Abstract
Researchers have long debated how salient-but-irrelevant features guide visual attention. Pure stimulus-driven theories claim that salient stimuli automatically capture attention irrespective of goals, whereas pure goal-driven theories propose that an individual’s attentional control settings determine whether salient stimuli capture attention. However, recent studies have suggested a hybrid model in which salient stimuli attract visual attention but can be actively suppressed by top-down attentional mechanisms. Support for this hybrid model has primarily come from event-related potential (ERP) studies demonstrating that salient stimuli which fail to capture attention also elicit a distractor positivity (PD) component, a putative neural index of suppression. Other support comes from a handful of behavioral studies showing that processing at the salient locations is inhibited compared to other locations. The current study was designed to link the behavioral and neural evidence by combining ERP recordings with an experimental paradigm that provides a behavioral measure of suppression. We found that when a salient distractor item elicited the PD component, processing at the location of this distractor was suppressed below baseline levels. Furthermore, the magnitude of behavioral suppression and the magnitude of the PD component covaried across participants. These findings provide a crucial connection between the behavioral and neural measures of suppression, which opens the door to using the PD component to assess the timing and neural substrates of the behaviorally observed suppression.
Keywords: attentional capture, visual attention, inhibition, suppression, event-relalted potentials
Salient signals, such as a lone red rose in a field of yellow tulips, seem to automatically “pop out” from visual scenes. The phenomenological experience is clear: the uniquely colored flower seems to capture our attention automatically. Indeed, industrialized societies use salient stimuli such as neon traffic cones and flashing beacons as warning devices, implicitly assuming that these salient signals will overpower other stimuli and guide our attentional resources to potential dangers. However, formal research on attention capture paints a more divided picture.
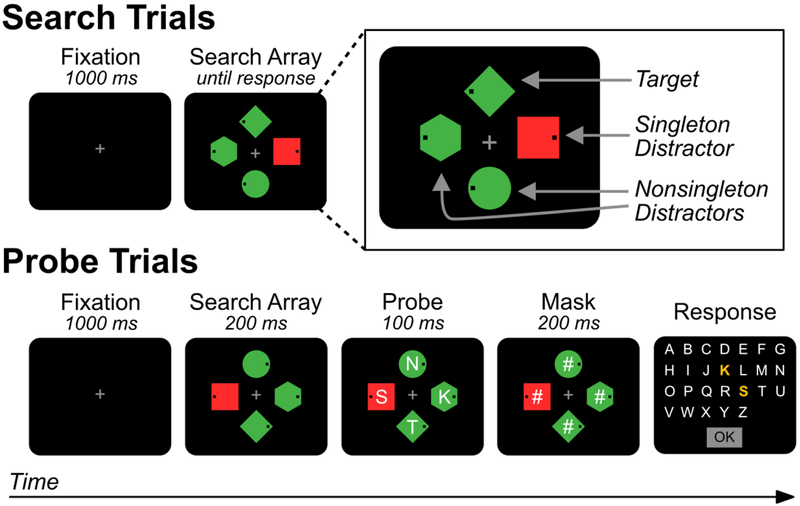
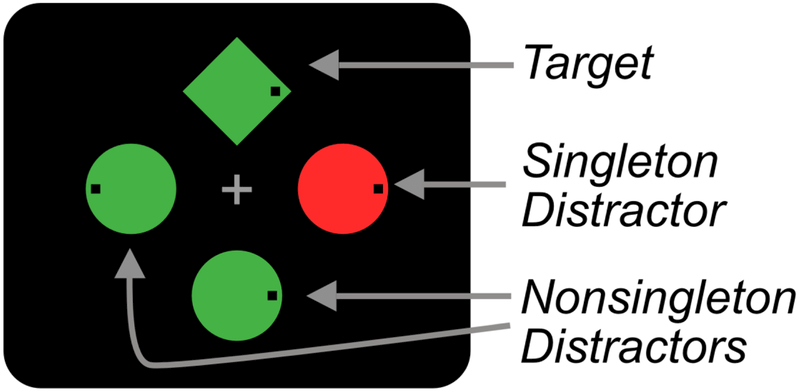
Traditionally, the field of attentional capture has been divided into two theoretical camps. According to stimulus-driven theories, salient stimuli have an automatic ability to capture visual attention, regardless of our current goals (Theeuwes, 1992, 2010). For example, Theeuwes (1992) had participants search displays for a circle target amongst distractor diamonds and report the orientation of a line inside the target. On half of trials, one of the distractor objects was a color singleton, a distractor that was drawn in a unique color; the other objects were all a different, uniform color (see Figure 1). Participants were slower on trials where the singleton was present than when it was absent (a singleton presence cost). This was initially taken as support for stimulus-driven theories – even though the singleton was task irrelevant, it interfered with target identification, presumably because it attracted attention.
Figure 1.
Schematic illustration of the capture-probe paradigm used in Experiment 1. On search trials, participants search for a target shape (e.g., green diamond) in a display of heterogeneous shapes and respond (via a speeded button press) to indicate whether a small black dot is on the left or right side of the target. On infrequent probe trials, an array of probe letters is superimposed on the search array for a brief period. Participants then report (via unspeeded mouse click) as many letters as they can recall. No response is made to the search array on probe trials.
Goal-driven theories, however, claim that salient stimuli do not automatically capture attention – instead, attention is captured only by stimuli that match features that are relevant for performing the task (Folk, Remington, & Johnston, 1992; Lien, Ruthruff, Goodin, & Remington, 2008). In other words, attentional guidance is dictated entirely by our attentional control settings. For example, if the target is a shape singleton (e.g., a lone diamond amongst multiple circles), this may encourage participants to look for singletons in general (called singleton-detection mode; Bacon & Egeth, 1994; Pashler, 1988, Experiment 7). Indeed, when participants are encouraged to develop an attentional set for a specific feature value and not search for singletons (called feature search mode), capture effects from irrelevant singletons disappear.
The Signal Suppression Hypothesis
We have proposed a potential reconciliation called the signal suppression hypothesis (Gaspelin & Luck, in press; Sawaki & Luck, 2010). According to this model, salient stimuli will capture attention unless they are suppressed by some top-down mechanism. This model can account for cases of capture by salient stimuli: If unsuppressed, salient stimuli will automatically capture attention. In this way, the signal suppression hypothesis is consistent with stimulus-driven theories but incompatible with pure goal-driven theories, which predict that capture will never occur unless a stimulus matches the attentional control settings.1 However, this model can also account for cases where salient stimuli fail to capture attention: If suppression succeeds, then attention can successfully avoid salient stimuli. In this way, the signal suppression hypothesis is consistent with goal-driven theories but incompatible with pure stimulus-driven theories, which predict that the initial shift of attention is determined solely by bottom-up salience.
Early support for the signal suppression hypothesis came from event-related potential (ERP) studies using the N2-posterior-contralateral (N2pc) component and the distractor positivity (PD) component. The N2pc component is an index of the covert deployment of visual attention (Eimer, 1996; Luck, 2012; Luck & Hillyard, 1994a, 1994b; Woodman & Luck, 2003), that appears as a negative-going deflection that is larger over the hemisphere contralateral to the attended location compared to the ipsilateral hemisphere. The N2pc typically occurs 200–300 ms after stimulus presentation, and combined data from magnetoencephalography, EEG, and functional magnetic resonance imaging indicate that the N2pc is generated in extrastriate areas of visual cortex (Hopf et al., 2000, 2006). The PD component is much like the inverse of the N2pc component: instead of presenting itself as a negative-going deflection contralateral to a to-be-attended item, the PD presents itself as a positive-going deflection contralateral to a to-be-suppressed item (Hickey, Di Lollo, & McDonald, 2009). Its generator source is currently unknown, but its scalp distribution is similar to that of the N2pc component.
Several studies suggest that the PD indexes covert suppression of salient stimuli. The PD often appears exclusively in response to to-be-ignored salient items in the absence of an N2pc component (e.g., Gaspar, Christie, Prime, Jolicoeur, & McDonald, 2016; Gaspar & McDonald, 2014; Jannati, Gaspar, & McDonald, 2013; Sawaki & Luck, 2010, 2011). The PD also has a larger amplitude on fast-response trials than slow-response trials (e.g., Gaspar & McDonald, 2014; Jannati, Gaspar, & McDonald, 2013; McDonald, Green, Jannati, & Di Lollo, 2013), suggesting that suppression of a salient item allows faster detection of the target stimulus. Another study of oculomotor capture found that a PD was present when the participant successfully avoided making an eye movement to a salient distractor but was absent when the distractor was fixated (Weaver, van Zoest, & Hickey, 2017), consistent with the hypothesis that the PD component reflects a suppressive process. However, eye tracking studies cannot determine whether covert attention was suppressed at the location of the salient distractor; it is possible that the salient distractor captured covert attention but not overt attention (see Gaspelin et al., 2017; Geng, 2014). In summary, there are many reasons to suspect that the PD reflects suppression of salient items. But no studies have directly shown that items eliciting a PD are covertly inhibited below baseline levels of processing.
The Current Study
The purpose of the current study was to provide definitive evidence that the PD component is associated with the inhibition of salient items. To accomplish this, we used a behavioral task — called the capture-probe paradigm – that makes it possible to compare processing at the singleton location to an appropriate baseline (Gaspelin, Leonard, & Luck, 2015). As illustrated in Figure 1, on most trials of this paradigm (search trials), participants perform a typical attentional capture task — they search for a target shape amongst heterogeneous shapes and make a speeded response to indicate the location of a dot inside the target. One of the distractors may be a color singleton. On a randomly intermixed subset of trials (probe trials), letters appear briefly inside each search location and then disappear. Participants attempt to report as many letters as possible on these trials (and are not required to respond to the search target). The probability that the probe letter at a given location is reported is used as an index of the level of processing at that location – participants should be more likely to report the letter at an attended location than to report the letter at an unattended location. The probability of reporting a letter at a nonsingleton distractor location provides a baseline level of performance, and the main question is whether the probability of reporting the letter at the color singleton distractor is reduced relative to this baseline. Consistent with the signal suppression hypothesis, experiments using this method have found that participants are less likely to report probe letters at the singleton distractor location than at a given nonsingleton distractor location (a probe suppression effect; Gaspelin et al., 2015; Gaspelin & Luck, in press). This indicates that the color singleton was suppressed below the most relevant baseline level of processing.
Experiment 1 simply determined whether the same conditions that result in a PD component also produce behavioral evidence of suppression. Specifically, participants performed the capture-probe paradigm, and we concurrently recorded ERPs to determine whether salient distractors produce both a PD component and a behavioral probe suppression effect. Experiment 2 provided an even stronger test of the hypothesis that the PD and the behavioral probe suppression effect reflect the same underlying neural mechanism by assessing whether these two measures were correlated across participants. In other words, we assessed whether individuals with larger PD amplitudes also had larger probe suppression effects 2 Experiment 3 ruled out a prominent alternative account which proposes that the early PD-like lateralized positivities may merely reflect an automatic salience detection process (called a Ppc effect).
Experiment 1
Participants performed a capture-probe paradigm similar to that used by Gaspelin et al. (2015). To discourage capture by color singletons, participants searched for a specific shape (e.g., diamond or circle) amongst heterogeneously shaped distractors. This prevented the target shape from popping out, thereby discouraging participants from using singleton detection mode, which has been previously shown to lead to the capture of attention by irrelevant singletons (Bacon & Egeth, 1994; Theeuwes, 1992). We concurrently measured ERPs while participants performed this task so that we could assess the N2pc and PD activity elicited by the target and by the color singleton.
The signal suppression hypothesis makes two clear predictions. First, on probe trials, participants should be less likely to report letters at the singleton distractor location than at nonsingleton distractor locations — a probe suppression effect (Gaspelin et al., 2015). Second, on search trials, the target shapes should elicit an N2pc component, indicating attentional allocation, whereas the color singletons should instead elicit a PD component. The combination of the behavioral and ERP measures will make it possible to determine whether the color singletons produce both a behavioral probe suppression effect and a PD component.
We also asked whether these two effects were correlated, with a greater PD in participants who exhibited a larger probe suppression effect. However, this experiment was not designed to produce robust participant-by-participant correlations, so the correlations should be treated with caution. Experiment 2 was designed to assess this correlation with greater power.
Method
Participants.
The final set of participants consisted of 20 University of California, Davis students who were paid for participating. Our sample size was determined on the basis of our prior behavioral research with the capture-probe paradigm (Gaspelin et al., 2015) and previous PD studies from our laboratory (Sawaki & Luck, 2010). This sample size was designed to provide adequate power to detect the presence of both the behavioral and ERP measures of suppression. However, the power for detecting a correlation between these measures was unknown because we had no way of estimating the magnitude of this correlation. All participants had normal/corrected-to-normal visual acuity and normal color vision as assessed by an Ishihara color vision test. The mean age was 20.8 years, and 17 of the 20 participants were female.
Stimuli & Procedure.
Stimuli were presented using PsychToolbox (Brainard, 1997) on a Hewlett-Packard ZR2440w LCD monitor with a black background that was placed at a viewing distance of 100 cm. A photosensor was used to measure the timing delay of the monitor (32 ms), and the event codes were shifted offline to compensate for this delay.
Figure 1 shows a schematic of the stimuli used. Each search display contained four items distributed at equal distances around a notional circle with a radius of 1 2°. The individual stimuli were a diamond (0.9° by 0.9°), a circle (1.1° diameter), a hexagon (1.1° in width and height), and a square (1.0° by 1.0°) drawn in red (23.3 cd/m2, x = .65, y = .34) or green (23.3 cd/m2, x = .29 , y = .63). Each shape contained a 0.1° black dot that was located 0.2° from the either the left or right edge of the shape. On probe trials, an uppercase letter (0.6° tall) was presented in white (132.0 cd/m2) Arial typeface at the center of each shape. A subsequent response screen displayed all letters in the English alphabet. A gray fixation cross (23.3 cd/m2, 0.5° by 0.5°) was continuously visible except during the probe screen and intertrial interval.
The search target was defined as the circle for half of the participants and the diamond for the other half. On search trials (70% of trials), the task was to report whether the black dot was on the left or right side of the target shape (by clicking either the left or right button on the mouse, using their preferred hand). The location of the target and the side of target on which the dot appeared varied randomly. The color of the target was counterbalanced across participants (red for half of the participants and green for the other half). All search items were the same color as the target on half of trials. On the remaining trials, two of the distractors were the same color as the target, and the remaining distractor (the color singleton) was the other color. Participants were explicitly told the color and shape of the target at the beginning of the experiment. They were also told the color of the singleton color and were instructed to ignore it.
Search trials began with the presenation of a blank screen for 500 ms followed by a fixation screen for 1000 ms. Next, the search array appeared until the participant responded. If the participant took too long to repsond (more than 2,000 ms), a time-out display (“Too Slow”) appeared for 500 ms. If the response was incorrect, a 200-Hz tone sounded for 500 ms. The interval between successive trials varied randomly between 0 and 500 ms.
On probe trials (30% of trials), one letter flashed briefly at each search location, and participants attempted to recall as many letters as possible when the response display appeared. The letters on a given trial were selected at random, without replacement, from the 26 letters of the English alphabet. Probe trials began just as search trials – with the presenation of a blank screen for 500 ms followed by a fixation screen for 1000 ms. Next, the search array appeared for 200 ms, followed immediately by a letter superimposed on each shape for 100 ms (the probe array). Then, the probe letters were replaced with masks (“#” characters) for 200 ms, which served to minimize shifts of attention within iconic memory following the offset of the probe array. Finally, the response display appeared; it disappeared when the participant clicked OK.
Participants first practiced the search task alone for one block of 144 trials. Then, they practiced the combined capture-probe paradigm for one block of 144 trials. The main experiment consisted of 6 blocks of 144 trials, for a total of 260 probe trials and 604 search trials. Half of these trials contained a color singleton. Each block was divided into 4 mini-blocks of 36 trials, with a 10-s rest break between mini-blocks. A longer break was provided at the end of each full block. Participants received block-by-block feedback on their mean RT and accuracy. We would like to emphasize that reliable results in this paradigm require extensive practice with the search task by itself and a limited proportion of probe trials (Gaspelin et al., 2015).
Trials were excluded from the probe analyses if the two or more preceding trials were also probe trials (10.8% of probe trials). Repeated probing could temporarily disrupt participants’ attentional set for the target. This would artificially distort probe report accuracy.
Electrophysiological Recording and Analysis.
The EEG was recorded using active Ag/AgCl electrodes (Brain Products actiCHamp system) from the left and right mastoids and 32 scalp sites (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P9, P7, P5, P3, P1, Pz, P2, P4, P6, P8, P10, PO7, PO3, POz, PO4, PO8, O1, Oz, O2, and Iz, according to the modified 10–20 system; American Electroencephalographic Society, 1994). To detect eye movements and blinks, the electrooculogram (EOG) was recorded from electrodes placed at the outer canthi of each eye and below the right eye. All signals were recorded in single-ended mode using a customized version of the PyCorder recording software and then referenced offline. The EEG was filtered online with a cascaded integrator-comb antialiasing filter with a half-power cutoff at 130 Hz and then digitized with a 500 Hz sampling rate.
All analyses after data acquisition were conducted using ERPLAB Toolbox (Lopez-Calderon & Luck, 2014) and EEGLAB Toolbox (Delorme & Makeig, 2004). The EEG signals were referenced to the average of the left and right mastoids, and the four EOG signals were referenced using bipolar vertical and horizontal EOG derivations. These signals were then filtered offline using a noncausal Butterworth high-pass filter (half-amplitude cutoff: 0.1 Hz, slope: 12 dB/octave). Averaged ERP waveforms were computed with a 600-ms epoch, beginning 200 ms before stimulus onset. To maximize temporal precision, a low-pass filter (half-amplitude cutoff: 30 Hz, slope: 12 dB/octave) was applied to the averaged ERPs only for plotting.
Unless otherwise noted, the EEG analyses focused on the search-only trials and excluded the probe trials. The appearance of the probe letters makes it difficult to examine the ERPs on these trials. Furthermore, probe trials were too rare to yield a sufficient number of trials to compute robust ERP waveforms, increasing the noise in the ERP waveforms. For these reasons, we focused most of the ERP analyses on the search trials only (which had no probe stimuli). This additionally also allowed us to better compare our ERP results with the existing attentional capture literature, which almost exclusively uses procedures analogous to our search trials (e.g., Gaspar & McDonald, 2014; Hickey, McDonald, & Theeuwes, 2006; Lien et al., 2008). However, we conducted supplementary ERP analyses on the probe trials simply to show that the initial allocation of attention was approximately equivalent on the search trials and the probe trials.
Trials were excluded if they contained an incorrect behavioral response, if the RTs were shorter than 200 ms or longer than 1500 ms, if unusually large voltage excursions were observed in any channel, and if a blink or eye movement was detected in the horizontal or vertical EOG channels (indexed by step-like voltage changes; see Luck, 2014). To assess residual eye movements, we computed averaged horizontal EOG waveforms for the left- and right-target trials, averaged across conditions (see Woodman & Luck, 2003). In N2pc/PD experiments, we always replace any participants whose residual eye movements in these waveforms differ on left- versus right-target trials by more than 3.2 μV between 100 and 300 ms poststimulus (i.e., during the N2pc and PD time range); we can be certain that the remaining participants had an average eye rotation of less than ± 0.1° in the direction of the target, with an estimated voltage propagation of less than 0.1 µV at the posterior scalp sites (Lins, Picton, Berg, & Scherg, 1993). Four participants were replaced for this reason. We also always replace any participants for whom more than 25% of trials are excluded due to EEG or EOG artifacts. One participant was replaced for this reason in the present experiment. In the final set of participants, an average of 5.2% of trials were excluded due to ocular and EEG artifacts (range = 0.2% to 24.5%).
The N2pc and PD components were measured from contralateral-minus-ipsilateral difference waves at the PO7 and PO8 electrode sites. These electrode sites were chosen a priori on the basis of our previous studies (Sawaki, Geng, & Luck, 2012; Sawaki & Luck, 2010). To minimize biases from the choice of measurement windows, we used a technique from Sawaki et al (2012): N2pc amplitude was quantified as the negative area between 200–400 ms and PD amplitude was quantified as the positive area between 100–300 ms (see also Luck, 2014). Note that the measurements of negative area excluded portions of the waveform above zero, and the measurements of positive area excluded portions of the waveform below zero. These components were obtained from the following stimulus display configurations: lateral target/singleton absent, lateral target/singleton midline, and lateral singleton/target midline. Because the N2pc and PD components reflect lateralized differences in stimulus processing, any neural activity for items presented on the midline will cancel out when these components are measured as a difference in amplitude between the contralateral and ipsilateral hemispheres (Hickey et al., 2009; Woodman & Luck, 2003).
ANOVAs with more than two levels of a factor used the Greenhouse–Geisser correction for nonsphericity, and only the corrected p values are reported. Also, because signed area is naturally biased away from zero, we could not use traditional statistical approaches to determine whether the area was greater than expected by chance (such as a one-sample t test comparing the mean to zero). We therefore used the nonparametric permutation approach developed by Sawaki et al. (2012), in which random permutations of the data are used to estimate the distribution of signed area values that would be expected by noise alone (for more information, see the Supplementary Materials). For all statistical tests, a p value less than .10, but greater than .05, was considered marginally significant (exact p values are provided so that readers can apply their own decision criteria).
Results
Behavioral Results from the Search Task
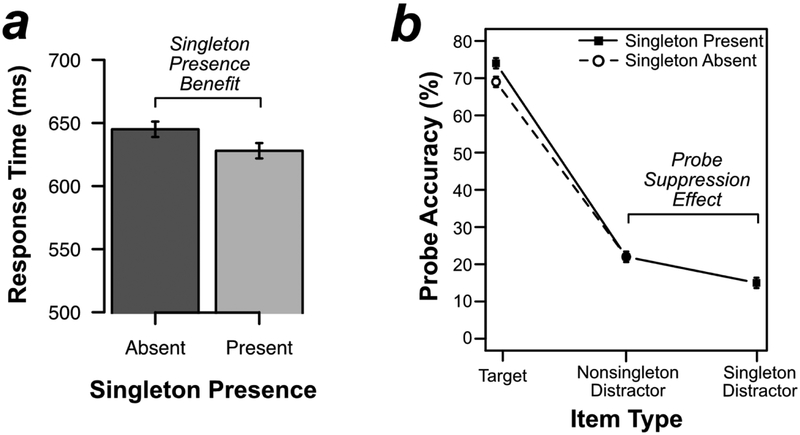
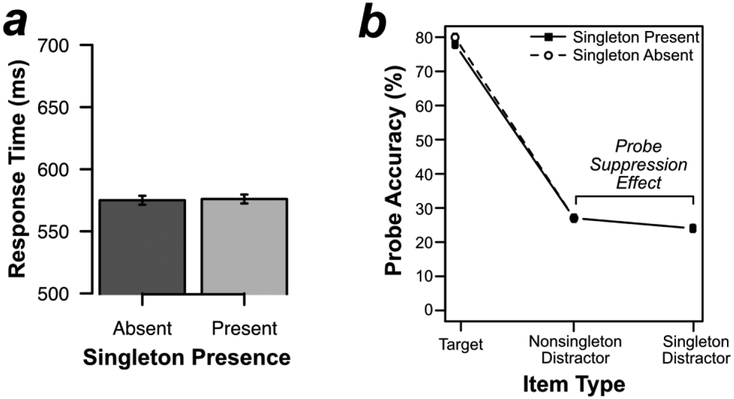
As shown in Figure 2a, responses in the search task were actually faster when the color singleton was present (628 ms) than when it was absent (645 ms) [t(19) = 4.033, p < .001, d = .902]. This 17-ms singleton presence benefit is exactly what one would expect if the singleton was suppressed, effectively reducing the relevant set size on singleton-present trials from 4 to 3 (see also Gaspelin et al., 2015, in press; Vatterott & Vecera, 2012). Error rates were not significantly different on singleton-present trials (1.7%) versus singleton-absent trials (1.5%) [t(19) = 1.037, p =.313, d = .232].
Figure 2.
Behavioral results from Experiment 1. (A) Mean response time on the search task by singleton presence. (B) Percentage of probe letters reported on probe trials by probed search item. Errors bars represent the within-subject 95% confidence interval (Loftus & Masson, 1994).
Behavioral Results from the Probe Task
Participants reported an average of 2.1 letters per trial, and 63.3% of reported letters were actually present in the probe array. Probe report accuracy at the nonsingleton locations was averaged to give a per-item estimate of probe report accuracy.
Figure 2b shows the results from the probe task. As predicted by the signal suppression hypothesis, participants were less likely to report probe letters at the singleton-distractor location (15%) than probe letters at the nonsingleton-distractor locations (22%) [t(19) = 4.448, p < .001, d = .995]. In other words, probe recall was impaired by 7% at the singleton-distractor location, replicating previous research (Gaspelin et al., 2015; Gaspelin & Luck, in press)
We also examined the effect of singleton presence on probe recall at the target and nonsingleton distractor locations. Planned t tests indicated that participants were more likely to recall probes at the target location on singleton-present trials (74%) than on singleton-absent trials (69%) [t(19) = 3.534, p = .002, d = .790]. This is exactly what would be expected if processing resources that would otherwise have been devoted to the singleton were instead allocated to the target. However, participants were not significantly more likely to recall probes at nonsingleton distractor locations on singleton-present trials (22%) than on singleton-absent trials (22%) [t(19) = .599, p = .556, d = .134].
Electrophysiological Results from Search Trials
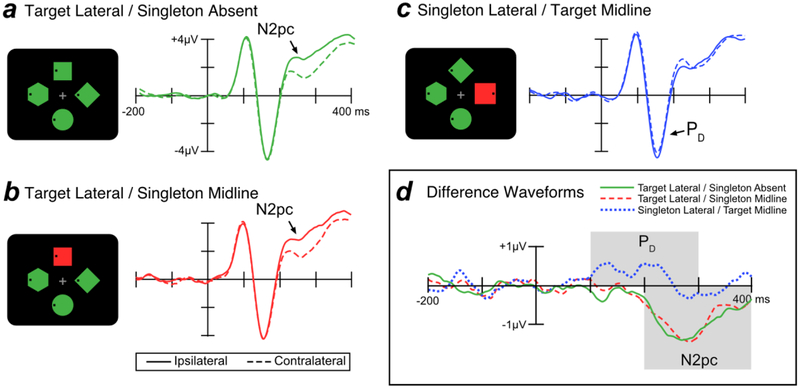
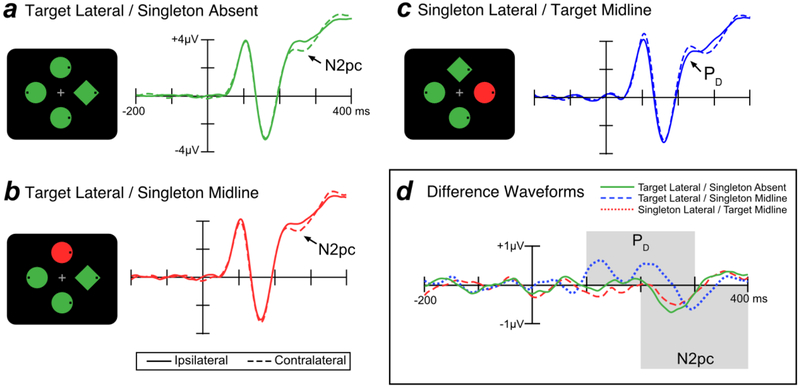
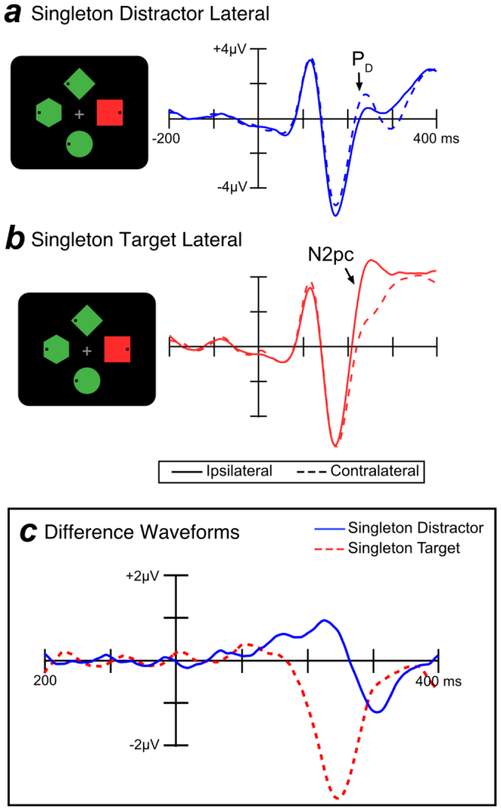
Figure 3 shows grand average ERP waveforms from lateral occipital scalp sites (PO7 and PO8) for targets and singleton distractors. Separate waveforms are shown for contralateral and ipsilateral sites, relative to the item of interest. For example, the contralateral waveform for the singleton distractor was the average of the left-hemisphere electrode when the singleton distractor was in the right visual field and the right-hemisphere electrode when the singleton distractor was in the left visual field (and the target was on the vertical meridian). The ipsilateral waveform for the singleton distractor was the average of the left-hemisphere electrode when the singleton distractor was in the left visual field and the right-hemisphere electrode when the singleton distractor was in the right visual field (and the target was on the vertical meridian). We excluded trials where both the target and the singleton distractor were lateralized; on these trials, the target N2pc and singleton PD summate, making it difficult to separately assess target processing and singleton processing.
Figure 3.
Electrophysiological results from search trials in Experiment 1. In the schematics of the search displays, the target was the green diamond and the singleton was the uniquely colored item. The waveforms in this and all subsequent figures were low-pass filtered to improve the visibility of the effects (Butterworth noncausal filter, half-amplitude cutoff = 30 Hz, slope = 12 dB/octave).
When the target was presented at a lateral location, the N2pc component was visible as a more negative (less positive) voltage at the contralateral scalp sites relative to the ipsilateral sites, beginning approximately 200 ms post-stimulus. This target-elicited N2pc was observed both when the singleton distractor was absent (Figure 3a) and when the singleton distractor was presented on the midline (Figure 3b). In contrast, when a singleton was presented at a lateral location and the target was presented on the midline—making it possible to isolate the lateralized response to the singleton—the singleton elicited a PD component, a more positive voltage in the ERP waveform at the contralateral relative to the ipsilateral sites (Figure 3c).
The time course of the N2pc and PD components can be difficult to evaluate when these components are superimposed on the non-lateralized ERP components, and it is easier to assess these components in contralateral-minus-ipsilateral difference waves, which subtract out all brain activity that is not lateralized with respect to the stimulus of interest. As shown in Figure 3d, these difference waves show that the PD effect for salient distractors began at approximately 100 ms whereas the N2pc for targets began at approximately 200 ms. Note that the PD component has two apparent peaks. Because this bifid waveshape was not anticipated, our main analyses were collapsed across the entire PD time window; a later section provides exploratory analyses that separate the early and late phases of the PD waveform.
The earlier onset latency of the PD relative to the N2pc component presumably reflects the greater salience of the singleton distractor relative to the target (see Luck et al., 2006, for evidence that N2pc onset time varies with salience). Consistent with this interpretation, previous studies that have equated the salience of the targets and distractors have found similar onset latencies for N2pc and PD (e.g., Gaspar & McDonald, 2014). Because we anticipated this latency difference, we used different time windows to measure the N2pc and PD components (100–300 ms for PD; 200–400 ms for N2pc). This strategy of using a slightly earlier time window for the PD component than the N2pc component is consistent with several previous studies (e.g., Sawaki & Luck, 2010; Weaver et al., 2017). Relative to the more common mean amplitude measures, positive and negative area measures are typically less impacted by latency differences between subjects (see Luck, 2014) and are therefore more appropriate for assessing correlations with behavioral measures (e.g., see Gaspar et al., 2016).
Statistical Analyses of the PD Time Window.
We first performed a one-way within-subject ANOVA comparing PD amplitude (measured from the contralateral-minus-ipsilateral difference waves) across the three trial types shown in Figure 3 (target lateral/singleton absent, target lateral/singleton midline, and singleton lateral/target midline). The finding of a substantial PD only for the singleton led to a significant effect of trial type [F(2, 38) = 15.85, p < .001, = .455]. Planned t tests showed that PD area was significantly larger for singleton lateral/target midline trials than for both target lateral/singleton absent trials [t(19) = 4.374, p < .001, d =.978] and target lateral/singleton midline trials [t(19) = 3.886, p < .001, d =.869]. The PD area did not significantly differ between target lateral/singleton absent and target lateral/singleton midline trials [t(19) = 1.366, p = .188, d =.305].
We also tested whether the area of the PD component was significantly greater than chance for each trial type using a permutation test (as described in the Method section). A significant PD was present for the singleton lateral/target midline trials [p < .001] but not for the target lateral/singleton absent trials [p = .998] or target lateral/singleton midline trials [p = .958].
Statistical Analyses of the N2pc Time Window.
We conducted the same analyses on the N2pc component. The presence of a large N2pc only for targets led to a significant main effect of trial type[F(2, 38) = 15.32, p < .001, η2 = .446]. Planned t tests showed that when the target was presented laterally, N2pc area did not differ significantly as a function of the presence or absence of a singleton on the midline [t(19) = .245, p = .809 d = .055]. However, N2pc area was significantly smaller for singleton lateral/target midline trials compared to both target lateral/singleton absent trials [t(19) = 4.085, p < .001, d = .913] and target lateral/singleton midline trials [t(19) = 4.672, p < .001, d = 1.045].
We also compared N2pc area to chance for each trial type using permutation tests. The N2pc was significant on both target lateral/singleton absent trials [p < .001] and target lateral/singleton midline trials [p < .001] but not on singleton latera/target midline trials [p < .231].
Together, the N2pc and PD results from this experiment replicate previous studies, showing that targets elicit an N2pc and salient distractors elicit a PD (Gaspar & McDonald, 2014; Hickey et al., 2009; Sawaki & Luck, 2010). However, the present study also provides behavioral evidence that the salient distractors were actually suppressed below baseline, consistent with the hypothesis that the PD effect reflects a suppressive process.
Correlations Between Behavioral and Electro-physiological Measures
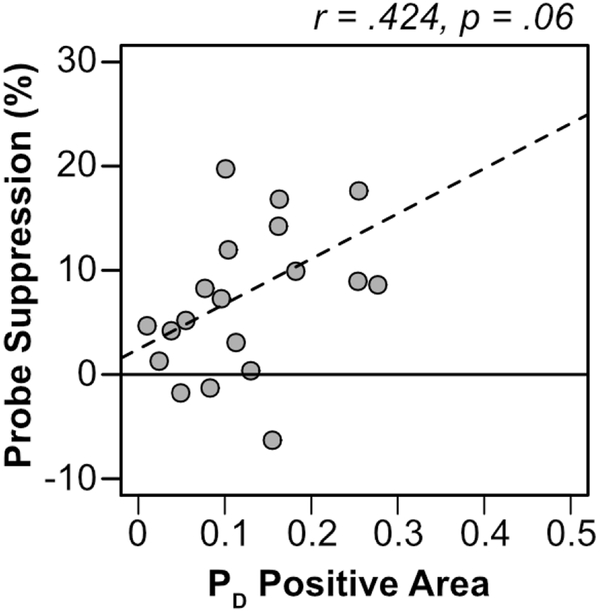
The sample size of this experiment was not chosen to provide the power needed to examine correlations between the behavioral and ERP measures. However, we conducted the correlational analyses to estimate the magnitude (as opposed to the statistical significance) of the correlation so that we could select an appropriate sample size for Experiment 2. We first examined the correlation between PD area and the behavioral probe suppression effect. The probe suppression effect was quantified for each participant as probe report accuracy for the average nonsingleton distractor minus probe report accuracy for the singleton distractor (yielding positive values when probe accuracy was suppressed at the singleton location compared to the nonsingleton distractor locations). As shown in Figure 4, there was a trend for the PD to be larger in participants who exhibited larger behavioral probe suppression effects [r(18) = .424, p = .062]. This trend was only marginally significant, but the correlation was moderately large and indicated that the need for a follow-up experiment with a larger N (see Experiment 2).
Figure 4.
Scatterplot of PD positive area on singleton lateral/target midline trials and probe suppression effects for each participant (gray dots). The dashed line is a regression line.
We also conducted the same correlational analysis on N2pc area. One might expect that people who have poor suppression would actually be captured by the singleton and therefore have an N2pc component rather than a PD component. However, participants who exhibited large suppression effects did not exhibit a smaller N2pc area [r(18) = .136, p = .567]. Note that in this experiment, we strongly encouraged suppression, so it is unlikely that many participants were actually captured by the singleton (eliciting an N2pc). This issue will be revisited in Experiment 2, which was designed to encourage more variation in the use of suppression.
Electrophysiological Results from Probe Trials
This study was designed to allow an analysis of the N2pc and PD components on the search trials, not on the probe trials, which were much less frequent (30% of trials) and were potentially distorted by the presence of probe-related ERP activity. However, we conducted post hoc analyses of the probe trials, which are provided in the Supplemental Materials. We found that probe trials produced the same general pattern of results as search trials: Trials with a lateralized target produced a large N2pc component, whereas trials with a lateralized singleton produced a large PD component. These results validate the assumption that the initial allocation of attention was the same on search trials and probe trials, justifying our comparison of the ERP effects on the search trials with the behavioral suppression effects on the probe trials.
Discussion
In this experiment, we measured ERPs in a capture-probe paradigm that encouraged feature search mode and suppression of the irrelevant color singleton. As in previous studies, the presence of a color singleton did not lead to a slowing of responses in the visual search task (Bacon & Egeth, 1994; Leber & Egeth, 2006). Instead, responses were faster when the singleton was present (a singleton presence benefit), consistent with the hypothesis that the singleton was suppressed, reducing the effective set size from 4 items to 3 items. Participants were also less likely to recall probes that appeared at the singleton location than at the average nonsingleton location (a probe suppression effect). In short, the behavioral result of this study nicely replicate previous studies of suppression (Gaspelin et al., 2015; Gaspelin & Luck, in press).
Importantly, the probe suppression effect was accompanied by the PD component, a putative electrophysiological index of suppression. Both the behavioral and ERP suppression effects have been observed before, but in separate experimental paradigms. The present results provide an initial link between the two measures of suppression: both can be observed in a single set of participants performing a single task.
An even stronger link between these effects would be implied by the presence of a correlation between the magnitude of the PD and the magnitude of the behaviorally measured probe suppression. The current experiment provided some evidence of an underlying association between these two measures. However, this experiment was not designed to provide a powerful test of this association because the sample size was chosen to detect differences across conditions and not to detect correlations between the ERP and behavioral effects. As a result, although the correlation between the electrophysiological and behavioral measures of suppression was moderately large, it was only marginally significant. Furthermore, the present experiment was not ideally suited to assess this correlation insofar as it strongly encouraged suppression, reducing the amount of participant-by-participant variation in probe suppression effects. Indeed, almost all participants exhibited suppression of the probe (see Figure 4). In the absence of substantial variation in the amount of suppression, there was little opportunity to observe a covariation between the amount of probe suppression and the magnitude of the PD component. Experiment 2 was designed to provide a better opportunity to detect an association between these variables.
Experiment 2
Experiment 2 was identical to Experiment 1 except for two changes. First, we modified the paradigm to increase variability across participants in the amount of probe suppression. Specifically, we decreased the incentive to suppress the singleton by using search displays that allowed participants to use either feature search mode or singleton detection mode (inspired by Leber & Egeth, 2006). In this “subject-option” version, the target was always the same shape (e.g., diamond) but it appeared amongst a homogenous set of distractor shapes (e.g., circles). This allowed participants to use either feature search mode (i.e., search for the diamond shape) or singleton detection mode (i.e., search for the singleton) to locate the target. Singleton detection mode should lead to capture of attention by the color singleton, whereas feature search mode should permit suppression of the color singleton. As a result, this version of the task should allow for a greater range of individual differences in capture/suppression, increasing our ability to detect a correlation between the PD and probe suppression effects.
In addition, we increased the sample size to 32 participants to increase the power for observing a significant correlation. Assuming the correlation coefficient value from Experiment 1 (r = .424), our increased sample size should have yielded approximately 75% power for detecting the correlation.
Method
All methods were identical to those of Experiment 1, except for the following changes. We tested 32 new participants [mean age = 20.7 years, 18 females and 14 males]. Five participants failed to meet the previously described artifact rejection criteria and were replaced.
As shown in Figure 5, the distractors shapes in this experiment were homogeneous (e.g., three circles). Target shape was counterbalanced across participants: half searched for a diamond target amongst circle distractors and the other half searched for a circle target amongst diamond distractors. Just as in Experiment 1, the target color and singleton color were held constant throughout the experiment for a given participant and were counterbalanced across participants.
Figure 5.
Schematic illustration of search displays used in Experiment 2. The target shape was held constant throughout the experimental session (e.g., diamond). The distractors shapes were homogenous (e.g., circles). This allowed participants to use either singleton-detection mode or feature-search mode to locate the target.
Results
Behavioral Results from the Search Task
This experiment was designed to encourage variability across participants in whether the color singleton was suppressed or captured attention, leading to little or no effect of singleton presence in the average across participants. As shown in Figure 6a, RTs in the search task were nearly identical when the color singleton was present (576 ms) or absent (575 ms) [t(31) = .223, p = .825, d = .039]. By contrast, we found significantly faster RTs when the singleton was present than when it was absent in Experiment 1, consistent with suppression of the singleton in that experiment. An across-experiment comparison indicated that the singleton presence benefit was significantly larger in Experiment 1 than in Experiment 2 [t(50) = 3.812, p < .001, d = 1.089]. Because the goal of this experiment to promote individual differences in suppression and capture, this pattern of results was ideal. Error rates were not significantly different on singleton-present trials (2.1%) and singleton-absent trials (2.1%) [t(31) = .178, p = .86, d = .031].
Figure 6.
Behavioral results from Experiment 2. (A) Mean response time on the search task by singleton presence. (B) Percentage of probe letters reported on probe trials by probed search item. Errors bars represent the within-subject 95% confidence interval (Loftus & Masson, 1994).
Behavioral Results from the Probe Task
Participants reported an average of 2.5 letters per trial, and 63% of reported letters were actually present in the probe array.
As shown in Figure 6b, probe letters at the singleton distractor location were slightly but significantly less likely to be reported (24%) than probe letters at the nonsingleton distractor locations (27%) [t(31) = 2.846, p = .008, d = .503]. In other words, there was a 3% probe suppression effect. This effect was significantly smaller than the 8% effect observed in Experiment 1 [t(50) = 2.611, p = .012, d = .744]. In fact, the 3% probe suppression effect was much smaller than that observed in any of our previous studies (typically between 6–12%; Gaspelin et al., 2015).
We formally assessed this trend by counting the number of participants who showed statistically significant probe suppression effects in each experiment (as assessed by performing single-subject statistical analyses on the individual trials for each participant). In Experiment 1, 55% of participants had a significant probe suppression effect, whereas only 19% of participants had significant suppression effects in Experiment 2.
Electrophysiological Results from Search Trials
Figure 7 shows the ERP waveforms from lateral occipital scalp sites (PO7 and PO8) for targets and singleton distractors. These grand averages are less meaningful than those from Experiment 1, because the current experiment was designed to encourage capture by the singleton for some participants (via singleton detection mode) and suppression of the singleton for others (via feature search mode). Thus, we expected the effects in grand averages to be weaker in Experiment 2 than in Experiment 1 (just as the behavioral results were).
Figure 7.
Electrophysiological results from Experiment 2. In the schematics of the search displays, the target was the green diamond and the singleton was the uniquely colored item. Difference waveforms were created by subtracting contralateral waveforms from ipsilateral waveforms.
Indeed, the ERP effects shown in Figure 7 were broadly similar to those of Experiment 1 (see Figure 3) but somewhat weaker. As in Experiment 1, the target elicited an N2pc component, whereas the singleton elicited a PD component (with two distinct peaks). The target-elicited N2pc was smaller in this experiment than in Experiment 1. This is presumably because the target was more salient in the current experiment than in Experiment 1, which reduces the size of the N2pc component (e.g., see the conjunction vs. feature search conditions of Luck, Girelli, McDermott, & Ford, 1997). The PD was also smaller in the present experiment, consistent with the reduction in behaviorally measured suppression.
Analyses of the PD Time Window.
We performed the same analyses as in Experiment 1. The presence of a PD only for the singleton lateral/target midline trials led to a significant main effect of trial type [F(2, 62) = 5.107, p = .020, ηp2 = .141]. The PD did not significantly differ between target lateral/singleton absent trials and target lateral/singleton midline trials [t(31) = .528, p = .601, d = .093]. The PD area was larger for the singleton lateral trials than for both target lateral/singleton absent trials [t(31) = 2.459, p = .020, d = .435] and target lateral/singleton absent trials [t(31) = 2.293, p = .029, d = .405]. Permutation tests showed that a significant PD was present for the singleton lateral/target midline trials [p < .001] but not for the target lateral/singleton absent [p = .467] or target lateral/singleton midline trials [p = .892].
Analyses of the N2pc Time Window.
Although the N2pc appeared to be larger for targets than for singletons (Figure 7d), there was at least some N2pc activity on singleton lateral/target midline trials, and the main effect of trial type on N2pc area was not significant [F(2, 62) = 2.472, p = .124, ηp2 = .073]. Consistent with this, planned comparisons of the three trial types yielded no significant pairwise differences in N2pc area [target lateral/singleton absent vs. target lateral/singleton midline: t(31) = .635, p = .530, d = .112; singleton lateral/target midline vs. target lateral/singleton absent: t(31) = 1.643, p = .111, d = .290; singleton lateral/target midline vs. target lateral/singleton midline: t(31) = 1.534, p = .135, d = .271]. Permutation tests indicated that a significant N2pc was present for both target lateral/singleton absent trials [p = .001] and target lateral/singleton midline trials [p = .044]. However, the N2pc was only marginally significant [p = .073] for singleton lateral/target midline trials.
Correlations Between Behavior and ERPs
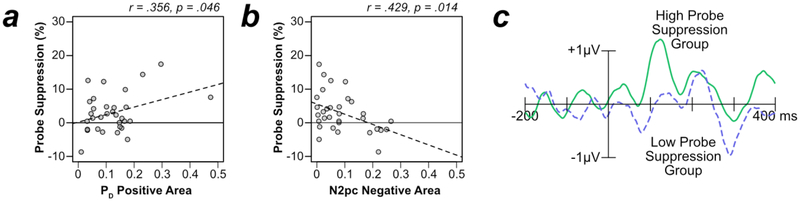
As in Experiment 1, we computed the correlation between the probe suppression effect and PD area. As a reminder, the probe suppression effect was calculated as the average probe report accuracy for the nonsingleton distractors minus probe report accuracy for the singleton. As shown in Figure 8a, these variables were significantly correlated [r(30) = .356, p = .046], indicating that participants who had larger probe suppression effects also had a larger PD. Although the trend for a positive correlation observed in Experiment 1 might justify a one-tailed test, we have provided the two-tailed p value to be conservative 3
Figure 8.
Relationship between ERP and behavioral measures of suppression in Experiment 2. (A) A scatter plot of PD positive area and probe suppression effects for each participant (gray dots). The dashed line is a regression line. (B) A scatter plot of N2pc negative area and probe suppression effects for each participant. (C) PD difference waveforms for two groups created by a median split of probe suppression effects. Difference waveforms were created by subtracting contralateral waveforms from ipsilateral waveforms on trials where the singleton was lateralized and the target was on the midline. The high probe suppression group shows a large early positivity (PD), but the low suppression group shows no such positivity.
We also computed the correlation between the probe suppression effect and N2pc area for the singleton distractor. As shown in Figure 8b, participants who exhibited a larger N2pc to the singleton distractor also had smaller probe suppression effects [r = −.429, p = .014]. Indeed, the probe suppression effects were negative for the participants with the largest N2pc amplitudes, indicating that the singleton actually captured attention in these participants. Thus, participants with low suppression scores on the behavioral task were more likely to be captured as measured by the N2pc component.
Correlation coefficients make it difficult to visualize how the whole waveform varies across participants. To visualize the relationship between the ERPs and behaviorally measured probe suppression, we divided the participants into two groups by performing a median split on the probe suppression effects. Note that this analysis is merely for illustrative purposes – median splits should generally be avoided in statistical analyses because they can drastically reduce power (MacCallum, Zhang, Preacher, & Rucker, 2002). The low probe suppression group had a 1.4% probe enhancement effect (i.e., they were more likely to report a probe at the location of the color singleton that at the location of a nonsingleton distractor), whereas the high suppression group had a 7.2% probe suppression effect (i.e., they were less likely to report the probe at the location of the color singleton). Figure 8c shows the contralateral-minus-ipsilateral difference waveforms for these two groups. In the high suppression group, the color singleton elicited a large early positivity (a PD), with no subsequent negativity (no N2pc). In the low suppression group, however, the color singleton did not elicit a large early positivity but did elicit a large subsequent negativity.
Early vs. Late PD Time Windows
As can be seen in Figure 8d, the PD component had two distinct peaks in this experiment. A similar but less distinct bifid PD waveform was present in Experiment 1 (see Figure 3d). The first phase lasted from approximately 100–175 ms, and the second phase lasted from approximately 175–250 ms. In the Supplemental Materials, we provide post hoc analyses of these two time windows. Note that these measurement windows were selected on the basis of the observed results, which can dramatically influence Type I error rates (Luck & Gaspelin, 2017), so these analyses should be treated as exploratory rather than confirmatory. Interestingly, in Experiment 2, we found that the early window was highly correlated with probe suppression effects [r(30)= .510, p = .003]. However, we urge caution in interpreting these results given that they were post hoc.
Discussion
In this experiment, we used displays that allowed participants to use either singleton detection mode (which should lead to capture by the color singleton) or feature search mode (which should lead to suppression of the color singleton). This allowed for a greater range of individual differences in capture/suppression, increasing our ability to detect a correlation between the PD and probe suppression effects. Indeed, we found exacty that: the magnitude of the behaviorally measured probe suppression effect was significantly correlated with the magnitude of the PD component. Although such a correlation is not by itself definitive evidence that the PD component and the behavioral suppression reflect the same underlying mechanism, this finding provides an important piece of converging evidence in support of this possibility.
Experiment 3
The results of Experiments 1 and 2 were consistent with the proposal that the PD component reflects suppression. However, one potential criticism is that the early positivity elicited by the color singleton in these experiments reflected some other cognitive process, such as an overall salience signal (a Ppc signal; Barras & Kerzel, 2017a; Kerzel & Barras, 2016; Pomerleau et al., 2014) or a larger contralateral P1 wave owing to lower levels of adaptation for the singleton color (Luck & Hillyard, 1994). To test this account, we used the same stimuli as in Experiment 1 but compared two different tasks, one in which the singleton was a distractor (as in Experiments 1 and 2) and one in which the singleton was the target. If the early lateralized positivity observed in Experiments 1 and 2 reflects suppression of the singleton, then it should be present only when the singleton is a distractor, and it should be replaced with an N2pc when the singleton is a target. However, if the early positivity reflects an early salience signal or some other purely stimulus-driven response, then it should be exactly the same whether the singleton is a target or a distractor.
Method
All methods were identical to those of Experiment 1, except for the following changes. We tested 16 new undergraduates from Binghamton University, State University of New York [mean age = 20.1 years, 6 females and 10 males]. A sample size of 16 was chosen because it should be sufficient to yield 90% power to detect a difference in PD between targets and distractors (assuming the same effect size observed in Experiment 1). One participant failed to meet the previously described artifact rejection criteria and was replaced. The configuration of the laboratory at Binghamton University was essentially identical to the laboratory at University of California, Davis (same stimulus presentation machines and EEG system).
The search task was identical to the task used in Experiment 1, except as follows. To improve statistical power, all trials contained a color singleton and probe trials were not included. For half of the session, the target was a nonsingleton color and shape (as in Experiment 1). For the other half, equivalent stimuli were generated, but the target was the singleton item (i.e., participants reported the position of the dot within the color singleton instead of the position of the dot within a specific shape). The order of these two tasks was counterbalanced across participants. Participants performed 10 blocks of 192 trials, of which 2 were practice blocks (1 practice block with each target type). This led to 768 trials with the singleton target and 768 with a nonsingleton target.
The analyses were analogous to those in the previous experiments. We focused on the ERPs for the trials on which the singleton was lateralized, which allowed us to test the central hypothesis of this experiment. The waveforms and analyses for trials with a lateralized nonsingleton are provided in Supplementary Materials.
Results
Behavioral Results
Participants responded significantly faster when the singleton was the target (510 ms) than when a nonsingleton shape was the target (570 ms), t(15) = 6.53, p < .001, d = 1.641. Error rates were also numerically lower when the singleton was the target (2.9%) than when a nonsingleton shape was the target, (3.3%), but this trend was not statistically significant, t(15) = 1.396, p = .183, d = .349. These results suggest that it was easier to find the singleton when it was the target than to find the appropriate nonsingleton shape when it was the target, as would be expected from the greater salience of the singleton.
Electrophysiological Results
Figure 9 shows the ERPs for trials on which the color singleton was lateralized, comparing the task in which this stimulus was a distractor (as in Experiments 1 and 2) and the task in which the same stimulus was the target. Generally speaking, the singleton produced a large N2pc when it was defined as the target for the task, whereas it produced a large and early PD when it was a distractor and participants were trying to select a nonsingleton shape. As a reminder, the goal of this experiment was to determine whether the singleton would produce the same early lateralized positivity when it was a target and when it was a distractor, which would indicate that this effect is an automatic stimulus-driven effect rather than a task-driven suppression effect. As can be seen in Figure 9, the singleton distractors yielded a robust lateralized positivity only when it was a distractor and not when it was the target.
Figure 9.
Electrophysiological results from Experiment 3. The target was always one of the nonsingleton items in the singleton distractor condition (a), whereas the target was always the singleton in the singleton target condition (b). Difference waveforms (c) were created by subtracting ipsilateral waveforms from contralateral waveforms.
It should be noted that the whole ERP waveform, including the P1 wave and the PD effect, appeared to be slightly later in this experiment than in Experiments 1 and 2. This may reflect some small difference in stimulus presentation hardware or participant population between experiments.
Statistical Analyses of the PD Time Window.
We first examined the PD component elicited by singletons using the same time window used in Experiments 1 and 2 (100–300 ms). A planned t test showed that PD area was significantly larger when a lateralized singleton was a distractor than when the same stimulus was the target, t(15) = 3.905, p = .001, d = .976. Permutation tests showed that the PD was significantly greater than chance when the singleton was a distractor [p < .001] but not when the singleton was the target.
Statistical Analyses of the Early PD Time Window (100–200 ms).
To demonstrate that the early phase of the PD effect was also greater when the singleton was a distractor than when it was the target, we repeated the statistical analyses on the positive area within the first half of the PD analysis window (i.e., 100–200 ms). A planned t test showed that this early portion of the positive area was significantly larger when the singleton was a distractor than when it was the target, [t(15) = 2.421, p = .029, d =.605]. Permutation tests again showed that this early portion of the PD was significantly greater than chance when the singleton was a distractor [p < .001]. Interestingly, the area during this early time window was also slightly but significantly different from chance when the singleton was the target [p = .024]. This may indicate the presence of a small but reliable automatic response elicited by the physical properties of the singleton.
Statistical Analyses of the N2pc Time Window.
We conducted the same analyses on the N2pc component, again focusing on trials with a lateral singleton. A planned t tests showed that N2pc area was significantly larger for the singleton when it was the target than when it was a distractor, t(15) = 3.694, p = .002, d = .924. Permutation tests showed that the N2pc area was significantly greater than chance when the singleton was a target [p < .001] and also when the singleton was a distractor [p = .013].
Discussion
The purpose of the present experiment was to determine whether the early lateralized positivity observed in Experiments 1 and 2 reflected suppression of to-be-ignored salient items or some sort of automatic response to the singleton. Using identical stimulus displays, we varied whether the singleton was a distractor or the target. If the PD component in Experiments 1 and 2 was an automatic response to the singleton (i.e., a Ppc), then the singleton should elicit the same early positivity whether it is a distractor or the target. However, we found that the singleton elicited a much larger contralateral positivity when it was a distractor than when it was a target. Moreover, this difference between tasks emerged early and was statistically significant within the first half of the PD measurement window (100–200 ms). These results provide clear evidence that the lateralized positivity in Experiments 1 and 2 was primarily a PD component that was triggered by the need to suppress the to-be-ignored color singleton and not a Ppc component that was triggered by the salience of the singleton.
Interestingly, the color singleton did elicit a small but statistically significant contralateral positivity that peaked at approximately 100 ms. This could reflect either a salience signal (a Ppc) or a larger sensory response resulting from less sensory adaptation to the singleton color than to the nonsingleton color (as described by Luck & Hillyard, 1994). In either case, most of the broad positivity elicited by the singleton when it was a distractor cannot be explained by automatic singleton processing and instead reflects a bona fide PD component.
General Discussion
Researchers have long debated the fundamental nature of attentional capture (Folk & Remington, 2010; Folk et al., 1992; Theeuwes, 1992, 2010). In the present study, we tested predictions of the signal suppression hypothesis, which attempts to bridge the traditional stimulus-driven and goal-driven theories of capture. According to this hypothesis, salient stimuli will automatically capture attention unless they are suppressed by means of a top-down control process. Some evidence has indirectly supported this account. But, as far as we know, no prior studies that have directly linked the PD component with behavioral index of covert attention suppression. The present study aimed to fill this gap by recording ERPs in a paradigm that allows performance at the singleton location to be compared with performance at the nonsingleton distractor locations.
In Experiment 1, we tested whether the PD and probe suppression effects could be observed in the same set of participants performing the same task. Participants searched for a target that had a constant shape (e.g., green diamond) and was presented amongst heterogeneous distractors. Consistent with previous behavioral studies (Gaspelin et al., 2015), we found a robust probe suppression effect: participants were less likely to recall probes at the singleton location than at baseline levels. We also found electrophysiological evidence of suppression: the singletons elicited a PD component without a subsequent N2pc component. In other words, we found that an electrophysiological index of suppression and a behavioral index of suppression co-occurred in the same experiment. This is consistent with the signal suppression hypothesis and provides the clearest evidence to date that the PD reflects suppression of covert attention.
If the PD and probe suppression effects measure the same underlying suppressive mechanism, then the two measures should be correlated. In Experiment 1, the magnitude of the PD component was correlated with the magnitude of the probe suppression effect, but the effect was only marginally significant. Experiment 2 was designed for the express purpose of detecting this correlation by using a larger sample size and a task that was designed to encourage greater participant-by-participant variation in the amount of singleton suppression. As expected, fewer participants showed probe suppression effects in Experiment 2 than in Experiment 1. Most importantly, we observed a significant correlation between the magnitude of the PD and the magnitude of the probe suppression effect. Specifically, participants with a larger probe suppression effect also had a larger PD component.
One potential concern is that the PD from Experiments 1 and 2—which appeared relatively early in stimulus processing (100–300 ms)—might instead reflect an automatic salience detection process (a Ppc; see Barras & Kerzel, 2017a; Kerzel & Barras, 2016; Pomerleau et al., 2014) or reduced adaptation of the singleton color (an increased P1; Luck & Hillyard, 1994). Experiment 3 directly tested this possibility using a search task similar to Experiment 1. The singleton was a distractor in one condition (just as in Experiment 1), but in a new condition the singleton was the target. If the lateralized positivity from Experiment 1 was an automatic response to the singleton, then it should be the same whether the singleton is the target or a distractor. However, we found that the lateralized positivity was much larger when the singleton was a to-be-suppressed distractor than when it was a to-be-attended target, beginning well before 200 ms. This result indicates that the contralateral positivity elicited by the singleton was primarily a suppression-related PD component and that this PD can be observed relatively early in processing. It is worth mentioning that the timing of the PD component varies considerably across studies (e.g., approximately 200–350 ms in Barras & Kerzel, 2016; 250–290 ms in Gaspar & McDonald, 2014; 148–158 ms in Weaver et al., 2017; 115–225 ms in Sawaki & Luck, 2010). It will be important for future research to determine whether the contralateral positivities observed in these studies actually reflect the same underlying process (e.g., see Livingstone, Christie, Wright, & McDonald, 2017).
One additional concern is that the behavioral probe suppression effect observed in Experiments 1 and 2 may reflect a postperceptual process (e.g., a memory bias). This is certainly possible. However, this interpretation seems unlikely for a few reasons. First, the size of the probe suppression effect was correlated with the size of the PD component (Experiment 2). Second, we have obtained comparable evidence for suppression of salient singletons using an eye-tracking paradigm. We found that eye movements avoided the singleton as early as 200 ms after stimulus onset (Gaspelin et al., 2017). It would be difficult to explain these rapid effects on oculomotor orienting in terms of a late, postperceptual memory bias. In summary, the signal suppression hypothesis is supported by several converging lines of evidence, which straightforwardly indicate the operation of an inhibitory process in the guidance of visual attention (for a review, see Gaspelin & Luck, in press). Any alternative explanation must account for these converging pieces of evidence.
In summary, the current study provides a crucial link between the PD component and the suppression of covert shifts of visual attention. It also corroborates existing evidence for the signal suppression hypothesis, which provides a plausible solution to the long-lasting attentional capture debate.
Footnotes
Supplementary Material
Acknowledgments
This study was made possible by National Research Service Award F32EY024834 to N.G. from the National Eye Institute and by NIH Grants R01MH076226 and R01MH065034 to S.J.L.
Footnotes
Pure goal-driven theories propose that attention is allocated solely on the basis of the match between the incoming stimulus and the attentional set. As a result, singletons would not have any automatic ability to capture attention. In other words, bottom-salience salience has no role in attentional guidance in a pure goal-driven model. We contend that if a model assumes that bottom-up salience has a potential role to guide visual attention, then it is a hybrid model of attentional guidance. Other researchers have proposed such models (e.g., Bacon & Egeth, 1994; Itti & Koch, 2001; Wolfe, 1994), but it was unclear how integration of bottom-up salience and top-down relevance was implemented. Signal suppression models offer a potential mechanism for such integration: inhibition of salient stimuli (see Gaspelin & Luck, in press).
Correlations of this nature are difficult to obtain because scalp-recorded ERP amplitudes can be influenced by individual differences in a variety of biophysical factors (e.g., skull thickness, cortical folding idiosyncrasies) that are unrelated to internal neural activity (Luck, 2014). Thus, a correlation between a behavioral measure and a scalp-recorded ERP amplitude will necessarily be weaker than the correlation between the behavioral measure and the internal neural activity that gives rise to the scalp-recorded component. However, if the underlying brain-behavior correlation is strong enough, it should be detectable with a sufficient sample size. Experiment 2 was designed for the express purpose of detecting this correlation.
One participant was objectively an outlier on the positive area measurement, being more than 2.5 standard deviations from the group mean (see right-most data point in Figure 8a). Even when this participant was removed, the correlation was still significant [r(30)= .357, p = .045].
References
- Bacon WF, & Egeth HE (1994). Overriding stimulus-driven attentional capture. Perception & Psychophysics, 55(5), 485–496. [DOI] [PubMed] [Google Scholar]
- Barras C, & Kerzel D (2017). Salient-but-irrelevant stimuli cause attentional capture in difficult, but attentional suppression in easy visual search. Psychophysiology, 54(12), 1826–1838. [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Eimer M (1996). The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology, 99(3), 225–234. [DOI] [PubMed] [Google Scholar]
- Folk CL, & Remington RW (2010). A critical evaluation of the disengagement hypothesis. Acta Psychologica, 135(2), 103–105. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, & Johnston JC (1992). Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance, 18(4), 1030–1044. [PubMed] [Google Scholar]
- Gaspar JM, Christie GJ, Prime DJ, Jolicœur P, & McDonald JJ (2016). Inability to suppress salient distractors predicts low visual working memory capacity. Proceedings of the National Academy of Sciences of the United States of America, 113(13), 3693–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar JM, & McDonald JJ (2014). Suppression of salient objects prevents distraction in visual search. The Journal of Neuroscience, 34(16), 5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, & Luck SJ (2015). Direct evidence for active suppression of salient-but-irrelevant sensory Iiputs. Psychological Science, 22(11), 1740–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, & Luck SJ (2017). Suppression of overt attentional capture by salient-but-irrelevant color singletons. Attention, Perception, & Psychophysics, 79(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, & Luck SJ (in press, a). Distinguishing among potential mechanisms of singleton suppression. Journal of Experimental Psychology: Human Perception and Performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, & Luck SJ (in press, b). The role of inhibition in avoiding distraction by salient stimuli. Trends in Cognitive Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ (2014). Attentional mechanisms of distractor suppression. Current Directions in Psychological Science, 23(2), 147–153. [Google Scholar]
- Hickey C, Di Lollo V, & McDonald JJ (2009). Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience, 21(4), 760–775. [DOI] [PubMed] [Google Scholar]
- Hickey C, McDonald JJ, & Theeuwes J (2006). Electrophysiological evidence of the capture of visual attention. Journal of Cognitive Neuroscience, 18, 604–613. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Luck SJ, Boelmans K, Schoenfeld MA, Boehler CN, Rieger J, & Heinze H-J (2006). The neural site of attention matches the spatial scale of perception. Journal of Neuroscience, 26(13), 3532–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, & Heinze HJ (2000). Neural sources of focused attention in visual search. Cerebral Cortex, 10(12), 1233–41. [DOI] [PubMed] [Google Scholar]
- Itti L, & Koch C (2001). Computational modelling of visual attention. Nature Reviews Neuroscience, 2(3), 194–203. [DOI] [PubMed] [Google Scholar]
- Jannati A, Gaspar JM, & McDonald JJ (2013). Tracking target and distractor processing in fixed-feature visual search: evidence from human electrophysiology. Journal of Experimental Psychology. Human Perception and Performance, 39(6), 1713–30. [DOI] [PubMed] [Google Scholar]
- Kerzel D, & Barras C (2016). Distractor rejection in visual search breaks down with more than a single distractor feature. Journal of Experimental Psychology: Human Perception and Performance, 42(5), 648–657. [DOI] [PubMed] [Google Scholar]
- Lien M-C, Ruthruff E, Goodin Z, & Remington RW (2008). Contingent attentional capture by top-down control settings: Converging evidence from event-related potentials. Journal of Experimental Psychology: Human Perception and Performance, 34(3), 509–530. [DOI] [PubMed] [Google Scholar]
- Lins OG, Picton TW, Berg P, & Scherg M (1993). Ocular Artifacts in EEG and event related potentials II: scalp topography. Brain Topography, 6(1), 51–63. [DOI] [PubMed] [Google Scholar]
- Livingstone AC, Christie GJ, Wright RD, & McDonald JJ (2017). Signal enhancement, not active suppression, follows the contingent capture of visual attention. Journal of Experimental Psychology: Human Perception and Performance, 43(2), 219–224. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8(April), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2012). Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In Luck SJ & Kappenman ES (Eds.) (pp. 329–360). New York, NY US: Oxford University Press. [Google Scholar]
- Luck SJ (2014). An Introduction to the Event-Related Potential Technique (2nd ed). Cambridge, MA: MIT Press. [Google Scholar]
- Luck SJ, Girelli M, McDermott MT, & Ford MA (1997). Bridging the gap between monkey neurophysiology and human perception: an ambiguity resolution theory of visual selective attention. Cognit Psychology, 33(1), 64–87. [DOI] [PubMed] [Google Scholar]
- Luck SJ, & Hillyard SA (1994a). Electrophysiological correlates of feature analysis during visual search. Psychophysiology. [DOI] [PubMed] [Google Scholar]
- Luck SJ, & Hillyard SA (1994b). Spatial filtering during visual search: evidence from human electrophysiology. Journal of Experimental Psychology. Human Perception and Performance, 20(5), 1000–14. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, & Rucker DD (2002). On the practice of dichotomization of quantitative variables. Psychological Methods, 7(1), 19–40. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Green JJ, Jannati A, & Di Lollo V (2013). On the electrophysiological evidence for the capture of visual attention. Journal of Experimental Psychology: Human Perception and Performance, 39(3), 849–860. [DOI] [PubMed] [Google Scholar]
- Pashler HE (1988). Cross-dimensional interaction and texture segregation. Perception & Psychophysics, 43(4), 307–318. [DOI] [PubMed] [Google Scholar]
- Pomerleau VJ, Fortier-Gauthier U, Corriveau I, McDonald JJ, Dell’Acqua R, & Jolioeur P (2014). The attentional blink freezes spatial attention allocation to targets, not distractors: Evidence from human electrophysiology. Brain Research, 1559, 33–45. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Geng JJ, & Luck SJ (2012). A common neural mechanism for preventing and terminating the allocation of attention. The Journal of Neuroscience, 32(31), 10725–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, & Luck SJ (2010). Capture versus suppression of attention by salient singletons: Electrophysiological evidence for an automatic attend-to-me signal. Attention, Perception, & Psychophysics, 72(6), 1455–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, & Luck SJ (2011). Active suppression of distractors that match the contents of visual working memory. Visual Cognition, 19(7), 956–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society AE (1994). Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology, 11, 111–113. [PubMed] [Google Scholar]
- Theeuwes J (1992). Perceptual selectivity for color and form. Perception & Psychophysics, 51(6), 599–606. [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2010). Top–down and bottom–up control of visual selection. Acta Psychologica, 135(2), 77–99. [DOI] [PubMed] [Google Scholar]
- Vatterott DB, & Vecera SP (2012). Experience-dependent attentional tuning of distractor rejection. Psychonomic Bulletin & Review, 19(5), 871–878. [DOI] [PubMed] [Google Scholar]
- Weaver MD, van Zoest W, & Hickey C (2017). A temporal dependency account of attentional inhibition in oculomotor control. NeuroImage, 147, 880–894. [DOI] [PubMed] [Google Scholar]
- Wolfe JM (1994). Guided search 2.0: A revised model of visual search. Psychonomic Bulletin & Review, 1(2), 202–238. [DOI] [PubMed] [Google Scholar]
- Woodman GF, & Luck SJ (2003). Serial deployment of attention during visual search. Journal of Experimental Psychology. Human Perception and Performance, 29(1), 121–138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.