Abstract
Distal hair segments collected at delivery may allow for the assessment of maternal cortisol secretion in early pregnancy, an important time window for fetal development. Therefore, an investigation of the validity of distal hair cortisol concentrations is warranted. We examined the concordance between proximal and distal hair cortisol concentrations (HCC), both representing the first trimester of pregnancy. The study population was comprised of a random sample of 97 women participating in the Pregnancy Outcomes Maternal and Infant Study, a prospective cohort study of pregnant women attending prenatal clinics in Lima, Peru. Each participant provided 2 hair samples: once at enrollment (mean gestational age (GA)=13.1 weeks) and again at full-term delivery (mean GA=39.0 weeks). Hair segments reflecting the first trimester were: 3cm hair segments closest to the scalp on the first hair sample (proximal) and 6-9cm from the scalp on the second hair sample (distal). HCC was determined using Luminescence Immunoassay. A subset (N=28) had both hair segments additionally analyzed using liquid chromatography tandem mass spectrometry (LC-MS/MS). HCC values were log-transformed (logHCC), and proximal-distal differences tested using paired sample t-tests. Concordance was evaluated within and across assay types. LogHCC, measured using immunoassay, in distal hair segments was lower compared to proximal hair segments (1.35 vs. 1.64 respectively; p-value=0.02). No difference was observed using LC-MS/MS (1.99 vs. 1.83, respectively; p-value=0.33). Proximal-distal concordance was low within assay (immunoassay: Pearson=0.27 and kappa=0.10; LC-MS/MS: Pearson= 0.37 and kappa=0.07). High correlation was observed across assays for both distal (Pearson=0.78, p-value <0.001; kappa=0.64) and proximal segments (Pearson=0.96, p-value <0.001; kappa=0.75). In conclusion, distal first trimester hair segments collected at delivery have lower absolute HCC compared to HCC in proximal first trimester hair segments collected in early pregnancy, and are poorly concordant with HCC in proximal segments. Findings may inform the design of future studies.
Keywords: Cortisol, Hair, Pregnancy, Stress
2-3 SENTENCE LAY SUMMARY:
On average, cortisol from hair segments farther from the scalp at delivery are lower and not in agreement with cortisol from hair segments closer to the scalp in early pregnancy. Therefore, researchers interested in early pregnancy hair cortisol concentrations, and its associations with pregnancy outcomes, are advised to restrict analyses to hair segments closer to the scalp in early pregnancy.
1. INTRODUCTION
Cortisol, a glucocorticoid hormone released by the hypothalamic-pituitary-adrenal (HPA) axis, plays a key role in maintaining homeostatic conditions and aids in the functioning of the metabolic, immune, and neurologic systems (McEwen and Seeman, 1999; Smith and Cidlowski, 2010; Van Londen et al., 1998). Chronically dysregulated cortisol concentrations have been associated with early miscarriage (Nepomnaschy et al., 2006), low birth weight delivery (Bolten et al., 2011), and depression in the perinatal period (Bjelanovic et al., 2015; Diego et al., 2009; Field et al., 2009; Hoffman et al., 2016; Lommatzsch et al., 2006; Murphy et al., 2015; O’Connor et al., 2014; O’Keane et al., 2011; Peer et al., 2013; Voegtline et al., 2013). Traditional cortisol monitoring techniques, such as saliva, serum and urine, have been useful in assessing how deviations in diurnal cortisol profiles are associated with such outcomes. However, these techniques are limited in their ability to monitor long-term cortisol secretion due to their reflection of cortisol concentrations in the past one to 24-hours, thereby requiring the collection of multiple hourly or daily samples over the course of many days.
Hair has emerged as a relatively non-invasive, stable, and easily stored biospecimen that represents a retrospective measure of integrated cortisol concentrations spanning months (Cirimele et al., 2000; D’Anna-Hernandez et al., 2011; Davenport et al., 2006; Gow et al., 2010; Kirschbaum et al., 2009; Raul et al., 2004; Sauve et al., 2007; Stalder and Kirschbaum, 2012; Wosu et al., 2013). Cortisol concentrations in hair are thought to result from the passive diffusion of unbound circulating cortisol from nearby blood vessels, sweat and sebaceous glands (Stalder and Kirschbaum, 2012). Hair cortisol concentrations (HCC) increase during pregnancy and correlate with salivary cortisol concentrations during pregnancy (D’Anna-Hernandez et al., 2011). The average hair growth in humans is approximately one centimeter per month (or 1.1 +/− 0.2cm) (Barman et al., 1965; Barth, 1986; Loussouarn et al., 2005; Pragst and Balikova, 2006). Despite increases in the percentage of scalp hairs in active growth during pregnancy (Conrad and Paus, 2004; Lynfield, 1960; Pecoraro et al., 1967) and increases to scalp hair diameter during pregnancy (Nissimov and Elchalal, 2003), similar rates are observed in scalp and pubic hair (Astore et al., 1979; Pecoraro et al., 1967). Therefore, researchers interested in assessing long-term cortisol secretion and release during the pregnancy period have collected hair samples of nine centimeters (cm) or more at delivery. However, due to washout of cortisol from distal hair segments over time, such samples may be limited in their ability to reflect early pregnancy cortisol synthesis and release. For researchers interested in early pregnancy cortisol secretion, and its role in fetal development and maternal health, an evaluation of the validity of distal segments collected at delivery is warranted.
Previous studies have observed increases in HCC during pregnancy when using 9cm hair samples collected at delivery, with segments representing the 1st trimester (6-9cm from the scalp), 2nd trimester (3-6cm from the scalp), and 3rd trimester (0-3cm nearest the scalp) (D’Anna-Hernandez et al., 2011; Kirschbaum et al., 2009). While this is consistent with observed increases in cortisol during pregnancy in saliva (Braithwaite et al., 2016; Davis et al., 2007; Giesbrecht et al., 2012; Lachelin, 2013), plasma (Burke and Roulet, 1970), and urine (Burke and Roulet, 1970), the lower cortisol concentrations in hair further from the scalp may be influenced by degradation or “washout effects” from prolonged environmental exposures (Hamel et al., 2011; Li et al., 2012). These exposures may lead to artificially low levels in hair segments beyond 6cm from the scalp, a commonly cited methodological limitation of long-term cortisol monitoring in hair (D’Anna-Hernandez et al., 2011; Hamel et al., 2011; Kirschbaum et al., 2009; Russell et al., 2012). However, the magnitude of such “washout effects” over time, and the extent to which distal segments are concordant with proximal segments presumed to reflect the same time period requires further investigation. Furthermore, assessing the concordance across laboratory methods of cortisol determination separately for proximal and distal hair segments would benefit researchers deciding between the two laboratory approaches. Combined, such findings could help inform the design of future studies, and aid in the interpretation of existing studies that utilize HCC. Therefore, using hair samples collected from a cohort of pregnant women in Lima, Peru, we evaluated the concordance between HCC in proximal hair segments collected in early pregnancy with HCC in distal hair segments collected at delivery, both presumed to reflect the first trimester of pregnancy (Figure 1).
Figure 1:
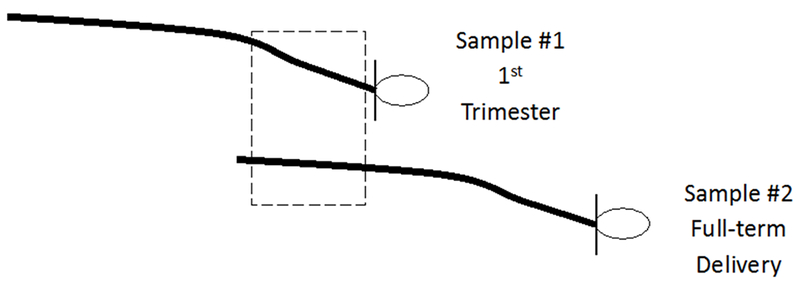
Diagram showing the proximal and distal first trimester hair segments for comparison
The dashed box indicates segments on each hair sample collection used for comparisons: 0-3cm from the scalp on hair sample 1 collected in the first trimester (proximal) and 6-9cm from the scalp on hair sample 2 collected at delivery (distal).
2. MATERIAL AND METHODS
2.1 STUDY PARTICIPANTS AND PROCEDURES
Data were gathered as part of the Pregnancy Outcomes, Maternal, and Infant Study (PrOMIS), a prospective cohort study consisting of pregnant women attending prenatal clinics at the Instituto Nacional Materno Perinatal (INMP) in Lima, Peru, the primary reference establishment for maternal and perinatal care operated by the Ministry of Health of the Peruvian government (Barrios et al., 2015). The institutional review boards of the Harvard T.H. Chan School of Public Health and INMP approved this study, and written informed consent was obtained from all participants. Recruitment for the PrOMIS study began in February of 2012, and scalp hair samples were collected from participants enrolled in the cohort during the period of October 2014 to November 2015. Participants who were 18 years of age or older, were able to speak and read in Spanish, and initiated prenatal care in early pregnancy were invited to participate (mean gestational age=13.1 weeks, standard deviation (SD)=3.9). Recruited participants were then followed from early pregnancy to delivery. Among enrolled PrOMIS cohort participants, 96% provided a first hair sample at enrollment in early pregnancy, 32% of which contributed a second hair sample at full-term delivery (mean gestational age 39.0 weeks, SD=1.0). Since it was neither necessary nor financially feasible to conduct biochemical analyses of all participants, we randomly selected 100 women from all eligible women with two hair samples. Selected women did not differ from non-selected women. The selection process for the present analysis is provided in Figure 2. Briefly, women were excluded if they failed to meet the following criteria: live singleton delivery, full term delivery (≥37 weeks), and two hair sample collections. Three women were excluded from the 100 randomly selected participants due to either undetectable cortisol values, or cortisol values that exceeded 100pg/mg (>4 standard deviations from the mean). Our final analytic sample consists of 97 participants, each providing two first-trimester hair segments: the proximal 3cm segment measured as 0-3cm from the scalp collected in early pregnancy, and the distal 3cm segment measured as 6-9cm from the scalp collected at full-term delivery (Figure 1). Assuming a two-tailed alpha of 0.05, a sample size of 100 participants had 80% power to detect a correlation of 0.28. The power for our sample size of 97 participants was 79.7%.
Figure 2:
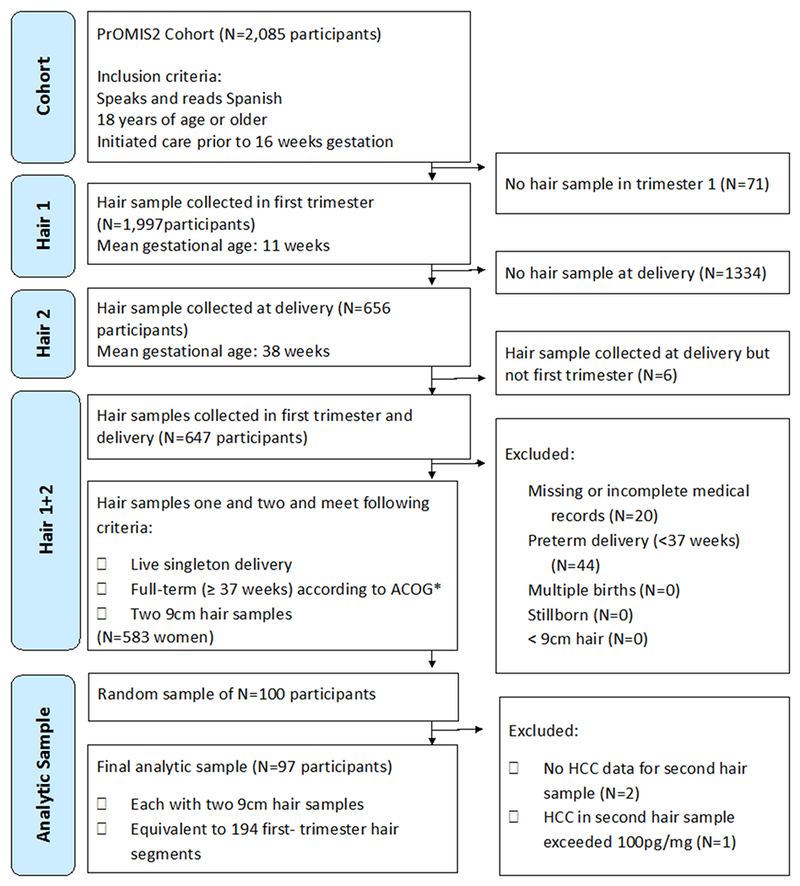
Flow chart showing selection into study
*ACOG= American Congress of Obstetricians and Gynecologists
Hair collection procedures were similar to those described elsewhere (Gao et al., 2013). In brief, trained research staff collected two hair samples from the posterior vertex region of the scalp as close to the scalp as possible twice during the perinatal period, first at enrollment and again at full-term delivery. Collected hair samples were then wrapped in aluminum foil, and stored in manila envelopes away from light and at room temperature using desiccants. Prior to assay, women’s hair samples were randomly ordered, and samples from the same woman assayed in the same immunoassay batch. From this sample of 97 women, approximately one third (N=28) were randomly selected for a sub-study validation using Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS). Assuming a two-tailed alpha of 0.05, a sample size of 28 participants was 80% powered to detect a correlation of 0.50.
2.2 LABORATORY ANALYSIS
Hair processing procedures for cortisol were as described in previous studies (Albar et al., 2013). First, both 9cm hair samples from each participant were segmented into three 3cm hair segments. This analysis was restricted to values from the first trimester hair segments only (Figure 1). Lab personnel used 7.5mg of whole non-pulverized hair per segment for analysis with the Cortisol Saliva Luminescence Immunoassay, IBL International ®. One immunoassay batch was defined as one 96-well plate, and all hair segments from the same woman were analyzed together in the same batch, thereby reducing the influence of variability across batches (Tworoger and Hankinson, 2006). For our sub-study validation, a subset of 28 randomly selected participants had both first trimester hair segments additionally analyzed using Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS), where samples were run consecutively rather than in batches. The same lot of reagents was used for all samples. Cortisol units of both techniques were reported in picograms per milligram (pg/mg), and the lower limit of detection was 0.1 pg/mg (Gao et al., 2016; Gao et al., 2013). Six blinded quality control (QC) samples were randomly dispersed to assess variability (Tworoger and Hankinson, 2006). For the immunoassay, inter-assay and intra-assay coefficients of variation (CV) were 19.4% and 11.9%, respectively. For the LC-MS/MS, the inter-assay CV was 8.1%. Since LC-MS/MS analyses did not employ batches, intra-assay CV’s are not reported. CV’s up to 20% are regarded as acceptable (Tworoger and Hankinson, 2006). In sensitivity analyses, immunoassay-derived cortisol concentrations were batch-corrected using the Rosner method (Rosner et al., 2008) to determine if batch-corrected agreement findings differed from original findings.
2.3 PARTICIPANT CHARACTERISTICS
At enrollment, structured interviewer-administered questionnaires were used to collect information on participants’ hair and sociodemographic characteristics, anthropometrics, and medical and reproductive history. Hair characteristics included: natural hair color, hair structure, hair washing frequency, shampoo and conditioner use, chemical hair treatment use, and hair cutting frequency. Sociodemographic characteristics included: age, educational attainment, smoking status prior to the study pregnancy, alcohol consumption prior to the study pregnancy, ethnicity, marital status, employment during the study pregnancy, and difficulty paying for basics. Early pregnancy body mass index (BMI) (kg/m2) was measured using the participants’ weight to the nearest 0.1kg and height to the nearest 0.1cm. Medical and reproductive history questions assessed whether the study pregnancy was planned, parity, gestational age at enrollment and full-term delivery using last menstrual period (in weeks), and asthma diagnosis before the study pregnancy. Psychological measures such as perceived stress, generalized anxiety disorder, depression, and post-traumatic stress disorder were assessed at enrollment using the 14-item Perceived Stress Scale (Cohen et al., 1983), the 7-item Generalized Anxiety Disorder scale (Zhong et al., 2015), the 9-item Patient Health Questionnaire (Zhong et al., 2014), and the Post-Traumatic Stress Disorder Checklist-Civilian version (Blanchard et al., 1996; Gelaye et al., 2017), respectively. Since cortisol concentrations in hair may be influenced by ultraviolet light (UV) exposure (Hansen et al., 2001; Li et al., 2012; Stalder et al., 2012), categories of UV exposure during the three-month time period of hair growth were estimated using meteorological UV levels. Hair growth occurring exclusively during the high UV Peru summer months of December to April were defined as ‘high’ (N=16), and hair growth occurring exclusively during the low UV Peru non-summer months of May to November were defined as ‘low’ (N=20). Hair growth occurring during both high UV summer months and low UV non-summer months was defined as ‘intermediate’ (N=61). Lastly, in order to assess whether differences in the time between the two hair sample collections (at enrollment and full-term delivery) influenced concordance, categories distinguishing participants whose hair samples were collected 5-6 months apart (N=58) vs. not (N=39) were created. If time between hair samples played a role, we hypothesized that participants whose hair samples were collected 5-6 months apart would be most concordant due to the average time between hair collections.
2.4 STATISTICAL ANALYSIS
The Shapiro-Wilk test statistic was used to test for skewness in HCC. Based on findings of right-skewness, HCC values were transformed on the natural logarithm scale (logHCC) to approximate normality. To facilitate comparison of HCC with other studies, we report geometric mean HCC and standard deviations (SD) for proximal and distal hair segments for all participants and according to participant characteristics. Student’s t-tests or analysis of variance (ANOVA) were used to evaluate differences in logHCC across maternal characteristics for proximal and distal hair segments. We then used paired sample t-tests to evaluate the mean difference between proximal and distal logHCC. Linear regression models were used to determine whether proximal-distal differences (deltas) varied according to maternal characteristics at enrollment. Bland-Altman plots were used to evaluate for evidence of systematic bias in differences. Concordance was further evaluated using scatter plots, Pearson correlation coefficients, Cohen’s weighted kappa test statistics using logHCC tertiles, and intra-class correlation coefficients (ICCs). In the subset of women whose first-trimester hair segments were analyzed using both laboratory methods (N=28), concordance across laboratory methods was evaluated separately for distal and proximal segments. All statistical analyses used SAS® version 9.4 software (SAS Institute, Inc., Cary, North Carolina) and p-values are two-sided at the alpha 0.05 level.
3. RESULTS
Participant ages ranged from 18 to 44 years (mean=26.5 years, SD=5.8), and the mean gestational ages at the two hair collections (i.e. early pregnancy and delivery) were 13.1 weeks (SD=3.9) and 39.0 weeks (SD=1.0), respectively. Participant sociodemographic characteristics, anthropometrics, medical history, and hair characteristics are described in Table 1. Participants reported no medication use or diabetes at time of interview. Differences in proximal HCC were observed across categories of education, asthma, generalized anxiety disorder, hair structure, and UV light exposure. Differences in distal HCC were observed in the similar directions as proximal HCC, albeit only statistically significantly for education and alcohol use.
Table 1:
Hair cortisol concentrations according to study population characteristics (N=97 participants, each with one distal and one proximal hair segment).
| Characteristic | Mean (SD) or % | Proximal 1st trimester hair (0-3cm) |
Distal 1st trimester hair (6-9cm) |
||
|---|---|---|---|---|---|
| Geometric Mean HCC in pg/mg (SD) | Pa | Geometric Mean HCC in pg/mg (SD) | Pa | ||
| All Participants (N=97) | 5.1 (2.6) | - | 3.4 (2.5) | - | |
| Maternal age (years), mean (SD) | 26.5 (5.8) | - | - | - | - |
| Maternal age (years), % | |||||
| 18-19 | 12.4% | 7.2 (2.4) | 0.35 | 5.9 (1.8) | 0.25 |
| 20-29 | 54.6% | 4.4 (2.5) | 3.7 (2.1) | ||
| 30-34 | 21.7% | 5.8 (3.1) | 3.1 (3.6) | ||
| ≥35 | 11.3% | 5.9 (2.2) | 4.5 (3.1) | ||
| Gestational age at enrollment (weeks), mean (SD) | 13.1 (3.9) | - | - | - | - |
| Gestational age at delivery (weeks), mean (SD) | 39.0 (1.0) | - | - | - | - |
| Early pregnancy body mass index (kg/m2), mean (SD) | 25.4 (3.6) | - | - | - | - |
| Early pregnancy body mass index (kg/m2), % | |||||
| 18.5-24.9 | 49.5% | 4.2 (2.5) | 0.11 | 3.4 (2.2) | 0.41 |
| 25.0-29.9 | 42.3% | 6.1 (2.7) | 4.2 (2.7) | ||
| ≥30.0 | 8.3% | 7.2 (2.1) | 5.0 (3.6) | ||
| Education (years), % | |||||
| 7-12 | 39.2% | 7.5 (2.0) | 0.002 | 5.0 (1.9) | 0.030 |
| >12 | 60.8% | 4.0 (2.8) | 3.3 (2.9) | ||
| Smoked prior to this pregnancy, % | |||||
| No | 85.6% | 5.3 (2.6) | 0.43 | 3.7 (2.6) | 0.44 |
| Yes | 14.4% | 4.3 (3.0) | 4.6 (2.1) | ||
| Alcohol consumption prior to this pregnancy, % | |||||
| No | 77.3% | 5.4 (2.3) | 0.28 | 4.3 (2.4) | 0.033 |
| Yes | 22.7% | 4.2 (3.7) | 2.7 (2.9) | ||
| Prior asthma diagnosis ever, % | |||||
| No | 89.7% | 5.7 (2.4) | 0.001 | 4.0 (2.5) | 0.37 |
| Yes | 10.3% | 2.1 (3.4) | 3.0 (3.0) | ||
| Ethnicity, % | |||||
| Mestizo | 85.6% | 5.0 (2.5) | 0.59 | 3.8 (2.7) | 0.57 |
| Other | 14.4% | 5.8 (3.4) | 4.4 (1.6) | ||
| Married or living with partner, % | |||||
| No | 20.6% | 6.4 (1.8) | 0.25 | 3.5 (3.0) | 0.63 |
| Yes | 79.4% | 4.9 (2.8) | 4.0 (2.4) | ||
| Employed during pregnancy, % | |||||
| No | 53.6% | 5.6 (2.4) | 0.34 | 3.4 (2.7) | 0.16 |
| Yes | 46.4% | 4.6 (2.9) | 4.5 (2.3) | ||
| Difficulty paying for basics, % | |||||
| No | 58.8% | 4.7 (2.7) | 0.26 | 3.7 (2.4) | 0.67 |
| Yes | 41.2% | 5.9 (2.5) | 4.1 (2.7) | ||
| Planned pregnancy, % | |||||
| No | 71.1% | 4.9 (2.7) | 0.42 | 3.7 (2.7) | 0.54 |
| Yes | 28.9% | 5.8 (2.4) | 4.2 (2.2) | ||
| First pregnancy, % | |||||
| No | 55.7% | 5.3 (2.7) | 0.78 | 3.8 (2.8) | 0.85 |
| Yes | 44.3% | 5.0 (2.6) | 3.9 (2.2) | ||
| Fair or poor health prior to this pregnancy, % | |||||
| No | 75.3% | 4.8 (2.8) | 0.26 | 3.7 (2.7) | 0.47 |
| Yes | 24.7% | 6.2 (2.1) | 4.4 (2.0) | ||
| Fair or poor health during this pregnancy, % | |||||
| No | 32.0% | 5.1 (3.1) | 0.98 | 3.9 (2.0) | 0.97 |
| Yes | 68.0% | 5.1 (2.4) | 3.9 (2.8) | ||
| Perceived stress score at enrollment, mean (SD) b | 29.0 (4.9) | - | - | - | - |
| Perceived stress score at enrollment, % | |||||
| Tertile 1 (Low) | 44.3% | 6.2 (2.4) | 0.21 | 4.0 (2.8) | 0.73 |
| Tertile 2 (Middle) | 27.8% | 4.2 (2.6) | 3.4 (1.9) | ||
| Tertile 3 (High) | 27.8% | 4.6 (2.8) | 4.1 (2.8) | ||
| Generalized anxiety disorder score, mean (SD) | 5.0 (3.4) | - | - | - | - |
| Generalized anxiety score ≥7, % | |||||
| No | 66.0% | 5.9 (2.4) | 0.051 | 3.9 (2.4) | 0.92 |
| Yes | 34.0% | 3.9 (2.9) | 3.8 (2.8) | ||
| Patient Health Questionnaire score, mean (SD) c | 6.5 (4.1) | - | - | - | - |
| Patient Health Questionnaire score ≥10, % | |||||
| No | 75.3% | 5.3 (2.7) | 0.39 | 3.7 (2.6) | 0.65 |
| Yes | 23.7% | 4.4 (2.2) | 4.1 (2.3) | ||
| Patient Health Questionnaire score, % c | |||||
| Minimal depression, score <5 | 34.4% | 5.1 (2.2) | 0.85 | 3.6 (2.4) | 0.94 |
| Moderate depression, score 5-9 | 41.7% | 5.5 (3.2) | 3.8 (2.8) | ||
| Moderately severe depression, score 10-14 | 17.7% | 4.3 (2.5) | 4.3 (2.6) | ||
| Severe depression, score ≥ 15 | 6.3% | 4.5 (1.5) | 3.6 (1.6) | ||
| Post-Traumatic Stress Disorder Checklist for Civilians score ≥26, % | |||||
| No | 80.4% | 5.0 (2.7) | 0.63 | 3.8 (2.6) | 0.67 |
| Yes | 19.6% | 5.6 (2.3) | 4.2 (2.1) | ||
| Hair color, % | |||||
| Black | 55.7% | 5.4 (2.4) | 0.65 | 3.9 (2.5) | 0.79 |
| Dark Brown | 42.3% | 4.9 (2.9) | 3.8 (2.6) | ||
| Light Brown | 2.1% | 3.0 (1.7) | 6.0 (1.6) | ||
| Hair structure, % | |||||
| Straight | 68.0% | 4.5 (2.7) | 0.040 | 3.7 (2.4) | 0.49 |
| Curly | 32.0% | 6.9 (2.2) | 4.3 (2.9) | ||
| Hair wash frequency (per week), % | |||||
| 1-2 times | 5.2% | 7.6 (3.6) | 0.28 | 5.2 (3.4) | 0.74 |
| 3-5 times | 78.4% | 4.7 (2.6) | 3.8 (2.6) | ||
| 6-7 times | 16.5% | 6.6 (2.2) | 4.1 (1.8) | ||
| Use of shampoo or conditioner when washing, % | |||||
| Shampoo only | 34.0% | 5.7 (2.5) | 0.43 | 4.7 (2.1) | 0.15 |
| Shampoo and conditioner | 66.0% | 4.9 (2.7) | 3.5 (2.7) | ||
| Chemical hair treatment use (tint, dye, or perm) | |||||
| No | 60.8% | 4.5 (2.8) | 0.12 | 3.8 (2.3) | 0.81 |
| Yes | 39.2% | 6.2 (2.3) | 4.0 (2.9) | ||
| Hair cutting frequency | |||||
| Every month | 10.3% | 5.7 (2.5) | 0.59 | 2.6 (2.5) | 0.75 |
| Every 3 months | 32.0% | 4.2 (3.6) | 3.5 (3.4) | ||
| Every 6 months | 30.9% | 5.3 (2.3) | 4.2 (2.3) | ||
| Once a year | 18.6% | 7.0 (1.9) | 4.5 (1.8) | ||
| Other | 8.3% | 4.9 (1.8) | 4.2 (1.8) | ||
| UV light exposure in first trimester d | |||||
| Low | 20.6% | 3.4 (2.6) | 0.046 | 4.4 (2.1) | 0.73 |
| Intermediate | 62.9% | 5.4 (2.6) | 3.6 (2.9) | ||
| High | 16.5% | 7.2 (2.3) | 4.1 (1.5) | ||
P-values in bold are statistically significant at the alpha 0.05 level
Abbreviations: HCC= hair cortisol concentration and SD= standard deviation
=Student’s t-test p-values for differences in mean logHCC across two categories, and ANOVA p-values for differences in mean logHCC across three or more categories.
=N=95 participants, each with one proximal and one distal hair segment
=N=96 participants, each with one proximal and one distal hair segment
=UV light categories were defined as “low” if hair growth occurred during the non-summer months of May through November only, “intermediate” if they occurred during both summer and non-Summer months, and “high” if occurred during the summer months of December to April only.
Mean logHCC, measured using immunoassay, in proximal hair segments were higher compared with distal segments,1.64 vs. 1.35 respectively (p-value=0.02) (Table 2). Bland-Altman plots showed no evidence of systematic bias in difference estimates (Figure 3). No statistical difference was observed using LC-MS/MS (1.99 vs. 1.83, p-value=0.33). The magnitudes of the delta values were investigated according to maternal characteristics in Table 1. Delta values statistically differed according to the following maternal characteristics at enrollment: employment status, UV exposure, and asthma diagnosis (Supplemental Table 1). Specifically, larger differences were observed among participants who were unemployed vs. employed, among participants exposed to higher UV in early pregnancy vs. low UV, and among participants with a history of asthma vs. participants without a history of asthma.
Table 2:
Comparisons of proximal and distal first-trimester hair cortisol concentrations by laboratory method of detection
| Method | N | Proximal (0-3cm) |
Distal (6-9cm) |
Difference in mean logHCC (95% CI) | P a | ||
|---|---|---|---|---|---|---|---|
| Mean logHCC | SD | Mean logHCC | SD | ||||
| Immunoassay | 97 | 1.64 | 0.96 | 1.35 | 0.93 | 0.28 (0.05 – 0.51) | 0.02 |
| LC-MS/MS | 28 | 1.99 | 0.78 | 1.83 | 0.67 | 0.16 (−0.16 – 0.47) | 0.33 |
Abbreviations: logHCC= log-transformed hair cortisol concentrations, LC-MS/MS= Liquid chromatography tandem mass spectrometry, SD = standard deviation, CI = confidence interval
Paired t-test p-values for paired data
Figure 3:
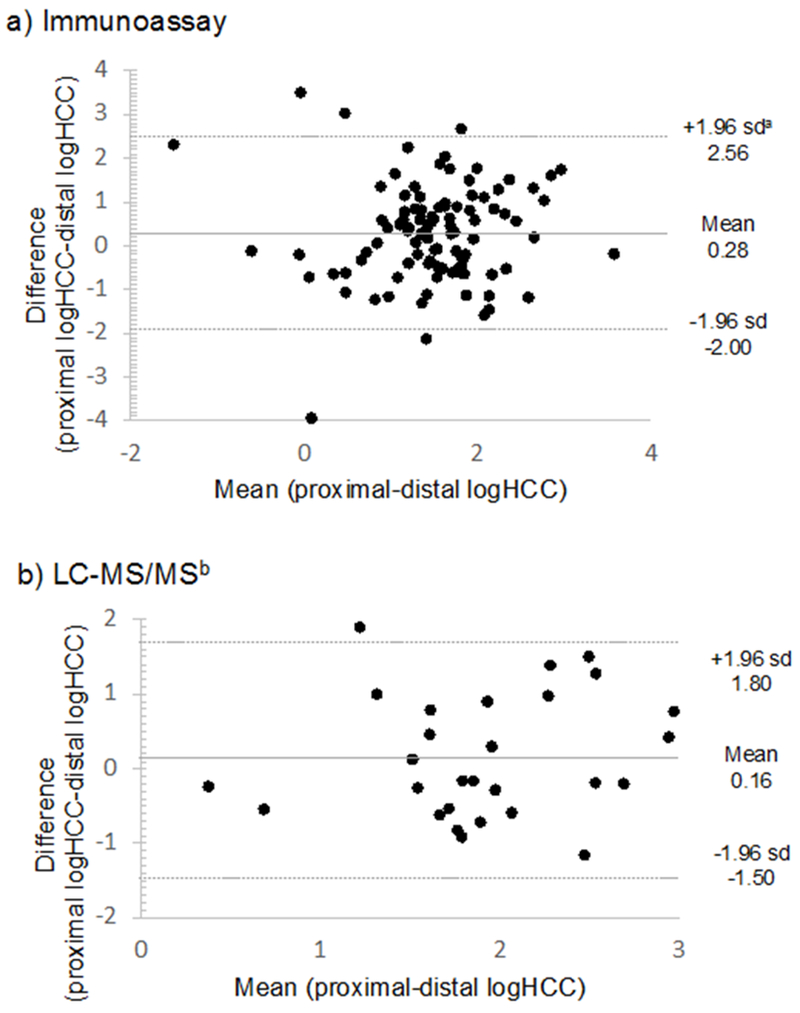
Bland-Altman plots comparing first trimester proximal (0-3cm hair sample 1) and distal (6-9cm on hair sample 2) log-transformed hair cortisol concentrations (logHCC), by laboratory method of detection, immunoassay (N=97) and liquid chromatography tandem mass spectrometry (LC-MS/MS) (N=28).
asd= standard deviation
The Pearson correlation between proximal and distal first trimester hair segments measured using immunoassay was 0.27 (p-value=0.008) (Figure 4). Correlation coefficients were highest among the subset of participants whose hair samples were collected 5-6 months apart (N=58, r=0.36, p-value 0.006) as compared to participants whose hair samples were not collected 5-6 months apart (N=39, r=0.14, p-value=0.39). The Pearson correlation between proximal and distal first trimester hair segments measured using LC-MS/MS was 0.37 (p-value=0.05) (Figure 4). The proportion of hair samples concordantly ranked as either low, middle, or high according to HCC tertiles was low comparing proximal and distal rankings (immunoassay weighted kappa= 0.10, LC-MS/MS weighted kappa= 0.07) (Table 3). Intra-class correlation (ICC) values were also low (immunoassay ICC: 0.46, LC-MS/MS ICC: 0.34) (Table 3). In the subset of participants whose proximal and distal hair samples were analyzed using both immunoassay and LC-MS/MS, concordance was high among distal segments (r=0.78, p-value <0.001; weighted kappa=0.64) and high among proximal segments (r=0.96, p-value <0.001; weighted kappa=0.75) (Figure 4). Batch-corrected immunoassay agreement measures did not substantially differ from the immunoassay agreement measures provided (Supplemental Table 2).
Figure 4:
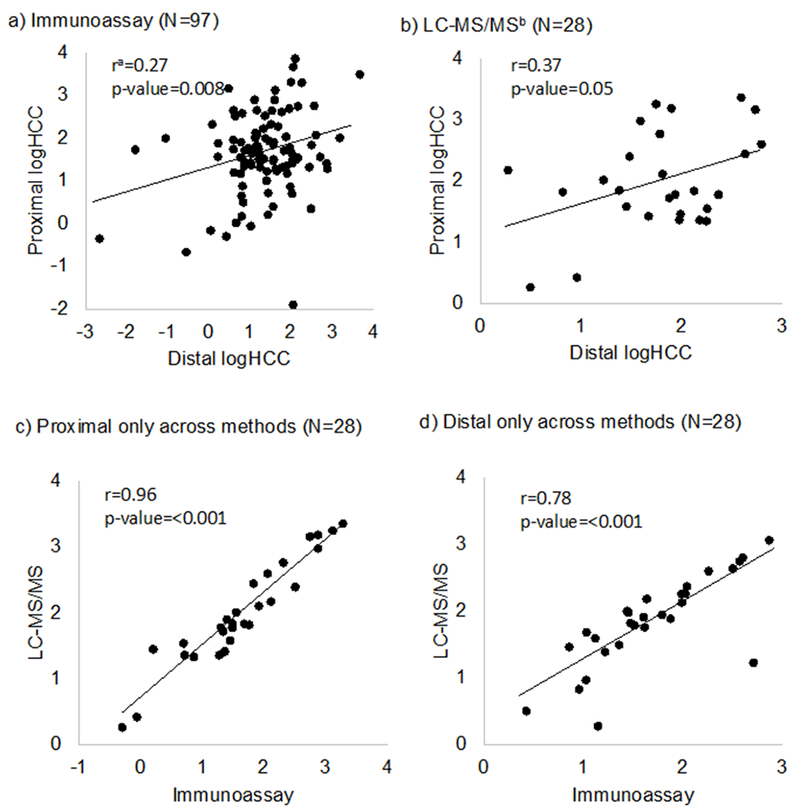
Scatterplots comparing proximal and distal log-transformed hair cortisol concentrations (logHCC) by laboratory method of detection.
a r= Pearson correlation coefficient, b LC-MS/MS=liquid chromatography tandem mass spectrometry
Table 3:
Agreement between proximal and distal first trimester hair cortisol concentrations according to method of cortisol analysis
| Immunoassay (N=97) | |||
| Proximal logHCC tertiles |
|||
| Distal logHCC tertiles | Low | Mid | High |
| Low | 13 (43.3%) | 12 (35.3%) | 7 (21.2%) |
| Mid | 8 (26.7%) | 11 (32.4%) | 14 (42.4%) |
| High | 9 (30.0%) | 11 (32.4%) | 12 (36.4%) |
| Total | 30 (100.0%) | 34 (100.0%) | 33 100.0%) |
| Weighted Kappa statistic = 0.10 | |||
| Intra-class correlation coefficient = 0.46 | |||
| Between-person variation = 0.40 | |||
| Total variation = 0.86 | |||
| Liquid Chromatography (N=28) | |||
| Proximal logHCC tertiles |
|||
| Distal logHCC tertiles | Low | Mid | High |
| Low | 3 (37.5%) | 5 (45.5%) | 2 (22.2%) |
| Mid | 2 (25.0%) | 3 (27.3%) | 3 (33.3%) |
| High | 3 (37.5%) | 3 (27.3%) | 4 (44.4%) |
| Total | 8 (100.0%) | 11 (100.0%) | 9 (100.0%) |
| Weighted Kappa statistic = 0.07 | |||
| Intra-class correlation coefficient = 0.34 | |||
| Between-person variation = 0.19 | |||
| Total variation = 0.55 | |||
Bolded values indicate the diagonal cells of agreement.
Abbreviations: logHCC = log-transformed hair cortisol concentrations
4. DISCUSSION
On average, HCC measured in distal hair segments collected at delivery were lower and had poor agreement with HCC measured in proximal hair segments collected in early pregnancy. This suggests that distal hair segments collected at the time of delivery cannot be used to reflect cortisol concentrations in the first trimester of pregnancy, and more generally that distal hair segments beyond 6cm from the scalp may not be appropriate for use in studies aiming to assess HCC over a 9-month period. Our findings also show that despite lower absolute measures of HCC using immunoassay as compared to LC-MS/MS, the two laboratory methods strongly agree and preserve relative rankings, as previously shown in an inter-laboratory round robin (Russell et al., 2015).
Reasons for why distal segments beyond 6cm from the scalp may not reflect cortisol concentrations in the presumed time period have been hypothesized to be due to “washout effects” over time (Russell et al., 2012). Successive laboratory washes of hair samples have been shown to result in the decreases in HCC due to potential leaching of cortisol from the hair (Davenport et al., 2006), mainly from water exposure rather than shampoo treatment (Hamel et al., 2011; Li et al., 2012). In our study, we observed lower HCC with increased self-reported hair washing frequency in early pregnancy; however these differences were not statistically significant. One study among non-pregnant women determined that HCC naturally declines by 30-40% as one moves from proximal to distal hair segments on the same hair sample (Kirschbaum et al., 2009). In comparison, we observed a smaller magnitude of difference comparing proximal and distal hair segments across different hair samples (mean logHCC values of 1.64 and 1.35, a 32% difference in HCC on the original scale).
We are aware of only one other study that compared early pregnancy cortisol concentrations in proximal and distal hair segments (D’Anna-Hernandez et al., 2011). In their study of 14 women recruited prior to 17 weeks gestational age with non-complicated pregnancies, D’Anna-Hernandez and colleagues reported no agreement in HCC proximal and distal segments reflecting early pregnancy (Pearson correlation coefficient=−0.2, p=0.29). Both our study and D’Anna-Hernandez et al. sampled hair from the posterior vertex of the scalp at two times (first in early pregnancy and again at delivery), used similar laboratory methods, recruited participants of comparable maternal age and at comparable times in pregnancy, and included participants with no medication use. Studies differed in sample size (14 vs. 97), region (USA vs. Peru), in exclusion criteria (our study included smokers (14.5%) and those who used hair treatments (39.2%)), and magnitude of correlation (−0.2, p-value 0.29 vs. 0.27, p-value 0.008). Despite differences in study design, geographic region, and behavioral characteristics, our present findings and that of D’Anna-Hernandez et al. indicate that distal hair segments collected at delivery are in poor agreement with proximal hair segments collected in early pregnancy.
Our study also builds upon previous findings by suggesting that differences in proximal and distal HCC values may vary according to participant characteristics. Specifically, we observed larger differences among participants who were unemployed compared to participants who were employed, among participants exposed to higher UV compared to participants exposed to low UV, and among participants with a history of asthma compared to participants without a history of asthma. Reasons for a larger difference among the non-employed as compared to the employed are unclear, and differences according to asthma status were based on a small sample of asthmatics (10.3%, N=10) requiring replication. However, given that asthma is commonly treated with corticosteroids, researchers interested in early pregnancy maternal cortisol levels should take this into consideration. Despite the fact that we observed higher HCC values in proximal hair segments that grew during seasons of higher UV, we observed larger proximal-distal differences at higher UV indices. This is plausible given that high UV irradiation is an environmental factor believed to facilitate washout of cortisol from hair over time (Dettenborn et al., 2012). If such differences across subgroups hold, differential measurement error of HCC in distal segments may result in biased estimates. Therefore, researchers interested in utilizing distal hair segments may need to consider how they will account for such differences in the study design and analytic phases.
Our findings of strong agreement across laboratory methods of cortisol analysis are consistent with earlier reports. A recent international inter-laboratory round robin of 15 hair samples showed excellent agreement comparing immunoassay and LC-MS/MS analyzed HCC levels (r’s ranged from 0.89-0.98) (Russell et al., 2015), though it is unclear if the hair samples used were proximal or distal segments. We show that correlations comparing proximal segments were stronger than correlations comparing distal segments (r’s=0.96 vs. 0.78), and that relative HCC rankings are preserved, albeit to a stronger extent in proximal segments compared to distal segments. Furthermore, ICC values indicated lower between-person and total variation using LC-MS/MS values compared to immunoassay values, a difference potentially due to the smaller sample size of the LC-MS/MS values. This information may be of importance for researchers deciding between the two laboratory methods.
In our investigation of the concordance between HCC in proximal and distal hair segments, both presumed to reflect the first trimester of pregnancy, our study had some limitations. First, our study assessed all maternal characteristics once at enrollment. Therefore, any changes that occurred later in pregnancy, such as hair washing frequency and chemical treatment, were not accounted for. Second, it is possible that morphological changes in hair (such as increased thickness) may have occurred during the second and third trimesters of pregnancy, potentially affecting porosity and leaching of cortisol from hair segments. However, the impact of these changes on distal first trimester hair segments is likely minimal given that first trimester hair growth was already complete. Third, the timing between hair collections was not exactly six months for all participants. According to the average hair growth (1cm/month), a six-month window between the two hair collections would have resulted in optimal overlap of the 3cm proximal and distal hair segments. Therefore, differences in time between hair collections may have impacted our findings. However, the mean timing between hair collections was 5.8 months (SD=1.1), close to this optimal window of overlap. To investigate the extent to which timing between hair collections impacted our findings, we evaluated concordance among a subset collected 5-6 months apart (N=58). In doing so, we observed stronger correlations (r=0.37). Therefore, the timing between hair collections may have had some impact on our findings. However, the magnitude of this influence does not appear substantial (r=0.27 among all participants vs. r=0.37 among this subset). Interestingly, slower underarm hair growth has been observed with pregnancy progression (Pecoraro et al., 1971). While this is not a major concern given our focus on scalp hair, we provide it as potential evidence for the impacts of pregnancy on general hair growth. Fourth, our CV’s are on the higher end of the acceptable range. However, batch-corrected findings were very similar. Lastly, we cannot extrapolate our findings of poor concordance of distal and proximal segments to non-pregnant populations.
Despite these limitations our study had many strengths. First, our study used standardized hair collection and extraction procedures. For example, the posterior vertex region of the scalp has the lowest intra-individual variation of HCC compared to samples obtained from other areas of the scalp (16% vs. 31%) (Sauve et al., 2007), and strong agreement has been observed comparing HCC across the posterior vertex region of the scalp during pregnancy (Pearson correlation = 0.85, p<0.001) (D’Anna-Hernandez et al., 2011). Second, our study assessed concordance using two laboratory methods of cortisol analysis, immunoassay and LC-MS/MS. Third, our study assessed multiple concordance measures, which yield similar conclusions. Fourth, our sample size of 97 participants, each of whom provided one proximal and one distal hair segment, exceeds the sample size of the only other known comparable study, although, an evaluation of concordance using a larger sample of participants or in non-pregnant populations is warranted. Lastly, our restriction to full-term deliveries helped to ensure that distal and proximal hair segments represented similar times during pregnancy.
5.0 CONCLUSION
HCC in distal hair segments collected at delivery do not appear to reflect HCC in proximal hair segments collected in early pregnancy. Therefore, in accordance with previous suggestions, we suggest that investigators interested in long-term maternal cortisol secretion in early pregnancy restrict analyses to proximal hair segments collected in early pregnancy, or if possible, perform a validation sub-study similar to our own study to determine the extent of cortisol degradation if any.
Supplementary Material
Acknowledgments
Source of funding and conflicts of interest:
Awards from the National Institutes of Health, National Institute of Minority Health and Health Disparities (T37-MD-001449) and Eunice Kennedy Shriver Institute of Child Health and Human Development (R01-HD-059835) supported this work. The National Institute of Health Training Grant in Psychiatric Epidemiology (T32-MH-017119) supported Olivia R. Orta, and a grant from the National Institutes of Health (ES000002) supported Brent A. Coull. The National Institutes of Health had no further role in the study design, data collection, analysis, interpretation of data, writing of the report, or in the decision to submit the paper for publication. Neither the authors nor the institution with which they are affiliated have any direct or indirect financial interest in the subject matter of our manuscript. The authors report no other conflict of interest in this work.
References
- Albar WF, Russell EW, Koren G, Rieder MJ, van Umm SH, 2013. Human hair cortisol analysis: comparison of the internationally-reported ELISA methods. Clinical and investigative medicine. Medecine clinique et experimentale 36, E312–316. [DOI] [PubMed] [Google Scholar]
- Astore IP, Pecoraro V, Pecoraro EG, 1979. The normal trichogram of pubic hair. The British journal of dermatology 101, 441–445. [DOI] [PubMed] [Google Scholar]
- Barman JM, Astore I, Pecoraro V, 1965. The Normal Trichogram of the Adult. The Journal of investigative dermatology 44, 233–236. [DOI] [PubMed] [Google Scholar]
- Barrios YV, Gelaye B, Zhong Q, Nicolaidis C, Rondon MB, Garcia PJ, Sanchez PA, Sanchez SE, Williams MA, 2015. Association of childhood physical and sexual abuse with intimate partner violence, poor general health and depressive symptoms among pregnant women. PloS one 10, e0116609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth JH, 1986. Measurement of hair growth. Clinical and experimental dermatology 11, 127–138. [DOI] [PubMed] [Google Scholar]
- Bjelanovic V, Babic D, Hodzic D, Bjelanovic A, Kresic T, Dugandzic-Simic A, Oreskovic S, 2015. Correlation of psychological symptoms with cortisol and CRP levels in pregnant women with metabolic syndrome. Psychiatria Danubina 27 Suppl 2, 578–585. [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA, 1996. Psychometric properties of the PTSD Checklist (PCL). Behaviour research and therapy 34, 669–673. [DOI] [PubMed] [Google Scholar]
- Bolten MI, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D, 2011. Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Archives of women’s mental health 14, 33–41. [DOI] [PubMed] [Google Scholar]
- Braithwaite EC, Murphy SE, Ramchandani PG, 2016. Effects of prenatal depressive symptoms on maternal and infant cortisol reactivity. Archives of women’s mental health 19, 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CW, Roulet F, 1970. Increased exposure of tissues to cortisol in late pregnancy. British medical journal 1, 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimele V, Kintz P, Dumestre V, Goulle JP, Ludes B, 2000. Identification of ten corticosteroids in human hair by liquid chromatography-ionspray mass spectrometry. Forensic science international 107, 381–388. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. Journal of health and social behavior 24, 385–396. [PubMed] [Google Scholar]
- Conrad F, Paus R, 2004. Estrogens and the hair follicle. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG 2, 412–423. [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML, 2011. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology & behavior 104, 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS, 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and comparative endocrinology 147, 255–261. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA, 2007. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry 46, 737–746. [DOI] [PubMed] [Google Scholar]
- Dettenborn L, Tietze A, Kirschbaum C, Stalder T, 2012. The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 15, 578–588. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH, 2009. Prenatal depression restricts fetal growth. Early human development 85, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Deeds O, Holder V, Schanberg S, Kuhn C, 2009. Depressed pregnant black women have a greater incidence of prematurity and low birthweight outcomes. Infant behavior & development 32, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Kirschbaum C, Grass J, Stalder T, 2016. LC-MS based analysis of endogenous steroid hormones in human hair. The Journal of steroid biochemistry and molecular biology 162, 92–99. [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C, 2013. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 928, 1–8. [DOI] [PubMed] [Google Scholar]
- Gelaye B, Zheng Y, Medina-Mora ME, Rondon MB, Sanchez SE, Williams MA, 2017. Validity of the posttraumatic stress disorders (PTSD) checklist in pregnant women. BMC psychiatry 17, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht GF, Campbell T, Letourneau N, Kooistra L, Kaplan B, 2012. Psychological distress and salivary cortisol covary within persons during pregnancy. Psychoneuroendocrinology 37, 270–279. [DOI] [PubMed] [Google Scholar]
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G, 2010. An assessment of cortisol analysis in hair and its clinical applications. Forensic science international 196, 32–37. [DOI] [PubMed] [Google Scholar]
- Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA, 2011. Effects of shampoo and water washing on hair cortisol concentrations. Clinica chimica acta; international journal of clinical chemistry 412, 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Skovgaard LT, Christensen JM, 2001. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clinica chimica acta; international journal of clinical chemistry 309, 25–35. [DOI] [PubMed] [Google Scholar]
- Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, Ross RG, 2016. Measures of Maternal Stress and Mood in Relation to Preterm Birth. Obstetrics and gynecology 127, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L, 2009. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34, 32–37. [DOI] [PubMed] [Google Scholar]
- Lachelin GC, 2013. Introduction to clinical reproductive endocrinology. Butterworth-Heinemann. [Google Scholar]
- Li J, Xie Q, Gao W, Xu Y, Wang S, Deng H, Lu Z, 2012. Time course of cortisol loss in hair segments under immersion in hot water. Clinica chimica acta; international journal of clinical chemistry 413, 434–440. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Hornych K, Zingler C, Schuff-Werner P, Hoppner J, Virchow JC, 2006. Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology 31, 388–394. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, El Rawadi C, Genain G, 2005. Diversity of hair growth profiles. International journal of dermatology 44 Suppl 1, 6–9. [DOI] [PubMed] [Google Scholar]
- Lynfield YL, 1960. Effect of pregnancy on the human hair cycle. The Journal of investigative dermatology 35, 323–327. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T, 1999. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences 896, 30–47. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Braithwaite EC, Hubbard I, Williams KV, Tindall E, Holmes EA, Ramchandani PG, 2015. Salivary cortisol response to infant distress in pregnant women with depressive symptoms. Archives of women’s mental health 18, 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG, 2006. Cortisol levels and very early pregnancy loss in humans. Proceedings of the National Academy of Sciences of the United States of America 103, 3938–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissimov J, Elchalal U, 2003. Scalp hair diameter increases during pregnancy. Clinical and experimental dermatology 28, 525–530. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER, 2014. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: short-term longitudinal study. Biological psychology 96, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, Moore R, Patrick K, 2011. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: a pilot study. Journal of affective disorders 130, 300–305. [DOI] [PubMed] [Google Scholar]
- Pecoraro V, Astore I, Barman JM, 1971. Growth rate and hair density of the human axilla. A. Comparative study of normal males and females and pregnant and post-partum females. The Journal of investigative dermatology 56, 362–365. [DOI] [PubMed] [Google Scholar]
- Pecoraro V, Astore IPL, Barman JM, 1967. The normal trichogram of pregnant women In: Advances in Biology of the skin (Ed. by Montagna W and Dobson JM), Vol. IX, New York. [Google Scholar]
- Peer M, Soares CN, Levitan RD, Streiner DL, Steiner M, 2013. Antenatal depression in a multi-ethnic, community sample of Canadian immigrants: psychosocial correlates and hypothalamic-pituitary-adrenal axis function. Canadian journal of psychiatry. Revue canadienne de psychiatrie 58, 579–587. [DOI] [PubMed] [Google Scholar]
- Pragst F, Balikova MA, 2006. State of the art in hair analysis for detection of drug and alcohol abuse. Clinica chimica acta; international journal of clinical chemistry 370, 17–49. [DOI] [PubMed] [Google Scholar]
- Raul JS, Cirimele V, Ludes B, Kintz P, 2004. Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical biochemistry 37, 1105–1111. [DOI] [PubMed] [Google Scholar]
- Rosner B, Cook N, Portman R, Daniels S, Falkner B, 2008. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American journal of epidemiology 167, 653–666. [DOI] [PubMed] [Google Scholar]
- Russell E, Kirschbaum C, Laudenslager ML, Stalder T, de Rijke Y, van Rossum EF, Van Uum S, Koren G, 2015. Toward standardization of hair cortisol measurement: results of the first international interlaboratory round robin. Therapeutic drug monitoring 37, 71–75. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S, 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37, 589–601. [DOI] [PubMed] [Google Scholar]
- Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SH, 2007. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and investigative medicine. Medecine clinique et experimentale 30, E183–191. [DOI] [PubMed] [Google Scholar]
- Smith LK, Cidlowski JA, 2010. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Progress in brain research 182, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, 2012. Analysis of cortisol in hair--state of the art and future directions. Brain, behavior, and immunity 26, 1019–1029. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C, 2012. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology 37, 602–610. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Hankinson SE, 2006. Use of biomarkers in epidemiologic studies: minimizing the influence of measurement error in the study design and analysis. Cancer causes & control : CCC 17, 889–899. [DOI] [PubMed] [Google Scholar]
- Van Londen L, Goekoop JG, Zwinderman AH, Lanser JB, Wiegant VM, De Wied D, 1998. Neuropsychological performance and plasma cortisol, arginine vasopressin and oxytocin in patients with major depression. Psychological medicine 28, 275–284. [DOI] [PubMed] [Google Scholar]
- Voegtline KM, Costigan KA, Kivlighan KT, Laudenslager ML, Henderson JL, DiPietro JA, 2013. Concurrent levels of maternal salivary cortisol are unrelated to self-reported psychological measures in low-risk pregnant women. Archives of women’s mental health 16, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosu AC, Valdimarsdottir U, Shields AE, Williams DR, Williams MA, 2013. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Annals of epidemiology 23, 797–811 e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Gelaye B, Rondon M, Sanchez SE, Garcia PJ, Sanchez E, Barrios YV, Simon GE, Henderson DC, Cripe SM, Williams MA, 2014. Comparative performance of Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale for screening antepartum depression. Journal of affective disorders 162, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong QY, Gelaye B, Zaslavsky AM, Fann JR, Rondon MB, Sanchez SE, Williams MA, 2015. Diagnostic Validity of the Generalized Anxiety Disorder - 7 (GAD-7) among Pregnant Women. PloS one 10, e0125096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


