and uptake of water, nutrients, and small molecules, however, it also performs preemptive and surveillance activities to protect the integrity of the system [23,44]. Thus, upon oral administration, vaccine formulations encounter a variety of biological and physicochemical mechanisms designed to prevent the entrance of foreign material to the body and mount immune responses towards them, if necessary. In order to develop next-generation vaccines that can overcome the aforementioned challenges in the generation of immunity (and avoidance of tolerance) pertinent to the oral route, it is necessary to consider the conditions within the gastrointestinal tract.
3.1. Organization of the GI tract
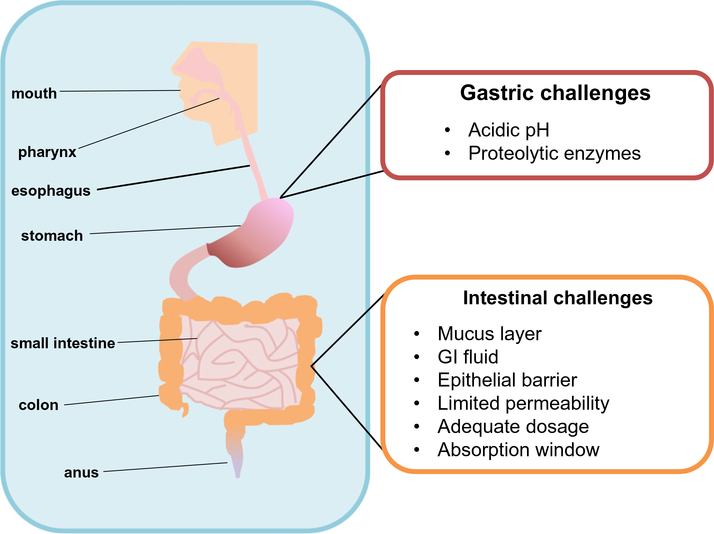
The GI tract is approximately 20 ft long and it consists of heterogeneous surfaces, mucosal thickness, pH levels, enzymatic conditions, residence times and cellular components [23,36,45,46]. As shown in Fig. 1, the GI tract is divided in two broad segments: The upper GI tract includes the oral cavity, pharynx, esophagus, and the stomach; while the lower GI tract involves the small intestine (with three sections: duodenum, jejunum, and ileum), the large intestine (also with three divisions: cecum, colon, and rectum), and anus [23]. Each one of these segments has different purposes and carries specific processes designed to absorb nutrients using either passive or active mechanisms.
Fig. 1.
Physiology of the gastrointestinal system and the challenges it presents for oral vaccines. The different segments of the GI tract present a variety of biological and physicochemical barriers to prevent the entrance of foreign material onto the body. The upper GI tract includes the mouth, pharynx, esophagus and the stomach. The lower GI tract encompasses the small intestine, colon, and anus. The stomach is designed to process and break down complex molecules (i.e. proteins) with the action of its acidic pH and proteolytic enzymes. Additionally, once vaccines have gone through the stomach and entered the small intestine, a different set of conditions need to be overcome in order for immunizations to be effective. The presence of a mucus layer, the composition of gastrointestinal fluid, and the action of epithelial barriers, limits the permeability of molecules to the lymphatic system. In order to design successful oral vaccines, careful considerations need to be taken to engineer adequate vaccine delivery vehicles.
The surface of the GI tract consists of a physical and chemical barrier formed by an impermeable layer of epithelial cells. This barrier functionality is critical as the GI tract is the initial point of contact between the body and the external environment and its first line of defense against pathogens [38,47]. The lining of the GI tract is composed of a heterogeneous population of cells with diverse roles based upon their location [47,48]. An overview of this organization is presented in Fig. 2.
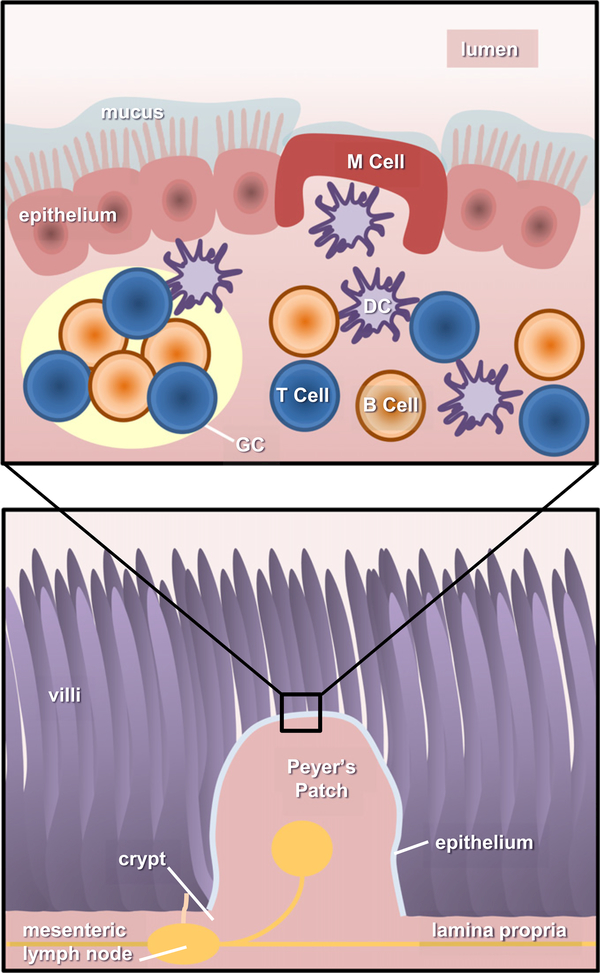
Fig. 2.
Anatomy of the gastrointestinal immune system. There are two main components in the gut-associated lymphoid tissue (GALT): inductive and effector sites. Inductive tissues include the action of Peyer’s patches, lymphoid follicles (within lymph nodes), and antigen presenting cells (APCs). Meanwhile, effector sites comprise the lamina propria and the surface epithelium. Upon entering to the intestinal lumen, antigens are transported across the intestinal epithelium barrier by sampling M cells, transcytosed and delivered to APCs (i.e. DCs). Activated DCs travel and prime CD4+ T cells in germinal centers (GCs), present in the Peyer’s Patches (PPs) and mesenteric lymph nodes, needed to initiate an immune response. Primed CD4+ T cells then activate B cells, which undergo isotype switching, thus generating IgA+ B cells. These B cells then leave the PPs using the lymphatic system to enter circulation and reach effector sites in the lamina propria, mature, and become IgA producing-plasma B cells.
The most populous cells in the GI tract are the enterocytes, which provide the primary barrier functionality of the intestine due to the formation of tight junctions between the cells. Additional intestinal cells include goblet cells, Paneth cells, microfold or M cells, intestinal epithelial stem cells (IESCs), and enteroendocrine cells [23,37,47]. In order to understand and select appropriate cellular targets for vaccine delivery a brief overview of their characteristics is presented in Table 1. Based on their cellular functions, enterocytes, goblet cells, and M cells are some of the key players involved in gut protection, transport, and immunity [43,49]. These three cell types are important in the elicitation of immune responses, since they are involved in antigen transport, uptake, and activation of immune cells. Specifically, M cells have become one of the targets for delivery vehicles to bypass the cellular barriers from the GI system more efficiently, and trigger immune responses towards their antigens [12,50–52].
Table 1.
Characteristics and functions of intestinal cells.
| Cell type | Characteristics | Function | References |
|---|---|---|---|
| Enterocytes | -Most abundant cells in the small intestine -Column-like shape -Have an apical membrane domain covered by microvilli and a carbohydrate glycocalyx |
-Nutrient digestion and absorption -Ion uptake from lumen to enterocyte cytoplasm -Important for innate immunity |
[37,45,47,53] |
| Goblet cells | -Mucus-secreting (especially MUC 2) | -Maintenance of the mucus protective layer -Regulation of intestinal wall |
[37,45,54–56] |
| Paneth cells | -Located in the deepest parts of the crypts of Lieberkühn (formed by the folding of the intestine) | -Shielding the epithelial wall by generation of antimicrobial proteins (AMPs) that disrupt pathogen integrity | [47,57–59] |
| M cells | -b1% of total cells in the intestinal lumen -Cover lymphatic bodies, including lymphoid follicles and Peyer’s patches -Short microvilli and thin mucus layer -Heavily invaginated -Have a protruding glycocalyx |
-Efficient transcytosis activity -Antigen sampling -Active transportation of pathogens -Receptor-mediated and non-specific antigen uptake -Delivery of these microbial parts to subepithelial dendritic cells |
[12,26,44,47,56,60] |
| Intestinal epithelial stem cells (IESCs) | -Located at the base of the crypts in the colon -Continuously migrate and mature to their final shedding into the lumen |
-Maintenance of healthy cellular populations in the intestine | [57,61,62] |
| Enteroendocrine cells | -Located in the mucosa -Placed between other epithelial cells |
-Secretion of hormones important for digestive functions -Mediation interactions between central and enteric endocrine systems | [37,63] |
In addition to the cellular organization, there is also a critical difference in the subjacent layers that constitute the physicochemical barrier of the GI tract. There are four layers that form this barrier: mucosa, submucosa, muscularis externa, and serosa (or adventitia) [23,48]. Each one of them has different characteristics and cellular composition depending on their corresponding roles [64,65], as described next.
-
a)
Mucosa: This is the surface layer of the GI tract and also responsible for mucus secretion. It is divided into three different sections: the epithelium, lamina propria, and muscularis mucosa. It is the primary point for absorption of food and drug molecules, therefore its cells secrete enzymes (e.g. pepsinogen) and chemical substances (e.g. hydrochloric acid) to process nutrients.
-
b)
Submucosa: This region is where the circulatory, nervous and lym-phatic systems interact with the gut tissues, specifically with the outer layers.
-
c)
Muscularis externa: This is an important muscular region, comprised by longitudinal and circular fibers that guide the food bolus through the GI tract.
-
d)
Serosa: The outermost layer of the intestine and consists of multiple epithelial sections
Besides this layered organization of the epithelia, there are also regional differences on the thickness and mucosal activity [48]. The characteristics of mucus and other physicochemical barriers of the GI tract also need to be taken into account when developing novel vaccine delivery vehicles to efficiently deliver across them into immune cells [38,48].
3.2. Biological and physicochemical barriers
Epithelial cells form the physical and biological barrier that prevents the permeation of pathogenic material into the human body, though there are also additional physicochemical barriers involved in the mucosae [66]. One of the most critical barriers is the presence of a robust mucus lining. Mucus is a hydrogel (N95% water) consisting of a mixture of proteins, carbohydrates, lipids, salts, and antibodies [38,54,55]. This complex fluid is primarily generated by mucins secreted by goblet cells. There are over twenty different mucin molecules in this family, of which the most abundant are MUC2, MUC5AC, and MUC6 [38]. These mucin molecules act as monomers, which are subsequently linked by disulfide bonds to synthesize larger molecules that can reach sizes of 0.5–40 MDa [38,66]. The resulting mucus lining is a tridimensional crosslinked network of the aforementioned mucins (2–5% w/ w), which forms a viscoelastic gel with shear-reducing properties that can vary depending on the composition, site, and physiological conditions [38,55].
The lubricating and shielding functions of mucus are essential in maintaining a healthy homeostasis [67]. The mucus lining contains both a firmly and a loosely adherent layer, the thicknesses of which are dependent upon their location along the GI tract. The presence of both the firmly and loosely adherent layers creates a slippage plane, which aids in the transport of undigested food and is essential for the protection and integrity of the GI tract [38,66]. Firm layers are formed by cell-bound mucins, glycolipids and glycoproteins that constitute the glycocalyx [38,54]. There are six cell-bound mucins that are known to be present in the GI tract, MUC1, MUC3, MUC4, MUC12, MUC13 and MUC17 [37]. The thickness and composition of the loose layers have shown to be dependent on the diet of the subject [68]. Since this mucus layer is focused on coating and lubricating undigested material, it protects the firmly bound layer through the peristaltic motion in the GI tract [55,66]. This structural composition is one of the reasons that mucus has such an important role maintaining the integrity of the GI system. Penetration of the mucus layer is one of the most important characteristics that orally administered vaccines need to have in order to reach the immunological sites within the gut.
One of the most formidable challenges that vaccine delivery vehicles need to overcome, specifically for protein antigens, is the composition of the gastrointestinal fluid [39,69]. This complex mixture is composed of water, bile salts, and enzymes (e.g. pepsin). and its hydrogen ion concentration changes its overall pH depending on its location [39,70]. Enzymatic degradation, in particular, poses one of the most significant threats to the stability of protein molecules delivered orally [39,45]. These enzymes are multiple proteases such as pepsin, trypsin, and lipase.
Pepsin, which is present in the stomach, is a proteinase that hydrolyses protein amide bonds. The activity of this enzyme is enhanced under acidic pH (b2.5), and it loses its activity at pH higher than 8 [50, 71]. This characteristic allows it to perform digestive functions at the low pH environment in the stomach. Another enzymatic protein present in the GI tract is trypsin. This protease is secreted by the pancreas and, together with carboxypeptidase and chymotrypsin, is present in the duodenum in large quantities (N1 g). The action of these three enzymes is responsible for 20% of the degradation of ingested proteins [50,72]. The remaining degradation is completed by the actions of the aforementioned elements of the gastrointestinal fluid.
Another physicochemical barrier that has a very critical role is the variation of pH throughout the GI tract. Most proteins are sensitive to their pH environment, with their stability at risk in acidic conditions due to the possible denaturation of their structure. The GI fluid is made of a mixture of saliva, ingested food and liquid, and refluxed liquid from the intestine, therefore the pH of each intestinal segment varies depending on the location, and the fasted or fed state of the host [23].
Overall, the pH range of the GI system varies from 1.0–7.0; in the stomach it is between 1.0 and 3.0, in the duodenum it fluctuates between 6.0 and 6.5; and in the colon it is 5.5–7.0. This gradient in pH is due to changes in the overall concentration of hydrogen ions caused by the presence of hydrochloric acid (HCl). In addition to affecting the stability and activity of delivered biomolecules, the pH directly impacts the dissolution of drugs and proteins [73]. It has been shown that the pH of the stomach is between 1.0 and 2.0 (with 0.01–0.1 M HCl) in the fasted state, and ranges from 3.0–7.0 (10−3–10−7 M HCl) after food ingestion [39]. The significant changes in the local pH of the GI tract have a measurable impact on orally delivered proteins and necessitate specially designed antigen delivery carriers to accomplish oral vaccination [39,70].
3.3. Gastrointestinal Immunity
The ultimate challenge of any antigenic administration is the elicitation of a robust response towards the immunogen. As previously discussed, there are multiple biological and physicochemical barriers that oral vaccine formulations have to overcome. However, if they succeed, they still need to stimulate the immune system by engaging their activation mechanisms. The intestine is the mucosal site that holds the highest number of immune cells in the body, and it is regulated by the gut-associated lymphoid tissue (GALT), that coordinates effector and inductive sites [21]. Inductive sites in the GI tract involve the coordinated action of Peyer’s patches, lymphoid follicles and antigen presenting cells (APCs), while effector sites mainly include the lamina propria (LP) and surface epithelium.
Following administration of oral vaccines, antigens travel through the GI tract. Upon entering the small intestine, M cells in the Peyer’s patches sample and transport the immunogens across to APCs. These materials are then taken up and processed by DCs that present antigenic fragments on their surface to activate naïve CD4+ T cells [56]. These helper cells further interact with antigen-specific B cells that then undergo class switching to become immunoglobulin-secreting cells. Upon maturation, B cells travel from the PPs through the lymphatic system to reach the mesenteric lymph node before entering systemic circulation. When these cells reach distant effector sites, they differentiate and maturate into plasma cells. In parallel, DCs migrate to the lymph nodes to activate humoral and cellular responses by interacting with germinal centers. A further analysis of the characteristics of the most relevant immune sites in the GALT is necessary to understand the process of generating gut immunity [21].
Peyer’s patches (PPs) are believed to be one of the largest lymphoid tissues in the GALT. They are formed by organized immune cells, and generally include B-cell rich follicles protected by a mesh-like formation known as the interfollicular region (IFR) made by T-cells [43]. They are slightly elevated lymphatic organs with a dome shaped structure that are located in the ileum within the small intestine. PPs have only efferent lymphatics, therefore they are protected by a follicle-associated epithelium (FAE). This FAE contains the previously described M cells that allow the sampling and transport of antigenic fragments from the intestinal lumen into the PPs [48]. These formations represent the main port of entry for antigens in order to elicit immunity in the gut and mucosae. Active targeting mechanisms towards M cells are being explored in order to efficiently deliver antigen into the Peyer’s patches [21,43]. Further exploration of these strategies is discussed in detail in a subsequent section.
Some of the most common and important APCs present in the GALT are dendritic cells (DCs). They take advantage of their location in the sub epithelial dome (SED) region below the FAE, where they can take up antigens directly from M cells [43,56,74]. DCs are specialized immune cells that process and present antigenic fragments to mucosal B and T cells to initiate antigen-specific immunity. There are three different DC subsets present in the GI tract: CD11c+ DCs in the SEDs, CD8α+ DCs in the IFRs, and CD11c−CD8α− DCs in both locations [75]. Finally, these cells play a significant role in the homing of activated T and B cells to the lamina propria, because of their processing ability of retinoic acid [43]. They express retinal dehydrogenase, an enzyme that can transform ingested vitamin A into retinoic acid [76]. This molecule induces gut imprinting molecules including α4β7 integrin and CCR9 [77]. Engagement of these APCs is critical in the initiation of local and systemic immunity; hence development of vaccine delivery carriers with targeting mechanisms towards these cell populations is important.
As previously mentioned, recruitment and activation of B and T cells are important in the generation of adaptive and long-lasting immunity towards an antigen. B cells make up the 75% of the cellular population of PPs, and are primarily located in the follicle region [43]. It is in these locations that germinal centers form, including during homeostatic conditions [43]. Germinal center formation is characteristic of strong thymus-dependent antibody responses, and the generation of GC B cells is an important part of triggering T helper cell responses [78]. These antibody-secreting lymphocytes are critical in the generation of serum and mucosal immunoglobulins and the host protection from bacterial and viral infections.
Cellular responses, generally performed by T cells, represent the other fundamental component of immunity. These populations are also involved in the development of robust humoral responses, via the initiation of B cell maturation. Follicular helper CD4+ T cells (TFH) provide essential co-stimulatory signals to B cells in germinal centers [29, 43,79]. Naïve T cells in the GALT are located mainly in the PPs, where they represent 20% of the total cell population [43]. However, there are also other phenotypes (e.g. Th1, Th2 and Treg) present in the gut [80,81]. Upon their activation, they can become tissue-resident memory T cells, or circulating-memory T cells, both of which may be more effective defense mechanisms than antibody-based responses. Memory cells control an infection by the secretion of cytokines and recruitment of other immune cells [79]. However, they can also cause tissue damage if there is a prolonged infiltration of such cell populations.
The barriers and challenges for antigen delivery in the GALT discussed in this section underline the need of novel design mechanisms for antigen delivery that can protect the cargo, penetrate the biological and physicochemical barriers, and possess adjuvant capabilities that can elicit robust and balanced immune responses. Optimal vaccination strategies would generate both humoral and cellular immunity with innate and adaptive components. An overview of the current oral vaccine strategies to enhance the ability of subunit antigens to elicit protective immunity, is discussed herein.
4. Types of vaccines
The history of vaccine development has largely followed Pasteur’s guiding principles of “isolate, inactivate, and inject” the causative microorganism, with the earliest successes resulting from trial and error [82]. However, advances in genetic engineering have enabled improvements in the design of vaccine technology and diversification of formulation types, thus expanding the number of diseases that can be prevented.
The earliest vaccines were live attenuated, meaning they contained a version of the living microbe that had been weakened or altered in the lab so as not to cause severe infection. Live-attenuated formulations most closely mimic natural infections, eliciting strong cellular and antibody responses that are likely to confer long-lived protective immunity [83]. Unfortunately, live, weakened vaccines can also pose risks such as inflammation, uncontrolled replication, and disease, particularly in immuno-compromised patients. Additionally, though extremely rare, attenuated pathogens have the potential to revert to a pathogenic form and cause the disease. For example, the live oral poliovirus vaccine (OPV) has not be administered in the United States since 2000 due to the risk of vaccine-associate paralytic poliomyelitis and the availability of a safer alternative in the form of an injected inactivated vaccine [84]. Advancements in genetic engineering have reduced the unpredictability of experimental attenuation and improved the safety associated with live attenuated viruses in a variety of ways, including manipulation or elimination of genes required for replication [85].
A safer alternative to live-attenuated vaccines is killed whole-cell vaccines, which consist of the disease-causing microbe inactivated by chemicals, heat, or radiation. Inactivated vaccines can still prompt an immune response, but cannot replicate. Consequently, these vaccines are safer and more stable options than live vaccines, but stimulate a weaker immune response, generally requiring additional doses or booster shots to maintain protection [86,87].
While vaccine development has traditionally focused on either live or killed whole organism vaccines, next-generation vaccine development has begun to focus on even safer and more cost-effective vaccine candidates: subunit vaccines. Subunit vaccines are considered the safest alternative as they do not contain any live components of the pathogen. They can be divided into four main categories: protein-based, polysaccharides, conjugates, and toxoids.
Protein-based subunit vaccines use a specific and isolated protein that is presented as an antigen to the immune system. These molecules can be harvested and purified from the cultured microbe or manufactured using recombinant DNA technology [88]. However, proteins are fragile structures and are easily denatured and degraded by changes in pH or presence of proteolytic enzymes [45,89]. Polysaccharide vaccines mimic the polysaccharide capsules associated with infectious bacteria, thereby eliciting an immune response. Similar to protein subunit vaccines, they are not very immunogenic, and, therefore, are associated with short-term immunological responses, not long-term memory. Conjugate vaccines also create a response against the pathogen’s protective polysaccharide capsule; however, they include a carrier protein in addition to the polysaccharides to improve generation of long-term protective immunity. Some of the commonly used protein carriers include diphtheria and tetanus toxoids and are, therefore, generally used against bacterial infections. Lastly, toxoid vaccines are used against pathogens in which bacterial toxin is the primary cause of illness, such as diphtheria and tetanus [88]. They are inactivated versions of the toxins and, therefore, are both safe and stable. However, most toxoid vaccines require the use of adjuvant, such as aluminum or calcium salts, for an effective immune response.
All subunit vaccines differ from inactivated immunizations by containing select antigenic parts of a pathogen that are required to elicit a protective immune response. These formulations provide excellent stability and safety profiles, but the process to find the appropriate combination of the aforementioned antigenic components in order to produce an effective immune response is extremely time-consuming [83,90]. Furthermore, subunit vaccines tend to be less immunogenic than their whole-cell counterparts. Current research has focused on the addition of adjuvants in order to enhance the immune response through inclusion of immunostimulatory molecules or design of antigen-delivery systems [83]. The remainder of this review will focus on the development of subunit vaccines for oral immunizations, including the challenges that these formulations need to overcome in order to promote protective immunity in the vaccinated individual.
5. Licensed oral vaccines
Despite the numerous challenges associated with oral immunizations, the presence of multiple licensed formulations demonstrates that oral immunization is a feasible goal as shown in Table 2. Current licensed vaccines in the U.S. address diseases caused by enteric pathogens, such as rotavirus, enterotoxigenic Escherichia coli (ETEC), Vibrio cholerae, and Shigella, as well as pathogens that invade via the intestinal mucosa to cause systemic diseases, such as Salmonella Typhi and polio-virus [21,91].
Table 2.
Licensed vaccines.
| Trade name | Disease | Antigen (s) | Indications | Formulation | Dosage | References |
|---|---|---|---|---|---|---|
| Sabin Live OPV |
Polio | Live-attenuated, trivalent OPV vaccine Sabin strains 1,2,3 |
*Not currently used in US | Solution | Three doses, taken at least four weeks apart | [8,90,92] |
| Vivotif | Typhoid Fever | Live-attenuated strain, Ty21a | Children (N6 years) | Enteric coated capsule | Four, taken on alternate days | [8,89,93–95] |
| Dukoral | Cholera | Recombinant B-subunit and inactivated whole cell Vibrio cholera | N2 years | Suspension | Two doses (75 mL children, 150 mL adults), 1 week apart | [29,98,99,107,109] |
| Vaxchora | Cholera | Live, oral, CVD-10-HgR | Adults (16–64), traveling | Suspension | Single, lyophilized dose reconstituted in 100 mL water | [101,102] |
| Rotarix | Gastroenteritis | Live-attenuated monovalent human rotavirus RIX4414 strain of G1P(8) type |
Infants (6–24 weeks) | Suspension | Two 1 mL doses, 4 weeks apart | [8,103,104] |
| RotaTeq | Gastroenteritis | Attenuated pentavalent live rotavirus reassortants: derived from human and bovine species | Infants (6–32 weeks) | Solution | Three 2 mL doses, 4–10 week intervals | [8,103,104] |
| None | Acute respiratory disease | Adenovirus, live | Military populations (ages 17–50) | Enteric coated tablets | Single dose | [105,106] |
The predominant vaccine strategy in these formulations involves the use of live-attenuated organisms to mimic natural infection. These oral vaccines have demonstrated the ability to elicit both broad and robust immune responses, including production of serum and mucosal antibodies, as well as synergistic effector and memory T cells. There has also been success with administering non-living vaccines via the oral route, which offers improved safety compared to live vaccines, but can also be less immunogenic.
5.1. Oral polio vaccine
The oral polio vaccine (OPV) was the first successful mucosal vaccine developed. OPV consists of a mixture of live attenuated poliovirus strains of each of the three infectious serotypes. Three spaced doses are required to generate protection via both humoral and mucosal immunity [92]. Serum antibodies prevent the spread of poliovirus to the nervous system, thereby protecting individuals from polio paralysis. Additionally, and uniquely to OPV as compared to the inactivated injectable polio vaccine (IPV), OPV produces a local SIgA immune response in the intestinal mucosa, which is the primary site for poliovirus entry and multiplication [8,93]. This local intestinal response is extremely effective at stopping person-to-person transmission of wild poliovirus.
However, with OPV administration there remains an extremely low but real risk of reversion to neurovirulence, occurring in approximately 1 in every 2.5 million cases [94]. An average of six to eight cases of vaccine-associated paralytic polio occurs annually in the United States. After successful eradication of the disease by widespread vaccination programs, the risk of acquiring the disease from the wild-type pathogen was lower than acquiring polio from OPV [94]. Accordingly, OPV has been replaced by IPV in most industrialized countries.
5.2. Live oral typhoid vaccine (Ty21a)
Typhoid fever is caused by Salmonella typhi, an invasive enteric bacterium generally ingested through contaminated food or water. For that reason, typhoid fever is extremely uncommon in industrialized countries but still endemic in less-developed regions, which typically lack access to treated water supplies and sanitary conditions [91]. One of the two licensed typhoid fever vaccines is an oral live attenuated Ty21a vaccine, developed by chemical mutagenesis of the Ty2 S. Typhi strain. Neither of the available vaccines is 100% effective, and Ty21a protection varies depending on vaccine formulation, number of doses and spacing between doses [95–97].
Currently, the formulation is available as either a liquid suspension or an enteric coated capsule and administered as three or four doses on alternate days. The vaccine confers protection seven days after the last dose, with up to 62% protection over a seven-year follow up period. The extent to which this vaccine mediates protection by systemic immunity or gut mucosal immunity is unclear, though Ty21a is associated with production of serum IgG, intestinal sIgA, as well as various cell-mediated immune responses such as T cell proliferation and Th1-type cytokines [91]. While the serum antibodies certainly help to achieve protection against S. Typhi, ongoing studies aim to elucidate the dominant immune mechanism to achieve long-term efficacy.
5.3. Cholera vaccines
Cholera is an extremely virulent and acute diarrheal disease spread through fecal contamination of food and water. Of the numerous enteric pathogens associated with diarrheal disease, V. cholera causes the most severe epidemic outbreaks, most often associated with natural disasters that interrupt access to clean water [98]. It remains endemic in regions with poor sanitation in impoverished and overcrowded areas. Acquired through ingestion of contaminated food and water, the bacteria V. cholerae colonizes the epithelial lining of the gut, resulting in profuse watery diarrhea that can kill within hours if untreated [91,99].
For many years, a killed whole cell cholera vaccine administered by injection was the only vaccine available, though protection was incomplete, short-lived, and associated with unpleasant side effects. It was considered unsatisfactory for general public health use, and has since been replaced by two improved oral vaccines. The more widely used is a recombinant cholera toxin B (CTB) subunit and inactivated whole cell V. cholera O1 called Dukoral ®, manufactured by Crucell (Leiden, The Netherlands) and administered as two doses fourteen days apart [29,100].
The recombinant cholera toxin B vaccine provides protection against different serotypes, is safe and stable, and provides approximately 65% protection against cholera for 2 years, including considerable herd protection [101,102]. The protection is mediated by local production of both antitoxic and antibacterial SIgA antibodies in the gut. Furthermore, the CTB component of the vaccine provides significant cross-protection against ETEC, which possesses a structurally and functionally similar heat-labile toxin. However, given that Dukoral® is administered with a buffer solution that requires 150 mL clean water for adults, it is primarily used for travelers as opposed to epidemic areas where clean water is often limited [101,102].
The second and more recently internationally licensed vaccine is an oral live attenuated cholera vaccine, CVD-10-HgR, containing a genetically manipulated V. cholera O1 Inaba strain (Vaxchora, PaxVax, USA). It is a reformulation of a previous CVD 103-HgR vaccine (Orochol; Mutachol), which was taken off the market for economic reasons [103]. It is available as a single dose, but is currently only indicated for adults 18–64 traveling to cholera-affected areas and meant to be administered at least ten days prior to potential exposure to V. cholera [104].
5.4. Rotavirus
Rotavirus is the leading cause of diarrheal mortality in infants and children under the age of 5 [8]. The virus is a triple-layered particle exhibiting diverse antigen types. Five serotypes are responsible for the majority of human rotavirus disease. There are currently two oral vaccine formulations available: a monovalent attenuated human rotavirus (RotaRix) and a pentavalent bovine-human rotavirus vaccine (RotaTeq). Though the composition differs, their effectiveness and mechanism of action are similar. Both are effective at preventing severe rotavirus gastroenteritis (N90%), but less effective against mild infections (60–75%) [105]. From a societal perspective, RotaRix has an improved cost-effectiveness ratio given the requirement of fewer doses, and therefore less storage space, as well as demonstrated thermostability [106].
5.5. Oral adenovirus vaccine
Acute respiratory disease, caused by adenovirus Type 4 and Type 7, used to be the leading cause of hospitalization for U.S. Army personnel [107]. Clinical symptoms are similar to the flu, including high fever, cough, chest pain, headache and congestion lasting 3–10 days. Adenovirus Type 4 and Type 7 vaccine began to be administered to military recruits in the 1960s as an enteric-coated capsule containing the live viruses. Oral administration allows selective asymptomatic infection in the lower intestinal tract, while conferring immunity in the upper respiratory tract. Protection is associated with the presence of serotype specific serum neutralizing antibodies, though there is no evidence that neutralizing antibodies are the sole source of protection [108].
6. Oral vaccine strategies: delivery systems
As previously mentioned, subunit vaccines require the use of delivery systems and/or immunostimulants to induce immune protection. In order to efficiently deliver stable antigens, it is necessary to: i) design carriers that can protect the payload through these conditions [110], ii) release the vaccine within the small intestine residence time to antigen presenting cells across the epithelial layers, and iii) enhance the immune responses elicited by the vaccine with the adjuvant capabilities of the delivery vehicles [28].
There are a multitude of parameters to be taken into account when designing delivery systems for the oral delivery of subunit vaccines. Among some of their controllable properties include size, geometry, antigen loading and release kinetic capabilities, and finally the ability to include functional molecules to improve their performance. Tailoring these characteristics can prolong the residence time of immunogens, enable the co-delivery of antigens and adjuvants to boost their immunogenicity and target immune cells (specifically APCs) for efficient transport, uptake and presentation. Furthermore, the material properties of these vehicles have the potential to act as immune-potentiators as well.
This section includes a brief overview of the most commonly explored delivery vehicles for oral vaccination, as shown in Fig. 3, their characteristics, and the responses obtained after their administration.
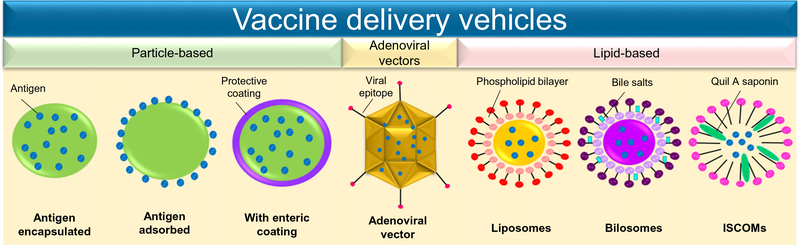
Fig. 3.
Oral vaccine delivery vehicles. Design of delivery vehicles for oral vaccination has been focused on three different types of carriers: particle-based, adenoviral vectors, and lipid-based technologies. Each of these alternatives has distinctive approaches to enhance the efficacy of the antigen upon its administration.
6.1. Polymeric/particulate vaccine design
Polymeric microparticles (MPs) and nanoparticles (NPs) have been extensively explored for the development of subunit-based vaccines. Proteins, DNA, and polysaccharide vaccine components are fragile molecules that could be structurally degraded during transition through the gastrointestinal tract or the mucosal layer, resulting in diminished bioactivity. Entrapment or encapsulation of the antigenic payload within polymeric particles affords protection, while also preventing antigen dilution over the large surface area of the GI tract. In addition to robust structural stability, particles can provide control over release site and profile for improved delivery of stable antigens. Furthermore, NP carriers have demonstrated the capability to efficiently deliver an antigenic payload directly to phagocytic APCs through passive or active targeting to stimulate cellular and humoral responses [111].
Particulate delivery systems passively provide characteristics of adjuvant behavior to weakly immunogenic subunit vaccines simply by virtue of APC recognition and internalization. However, NPs enable the incorporation of enhanced adjuvant strategies through co-delivery of immunomodulators or by manipulation of surface properties for enhanced or targeted uptake by immune cells, a strategy that is further discussed in Section 7 [112]. Furthermore, there is a diversity of both synthetic and natural materials with desirable physicochemical properties capable of responding to physiological changes, making polymeric particles a versatile option for rational vaccine design [113].
6.1.1. Synthetic polymers
Polyester nanoparticles, in particular poly(lactic) acid (PLA) and poly(lactic-co-glycolic) acid (PLGA), are the leading synthetic polymers explored in preclinical studies for oral vaccine administration due to their biocompatibility, biodegradability, and controlled sustained release patterns of encapsulated antigens for up to several months [114–116]. Additionally, both PLA and PLGA are FDA-approved materials, which can expedite the development and approval of the delivery carriers.
Biodegradable vaccine delivery systems allow for prolonged antigen release and are a viable strategy to achieve single-dose administration, which is particularly desirable in the context of reducing number of repeated administrations required for long-term protection and the cost implications for mass vaccination campaigns. For several antigens studied, including plasmid DNA and protein antigen payloads, single administration significantly improved long-term IgA and IgG antibody titers in comparison to soluble antigen, attributed to sustained antigen release mediated by particles [117–120].
Antibody responses can be further improved compared to encapsulation of the antigen alone by co-delivery of antigens with immunostimulants, such as the TLR4-adjuvant monophosphoryl lipid a (MPLA) [121], or co-polymerization with PEG as a stabilizer to improve NP stability in gastrointestinal conditions and antigenicity of encapsulated antigen [122]. Zhu et al. designed a PLGA-based system containing both immunostimulant and GI stabilizing strategies for an effective HIV peptide vaccine. PLGA nanoparticles containing three different TLR ligands and the HIV Env epitope were coated with the methacrylate-based polymer Eudragit FS30D [123]. This pH-responsive polymer was chosen to selectively deliver the antigenic payload to the large intestine. While orally administered PLGA nanoparticles induced local responses in the small intestine, the two-part PLGA/Eudragit microparticle formulations induced immunity in the rectal and vaginal mucosa, ultimately protecting against rectal or vaginal viral challenge. The study demonstrates the possibility to target regions of the GI tract in a pH-dependent manner.
While these polyesters provide several advantages for vaccine design, acidification of the microenvironment within the delivery vehicles upon degradation can prove to be unfavorable for the encapsulated agents, causing disruption to tertiary structure and protein degradation [124,125]. Efforts have been made to optimize PLGA particle fabrication methods and add stabilizing agents, which have helped to alleviate deleterious effects of local acidification [118]. In a strategy to circumvent the stability issues associated with encapsulation, PLGA has been evaluated as an adjuvant for particulate delivery of surface-adsorbed antigens [126,127]. This strategy does not afford controlled release of the antigen, but could provide an organic and biodegradable alternative to inorganic adjuvants such as alum.
Polyanhydride NPs are another class of biodegradable materials being investigated for mucosal vaccine design. In contrast to polyesters, the degradation products of polyanhydrides are less acidic and therefore can improve the stability of encapsulated antigens [128]. In addition, polyanhydride materials have been shown to modulate immune response without the requirement of supplementary adjuvants. Copolymers of methyl vinyl ether (PVM) and maleic anhydride (designated as P(VM-g-MA)) have demonstrated Th1-adjuvant activity. Upon immunization with ovalbumin-loaded ligand-coated P(VM-g-MA) NPs, a balanced IgG1 (Th2) and IgG2 (Th1) response was generated [129, 130]. Th1-adjuvant capacity is mediated by NPs promoting a close interaction between the antigen and APCs, which can be further enhanced with targeting strategies, and also by acting as an agonist of various TLRs [131]. Single dose immunization with P(VM-g-MA) NPs containing outer membrane vesicles (OMVs) derived from Shigella induced antibody protection (IgG1, IgG2, and IgA) as well as Th1 cytokines, ultimately protecting mice against lethal challenge [132], demonstrating the potential of polyanhydrides as a platform for protection by subunit antigens.
Additionally, other synthetic pH-responsive materials have been explored for oral vaccination due to their ability to protect antigens from degradation and provide targeted release. Mannan-coated methacrylic acid-based copolymers demonstrated both pH-dependent release of encapsulated antigens as well as uptake and activation of APCs [133], while co-delivery of antigen with the mucosal adjuvant cholera toxin (CT) elicited significantly increased IgG and IgA antibodies as compared to soluble dosages [134].
Overall, the variety of synthetic polymers that have been used to design oral vaccines have shown diverse abilities to elicit mucosal and systemic responses. Even though selected formulations have shown to generate protection against lethal challenge, the majority of synthetic platforms have been validated using robust model antigens. Despite the successes in pre-clinical evaluation, there is still a need to elucidate the mechanism and optimal characteristics of such vehicles to tailor efficacious formulations that can proceed to clinical trials. The next step for these systems is their optimization for efficacious oral vaccines using relevant fragile immunogens.
6.1.2. Natural polymers
Natural polymers (e.g. polysaccharides) tend to be non-toxic, biocompatible, and biodegradable as well as possess mild gelation conditions for encapsulation of sensitive macromolecules. They have been extensively explored in drug delivery applications. Additionally, carbohydrates are desirable oral vaccine components given the numerous lectin-receptors expressed by M cells in the intestinal mucosa.
Chitosan is a cationic polysaccharide with advantageous properties for oral delivery including mucoadhesion and an ability to reversibly disrupt epithelial tight junctions [135,136]. However, chitosan particles are limited by their high solubility in acidic conditions, risking the integrity of the sensitive payload. Strategies to overcome chitosan’s dissolution in acidic pH include encapsulation of antigen-loaded chitosan particles within liposomes to protect transit through the stomach [137], stabilization by crosslinking with tripolyphosphate and glutaraldehyde [138], and electrostatic coating with the anionic polysaccharide alginate [139,140]. These strategies significantly improve particle stability and payload retention in acidic environment to protect the antigen, and induced significantly higher antibody titers in vivo as compared to unmodified chitosan particles.
These results from natural polymeric delivery systems using in vitro and in vivo models are promising. However, the limited control over their chemical structure requires better engineering of formulations using natural polymers to continue the progress for their evaluation in clinical studies. Additionally, the assessment of natural platforms has been restricted to evaluation of antibody-driven responses. Humoral responses are important in the generation of robust immunity, thus further analysis of these systems in this area is needed.
6.2. Lipid-based vehicles
Lipid-based vaccine delivery carriers are some of the most commonly used vehicles for oral administration. Among these are included liposomes, bilosomes, and ISCOMs. They are based on the separate encapsulation of hydrophilic and lipophilic agents using lipid bilayers.
6.2.1. Liposomes
Liposomes are spherical vesicles formed by one or more phospholipid bilayers synthesized from cholesterol and other non-toxic lipids. The properties of these systems vary depending on their composition (i.e. their size, charge, and protein compatibility), and can be optimized by changing their fabrication parameters. These liposomal systems also offer the ability to deliver multiple active agents with vastly different properties, since they can be located in different compartments of the carrier. Specifically, water-soluble molecules, such as proteins, RNA, carbohydrates, or peptides are encapsulated in the inner layer of these vehicles; meanwhile lipophilic compounds can be included in the external section of the formulation [141,142].
A variety of liposome-based vaccines for oral administration have been previously synthesized to target a wide range of viral and bacterial diseases. For example, an influenza A viral vaccine was produced using a construct DNA vaccine with a pcDNA 3.1(+) plasmid encapsulated in cationic liposomes. Oral immunization with this formulation induced humoral and cellular immune responses, in addition to increasing cytokine production [143]. Liposomes have also been used to prevent bacterial infections, such as Salmonella Enteritidis. A vaccine to prevent this disease was created using a liposome-associated carrier with the recombinant SefA protein by Pang and collaborators. This oral vaccine was able to generate protective immunity in chickens and a significant reduction of intestinal bacterial load was observed after oral challenge with 2 × 106 CFUs of live Salmonella Enteriditis [144].
Liposomes have demonstrated their ability to deliver diverse antigens, including DNA, peptides, and proteins. For example, encapsulation of a DNA-based antigen (Mycobacterium pcDNA3.1+/Ag85A) in liposomal formulations enhanced its presence in the epithelium, M cells, DCs and PPs within the small intestine of C57BL/6 mice after three oral immunizations. The ability of the system to induce antigen-specific mucosal immunity made this formulation a potential vaccine carrier [145]. In a different study, delivery of antigenic peptides and CTL epitopes within liposomes allowed their efficacious transport to APCs and improved the host response towards these antigens [146–148]. Additionally, the adjuvant capabilities of these formulations have been tested using model antigens (e.g. ovalbumin, bovine serum albumin). These experiments have shown that liposomes can effectively load and release stable protein. They are also able to elicit Th1/Th2 immunity, reflected by the generation of mucosal and systemic antibody responses [21, 141,149,150]. Finally, these systems can also be decorated with targeting molecules (e.g. carbohydrates) to enhance their efficacy. In oral immunization experiments, lectinized liposomes were able to effectively target M cells in the PPs, resulting in elicited mucosal responses with high antibody titers [141,151].
The ability of traditional liposomal vehicles to elicit immune responses is key in the development of oral vaccine delivery systems. However, these platforms need to be further-engineered to be stable under the harsh conditions in the GI tract and protect fragile antigens [152]. Additionally, encapsulation efficiency of proteins within liposomes is highly dependent on the antigen charge and size, which can limit their potential when high protein doses are needed to elicit strong immunity [153]. Overall, liposomes have shown promising properties for vaccine delivery applications. They require further investigation and optimization to result in efficacious formulations for human vaccination.
6.2.2. Bilosomes
A different lipid-based carrier being explored for oral immunization is bilosomes. These non-ionic surfactant vesicles have adjuvant functionalities and incorporate bile salts in their formulation. Bilosomes are typically synthesized with monopalmitoyl glycerol (MPG), cholesterol (CH), and dicetyl phosphate (DCP); and surfactants such as sodium deoxycholate (SDC), or sorbitan tristearate (STS). Similar to liposomes, bilosomes also have a bilayer with polar and non-polar ends, permitting the integration of vaccine elements with significantly different properties.
Traditional liposomal vesicles can be disrupted by bile salts, however, if vesicles are fabricated in the presence of bile sales, such as bilosomes, they are no longer affected by their action and remain stable. These systems are able to stimulate humoral and cellular immune responses and the inclusion of bile salts allow the protection of the cargo from the harsh environment from the GI tract [152,154,155]. One of the main advantages of bilosomal formulations is the improved stability that they can confer to fragile antigens. In previous studies it has been shown that bilosomes are able to entrap and stabilize a variety of fragile antigens, including tetanus toxoid (TT), A/Panama (influenza A immunogen), diphtheria toxoid, Bac-VP1 (hand, foot and mouth disease vaccine candidate) [154–160].
Additionally, adjuvant and drug release studies with bilosomes have been carried out using model antigens such as bovine serum albumin (BSA) and cholera toxin subunit B [152,161]. Their immunogenic abilities have also been explored using various disease models. Previously, mannosylated bilosomes targeting DCs for oral immunization against hepatitis B virus generated both systemic and local immunity, including in the mucosa [157].
The use of these formulations induced production of soluble immunoglobulin A at all local and distal sites of the GI tract. A different set of studies performed using a subunit vaccine against influenza in an orally administered formulation also elicited high antibody titers and cellular responses. Specifically, Th1 and Th2 responses were successfully produced [152,157]. These results are very promising, since these systems have shown capabilities to stimulate balanced mucosal and systemic immunity. Despite this, their ability to confer long-term immunity and protection against lethal challenge still need to be further studied. As summarized here, the aforementioned benefits provided to different antigens because of bilosomal entrapment, make this system a feasible vaccine delivery platform for oral immunizations. Further evaluation using clinical trials is the next step in the development of bilosomal oral vaccines.
6.2.3. ISCOMs
Immune-stimulating complexes (ISCOMs) are second-generation liposomes regarded as both a carrier and as an immunostimulant for vaccine delivery. Synthesized using colloidal saponin (often QuilA extracted from the tree Quillaja saponaria), cholesterol and other phospholipids (generally phosphatidylethanolamine or phosphatidylcholine), these nano-sized vectors (~40 nm) with self-adjuvant abilities are organized in open-caged structures [15,21,162,163]. These vehicles have been used to entrap bacterial and viral envelope proteins to prompt vaccines against such pathogens. Classical ISCOMs are self-assembling systems fabricated in the presence of a non-ionic detergent that is removed post-synthesis [162].
These formulations have been shown to have a great breadth of applications, incorporating antigens to prevent herpes simplex virus 1, hepatitis B, respiratory syncytial virus, Escherichia coli, Brucella abortus, and Plasmodium falciparum infections [162,164–166]. ISCOM-based vaccines have shown to be highly immunogenic, generating balanced humoral and cellular responses in different animal models [162]. The properties of this system engage components of both the innate and adaptive immune systems. This characteristic makes this platform a highly desirable delivery methodology, although their intricate action mechanisms remain to be fully elucidated. However, it is also important for oral applications the elicitation of mucosal immunity (i.e. secretory IgA) to prevent enteric infections. The development of ISCOM-based vaccines requires further evaluation in pre-clinical studies to optimize the adjuvant properties of this platform.
6.3. Adenoviral vectors
Traditional vaccines were based on the use of killed or attenuated pathogens, but as previously discussed there are risks in the immunization of vulnerable populations with such platforms due to the potential reversal of their pathogenicity. However, with advances in genetic engineering and molecular virology, there are some alternatives for the use of such microbial structures without their detrimental side effects. Adenoviruses are double-stranded DNA viruses, with a ~40 kb genome, they are species-specific and have different serotypes. While this platform was initially devised for gene delivery, due to its highly immunogenic nature it became less attractive for therapeutic use.
However, based on the advancement and optimization in the synthesis of adenoviral vectors, they have become interesting possibilities as vaccine delivery carriers. The adenoviral genome is well studied and can be readily manipulated, thus allowing the synthesis of nonpathogenic vectors. Another advantage of these systems is that most of these viruses in their original form only induce mild diseases in immunocompetent human adults. These systems can also be modified to nullify their replication mechanism, further reducing their ability to infect a host.
The previously mentioned features have prompted the use of adenoviral vectors as vaccine delivery vehicles for the treatment of viral diseases. Vaccines using adenoviral vectors have targeted a wide range of some of the most challenging diseases, including HIV, influenza, rabies, botulism, dengue, SARS and Ebola [167–173]. They are able to generate robust cellular and humoral immune responses. Oral immunization with the most common adenoviral vaccine vectors (AdHu5) have been shown to induce potent CD8+ T cell responses and antibody responses, but not engage CD4+ T cell responses [167,174–176]. The multiple isotypes (e.g. IgG2a, IgG1) generated by such vectors indicates the elicitation of a Th1/Th2 response, however it is predominantly skewed towards the first one [167].
Additionally, adenoviral vectors activate innate immunity mechanisms by the expression of pathogen-associated molecular patterns (PAMPs) on their surface, initiating the secretion of pro-inflammatory cytokines, activation of complement, and the differentiation of APCs. One of the important considerations during the development of novel delivery vehicles is their ability to induce strong responses in relevant models for clinical application. These systems have been used for administration of vaccines in multiple animal models including rodents, dogs, non-human primates, and most importantly, they have reached human clinical trials [167]. By taking advantage of their immunogenic characteristics, adenoviral vectors represent an alternative to killed or attenuated vaccines, and their further use and optimization remains as a valuable option for pathogen-mimicking delivery vehicles.
7. Approaches to enhance oral vaccination
Development of targeting strategies could lead to development of more rational oral vaccine design. Physicochemical characteristics of antigen delivery systems, including size, shape, surface charge, and hydrophobicity can be tailored to achieve passive targeting of desired cells [177–179]. However, active targeting strategies have been explored to more specifically direct particulate delivery systems, thereby potentially lowering the dosage required to elicit an immune response. Receptors on intestinal epithelial cells, M cells and APCs have all been explored for targeting vaccine delivery using a variety of ligands, including bacterially derived moieties, lectins, PAMPs, and antibodies [180,181].
7.1. M cell targeting
An important mechanism of particle transport from the intestinal lumen into the GALT is via M cells. These specialized transcytotic cells efficiently internalize and transport particulate matter (e.g. bacteria, viruses) to the underlying Peyer’s Patches, and are therefore extremely desirable targets for oral vaccine design [182]. M cells express unique carbohydrate receptors that provide selective targets for mucosal vaccine delivery. Lectins are among the most studied bioadhesive, consisting of proteins and glycoproteins that can bind reversibly to specific carbohydrate residues. Both Ulex europaeus agglutinin-1 (UEA-1) and Aleuria auranitia target the α-L-fucose resides expressed apically on M cells. Oral immunization of particles surface decorated with either lectin results in significantly higher SIgA as compared to untargeted particles [151,183–185]. Additionally, these particles have also demonstrated enhanced cellular immunity, indicated by substantial increases in Th1-cytokines IL-2 and IFN-γ. These results indicate the potential for lectin-targeted strategies to improve mucosal immune response. However, it should also be noted that some lectins are toxic and can be inherently immunogenic. The immunostimulatory capacity could be advantageous in using lectins as mucosal adjuvants, but also poses the risk of eliciting a response against the targeting molecule and ultimately preventing uptake [186].
Other protein receptors expressed on M cells have been exploited for targeted delivery. For example, RGD is a ubiquitous peptide for cellular attachment, but due to overexpression of the β1 integrin on the apical side of M cells has also been used to target M cell mediated transport, increasing humoral response with reduced doses of antigen [51]. Claudin 4 is a tight junction transmembrane protein highly expressed in M cells which can be targeted with surface-conjugated peptides to mediate enhanced SIgA response [187]. Additionally, elucidation of markers specific to M cells could enable the development of antibody-mediated targeting, as demonstrated by Nochi, et al., with a novel monoclonal antibody (NKM 16–2-4) that distinguished M cells from goblet cells for a highly effective vaccine capable of protecting against lethal challenge [188].
Finally, strategies have been borrowed from enteric pathogens, which exploit M cells to gain host entry, ranging from bacterial adhesins and toxins to viral proteins. Glycoprotein 2 (GP2) is an M cell receptor expressed in humans and mice that interacts with FimH, an outer membrane component associated with type I piliated bacteria (E. coli, Yersinia, Salmonella). FimH or other GP2 ligands could represent a strategy to hijack M-cell mediated bacterial transcytosis and the subsequent induction of mucosal immune response [189,190]. Yersinia also binds with the β1 integrins. Conjugation of the invasion protein [191], and, more recently, recombinant bacterial strains expressing the Yersinia invasion have been investigated to target M cells [192].
While M cell targeting strategies have been demonstrated to be effective in animal models, challenges still remain, including the identification of M cell target receptors that will translate from mice to humans as well as ensuring the induction of immunity instead of tolerance [187, 193]. Work is being done to better understand M cell biology using an in vitro M cell culture model, comprised of Caco-2 human colon adenocarcinoma cells and Raji B human cell line [51,194], and more recently with the addition of HT29-MTX mucus secreting goblet cells [195,196]. This model has the potential to elucidate the mechanisms of antigen transport across M cells, accelerating identification of M cell specific receptors and improving rational oral vaccine design.
7.2. Next generation adjuvants
M cells are such attractive targets for oral vaccine design due to their specific association with the lymphoid tissues. However, M cells comprise b5% of the FAE [197]. Additionally, evaluation of targeting strategies can be made extremely difficult in vivo due to highly variable proportion and phenotype among species. Therefore, targeting receptors expressed on normal gut epithelial cells is an alternate strategy to M-cell targeting. For example, epithelial cells also express a variety of lectins which can be exploited to enhance transepithelial transport [9, 181,198].
Ligands such as wheat germ agglutinin (WGA) which targets N-acetyl-D-glucosamine and sialic acid residues that are expressed by enterocytes throughout the GI tract have been well explored for oral drug delivery. Pattern recognition receptors (PRRs) are also expressed on various cell types, including epithelial cells and APCs, and recognize microorganism-associated molecular patterns (MAMPs) to enhance phagocytosis of microorganisms. PRR ligands, therefore, possess innate adjuvant properties by activating key innate immune signals [199]. TLR agonists represent the majority of the PRR ligands used as supplementary adjuvants or targeting moieties in oral vaccine development, most of which are derived from pathogens. Co-delivery of toll-like receptor agonists, particularly TLR-2 and TLR-4, have been demonstrated to effectively enhance transport across intestinal lumen [200–202].
CpG oligodeoxy-nucleotides (ODN) are a common element in bacterial and viral DNA that are recognized by TLR9 and possess strong immunomodulatory properties [123,203]. Flagellin, a major protein associated with bacteria, are recognized by TLR5, which is expressed by epithelial cells, B cells, and dendritic cells among others [204], as well as a nod-like receptor (NLRC4) to potentially activate two PRR systems to enhance immune response [205]. Flagellin loaded particles have demonstrated potent humoral response to a model antigen, as well as maturation of intestinal DCs and activation of helper T cell response in vivo [129,206]. LPS is another bacterially derived endotoxin that acts as an agnostic of TLR4 and can be encapsulated [207,208] or immobilized onto particle surfaces [209], resulting in preferential uptake by DCs and generation of potent humoral and cellular immunity. However, the inherent toxicity associated with LPS can be problematic for vaccine design.
MPLA is a derivative of lipid A from Salmonella and alternate TLR4 agonist considered to be safer although often less effective than LPS [207,210]. Similarly, Cholera toxin (CT) from Vibrio Cholerae and the heat-labile enterotoxin (LT) from ETEC are two of the most promising mucosal adjuvants derived from bacteria but limited by the potential issues associated with the use of native toxins [8]. Both consist of enzymatically active A subunits that mediate toxicity and cell-penetrating B subunits. Substantial work has been dedicated to deriving toxin mutants that either eliminate or reduce the toxicity of the A subunits while still providing mucosal adjuvant capacity [181].
8. Future directions
The development of successful oral vaccines using subunit antigens requires careful design of delivery vehicles and incorporation of molecules that can potentiate their effect to elicit strong and balanced immune responses. As described in this review, there are several advantages in the use of the oral route to improve vaccination efficacy; however, there are challenges including the protection of these fragile proteins, their release, and the adjuvant ability of their carriers. The characteristics of some of these strategies have been briefly described in this work, but there are still alternatives that should be explored in order to achieve optimal systems for oral vaccination.
While the physiological and biological structure of the GI system has been widely studied, questions about biomaterial interactions with the GALT remain unanswered. A variety of studies using other mucosal routes have shown that there is a dependence between prolonged immunogen presentation and production of long-term protective immunity. Due to its nature and role, the GI tract presents a tougher challenge since this can cause tolerance instead [211,212]. A better understanding of the dose and the antigen release kinetics better suited for orally administered vaccines is necessary to aid in the selection of biomaterial candidates to be used as efficacious delivery systems.
Variation on the immunity generated by oral vaccines has been shown to be dependent on the nutrition and health of the GI system of the patient. In particular, tropical enteropathy can cause child undernutrition, intestinal absorption, and inflammatory disorders that diminish the efficacy of oral immunization [213–215]. Addition of certain molecules, such as co-factors (i.e. retinoic acid) in these formulations can improve the response from the vaccinated individual. Especially for their use in low-income countries, the presence of these nutrients would represent an important component of oral immunizations.
The ultimate goal of vaccination is the generation of protective immunity. As presented in this work, there are a myriad of adjuvant/carrier systems that are being currently explored for this use. Additionally, next-generation vaccines include biomolecules that can target or enhance their efficacy. Despite this, live and attenuated vaccines are the only licensed products in the US for these applications. In order to have pathogen-mimicking capabilities and generate similar responses to microbial infections, the combination of two or more of these approaches can improve their individual abilities. Assembly of these structures, if working in unison, will allow to take advantage of their strengths, while minimizing their limitations.
Vaccine technology has continuously evolved since its inception. Advances on genetic and metabolic engineering, among others, have allowed the fabrication of novel molecules that are safe and can generate immune responses. It is time that the biomaterial field can catch up with physicochemical and biological tools, in order to design appropriate delivery vehicles for such antigens. Design and development of these carriers that may include immune-potentiators, mucus-penetrating strategies, adjuvants, and other approaches, will certainly help in the production of subunit oral vaccines for mucosal diseases.
Acknowledgements
The authors acknowledge the Cockrell Family Regents Chair for their generous support. During this work, L.A.S. was supported by a National Science Foundation Graduate Research Fellowship (DGE-1610403). L.A.S. would like to acknowledge the generous support from the Philanthropic Educational Organization Scholar Awards.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Immuno-engineering”.
References
- [1].Plotkin SA, Vaccines: the fourth century, Clin. Vaccine Immunol 16 (2009) 1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Doherty M, Buchy P, Standaert B, Giaquinto C, Prado-Cohrs D, Vaccine impact: benefits for human health, Vaccine 34 (2016) 6707–6714. [DOI] [PubMed] [Google Scholar]
- [3].Rappuoli R, Miller HI, Falkow S, The intangible value of vaccination, Science 297 (2002) 937–939. [DOI] [PubMed] [Google Scholar]
- [4].WHO, WHO The Top 10 Causes of Death, World Health Organization, 2014. [Google Scholar]
- [5].Irvine DJ, Swartz MA, Szeto GL, Engineering synthetic vaccines using cues from natural immunity, Nat. Mater 12 (2013) 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].WHO, World Health Statistics, World Health Organization, 2012. [Google Scholar]
- [7].Belyakov IM, Ahlers JD, What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J. Immunol 183 (2009) 6883–6892. [DOI] [PubMed] [Google Scholar]
- [8].Holmgren J, Czerkinsky C, Mucosal immunity and vaccines, Nat. Med 11 (2005) S45–S53. [DOI] [PubMed] [Google Scholar]
- [9].Russell-Jones GJ,Oral vaccine delivery, J. Control. Release 65 (2000) 49–54. [DOI] [PubMed] [Google Scholar]
- [10].Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z, Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis, J. Immunol 173 (2004) 6357–6365. [DOI] [PubMed] [Google Scholar]
- [11].Lycke N, Bemark M, Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins, Mucosal Immunol. 3 (2010) 556–566. [DOI] [PubMed] [Google Scholar]
- [12].Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J, Enhancing oral vaccine potency by targeting intestinal M cells, PLoS Pathog. 6 (2010), e1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Newsted D, Fallahi F, Golshani A, Azizi A, Advances and challenges in mucosal adjuvant technology, Vaccine 33 (2015) 2399–2405. [DOI] [PubMed] [Google Scholar]
- [14].Mitragotri S, Burke PA, Langer R, Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies, Nat. Rev. Drug Discov 13 (2014) 655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang S, Liu H, Zhang X, Qian F, Intranasal and oral vaccination with proteinbased antigens: advantages, challenges and formulation strategies, Protein Cell 6 (2015) 480–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, Sovran J, Stephens D, Katz J, Survey of the prevalence of immunization non-compliance due to needle fears in children and adults, Vaccine 30 (2012) 4807–4812. [DOI] [PubMed] [Google Scholar]
- [17].Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger JA, Vaccine hesitancy, Hum. Vaccin. Immunother 9 (2013) 1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dubé E, Gagnon D, MacDonald NE, Strategies intended to address vaccine hesitancy: Review of published reviews, Vaccine 33 (2015) 4191–4203. [DOI] [PubMed] [Google Scholar]
- [19].Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P, Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012, Vaccine 32 (2014) 2150–2159. [DOI] [PubMed] [Google Scholar]
- [20].Gessner BD, Feikin DR, Vaccine preventable disease incidence as a complement to vaccine efficacy for setting vaccine policy, Vaccine 32 (2014) 3133–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marasini N, Skwarczynski M, Toth I, Oral delivery of nanoparticle-based vaccines, Expert Rev. Vaccines 13 (2014) 1361–1376. [DOI] [PubMed] [Google Scholar]
- [22].Webster DE, Gahan ME, Strugnell RA, Wesselingh SL, Advances in oral vaccine delivery options, Am. J. Drug Deliv 1 (2003) 227–240. [Google Scholar]
- [23].Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK, Approaches for enhancing oral bioavailability of peptides and proteins, Int. J. Pharm. 447 (2013) 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].DeVane LC, Principles of pharmacokinetics and pharmacodynamics, in: Schatzberg AF, Nemeroff CB (Eds.), The American Psychiatric Publishing Textbook of Psychopharmacology, American Psychiatric Pub, Washington, DC: 2004, pp. 181–200. [Google Scholar]
- [25].Ibraheem D, Elaissari A, Fessi H, Administration strategies for proteins and peptides, Int. J. Pharm 477 (2014) 578–589. [DOI] [PubMed] [Google Scholar]
- [26].Pavot V, Rochereau N, Genin C, Verrier B, Paul S, New insights in mucosal vaccine development, Vaccine 30 (2012) 142–154. [DOI] [PubMed] [Google Scholar]
- [27].Giudice EL, Campbell JD, Needle-free vaccine delivery, Adv. Drug Deliv. Rev 58 (2006) 68–89. [DOI] [PubMed] [Google Scholar]
- [28].Correia-Pinto JF, Csaba N, Alonso MJ, Vaccine delivery carriers: insights and future perspectives, Int. J. Pharm 440 (2013) 27–38. [DOI] [PubMed] [Google Scholar]
- [29].Davitt CJH, Lavelle EC, Delivery strategies to enhance oral vaccination against enteric infections, Adv. Drug Deliv. Rev 91 (2015) 52–69. [DOI] [PubMed] [Google Scholar]
- [30].Levine MM, Dougan G, Optimism over vaccines administered via mucosal surfaces, Lancet 351 (1998) 1375–1376. [DOI] [PubMed] [Google Scholar]
- [31].Hutton G, Tediosi F, The costs of introducing a malaria vaccine through the expanded program on immunization in Tanzania, Am.J.Trop. Med. Hyg 75 (2006) 119–130. [DOI] [PubMed] [Google Scholar]
- [32].Talaat M, Kandeel A, El-Shoubary W, Bodenschatz C, Khairy I, Oun S, Mahoney FJ, Occupational exposure to needlestick injuries and hepatitis B vaccination coverage among health care workers in Egypt, Am. J. Infect. Control 31 (2003) 469–474. [DOI] [PubMed] [Google Scholar]
- [33].Amorij J-P, Kersten GFA, Saluja V, Tonnis WF, Hinrichs WLJ, Slütter B, Bal SM, Bouwstra JA, Huckriede A, Jiskoot W, Towards tailored vaccine delivery:needs, challenges and perspectives, J. Control. Release 161 (2012) 363–376. [DOI] [PubMed] [Google Scholar]
- [34].Emmanuel J, Ferrer M, Ferrer F, Waste Management and Disposal During the Philippine Follow-Up Measles Campaign 2004, Health Care Without Harm and the Philippine Department of Health, 2004. [Google Scholar]
- [35].Holmgren J, Svennerholm A-M, Vaccines against mucosal infections, Curr. Opin. Immunol 24 (2012) 343–353. [DOI] [PubMed] [Google Scholar]
- [36].McConnell EL, Fadda HM, Basit AW, Gut instincts: explorations in intestinal physiology and drug delivery, Int. J. Pharm 364 (2008) 213–226. [DOI] [PubMed] [Google Scholar]
- [37].Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EEL, Wising C, Johansson MEV, Hansson GC, The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system, Immunol. Rev 260 (2014) 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ensign LM, Cone R, Hanes J, Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers, Adv. Drug Deliv. Rev 64 (2012) 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mudie DM, Amidon GL, Amidon GE, Physiological parameters for oral delivery and in vitro testing, Mol. Pharm 7 (2010) 1388–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mestecky J, Russell MW, Elson CO, Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J. Immunol. 179 (2007) 5633–5638. [DOI] [PubMed] [Google Scholar]
- [41].Ogra PL, Faden H, Welliver RC, Vaccination strategies for mucosal immune responses, Clin. Microbiol. Rev 14 (2001) 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B, Vaccine adjuvants: current challenges and future approaches, J. Pharm. Sci 98 (2009) 1278–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kunisawa J, Kurashima Y, Kiyono H, Gut-associated lymphoid tissues for the development of oral vaccines, Adv. Drug Deliv. Rev 64 (2012) 523–530. [DOI] [PubMed] [Google Scholar]
- [44].Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A, Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium, Mucosal Immunol. 6 (2013) 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pawar VK, Meher JG, Singh Y, Chaurasia M, Surendar Reddy B, Chourasia MK, Targeting of gastrointestinal tract for amended delivery of protein/peptide therapeutics: strategies and industrial perspectives, J. Control. Release 196 (2014) 168–183. [DOI] [PubMed] [Google Scholar]
- [46].Kompella UB, Lee VHL, Delivery systems for penetration enhancement of peptide and protein drugs: design considerations, Adv. Drug Deliv. Rev 46 (2001) 211–245. [DOI] [PubMed] [Google Scholar]
- [47].Peterson LW, Artis D, Intestinal epithelial cells: regulators of barrier function and immune homeostasis, Nat. Rev. Immunol 14 (2014) 141–153. [DOI] [PubMed] [Google Scholar]
- [48].Mowat AM, Agace WW, Regional specialization within the intestinal immune system, Nat. Rev. Immunol 14 (2014) 667–685. [DOI] [PubMed] [Google Scholar]
- [49].Yun Y, Cho YW, Park K, Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery, Adv. Drug Deliv. Rev 65 (2013) 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bruno BJ, Miller GD, Lim CS, Basics and recent advances in peptide and protein drug delivery, Ther. Deliv 4 (2013) 1443–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Garinot M, Fiévez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, Theate I, Freichels H, Jérôme C, Marchand-Brynaert J, Schneider Y-J, Préat V, PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination, J. Control. Release 120 (2007) 195–204. [DOI] [PubMed] [Google Scholar]
- [52].Fievez V, Plapied L, des Rieux A, Pourcelle V, Freichels H, Wascotte V, Vanderhaeghen M-L, Jerôme C, Vanderplasschen A, Marchand-Brynaert J, Schneider Y-J, Préat V, Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination, Eur. J. Pharm. Biopharm 73 (2009) 16–24. [DOI] [PubMed] [Google Scholar]
- [53].Gallo RL, Hooper LV, Epithelial antimicrobial defence of the skin and intestine, Nat. Rev. Immunol 12 (2012) 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lai SK, Wang Y-Y, Hanes J, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues, Adv. Drug Deliv. Rev 61 (2009) 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lai SK, Wang Y-Y, Wirtz D, Hanes J, Micro- and macrorheology of mucus, Adv. Drug Deliv. Rev 61 (2009) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Brayden DJ, Jepson MA, Baird AW, Keynote review: intestinal Peyer’s patch M cells and oral vaccine targeting, Drug Discov. Today 10 (2005) 1145–1157. [DOI] [PubMed] [Google Scholar]
- [57].Marshman E, Booth C, Potten CS, The intestinal epithelial stem cell, BioEssays 24 (2002) 91–98. [DOI] [PubMed] [Google Scholar]
- [58].Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ, Secretion of microbicidal [alpha]-defensins by intestinal Paneth cells in response to bacteria, Nat. Immunol 1 (2000) 113–118. [DOI] [PubMed] [Google Scholar]
- [59].Wilks J, Beilinson H, Golovkina TV, Dual role of commensal bacteria in viral infections, Immunol. Rev 255 (2013) 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yuki Y, Kiyono H, Mucosal vaccines: novel advances in technology and delivery, Expert Rev. Vaccines 8 (2009) 1083–1097. [DOI] [PubMed] [Google Scholar]
- [61].Booth C, Potten CS, Gut instincts: thoughts on intestinal epithelial stem cells, J. Clin. Invest 105 (2000) 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Potten CS, Booth C, Pritchard DM, The intestinal epithelial stem cell: the mucosal governor, Int. J. Exp. Pathol 78 (1997) 219–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gunawardene AR, Corfe BM, Staton CA, Classification and functions of enteroendocrine cells of the lower gastrointestinal tract, Int. J. Exp. Pathol 92 (2011) 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Granger J, Jaladanki RN, Wang J-Y, Granger DN, Intestinal Architecture and Development, Regulation of Gastrointestinal Mucosal Growth, Morgan & Claypool Publishers, US, 2011. [Google Scholar]
- [65].Emmanuel A, Current management of the gastrointestinal complications of systemic sclerosis, Nat. Rev. Gastroenterol. Hepatol 13 (2016) 461–472. [DOI] [PubMed] [Google Scholar]
- [66].Cone RA, Barrier properties of mucus, Adv. Drug Deliv. Rev 61 (2009) 75–85. [DOI] [PubMed] [Google Scholar]
- [67].Atuma C, Strugala V, Allen A, Holm L, The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo, Am. J. Physiol. Gastrointest. Liver Physiol 280 (2001) G922–G929. [DOI] [PubMed] [Google Scholar]
- [68].Yildiz HM, Speciner L, Ozdemir C, Cohen DE, Carrier RL, Food-associated stimuli enhance barrier properties of gastrointestinal mucus, Biomaterials 54 (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dressman JB, Amidon GL, Reppas C, Shah VP, Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms, Pharm. Res 15 (1998) 11–22. [DOI] [PubMed] [Google Scholar]
- [70].Choonara BF, Choonara YE, Kumar P, Bijukumar D, du Toit LC, Pillay V, A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules, Biotechnol. Adv 32 (2014) 1269–1282. [DOI] [PubMed] [Google Scholar]
- [71].Liu Y, Zhang Y, Dong P, An R, Xue C, Ge Y, Wei L, Liang X, Digestion of nucleic acids starts in the stomach, Sci. Rep 5 (2015) 11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mahato RI, Narang AS, Thoma L, Miller DD, Emerging trends in oral delivery of peptide and protein drugs, Crit. Rev. Ther. Drug Carrier Syst 20 (2003) 153–214. [DOI] [PubMed] [Google Scholar]
- [73].Van de Graaff KM, Anatomy and physiology of the gastrointestinal tract, Pediatr. Infect. Dis 5 (1986) S11–S16. [DOI] [PubMed] [Google Scholar]
- [74].Neutra MR, Kozlowski PA, Mucosal vaccines: the promise and the challenge, Nat. Rev. Immunol 6 (2006) 148–158. [DOI] [PubMed] [Google Scholar]
- [75].Milling S, Yrlid U, Cerovic V, MacPherson G, Subsets of migrating intestinal dendritic cells, Immunol. Rev. 234 (2010) 259–267. [DOI] [PubMed] [Google Scholar]
- [76].Mora JR, Iwata M, Eksteen B, Song S-Y, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH, Generation of gut-homing IgA-secreting b cells by intestinal dendritic cells, Science 314 (2006) 1157–1160. [DOI] [PubMed] [Google Scholar]
- [77].Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y, Retinoic acid imprints gut-homing specificity on T cells, Immunity 21 (2004) 527–538. [DOI] [PubMed] [Google Scholar]
- [78].Vela Ramirez JE, Tygrett LT, Hao J, Habte HH, Cho MW, Greenspan NS, Waldschmidt TJ, Narasimhan B, Polyanhydride nanovaccines induce germinal center B cell formation and sustained serum antibody responses, J. Biomed. Nanotechnol 12 (2016) 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lycke N, Recent progress in mucosal vaccine development: potential and limitations, Nat. Rev. Immunol 12 (2012) 592–605. [DOI] [PubMed] [Google Scholar]
- [80].McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H, The mucosal immune system: from fundamental concepts to vaccine development, Vaccine 10 (1992) 75–88. [DOI] [PubMed] [Google Scholar]
- [81].Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S, Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches, Science 323 (2009) 1488–1492. [DOI] [PubMed] [Google Scholar]
- [82].Rappuoli R, Bridging the knowledge gaps in vaccine design, Nat. Biotechnol 25 (2007) 1361–1366. [DOI] [PubMed] [Google Scholar]
- [83].Karch CP, Burkhard P, Vaccine technologies: from whole organisms to rationally designed protein assemblies, Biochem. Pharmacol 120 (2016) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, Strebel PM, Cono J, Wharton M, Orenstein WA, Sutter RW, Vaccine policy changes and epidemiology of poliomyelitis in the United States, JAMA 292 (2004) 1696–1701. [DOI] [PubMed] [Google Scholar]
- [85].Lauring AS, Jones JO, Andino R, Rationalizing the development of live attenuated virus vaccines, Nat. Biotechnol 28 (2010) 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Raghavan S, Hjulström M, Holmgren J, Svennerholm A-M, Protection against experimental Helicobacter pylori infection after immunization with inactivated H. pylori whole-cell vaccines, Infect. Immun 70 (2002) 6383–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Summerton NA, Welch RW, Bondoc L, Yang H-H, Pleune B, Ramachandran N, Harris AM, Bland D, Jackson WJ, Park S, Clements JD, Nabors GS, Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin, Vaccine 28 (2010) 1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].NIAID, Types of vaccines, HHS (Ed.)vaccines.gov, 2013. [Google Scholar]
- [89].Morishita M, Peppas NA, Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today 11 (2006) 905–910. [DOI] [PubMed] [Google Scholar]
- [90].Bobbala S, Hook S, Is there an optimal formulation and delivery strategy for subunit vaccines? Pharm. Res 33 (2016) 2078–2097. [DOI] [PubMed] [Google Scholar]
- [91].Pasetti MF, Simon JK, Sztein MB, Levine MM, Immunology of gut mucosal vaccines, Immunol. Rev 239 (2011) 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ogra PL, Fishaut M, Gallagher MR, Viral vaccination via the mucosal routes, Rev. Infect. Dis 2 (1980) 352–369. [DOI] [PubMed] [Google Scholar]
- [93].Parker EP, Molodecky NA, Pons-Salort M, O’Reilly KM, Grassly NC, Impact of inactivated poliovirus vaccine on mucosal immunity: implications for the polio eradication endgame, Expert Rev. Vaccines 14 (8) (2015) 1113–1123, 10.1586/14760584.2015.1052800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Strebel PM, Sutter RW, Cochi SL, Biellik RJ, Brink EW, Kew OM, Pallansch MA, Orenstein WA, Hinman AR, Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease, Clin. Infect. Dis 14 (1992) 568–579. [DOI] [PubMed] [Google Scholar]
- [95].Ferreccio C, Levine MM, Rodriguez H, Contreras R, Comparative efficacy of two, three, or four doses of TY21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area, J. Infect. Dis 159 (1989) 766–769. [DOI] [PubMed] [Google Scholar]
- [96].Black RE, Levine MM, Ferreccio C, Clements ML, Lanata C, Rooney J, Germanier R, Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Chilean Typhoid Committee, Vaccine 8 (1990) 81–84. [DOI] [PubMed] [Google Scholar]
- [97].Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S, Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine, Vaccine 17 (1999) S22–S27. [DOI] [PubMed] [Google Scholar]
- [98].WHO, WHO | Cholera, World Health Organization, 2016. [Google Scholar]
- [99].Levine MM, Kaper JB, Black RE, Clements ML, New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development, Microbiol. Rev 47 (1983) 510–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sánchez J, Holmgren J, Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea, Curr. Opin. Immunol 17 (2005) 388–398. [DOI] [PubMed] [Google Scholar]
- [101].Holmgren J, Svennerholm AM, Jertborn M, Clemens J, Sack DA, Salenstedt R, Wigzell H, An oral B subunit: whole cell vaccine against cholera, Vaccine 10 (1992) 911–914. [DOI] [PubMed] [Google Scholar]
- [102].WHO, Weekly epidemiological record Relevé épidémiologique hebdomadaire Cholera vaccines, WHO position paper, 85, World Health Organization; 2010, pp. 117–128. [PubMed] [Google Scholar]
- [103].Herzog C, Successful comeback of the single-dose live oral cholera vaccine CVD 103-HgR, Travel Med. Infect. Dis. 14 (2016) 373–377. [DOI] [PubMed] [Google Scholar]
- [104].Freedman DO, Re-born in the USA: another cholera vaccine for travellers, Travel Med. Infect. Dis. 14 (2016) 295–296. [DOI] [PubMed] [Google Scholar]
- [105].Vesikari T, Rotavirus vaccination: a concise review, Clin. Microbiol. Infect. 18 (2012) 57–63. [DOI] [PubMed] [Google Scholar]
- [106].van Hoek AJ, Ngama M, Ismail A, Chuma J, Cheburet S, Mutonga D, Kamau T, Nokes DJ, A cost effectiveness and capacity analysis for the introduction of universal rotavirus vaccination in Kenya: comparison between Rotarix and RotaTeq vaccines, PLoS One 7 (2012), e47511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Top FH Jr., Control of adenovirus acute respiratory disease in U.S. army trainees, Yale J. Biol. Med 48 (1975) 185–195. [PMC free article] [PubMed] [Google Scholar]
- [108].FDA, Summary Basis of Regulatory Action Template Version 2.0 Effective Date: April 6, 2009, Food and Drug Administration, 2009. [Google Scholar]
- [109].WHO, Weekly Epidemiological Record Relevé épidémiologique hebdomadaire Cholera Vaccines, WHO position paper, 85, 2010. 117–128. [PubMed] [Google Scholar]
- [110].McHugh KJ, Guarecuco R, Langer R, Jaklenec A, Single-injection vaccines: progress, challenges, and opportunities, J. Control. Release 219 (2015) 596–609. [DOI] [PubMed] [Google Scholar]
- [111].Narasimhan B, Goodman JT, Vela Ramirez JE, Rational design of targeted nextgeneration carriers for drug and vaccine delivery, Annu. Rev. Biomed. Eng 18 (2016) 25–49. [DOI] [PubMed] [Google Scholar]
- [112].Brannon-Peppas L, Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery, Int. J. Pharm 116 (1995) 1–9. [Google Scholar]
- [113].Singh A, Peppas NA, Hydrogels and scaffolds for immunomodulation, Adv. Mater 26 (2014) 6530–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Peek LJ, Middaugh CR, Berkland C, Nanotechnology in vaccine delivery, Adv. Drug Deliv. Rev 60 (2008) 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V, PLGA-based nanoparticles: an overview of biomedical applications, J. Control. Release 161 (2012) 505–522. [DOI] [PubMed] [Google Scholar]
- [116].Pavot V, Berthet M, Rességuier J, Legaz S, Handké N, Gilbert SC, Paul S, Verrier B, Poly(lactic acid) and poly(lactic-co-glycolic acid) particles as versatile carrier platforms for vaccine delivery, Nanomedicine (Lond.) 9 (2014) 2703–2718. [DOI] [PubMed] [Google Scholar]
- [117].He X-W, Wang F, Jiang L, Li J, Liu S.-k., Xiao Z-Y, Jin X-Q, Zhang Y-N, He Y, Li K, Guo Y-J, Sun S-H, Induction of mucosal and systemic immune response by single-dose oral immunization with biodegradable microparticles containing DNA encoding HBsAg, J. Gen. Virol 86 (2005) 601–610. [DOI] [PubMed] [Google Scholar]
- [118].Nayak B, Panda AK, Ray P, Ray AR, Formulation, characterization and evaluation of rotavirus encapsulated PLA and PLGA particles for oral vaccination, J. Microencapsul 26 (2009) 154–165. [DOI] [PubMed] [Google Scholar]
- [119].Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, Fahmy TM, Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype , Biomaterials 33 (2012) 4957–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Carreño JM, Perez-Shibayama C, Gil-Cruz C, Printz A, Pastelin R, Isibasi A, Chariatte D, Tanoue Y, Lopez-Macias C, Gander B, Ludewig B, PLGA-microencapsulation protects Salmonella typhi outer membrane proteins from acidic degradation and increases their mucosal immunogenicity, Vaccine 34 (2016) 4263–4269. [DOI] [PubMed] [Google Scholar]
- [121].Sarti F, Perera G, Hintzen F, Kotti K, Karageorgiou V, Kammona O, Kiparissides C, Bernkop-Schnürch A, In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A, Biomaterials 32 (2011) 4052–4057. [DOI] [PubMed] [Google Scholar]
- [122].Jain AK, Goyal AK, Mishra N, Vaidya B, Mangal S, Vyas SP, PEG–PLA–PEG block copolymeric nanoparticles for oral immunization against hepatitis B, Int. J. Pharm 387 (2010) 253–262. [DOI] [PubMed] [Google Scholar]
- [123].Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, Kirk J, Eppler B, Klinman DM, Sui Y, Gagnon S, Belyakov IM, Mumper RJ, Berzofsky JA, Large intestine–targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection, Nat. Med 18 (2012) 1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Fu K, Pack DW, Klibanov AM, Langer R, Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres, Pharm. Res 17 (2000) 100–106. [DOI] [PubMed] [Google Scholar]
- [125].van de Weert M, Hennink WE, Jiskoot W, Protein instability in poly(lactic-coglycolic acid) microparticles, Pharm. Res 17 (2000) 1159–1167. [DOI] [PubMed] [Google Scholar]
- [126].Singh M, Kazzaz J, Ugozzoli M, Malyala P, Chesko J, O’Hagan DT, Polylactide-coglycolide microparticles with surface adsorbed antigens as vaccine delivery systems, Curr. Drug Deliv 3 (2006) 115–120. [DOI] [PubMed] [Google Scholar]
- [127].Zhang Q, Zhao Q, Zhang Y, Han N, Hu L, Zhang C, Jiang T, Wang S, Investigation of 3-D ordered materials with a high adsorption capacity for BSA and their potential application as an oral vaccine adjuvant, J. Colloid Interface Sci 434 (2014) 113–121. [DOI] [PubMed] [Google Scholar]
- [128].Mallapragada SK, Narasimhan B, Immunomodulatory biomaterials, Int. J. Pharm 364 (2008) 265–271. [DOI] [PubMed] [Google Scholar]
- [129].Salman HH, Irache JM, Gamazo C, Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination, Vaccine 27 (2009) 4784–4790. [DOI] [PubMed] [Google Scholar]
- [130].Irache JM, Salman HH, Gomez S, Espuelas S, Gamazo C, Poly(anhydride) nanoparticles as adjuvants for mucosal vaccination, Front. Biosci. (Schol. Ed.) 2 (2010) 876–890. [DOI] [PubMed] [Google Scholar]
- [131].Tamayo I, Irache JM, Mansilla C, Ochoa-Repáraz J, Lasarte JJ, Gamazo C, Poly(anhydride) nanoparticles act as active Th1 adjuvants through toll-like receptor exploitation, Clin. Vaccine Immunol 17 (2010) 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Camacho AI, Irache JM, de Souza J, Sánchez-Gómez S, Gamazo C, Nanoparticlebased vaccine for mucosal protection against Shigella flexneri in mice, Vaccine 31 (2013) 3288–3294. [DOI] [PubMed] [Google Scholar]
- [133].Durán-Lobato M, Carrillo-Conde B, Khairandish Y, Peppas NA, Surfacemodified P(HEMA-co-MAA) nanogel carriers for oral vaccine delivery: design, characterization, and in vitro targeting evaluation, Biomacromolecules 15 (2014) 2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Yoshida M, Kamei N, Muto K, Kunisawa J, Takayama K, Peppas NA, TakedaMorishita M, Complexation hydrogels as potential carriers in oral vaccine delivery systems, Eur. J. Pharm. Biopharm (2017) 138–142. [DOI] [PubMed] [Google Scholar]
- [135].Bowman K, Leong KW, Chitosan nanoparticles for oral drug and gene delivery, Int. J. Nanomedicine 1 (2006) 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Yeh T-H, Hsu L-W, Tseng MT, Lee P-L, Sonjae K, Ho Y-C, Sung H-W, Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening, Biomaterials 32 (2011) 6164–6173. [DOI] [PubMed] [Google Scholar]
- [137].Jain S, Sharma RK, Vyas SP, Chitosan nanoparticles encapsulated vesicular systems for oral immunization: preparation, in-vitro and in-vivo characterization, J. Pharm. Pharmacol 58 (2006) 303–310. [DOI] [PubMed] [Google Scholar]
- [138].Harde H, Agrawal AK, Jain S, Development of stabilized glucomannosylated chitosan nanoparticles using tandem crosslinking method for oral vaccine delivery, Nanomedicine 9 (2014) 2511–2529. [DOI] [PubMed] [Google Scholar]
- [139].Borges O, Tavares J, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A, Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles, Eur. J. Pharm. Sci 32 (2007) 278–290. [DOI] [PubMed] [Google Scholar]
- [140].Biswas S, Chattopadhyay M, Sen KK, Saha MK, Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice, Carbohydr. Polym. 121 (2015) 403–410. [DOI] [PubMed] [Google Scholar]
- [141].Schwendener RA, Liposomes as vaccine delivery systems: a review of the recent advances, Ther. Adv. Vaccines 2 (2014) 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K, Liposome: classification, preparation, and applications, Nanoscale Res. Lett 8 (2013) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Liu J, Wu J, Wang B, Zeng S, Qi F, Lu C, Kimura Y, Liu B, Oral vaccination with a liposome-encapsulated influenza DNA vaccine protects mice against respiratory challenge infection, J. Med. Virol 86 (2014) 886–894. [DOI] [PubMed] [Google Scholar]
- [144].Pang Y, Zhang Y, Wang H, Jin J, Piao J, Piao J, Liu Q, Li W, Reduction of Salmonella enteritidis number after infections by immunization of liposome-associated recombinant SefA, Avian Dis. 57 (2013) 627–633. [DOI] [PubMed] [Google Scholar]
- [145].Wang D, Xu J, Feng Y, Liu Y, McHenga SSS, Shan F, Sasaki J.-i., Lu C, Liposomal oral DNA vaccine (mycobacterium DNA) elicits immune response, Vaccine 28 (2010) 3134–3142. [DOI] [PubMed] [Google Scholar]
- [146].Torchilin VP, Recent advances with liposomes as pharmaceutical carriers, Nat. Rev. Drug Discov 4 (2005) 145–160. [DOI] [PubMed] [Google Scholar]
- [147].Chikh G, Schutze-Redelmeir M-P, Liposomal delivery of CTL epitopes to dendritic cells, Biosci. Rep 22 (2002) 339–353. [DOI] [PubMed] [Google Scholar]
- [148].Copland MJ, Baird MA, Rades T, McKenzie JL, Becker B, Reck F, Tyler PC, Davies NM, Liposomal delivery of antigen to human dendritic cells, Vaccine 21 (2003) 883–890. [DOI] [PubMed] [Google Scholar]
- [149].Wang N, Wang T, Zhang M, Chen R, Niu R, Deng Y, Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system, Eur. J. Pharm. Biopharm 88 (2014) 194–206. [DOI] [PubMed] [Google Scholar]
- [150].Liau JJ, Hook S, Prestidge CA, Barnes TJ, A lipid based multi-compartmental system: liposomes-in-double emulsion for oral vaccine delivery, Eur. J. Pharm. Biopharm 97 (Part A) (2015) 15–21. [DOI] [PubMed] [Google Scholar]
- [151].Gupta PN, Vyas SP, Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization, Colloids Surf. B: Biointerfaces 82 (2011) 118–125. [DOI] [PubMed] [Google Scholar]
- [152].Conacher M, Alexander J, Brewer JM, Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes), Vaccine 19 (2001) 2965–2974. [DOI] [PubMed] [Google Scholar]
- [153].Bozzuto G, Molinari A, Liposomes as nanomedical devices, Int. J. Nanomedicine 10 (2015) 975–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Shukla A, Katare OP, Singh B, Vyas SP, M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes, Int. J. Pharm 385 (2010) 47–52. [DOI] [PubMed] [Google Scholar]
- [155].Mann JFS, Scales HE, Shakir E, Alexander J, Carter KC, Mullen AB, Ferro VA, Oral delivery of tetanus toxoid using vesicles containing bile salts (bilosomes) induces significant systemic and mucosal immunity, Methods 38 (2006) 90–95. [DOI] [PubMed] [Google Scholar]
- [156].Jain S, Harde H, Indulkar A, Agrawal AK, Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration, Nanomed. Nanotechnol. Biol. Med 10 (2014) 431–440. [DOI] [PubMed] [Google Scholar]
- [157].Shukla A, Mishra V, Kesharwani P, Bilosomes in the context of oral immunization: development, challenges and opportunities, Drug Discov. Today 21 (2016) 888–899. [DOI] [PubMed] [Google Scholar]
- [158].Wilkhu JS, McNeil SE, Anderson DE, Perrie Y, Characterization and optimization of bilosomes for oral vaccine delivery, J. Drug Target 21 (2013) 291–299. [DOI] [PubMed] [Google Scholar]
- [159].Mann JFS, Ferro VA, Mullen AB, Tetley L, Mullen M, Carter KC, Alexander J, Stimson WH, Optimisation of a lipid based oral delivery system containing A/Panama influenza haemagglutinin, Vaccine 22 (2004) 2425–2429. [DOI] [PubMed] [Google Scholar]
- [160].Premanand B, Prabakaran M, Kiener TK, Kwang J, Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice, PLoS One 8 (2013), e55536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Singh P, Prabakaran D, Jain S, Mishra V, Jaganathan KS, Vyas SP, Cholera toxin B subunit conjugated bile salt stabilized vesicles (bilosomes) for oral immunization, Int. J. Pharm 278 (2004) 379–390. [DOI] [PubMed] [Google Scholar]
- [162].Sanders MT, Brown LE, Deliyannis G, Pearse MJ, ISCOMTM-based vaccines: the second decade, Immunol. Cell Biol 83 (2005) 119–128. [DOI] [PubMed] [Google Scholar]
- [163].Petrovsky N, Aguilar JC, Vaccine adjuvants: current state and future trends, Immunol. Cell Biol 82 (2004) 488–496. [DOI] [PubMed] [Google Scholar]
- [164].Morein B, Simons K, Subunit vaccines against enveloped viruses: virosomes, micelles and other protein complexes, Vaccine 3 (1985) 83–93. [DOI] [PubMed] [Google Scholar]
- [165].Gregory A, Williamson D, Titball R, Vaccine delivery using nanoparticles, Front. Cell. Infect. Microbiol 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Hu K-F, Lövgren-Bengtsson K, Morein B, Immunostimulating complexes (ISCOMs) for nasal vaccination, Adv. Drug Deliv. Rev 51 (2001) 149–159. [DOI] [PubMed] [Google Scholar]
- [167].Tatsis N, Ertl HCJ, Adenoviruses as vaccine vectors, Mol. Ther 10 (2004) 616–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Peters W, Brandl JR, Lindbloom JD, Martinez CJ, Scallan CD, Trager GR, Tingley DW, Kabongo ML, Tucker SN, Oral administration of an adenovirus vector encoding both an avian influenza A hemagglutinin and a TLR3 ligand induces antigen specific granzyme B and IFN-γ T cell responses in humans, Vaccine 31 (2013) 1752–1758. [DOI] [PubMed] [Google Scholar]
- [169].Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, Ervin JE, Greenberg RN, Strout C, Treanor JJ, Webby R, Wright PF, Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study, Lancet Infect. Dis 13 (2013) 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Thippeshappa R, Tian B, Cleveland B, Guo W, Polacino P, Hu S-L, Oral immunization with recombinant vaccinia virus prime and intramuscular protein boost provides protection against intrarectal Simian-human immunodeficiency virus challenge in Macaques, Clin. Vaccine Immunol 23 (2016) 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Khurana S, Coyle EM, Manischewitz J, King LR, Ishioka G, Alexander J, Smith J, Gurwith M, Golding H, Oral priming with replicating adenovirus serotype 4 followed by subunit H5N1 vaccine boost promotes antibody affinity maturation and expands H5N1 cross-clade neutralization, PLoS One 10 (2015), e0115476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Zhou D, Cun A, Li Y, Xiang Z, Ertl HCJ, A chimpanzee-origin adenovirus vector expressing the rabies virus glycoprotein as an oral vaccine against inhalation infection with rabies virus, Mol. Ther 14 (2006) 662–672. [DOI] [PubMed] [Google Scholar]
- [173].Kim L, Martinez CJ, Hodgson KA, Trager GR, Brandl JR, Sandefer EP, Doll WJ, Liebowitz D, Tucker SN, Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule, Sci. Rep 6 (2016) 37295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Xiang ZQ, Yang Y, Wilson JM, Ertl HCJ, A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier, Virology 219 (1996) 220–227. [DOI] [PubMed] [Google Scholar]
- [175].Prevec L, Campbell JB, Christie BS, Belbeck L, Graham FL, A recombinant human adenovirus vaccine against rabies, J. Infect. Dis 161 (1990) 27–30. [DOI] [PubMed] [Google Scholar]
- [176].Casimiro DR, Chen L, Fu T-M, Evans RK, Caulfield MJ, Davies M-E, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu D-M, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW, Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene, J. Virol 77 (2003) 6305–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Albanese A, Tang PS, Chan WCW, The effect of nanoparticle size, shape, and surface chemistry on biological systems, Annu. Rev. Biomed. Eng 14 (2012) 1–16. [DOI] [PubMed] [Google Scholar]
- [178].Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S, Shape and sizedependent immune response to antigen-carrying nanoparticles, J.Control. Release 220 (Part A) (2015) 141–148. [DOI] [PubMed] [Google Scholar]
- [179].Bachmann MF, Jennings GT, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns, Nat. Rev. Immunol 10 (2010) 787–796. [DOI] [PubMed] [Google Scholar]
- [180].Cruz LJ, Tacken PJ, Rueda F, Domingo JC, Albericio F, Figdor CG, Chapter eight – targeting nanoparticles to dendritic cells for immunotherapy, Methods Enzymol. (2012) 143–163. [DOI] [PubMed] [Google Scholar]
- [181].Devriendt B, De Geest BG, Goddeeris BM, Cox E, Crossing the barrier: Targeting epithelial receptors for enhanced oral vaccine delivery, J. Control. Release 160 (2012) 431–439. [DOI] [PubMed] [Google Scholar]
- [182].Kim WS, Mooney DJ, Arany PR, Lee K, Huebsch N, Kim J, Adipose tissue engineering using injectable, oxidized alginate hydrogels, Tissue Eng. A 18 (2012) 737–743. [DOI] [PubMed] [Google Scholar]
- [183].Ann Clark M, Blair H, Liang L, Brey RN, Brayden D, Hirst BH, Targeting polymerised liposome vaccine carriers to intestinal M cells, Vaccine 20 (2001) 208–217. [DOI] [PubMed] [Google Scholar]
- [184].Roth-Walter F, Bohle B, Schöll I, Untersmayr E, Scheiner O, Boltz-Nitulescu G, Gabor F, Brayden DJ, Jensen-Jarolim E, Targeting antigens to murine and human Mcells with Aleuria aurantia lectin-functionalized microparticles, Immunol. Lett 100 (2005) 182–188. [DOI] [PubMed] [Google Scholar]
- [185].Gupta PN, Khatri K, Goyal AK, Mishra N, Vyas SP, M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B, J. Drug Target 15 (2007) 701–713. [DOI] [PubMed] [Google Scholar]
- [186].Lavelle EC, O’Hagan DT, Delivery systems and adjuvants for oral vaccines, Expert Opin. Drug Deliv 3 (2006) 747–762. [DOI] [PubMed] [Google Scholar]
- [187].Lo DD, Ling J, Holly Eckelhoefer A, M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses, BMC Biotechnol 12 (2012) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Nochi T, Yuki Y, Matsumura A, Mejima M, Terahara K, Kim D-Y, Fukuyama S, Iwatsuki-Horimoto K, Kawaoka Y, Kohda T, Kozaki S, Igarashi O, Kiyono H, A novel M cell–specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses, J. Exp. Med 204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, Yabashi A, Waguri S, Nakato G, Kimura S, Murakami T, Iimura M, Hamura K, Fukuoka S-I, Lowe AW, Itoh K, Kiyono H, Ohno H, Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response, Nature 462 (2009) 226–230. [DOI] [PubMed] [Google Scholar]
- [190].Shima H, Watanabe T, Fukuda S, Fukuoka S-I, Ohara O, Ohno H, A novel mucosal vaccine targeting Peyer’s patch M cells induces protective antigen-specific IgA responses, Int. Immunol 26 (2014) 619–625. [DOI] [PubMed] [Google Scholar]
- [191].Hussain N, Florence AT, Utilizing bacterial mechanisms of epithelial cell entry: invasin-induced oral uptake of latex nanoparticles, Pharm. Res 15 (1998) 153–156. [DOI] [PubMed] [Google Scholar]
- [192].Critchley-Thorne RJ, Stagg AJ, Vassaux G, Recombinant Escherichia coli expressing invasin targets the Peyer’s patches: the basis for a bacterial formulation for oral vaccination, Mol. Ther 14 (2006) 183–191. [DOI] [PubMed] [Google Scholar]
- [193].Rynda A, Maddaloni M, Mierzejewska D, Ochoa-Repáraz J, Maslanka T, Crist K, Riccardi C, Barszczewska B, Fujihashi K, McGhee JR, Pascual DW, Low-dose tolerance is mediated by the microfold cell ligand, reovirus protein sigma1, J. Immunol 180 (2008) 5187–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].des Rieux A, Fievez V, Théate I, Mast J, Préat V, Schneider Y-J, An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells, Eur. J. Pharm. Sci 30 (2007) 380–391. [DOI] [PubMed] [Google Scholar]
- [195].Antunes F, Andrade F, Araújo F, Ferreira D, Sarmento B, Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs, Eur. J. Pharm. Biopharm 83 (2013) 427–435. [DOI] [PubMed] [Google Scholar]
- [196].Schimpel C, Teubl B, Absenger M, Meindl C, Fröhlich E, Leitinger G, Zimmer A, Roblegg E, Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles, Mol. Pharm 11 (2014) 808–818. [DOI] [PubMed] [Google Scholar]
- [197].Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR, Human intestinal M cells display the Sialyl Lewis A antigen, Infect. Immun 67 (1999) 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chen H, Torchilin V, Langer R, Lectin-bearing polymerized liposomes as potential oral vaccine carriers, Pharm. Res 13 (1996) 1378–1383. [DOI] [PubMed] [Google Scholar]
- [199].Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM, Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines, Trends Biotechnol. 29 (2011) 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Cario E, Gerken G, Podolsky DK, Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C, Gastroenterology 127 (2004) 224–238. [DOI] [PubMed] [Google Scholar]
- [201].Chabot S, Wagner JS, Farrant S, Neutra MR, TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium, J. Immunol 176 (2006) 4275–4283. [DOI] [PubMed] [Google Scholar]
- [202].Chabot SM, Chernin TS, Shawi M, Wagner J, Farrant S, Burt DS, Cyr S, Neutra MR, TLR2 activation by proteosomes promotes uptake of particulate vaccines at mucosal surfaces, Vaccine 25 (2007) 5348–5358. [DOI] [PubMed] [Google Scholar]
- [203].Harandi AM, Holmgren J, CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections, Curr. Opin. Investig. Drugs 5 (2004) 141–145. [PubMed] [Google Scholar]
- [204].Honko AN, Mizel SB, Effects of flagellin on innate and adaptive immunity, Immunol. Res 33 (2005) 083–102. [DOI] [PubMed] [Google Scholar]
- [205].Franchi L, Amer A, Body-Malapel M, Kanneganti T-D, Özören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Núñez G, Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages, Nat. Immunol 7 (2006) 576–582. [DOI] [PubMed] [Google Scholar]
- [206].Uematsu S, Fujimoto K, Jang MH, Yang B-G, Jung Y-J, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S, Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5, Nat. Immunol 9 (2008) 769–776. [DOI] [PubMed] [Google Scholar]
- [207].Arigita C, Luijkx T, Jiskoot W, Poelen M, Hennink WE, Crommelin DJA, Ley PVD, Els CV, Kersten GFA, Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives, Vaccine 23 (2005) 5091–5098. [DOI] [PubMed] [Google Scholar]
- [208].Jain V, Sahu R, Misra-Bhattacharya S, Vyas SP, Kohli D, Enhancement of T-helper type I immune responses against hepatitis B surface antigen by LPS derivatives adjuvanted liposomes delivery system, J. Drug Target 16 (2008) 706–715. [DOI] [PubMed] [Google Scholar]
- [209].Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Saltzman WM, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM, Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy, Vaccine 27 (2009) 3013–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Casella CR, Mitchell TC, Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant, Cell. Mol. Life Sci 65 (2008) 3231–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Mann JFS, Acevedo R, Campo JD, Pérez O, Ferro VA, Delivery systems: a vaccine strategy for overcoming mucosal tolerance? Expert Rev. Vaccines 8 (2009) 103–112. [DOI] [PubMed] [Google Scholar]
- [212].Faria AMC, Weiner HL, Oral tolerance, Immunol. Rev 206 (2005) 232–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Humphrey JH, Child undernutrition, tropical enteropathy, toilets, and handwashing, Lancet 374 (2009) 1032–1035. [DOI] [PubMed] [Google Scholar]
- [214].Korpe PS, Petri WA Jr, Environmental enteropathy: critical implications of a poorly understood condition, Trends Mol. Med 18 (2012) 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Prendergast A, Kelly P, Enteropathies in the developing world: neglected effects on global health, Am.J.Trop. Med. Hyg 86 (2012) 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]