Abstract
Engagement in cognitive demanding everyday activities has been shown to benefit cognitive health in later life. We investigated the factors that influence engagement, with specific interest in determining the extent to which the costs of engaging cognitive resources are associated with intrinsic motivation and, ultimately, participation in everyday activities. Older adults (N = 153) aged from 65 to 81 years completed a challenging cognitive task, with the costs of cognitive engagement—operationalized as the effort required to maintain performance—assessed using systolic blood pressure responses (SBP-R). We also assessed participation in everyday activities using both two-year retrospective reports and five daily reports over a five-week period. Structural models revealed that lower levels of costs were associated with more positive attitudes about aging, which in turn were associated with higher levels of intrinsic motivation. Motivation was subsequently predictive of everyday activity engagement, with the effect being specific to those activities thought to place demands on cognitive resources. The measure of engagement had minimal impact on the nature of the observed effects, suggesting that the retrospective and weekly assessments were tapping into similar constructs. Taken together, the results are consistent with expectations derived from Selective Engagement Theory (Hess, 2014), which argues that engagement in demanding activities is related to the cost associated with such engagement, which in turn leads to selective participation through changes in motivation.
Keywords: Aging, motivation, engagement, aging attitudes, cognition
Research is increasingly demonstrating the beneficial effects of activity participation on cognitive health in later life, particularly those activities that involve physical or mental demands (see Hertzog, Kramer, Wilson, & Lindenberger, 2008). There is also evidence, however, that activity participation decreases in old age, especially for those activities that are non-obligatory in nature or cognitively demanding (e.g., M.M. Baltes & Lang, 1997; Jopp & Hertzog, 2007; Mitchell et al., 2012). Given the benefits of such activity participation, an important question concerns the factors that determine engagement. That is, what distinguishes those older adults who engage in these activities from those who do not? In addition, how do the characteristics of the individual that determine engagement interact with the specific situation to influence activity participation? Surprisingly, given the importance of activity participation, there has been relatively little research examining the factors that are predictive of engagement.
Selective Engagement
One perspective that may prove helpful in understanding participation is selective engagement theory (SET; Hess, 2014). This theory argues that aging is associated with a negative impact on the resources underlying participation in cognitively demanding activities, effectively increasing the costs of engagement in later life. At any given level of perceived benefits of an activity, these increased costs essentially reduce the ratio of benefits to costs for that activity for older relative to younger adults. This, in turn, has a negative impact on older adults’ willingness (i.e., motivation) to engage cognitive resources. These enhanced costs also increase the salience of attributes such as the meaningfulness of the activity (e.g., congruence with personal goals), which factor into the perceived benefits that are eventually weighed against these costs in determining engagement. For example, younger adults may be willing to engage in a moderately challenging activity even if the benefits are not great due to the relatively low demands on their cognitive resources (i.e., costs) relative to these benefits. In contrast, due to the hypothesized normative increase in costs of resource engagement, the perceived benefits in this same situation will need to be greater to encourage engagement in older adults.
Past research has provided support for this theory by demonstrating: (a) normative increases in later life in the costs associated with engagement (e.g., Cappell, Gmeindl, & Reuter-Lorenz, 2010; Ennis, Hess, & Smith, 2013; Hess & Ennis, 2012); (b) increased selectivity in old age relating to situational factors affecting the perceived importance of the task (e.g., Smith & Hess, 2015; Zhang, Fung, Stanley, Isaacowitz, & Ho, 2013); and (c) the mediating role of motivation in determining the linkages between resources thought to underlie cognitive costs (e.g., health) and engagement in everyday activities (e.g., Hess, Emery, & Neupert, 2011; Queen & Hess, 2018).
Of central importance to this framework is the linkage between costs and motivation. Within SET, costs are operationalized in terms of the resources or effort required to achieve a specific level of objective task performance, along with the consequences associated with effort expenditure (e.g., fatigue). Ideally, such costs are assessed within the context of actual task engagement under constrained conditions designed to encourage participants to expend the effort necessary to achieve successful performance. In previous studies, based on ideas by Obrist (1981) and Wright (1996), we have used systolic blood pressure response (SBP-R) as an index of engagement to monitor costs under such conditions. This research has found that SBP-R varies systematically with both task difficulty and situational factors designed to affect motivation. Importantly, evidence for an age-related increase in costs is found in the facts that (a) older adults exhibit higher SBP-R—thought to reflect effort—than young adults at all levels of objective task difficulty (e.g., Ennis et al., 2013; Hess, Smith, & Sharifian, 2016), and (b) the higher level of effort accounts for the greater fatigue effects observed in older adults (Hess & Ennis, 2012; Smith & Hess, 2015).
The previously discussed research exploring the relationship between costs and motivation focused only on factors assumed to underlie these costs as opposed to a more direct index. For example, using data from the Health and Retirement Study, Queen and Hess (2018) found that intrinsic motivation to engage in cognitively challenging activities—as assessed at the trait level by Need for Cognition (Cacioppo, Petty, Feinstein, & Jarvis, 1996)—partially mediated the relationship between resources associated with both health and cognitive ability and the frequency of engagement in cognitively demanding everyday tasks (see also Hess et al., 2011). In the present study, we build on this research by using a more direct assessment of costs as reflected in SBP-R assessed during a laboratory-based memory-scan task similar to that used in previous research (e.g., Ennis et al., 2013). Individual indices of costs associated with engagement of resources to support task performance were extracted based on changes in responses to task demands. We then modeled the extent to which intrinsic motivation (i.e., Need for Cognition) mediated the relationship between costs and assessments of engagement in everyday activities. We hypothesized that costs would be negatively associated with need for cognition, which in turn would be positively associated with activity participation. To be consistent with our past research (e.g., Queen & Hess, 2018), we also included measures of health and ability as predictors of intrinsic motivation. We were specifically interested in whether these factors undergird individual differences in costs or accounted for independent variance in Need for Cognition.
We further predicted that this mediating relationship would be strongest for those activities that are assumed to involve some degree of challenge and place demands on cognitive resources (e.g., learning a new language) than for those considered to be more passive and less demanding (e.g., watching TV). To test this, we expanded upon our previous attempts to assess activity by using a modified version of the Victoria Longitudinal Study Activity Questionnaire (VLSAQ; Jopp & Hertzog, 2010). Specifically, we examined several subcategories of activities across which the cognitive demands or costs associated with participation were assumed to vary based upon the correlation between participation levels and fluid intelligence identified by Jopp and Hertzog.
The extraction of a valid index of cognitive costs—in this case, conceptualized in terms of effort expenditure—was critical to the present analysis. Although previous research with older adults has suggested that SBP-R is a valid index of effort (see Hess & Ennis, 2014), aging is also associated with a general increase in SBP reactivity to emotionally evocative situations in later life (Uchino, Birmingham, & Berg, 2010). This suggests the possibility that previously observed age differences in SBP response thought to reflect engagement or effort expenditure (e.g., Ennis et al., 2013) may simply be indicative of normative changes in physiological reactivity to stress. We argue, however, that there is a fundamental difference between reactivity in passive coping situations (e.g., unavoidable exposure to stressors) versus responses in situations that involve active coping on the part of the individual (e.g., performance on a cognitive test) (see Obrist, 1981). Thus, we distinguish between reactivity in the former, and responsivity in the latter. To ensure that our assessment of costs in the present study reflected individual differences in effort expenditure are independent of general physiological reactivity, we also measured general reactivity using a cold pressor task—a common means of assessing reactivity in situations involving passive coping (e.g., Huisman et al., 2002). A measure of reactivity from this task was then entered as a covariate in the analyses of SBP responsivity in the memory-scan task.
Aging Attitudes
We were also interested in examining the possibility that attitudes about aging influence the relationship between costs, motivation, and activity participation. A growing body of research indicates that attitudes are predictive of important functional outcomes in later life (e.g., Levy, Zonderman, Slade, & Ferrucci, 2009, 2012). In addition, attitudes are positively associated with engagement in physical and social activities in older adults (e.g., Emile, Chalabaev, Stephan, Corrion, & d’Arripe-Longueville, 2014; Hicks & Siedlecki, 2017; Palacios, Torres, & Mena, 2009; Wurm, Tomasik, & Tesch-Römer, 2009), suggesting that attitudes may influence the motivation to engage in the types of activities that promote optimal functioning. Little is known, however, about either the determinants of individual differences in attitudes or the specific mechanisms through which attitudes have their impact. One possibility is that declines in the resources supporting performance of demanding activities reinforce negative expectations about the impact of aging on the cognitive costs of, and the associated ability to perform such activities.
Consistent with Obrist (1981), SET originally assumed that effort expenditure (e.g., SBP-R) was a relatively direct reflection of sympathetic nervous system activation associated with active coping in response to task demands. However, we have also shown that subjective perceptions of task difficulty account for significant variance in engagement levels in older adults above-and-beyond that accounted for by objective task demands (e.g., Hess et al., 2016). In addition, Zafeiriou and Gendolla (2017) recently observed that implicit activation of aging stereotypes in young adults resulted in elevated levels of effort expenditure. They attributed this to fact that cognitive difficulties are an important component of aging stereotypes, and that the increased accessibility of this belief following stereotype activation subtly influenced perceptions of the effort required to support task performance. Together, these findings suggest that negative beliefs about aging may be associated with increased salience of cognitive costs, potentially influencing perceptions of task demands and subsequent motivation to engage.
We examined two possible ways in stereotypes might exert their influence. First, individuals with negative attitudes might interpret the high levels of effort they must exert while performing a cognitively challenging task as a reflection of the negative impact of aging on cognitive resources as opposed to simply being due to the actual demands of the task. This results in a calculation of costs that is reflective of both objective task demands and subjective interpretations of task demands, with negative attitudes potentially having a negative influence on motivation through their magnification of costs. Alternatively, much like physical abilities, health, and well-being (e.g., Bryant et al., 2016; Kornadt & Rothermund, 2012), the costs of cognitive engagement experienced by older adults might have a more direct impact on attitudes (e.g., greater costs associated with more negative attitudes), which in turn would mediate the relationship between costs and motivation.
This examination of aging attitudes within the context of SET also highlights a potential pathay through which attitudes may have an impact on behavior. Although there is much evidence associating negative attitudes about aging with negative outcomes in everyday functioning, the specific mechanisms through which attitudes exert their influence are relatively obscure. Levy (2009) suggests that attitudes have their impact through processes of internalization, which then presumably impact behavior in ways that eventually have either positive or negative consequences, depending on one’s attitudes. Research demonstrating an increase in outcomes consistent with negative aging stereotypes (e.g., poorer memory performance) due to stereotype threat or implicit priming of negative stereotypes (e.g., Hess, Auman, Colcombe, & Rahhal, 2003; Levy, 1996) is also suggestive of potential mechanisms. There has not, however, been much research examining linkages between attitudes and behaviors in everyday life. The previously discussed association between costs, attitudes, and motivation, however, suggests a potential avenue of influence. For example, an increase in the experienced costs associated with engaging in demanding tasks (e.g., learning to use a computer) in later life by older adults may negatively affect expectations or attitudes regarding the impact of aging on their ability to perform these activities. The increased salience of the costs of cognitive activity brought about by these negative expectations may, in turn, reduce intrinsic motivation to engage in cognitively challenging tasks and reduce participation in everyday activities that place demands on cognitive resources. To examine these ideas, we tested the hypothesis that attitudes would influence participation in cognitively demanding activities through their effect on intrinsic motivation to engage in cognitively demanding activities.
Methods
Participants
Our sample included 153 older adults (82 women; age range = 64 to 81) recruited to participate in a 5-year longitudinal study through advertisements in the local newspaper and appeals through senior citizens clubs run by the Department of Parks and Recreation in Raleigh, North Carolina. The data reported here were from the first wave of testing during the Spring of 2016. Participants were screened for potential cognitive impairment using a cut-off score of 6 on the Short Blessed (Katzman et al., 1983). Potential participants were also excluded if their self-reported blood pressure (BP)—treated or untreated—was greater than 160 for systolic or 100 for diastolic, or if their BP assessed in the laboratory prior to testing was above these cutoffs. (If an initial reading was unacceptable, we allowed the participant to relax for 5 min and then took a second measurement.) Individuals on anti-hypertensives were allowed to participate if their treated BP fell below the cutoff. In addition, participants were also excluded if they had medical conditions that could potentially affect cardiovascular responses, including diabetes, congestive heart failure, and coronary heart disease. Participants received an incentive of $40 for their time and effort, including that associated with completing the weekly assessments.
This research project was reviewed and approved by the North Carolina State University IRB.
Materials and Equipment
Pretest questionnaires.
Prior to coming into the lab, participants completed paper-and-pencil or on-line versions of questionnaires assessing critical constructs for the project. Attitudes about aging were assessed using the 12-item Expectations Regarding Aging survey (ERA-12; Sarkisian, Steers, Hays, & Mangione, 2005), which included three subscales of four items each assessing expectations regarding physical health, mental health, and cognitive functioning. The scale has been shown to have good reliability and validity (Sarkisian et al.). The 18-item Need for Cognition scale (NFC; Cacioppo, Petty, & Kao, 1982) was used to assess intrinsic motivation. This measure assesses the degree of enjoyment associated with engaging in cognitively demanding activities and is associated with engagement in complex thought (Cacioppo et al., 1996). The Geriatric Depression Scale (GDS; Sheikh & Yesavage, 1986) was used to help assess mental health. We also included seven items that focus on cognitive activity from the Mental Fatigue scale (MFS; Johansson, Starmark, Berglund, Rödholm, & Rönnbäck, 2010) to assess perceptions of chronic mental fatigue.
Finally, a modified 28-item version of the Victoria Longitudinal Study Activities Questionnaire (VLSAQ; Jopp & Hertzog, 2010) was included to assess everyday activity engagement. The original VLSAQ included 3 – 7 specific activities within each of 11 categories of activities. The 11-category structure was validated by Jopp and Hertzog in two large independent samples. Our modification included the four activities with the highest factor loadings from each of seven subscales (developmental, experiential, games, physical, social, technical, and TV). These activity categories were chosen because they exhibited significant associations with a measure of fluid intelligence—including a negative association for TV—the strength of which we assumed was a proxy for the cognitive demands or costs associated with that activity category. Participants reported their frequency of engagement in each activity over the past two years using the original 9-point scale (1 = never, 9 = daily). Jopp and Hertzog reported Cronbach’s α for these seven categories ranging from .58 to .79, and test-retest reliabilities ranging from .65 to .88.
Cognitive engagement.
Cognitive costs were assessed using a computerized memory-scan task. In this task, a string of consonants (i.e., the memory set) appeared on the screen, followed by a screen containing a single consonant (i.e., the target). Participants used a serial response box to indicate whether the target letter was present in the memory set by pressing the “Yes” or “No” button. The task had four levels of increasing difficulty with 1, 3, 5, and 7 letters being presented in the memory set for 250, 750, 1250, and 1750 ms, respectively. Each level of difficulty had 40 trials, and participants had unlimited time to respond on each one. Performance data regarding accuracy and response time were recorded using E-Prime 2.0 computerized testing software.
A letter-comparison task was also given, in which participants had to decide whether two horizontal strings of letters presented side-by-side on the computer screen were the same or different. The data from this task are not used in the present study.
Cardiovascular monitoring.
To assess engagement while completing these tasks, participants were connected to a noninvasive arterial BP device, CNAP Monitor 500 HD (CNSystems Medizintechnik AG, Graz, Austria), which provides beat-to-beat measurement of BP, and has demonstrated reliability and validity (e.g., Jeleazcov et al., 2010). This device uses two finger cuffs that are placed around the index and middle fingers of the non-dominant hand to record continuous measures of finger arterial pressure. The device also incorporates a standard BP measurement cuff around the non-dominant upper arm, which is used to calibrate finger arterial pressure and automatically align it with brachial artery BP as the reference. These continuous BP data were acquired and transferred to a BIOPAC MP150 system (BIOPAC Systems, Inc., Goleta, CA) using AcqKnowledge software.
Subjective perceptions of tasks.
The NASA Task Load Index (TLX; Hart & Staveland, 1988) was used to assess perceptions of the cognitive tasks at each level of difficulty. The scale includes items assessing mental demand, physical demand, temporal demand, performance, effort, and frustration. For the purposes of our study, we added an additional item to measure engagement (i.e., “How engaged were you in the task?”). Participants completed the NASA TLX on the computer using a slider scale to indicate their responses.
Situational motivation.
A computerized version of the intrinsic motivation inventory (IMI; McAuley, Duncan, & Tammen, 1989) was used as a type of manipulation check to assess whether our instructions positively affected participants’ intrinsic motivation to engage and perform well in the memory scan task. High levels of engagement are seen as critical to obtaining valid assessments of effort expenditure that reflect individual differences in personal resources. The IMI includes 30 items rated on a Likert scale of 1 (not at all true of me) to 7 (very true of me), and five subscales. Of particular interest for the present study were the two subscales most closely aligned with task-specific intrinsic motivation: Interest/Enjoyment and Effort/Importance (Cronbach’s α = .75 and .68, respectively).
Cognitive ability measures.
We used five tests to assess cognitive ability to provide additional background information on our participants and to assess the role of ability in influencing activity relative to the other variables of interest. We assessed processing speed and working memory using the Digit-Symbol Substitution and Letter-Number Sequencing subtests from the Wechsler Adult Intelligence Scale, Third edition (WAIS-III; Wechsler, 1997). Verbal ability was assessed using Vocabulary Test V-2 from the Kit of Factor-Referenced Cognitive Tests (Ekstrom, French, Harman, & Derman, 1976). The plus-minus task was used to assess task-switching. Participants were given a sheet of paper with three columns of 30 numbers. They were timed while performing arithmetic operations on the numbers in each column, which involved adding 3 to each number in the first column, subtracting 3 in the second column, and alternating between adding 3 and subtracting 3 in the last column. To assess inhibitory control, participants completed the Stroop task, which involved three parts: (a) naming the colors of a series of blue, black, green, and red colored blocks; (b) reading the names of these colors printed in black ink; and (c) naming the ink colors used in a series of color names, which were incongruent with the color of ink. Participants were told to work as quickly and as accurately as possible, and were timed during each of the three parts of the task.
Activity assessment.
As a further measure of everyday activities, a posttest assessment was developed that was designed to assess participation for specific days (as opposed to over an extended time period) during the five weeks following testing in the lab. This provided an alternative and more direct assessment that may be less subject to errors of recollection when compared to the more traditional retrospective format used in the VLSAQ. The instrument used the same seven VLSAQ categories as the pretest measure, but included an additional 14 activities. We did this more extensive sampling to increase the probability of capturing activities included within each category over the shorter assessment period (5 individual days over 5 weeks vs. 2-year retrospective report).
Procedure
After agreeing to participate and prior to the laboratory test session, individuals completed the pretest survey over the internet or phone. Upon entering the lab, and after giving informed consent, an initial BP assessment was made using a BP-785 automatic monitor (Omron Health Care, Inc., Kyoto Japan) to ensure that the participant did not exceed the previously described exclusion criteria. If the limits were exceeded, the participant was allowed 5 min to rest quietly, followed by an additional BP reading. Individuals who did not fall below our cutoff still received full compensation, but did not participate in the study (n = 3).
Next, participants were connected to the CNAP monitor. They sat and relaxed silently for 10 min while we recorded cardiovascular data, with the last 5 min of this period being used as a baseline assessment. Before beginning the computerized tests, a motivational prompt was given stating that the computer would keep track of results and display them after each set of trials. Additionally, participants were informed that the experimenter would examine the results of their performance and discuss with them how well they performed at the end. This accountability manipulation has been used successfully in previous work with older adults using a similar task (e.g., Smith & Hess, 2016). In the present study, we used it in order to optimize performance and to ensure high levels of engagement, thereby increasing the validity of our assessments of cognitive costs for comparisons across participants. That is, we wanted SBP responses to accurately reflect individual differences in the effort necessary to successfully cope with a specific objective level of task demands. Participants then completed the four levels of the memory-scan task, receiving feedback about the accuracy of their responses after each trial as well as a summary of performance at the end of each block of trials at each difficulty level. In between each level of difficulty, subjective perceptions of the task were collected using the TLX.
The letter-comparison task was given next, followed by administration of the IMI. Participants then completed the cold pressor task in order to measure physiological reactivity. They placed their dominant hand into a container of ice water (0–5°C) and kept it immersed for 90 s while the CNAP continued recording cardiovascular responses from the non-dominant hand. Participants were disconnected from the CNAP afterwards.
In the remaining portion of the study session, participants completed the five cognitive tasks described above, as well as a demographics questionnaire, which included the SF-36 health survey (Ware, 1993) and a chronic disease checklist based on that used in the Midlife in the United States study (http://midus.wisc.edu).
After receiving compensation, participants were contacted either through email or by phone once a week, for five weeks, to fill out a questionnaire about their activities during the previous day. An attempt was made to have participants report on five different days of the week over the five-week period, including two weekend days. For each survey, participants indicated whether or not they participated in each of the 42 items included in the posttest activity assessment.
Results
Assessment of Costs
Our initial set of analyses focused on obtaining individual assessments of costs for use in predicting activity. An essential aspect of our obtaining individual indices of costs relates to participants being highly motivated to do well, and thus exerting the effort necessary to support successful performance. Examination of responses in the memory-scan task also allowed us to address our second research question dealing with the impact of general CV reactivity.
Memory-scan task: Performance.
We first examined performance as a function of task demands, as reflected in response times (RT) and accuracy, using multilevel models (MLM) that allowed us to deal with missing data as well as incorporate both linear and quadratic components of demands (grand-mean centered at 4). Null models performed on RTs revealed significant (ps < .0001) within- (38.7%) and between-person (61.3%) variance. In addition, significant linear, b = 48.25, t(442) = −17.01, p < .0001, and quadratic, b = −6.21, t(442) = −7.18, p < .0001, effects of demands were observed. RT estimates (SE) for memory-set sizes of 1, 3, 5, and 7—868 (18), 1014 (18), 1111 (20), and 1158 (22), respectively—revealed the typical pattern of increasing RTs with an increase in difficulty.
A similar analysis of accuracy scores (i.e., proportion correct) revealed that almost all of the variance (99.8%; p < .0001) was between persons. The subsequent MLM examining accuracy revealed significant linear, b = −.024, t(442) = −17.38, p < .0001, and quadratic, b = −.022, t(442) = −11.65, p < .0001, effects of demands, with score estimates (SE) based on this model being .97 (.01), .97 (.01), .92 (.01), and .83 (01) memory sets of 1, 3, 5, and 7 items, respectively. This relatively high level of performance along with the gauging of RTs to task demands suggest that participants were exhibiting high levels of task engagement.
Subjective reports of engagement.
We next examined self-reported engagement using two separate indices. The first was the IMI Enjoyment and Effort subscales, which are thought to reflect task-specific intrinsic motivation. Notably, scores for the group were in the moderately high range (on a scale of 1 – 7): Enjoyment—M= 5.4, SD = 0.9; Effort—M = 6.1, SD = 0.8. The second index was the rating of engagement participants completed along with the TLX assessment following each set of trials in the memory-scan task. Once again, these scores were all in the moderately high range (on a scale of 0 – 100), with mean scores (SD) for levels 1, 3, 5, and 7 being 72.1 (22.4), 71.8 (21.7), 75.5 (18.4), and 79.1 (15.5), respectively. Taken together, these subjective measures along with the performance data provide strong evidence of participants being engaged in the task, and thus fulfill the prerequisite conditions necessary for our SBP measures to be interpreted as reflections of individual differences in cognitive costs.
Costs.
We next calculated SBP-R by subtracting mean baseline SBP from the mean SBP during each block of trials in the memory-scan task, which we then examined using similar analyses to those used to examine performance. The null model revealed both significant (ps < .0001) within- (30.5%) and between-person (69.5%) variance. We then ran a multi-level model examining the impact of task demands—linear and quadratic—on SBP-R. We included grand-mean centered baseline SBP to control for the possibility that range of response is related to this measure. We also controlled for grand-mean centered accuracy to assess effort at a constant, relatively high level of performance. Both covariates were significant (ps < .05), as was the linear component of demands, b = 1.12, t(427) = 2.89, p = .004. Thus, consistent with past findings, SBP-R increased systematically with task demands in our sample of older adults.
We subsequently controlled for the potential confounding impact of individual differences in intrinsic physiological reactivity on the results. To do so, we modeled change in the cold-pressor task from initial immersion to the end of the assessment period, and used the resulting slope as an index of physiological reactivity.1 When included as a covariate in our model, this reactivity index was significant (b = .17, p < .0001), but the impact of task demands on SBP-R remained unaltered.2 This suggests that the systematic response associated with memory load and individual differences in the degree of response could not simply be attributed to individual variation in physiological reactivity.
Finally, we calculated intercepts and regression coefficients associated with each participant’s responses as estimates of costs associated with task demands for use as predictors in our structural models. It was assumed that greater costs would be associated with greater intercepts and linear slopes, and smaller (i.e., more negative) quadratic slopes representing disengagement as the task presumably became too demanding.
Prediction of Activity Participation
Our primary analyses focused on prediction of activity participation, with a particular emphasis on understanding the impact of personal resources and cognitive costs on attitudes of aging and motivation as predictors. As already described, we had two VLSAQ-based indices of participation: the pretest retrospective assessments and the posttest weekly assessments. As an initial examination of consistency of these assessments, we calculated correlations between scores for each category of activity on the retrospective assessment with the total activities engaged in within that same category over the 5-week period. All correlations were significant (ps < .001), ranging from .38 to .79. The relatively wide range and moderate level of association, however, suggests that the two measures might be capturing slightly different aspects of behavior. Thus, we performed all analyses twice, once using the retrospective reports and one using the weekly assessments.
To begin, we constructed a measurement model that included latent variables reflecting the constructs of interest. These included: (a) subjective reports of health (SF36 physical health, SF36 mental health, number of chronic conditions, MFS fatigue scores, and depressive symptoms [GDS]); (b) ability (digit-symbol substitution, letter-number sequencing, Stroop interference, and plus-minus); (c) cognitive costs (SBP-R intercept, SBP-R linear slope, and SBP-R quadratic slope obtained from the previously described modeling of each individual’s responses); and (d) attitudes about aging (ERA physical health, mental health, and cognitive functioning subscores). Based on modification indices, we added error covariance paths between SF36 mental health and both SF36 physical health and the ERA physical subscale. This resulted in an excellent fit for the measurement model (Table 1): CFI = .982, TLI = .977, RMSEA = .029, SRMR = .056. Even though the latent construct representing costs was not significantly correlated with those for either health (−.076) or ability (−.083), we examined the possibility that cognitive costs might simply be reflections of either health or ability by testing three additional models in which variables related to costs were included as indicators of the individual latent constructs of health or ability or of both. All models were poor fits (e.g., CFIs < .909), supporting inclusion of costs as an independent construct.
Table 1.
Standardized Measurement Model
| Factor | Indicators | B (SE) |
|---|---|---|
| Health | SF36 Physical Health | 0.31 (0.10)** |
| # chronic illnesses | −0.42 (0.08)** | |
| SF36 Mental Health | 0.61 (0.08)** | |
| Depression (GDS) | −0.68 (0.06)** | |
| Fatigue (MFS) | −0.43 (0.08)** | |
| Ability | Digit Symbol Substitution | 0.81 (0.14)** |
| Letter-Number Sequencing | 0.47 (0.11)** | |
| Stroop | −0.27 (0.09)* | |
| Plus-Minus | −0.16 (0.10) | |
| Costs | Intercept | 0.95 (0.03)** |
| Linear slope | 0.79 (0.04)** | |
| Quadratic slope | −0.81 (0.04)** | |
| Aging attitudes | ERA Cognition | 0.71 (0.05)** |
| ERA Mental | 0.73 (0.06)** | |
| ERA Physical | 0.76 (0.05)** |
p < .01
p < .001
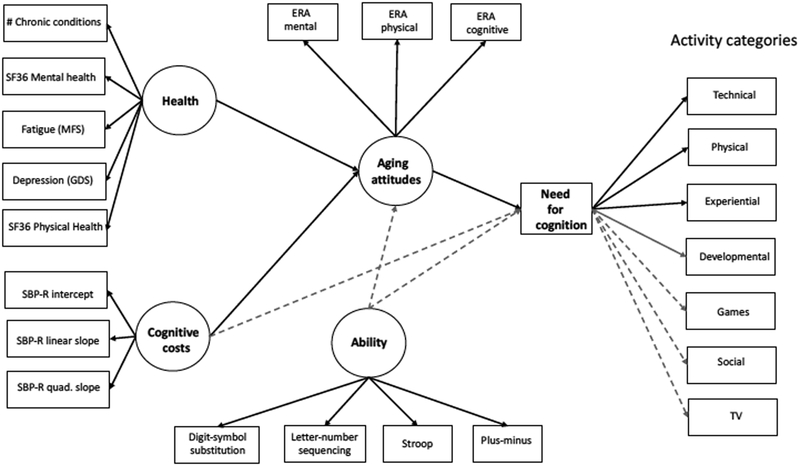
We next constructed a structural model, with cognitive ability, costs, and health as exogenous predictors of aging attitudes and motivation—as assessed by NFC scores. In addition, aging attitudes and motivation served as mediators of the relationship between these predictors and activity, with the impact of attitudes also modeled through motivation. This model resulted in satisfactory fits for both types of activity assessments: retrospective assessments—CFI = .944, TLI = .928, RMSEA = .037, SRMR = .063; weekly assessments—CFI = .933, TLI = .914, RMSEA = .043, SRMR = .070. (Standardized path coefficients are presented in Table 2, and variance in activity participation explained by the models is presented in Table 3)3. Focusing on significant pathways (Figure 1), several notable results can be seen. First, health was positively associated with attitudes about aging, with healthier individuals having more positive attitudes. In contrast, greater costs were associated with more negative attitudes. Positive attitudes, in turn, were associated with higher levels of intrinsic motivation and mediated the relation between both health and costs and motivation. As expected, intrinsic motivation was positively associated with activity engagement, although the strength of this association varied across activity categories. Notably, the strongest association was with technical activities, which Joop and Hertzog (2010) also found to have the strongest relationship with cognitive abilities, such as working memory and fluid intelligence. Similarly, a nonsignificant negative association was observed for TV watching, which Jopp and Hertzog found to have significant negative correlations with ability. Positive associations were observed for the remaining five activity categories, with these associations being significant for physical, experiential, and developmental (retrospective analysis only) activities. Jopp and Hertzog found that reported participation in each of these categories was positively associated with ability, with the strength of the relationship varying by ability domain. Thus, there is no clear way at present to extrapolate from their data and rank-order activity categories by presumed cognitive demands. Most importantly, however, and consistent with expectations, the influence of costs—through attitudes and motivation—was selective, with some evidence of this being related to the inferred cognitive demands of the activities in each category.
Table 2.
Standardized Path Coefficients from Structural Models Predicting Activity Participation
| Weekly assessments | Retrospective assessments | |
|---|---|---|
| Path | B (SE) | B (SE) |
| Health -> Aging Attitudes | 0.62 (0.15)*** | 0.62 (0.15)*** |
| Costs -> Aging Attitudes | −0.17 (0.09)* | −0.17 (0.09)* |
| Ability -> Aging Attitudes | 0.031 (0.14) | 0.031 (0.14) |
| Costs -> Need for Cognition | 0.17 (0.09) | 0.17 (0.09) |
| Ability -> Need for Cognition | 0.16 (0.16) | 0.16 (0.16) |
| Aging Attitudes -> Need for Cognition | 0.43 (0.10)*** | 0.43 (0.10)*** |
| Need for Cognition -> Technical Activity | 0.32 (0.07)*** | 0.40 (0.07)*** |
| Need for Cognition -> Physical Activity | 0.22 (0.07)** | 0.33 (0.08)*** |
| Need for Cognition -> Experiential Activity | 0.24 (0.07)** | 0.20 (0.09)* |
| Need for Cognition -> Developmental Activity | 0.12 (0.09) | 0.31 (0.07)*** |
| Need for Cognition -> Games | 0.05 (0.08) | 0.10 (0.08) |
| Need for Cognition -> Social Activity | 0.01 (0.08) | 0.11 (0.08) |
| Need for Cognition -> TV | −0.11 (0.08) | −0.06 (0.08) |
p < .05
p < .01
p < .001
Table 3.
R2(SE) for Outcome Measures
| Activity Category | Weekly assessments | Retrospective assessments |
|---|---|---|
| Technical Activity | 0.102 (0.44) | 0.161 (0.06) |
| Physical Activity | 0.049 (0.32) | 0.109 (0.05) |
| Experiential Activity | 0.059 (0.35) | 0.041 (0.04) |
| Developmental Activity | 0.015 (0.02) | 0.095 (0.07) |
| Games | 0.002 (0.01) | 0.010 (0.02) |
| Social Activity | 0.000 (0.00) | 0.012 (0.02) |
| TV | 0.012 (0.02) | 0.003 (0.01) |
Figure 1.
Final model depicting significant pathways (solid black lines). Solid gray lines depict additional pathways that were only significant for the retrospective activity assessments, whereas the dashed gray lines depict nonsignificant pathways.
Several other findings are of interest. First, costs did not directly influence motivation, but operated through aging attitudes. Second, although we did not include direct effects from the costs, health, and aging attitudes to activity categories in the current model due to limitations associated with our sample size, exploratory analyses indicated that costs did not directly influence activity, but rather appeared to operate through attitudes and motivation. Finally, although the pattern of results was similar across the two activity assessment methods, the associations with motivation were somewhat stronger for the retrospective assessments.
It is interesting that ability did not have any significant relationships within the model. We thus explored alternative relationships involving ability. In one set of models, we allowed costs to predict ability under the assumption that scores would in part be based on the degree of effort that individuals need to exert in order to support performance. An alternative set modeled ability and health influencing costs. Although somewhat less conceptually sound, we also considered the possibility that NFC predicted aging attitudes, and reran all of our models—including the aforementioned alternatives—using this ordering. All models for both sets of activity measures resulted in poorer fitting models (CFI = .883 – .940; TLI = .842 – .924; RMSEA = .038 – .059; SRMR = .068 – .092) than the original model, with no change in the associations involving ability. Finally, we also tested models in which ability moderated the impact of costs, but these models failed to converge.
Finally, we created structural models in which aging attitudes moderated the impact of costs on motivation to explore our alternative hypothesis that attitudes may influence motivation through the accentuation of the perceptions of costs. These models also failed to result in satisfactory solutions, suggesting that aging attitudes had a more direct effect on motivation as opposed to one that operated through its effect on costs.
In sum, consistent with expectations, we found that health and cognitive costs were predictive of motivation, with the novel finding that attitudes about aging mediated this relationship. Motivation, in turn, was the primary predictor of activity, with the impact of health, costs, and attitudes filtered through this variable. In addition, the impact of motivation on activity participation was selective, with stronger associations observed with those activities presumed to have stronger cognitive demands.
Discussion
The primary goal of this research was to examine a basic tenet of SET (Hess, 2014); specifically, that the costs of cognitive engagement should influence intrinsic levels of motivation that ultimately affect engagement in cognitively demanding everyday activities. Previous research focused on either characterizing age effects on costs within the laboratory or examining relations involving presumed correlates of costs and both motivation and everyday activity. The first step in attempting to link costs with motivation and behavior was to identify a means for obtaining an index of costs that could be used as a measure of individual differences. Within SET, costs are defined in terms of the amount of effort required to achieve successful performance, which has been assessed by examining systematic changes in SBP in response to objective task demands (e.g., Ennis et al., 2013; Smith & Hess, 2015). Thus, we employed a memory scan task and examined changes in SBP responses as the size of the memory set increased. To help ensure the validity of responses for accurately representing individual differences in effort, we used a motivational manipulation that resulted in participants both reporting relatively high levels of engagement and exhibiting high levels of performance.
Examination of performance revealed patterns consistent with those observed in prior work (e.g., Smith & Hess, 2015), with SBP increasing systematically with objective task demands. We assume that this pattern of performance is reflective of participants’ attempts at active coping. However, to investigate the possibility that individual differences in levels of SBP reflect variations in age-related increase in general CV reactivity (see Uchino et al., 2010), we also assessed SBP reactivity to a passive coping cold pressor exposure and then examined responses in the memory-scan task controlling for this reactivity. Importantly, this did not alter the impact of task demands on SBP responses, providing support for the contention that these responses specifically are indicative of effort associated with attempts at active coping.
Using data from the memory-scan task, we calculated measures of SBP responsivity from individual response functions and created a latent factor score representing the costs of cognitive engagement. When entered into a structural model to address our research questions regarding the impact of costs on aging attitudes, intrinsic motivation, and activity engagement, several important findings emerged. Foremost among these was that costs had an indirect effect on engagement through aging attitudes and motivation. These findings support the SET-based contention that aging-associated changes in the costs of cognitive engagement have a negative impact on both motivation and, subsequently, engagement in everyday activities, potentially accounting for normative declines in activity levels in later life.
Our analyses also support the contention that these effects are selective, with the strength of these associations varying across categories of activities. We assume that the strength of these effects in part reflects the general cognitive demands associated with the activities in each category. At present, we have no way of actually verifying these demands, but the relationships between activity and cognitive ability—particularly fluid intellectual skills—observed by Jopp and Hertzog (2010) is suggestive. If it is assumed that the stronger this relationship is, the greater the cognitive demands, then our results align nicely with the hypothesized selectivity effects. For example, participation in technical activities had the strongest positive association with fluid ability in Jopp and Hertzog, whereas activities centered around television actually had negative associations. The strength of associations between motivation and activity levels observed in the present study are similar in nature. Further, the correlation between (a) the mean age-adjusted correlations with ability across the two samples in Jopp and Hertzog and (b) the mean standardized coefficients obtained across the two sets of analyses presented in Table 3 for the activity categories assessed in our study was .77. Although caution must be exercised in inferring that ability relations reflect cognitive task demands, this pattern of results suggests that motivation-based selectivity is greatest for those activities that are most demanding.
Another goal of the present study was the exploration of aging attitudes within SET. We examined two specific hypotheses, one in which costs were proposed to have a direct impact on attitudes (i.e., greater costs would be associated with more negative attitudes) versus another in which attitudes moderated the impact of costs. Our results most clearly aligned with the former. The relations observed in our structural model were also interesting in terms of highlighting the potential role of attitudes about aging on important functional outcomes. Specifically, we observed that health and costs were predictive of attitudes, with their impact on motivation being filtered through attitudes. Consistent with recent research (Zafeiriou & Gendolla, 2017), we hypothesize that negative aging attitudes increase the salience of cognitive costs associated with engaging in cognitively demanding activities, thereby reducing the motivation to engage in such activities. Of course, the cross-sectional nature of the current data precludes making strong causal assumptions, but our results are consistent with recent work by Bryant et al. (2012; 2016) demonstrating that changes in health status and well-being over an approximately nine-year period predicted attitudes about aging. The association between aging attitudes, motivation, and activity in the present study is particularly interesting, highlighting a mechanism through which aging attitudes may influence important functional outcomes.
The results of this study also provide potential insights into the nature of costs. Somewhat unexpectedly, we observed that costs were predictive independently of both physical health and cognitive ability. Given that we screened for health issues that were likely to compromise cardiovascular reactivity, the independence of the health factors and costs may not be that surprising. The seeming independence of cognitive ability and costs is perhaps a bit more unexpected, but may simply underscore the importance of motivation in determining performance on standardized cognitive ability tests. In other words, performance may not only reflect ability, but the motivation to engage resources (e.g., Hess et al., 2011). It should also be recognized that that the same level of performance on tests of ability may be achieved with different costs, and that the impact of the effort necessary to support performance may be a more important determinant of motivation than actual performance (or scores on an ability test). Most importantly, the present analyses suggest that individual differences in costs are reliably related to important psychological and functional outcomes. Given previously observed normative age-related effects in effort and fatigue associated with cognitive engagement (e.g., Ennis et al., 2013; Hess & Ennis, 2012), these findings support the possibility of changes in costs with age accounting for normative changes in activity participation through the impact on aging attitudes and motivation.
From a measurement perspective, it also noteworthy that we got similar results using two different methods of assessing the same activities. There are several different ways in which self-reports of activities have been obtained in the literature (e.g., Bielak, 2017), each with different validity and reliability issues. The standard format of the VLSAQ depends heavily on the reporting of events over an extended period of time, with inaccuracies in memory potentially affecting the validity of these assessments. We reasoned that our weekly reports on activities during the previous day might be less subject to memory bias. We also expected that our sampling across a reasonably wide timespan on different days would do a good job of capturing both the frequency and variety of everyday behaviors. Finally, we thought that the assessment of activity levels concurrent with our other assessments would also increase the validity and reliability of associations observed with these weekly assessments. Thus, the finding that somewhat stronger associations were observed with the retrospective reports was a bit surprising. One possibility is that even though we had five different assessments and increased the variety of activities sampled, we still may not have been able to capture the richness of the participants’ activity that is potentially reflected in the retrospective reports.
In conclusion, the present results are generally supportive of ideas drawn from SET, and extend this theory by elucidating the potential role of attitudes toward aging. Of particular note is the linking of the costs of cognitive engagement (i.e., effort) to intrinsic motivation through attitudes. Age-related selectivity is subsequently manifested in terms of these influences on motivation having their ultimate effect on a specific set of everyday activity: those presumed to put demands on cognitive resources. Given the potentially beneficial effect of such activities on cognitive health in later life, our findings provide some important clues regarding factors that determine individual differences in activity engagement patterns in everyday life. Naturally, several cautions need to be provided in considering our results and interpretations. Principal among these is the reliance on cross-sectional data to test the hypothesized relationships. Verification regarding the validity of these linkages awaits further testing using longitudinal data, with subsequent years of data collection in the present project providing the opportunity to provide such tests. Concerns could also be raised regarding the assessment of everyday activity given the lack of clarity in the literature regarding the best means of assessment (e.g., temporal assessment, activity sampling). However, the reliance on an instrument based in the psychometrically sound VLSAQ, the use of retrospective and weekly assessments, and measurement of activities presumed to vary in cognitive demands—validated in part through our model—help to allay some of these concerns.
Acknowledgments
The research was supported by grant AG05552 from NIH/NIA awarded to Thomas M. Hess.
We gratefully acknowledge the assistance of Sherri Hutchinson, John Rucker, Yolanda Cabrerra, Navodya Denuwara, Gabriella Ragouzeos, Madeline Afshar, and Richard Earnhardt in various phases of this project.
Appendix
Correlations between variables included in models.
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Chronic conditions | 1 | ||||||||||
| 2. SF36 Physical Health | 0.005 | 1 | |||||||||
| 3. SF36 Mental Health | −0.359 | −0.248 | 1 | ||||||||
| 4. Geriatric Depression Scale | 0.252 | −0.184 | −0.462 | 1 | |||||||
| 5. Mental Fatigue Scale | 0.231 | −0.121 | −0.251 | 0.239 | 1 | ||||||
| 6. Digit-Symbol Substitution | −0.134 | 0.151 | 0.125 | −0.222 | −0.217 | 1 | |||||
| 7. Letter-Number Sequencing | −0.144 | 0.090 | 0.095 | −0.068 | −0.127 | 0.371 | 1 | ||||
| 8. Stroop Task | 0.089 | 0.033 | −0.146 | 0.094 | 0.146 | −0.271 | −0.102 | 1 | |||
| 9. Plus-Minus Task | 0.054 | −0.088 | 0.026 | 0.019 | 0.085 | −0.013 | −0.093 | −0.113 | 1 | ||
| 10. SBP-R intercept | −0.019 | 0.017 | 0.040 | −0.107 | −0.065 | −0.044 | −0.135 | −0.103 | −0.041 | 1 | |
| 11. SBP-R linear | −0.035 | −0.053 | 0.014 | 0.026 | 0.066 | −0.047 | −0.213 | −0.109 | −0.091 | 0.721 | 1 |
| 12. SBP-R quadratic | −0.020 | −0.063 | −0.051 | 0.071 | −0.015 | −0.005 | 0.126 | 0.105 | −0.038 | −0.771 | −0.655 |
| 13. ERA Mental | −0.121 | 0.174 | 0.237 | −0.395 | −0.277 | 0.247 | 0.142 | −0.172 | 0.045 | −0.067 | 0.004 |
| 14. ERA Physical | −0.136 | 0.319 | 0.063 | −0.240 | −0.305 | 0.161 | 0.084 | −0.102 | 0.004 | −0.091 | −0.006 |
| 15. ERA Cognitive | −0.110 | 0.249 | 0.185 | −0.253 | −0.294 | 0.122 | 0.108 | −0.038 | 0.038 | −0.110 | −0.093 |
| 16. Need for Cognition | −0.171 | 0.184 | 0.146 | −0.231 | −0.324 | 0.145 | 0.205 | −0.197 | −0.070 | 0.106 | 0.087 |
| 17. Technical Activity (R)a | −0.113 | 0.058 | 0.098 | −0.131 | −0.102 | 0.155 | 0.171 | −0.146 | 0.133 | 0.029 | −0.037 |
| 18. Physical Activity (R) | −0.181 | −0.075 | 0.177 | −0.237 | −0.153 | 0.195 | 0.091 | −0.150 | 0.053 | 0.092 | 0.046 |
| 19. Experiential Activity (R) | 0.014 | −0.105 | 0.044 | −0.123 | −0.022 | 0.120 | 0.162 | −0.098 | −0.063 | −0.016 | −0.029 |
| 20, Decvelopmental Activity (R) | −0.081 | −0.032 | 0.141 | −0.156 | −0.109 | 0.073 | 0.042 | −0.088 | 0.068 | −0.111 | −0.079 |
| 21. Game Activity (R) | 0.061 | 0.122 | 0.121 | −0.070 | −0.036 | 0.159 | 0.103 | 0.032 | −0.048 | −0.047 | −0.016 |
| 22. Social Activity (R) | −0.076 | −0.141 | 0.208 | −0.236 | −0.185 | 0.043 | −0.041 | 0.069 | 0.116 | 0.068 | 0.070 |
| 23. TV Activity (R) | 0.086 | 0.059 | −0.004 | 0.029 | 0.154 | −0.147 | −0.038 | 0.074 | −0.102 | 0.037 | 0.161 |
| 24. Technical Activity (W)a | −0.066 | 0.055 | 0.059 | −0.109 | −0.078 | 0.202 | 0.149 | −0.190 | 0.003 | 0.087 | 0.030 |
| 25. Physical Activity (W) | −0.171 | 0.037 | 0.198 | −0.252 | −0.156 | 0.201 | 0.011 | −0.209 | 0.070 | 0.124 | 0.095 |
| 26. Experiential Activity (W) | 0.031 | 0.138 | −0.048 | −0.097 | −0.030 | 0.219 | 0.101 | −0.057 | 0.095 | −0.011 | 0.007 |
| 27, Developmental Activity (W) | −0.136 | 0.040 | 0.116 | −0.129 | −0.129 | 0.030 | −0.019 | −0.071 | 0.063 | −0.124 | −0.064 |
| 28. Game Activity (W) | −0.066 | 0.151 | 0.116 | −0.146 | −0.082 | 0.243 | 0.206 | −0.004 | −0.025 | −0.062 | 0.007 |
| 29. Social Activity (W) | −0.085 | −0.178 | 0.073 | −0.084 | 0.084 | 0.085 | 0.046 | 0.048 | 0.009 | −0.069 | −0.087 |
| 30. TV Activity (W) | 0.029 | 0.043 | 0.010 | 0.019 | 0.105 | −0.047 | 0.092 | 0.090 | −0.104 | 0.002 | 0.051 |
| Variable | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | 20. | 21. | 22. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Chronic conditions | |||||||||||
| 2. SF36 Physical Health | |||||||||||
| 3. SF36 Mental Health | |||||||||||
| 4. Geriatric Depression Scale | |||||||||||
| 5. Mental Fatigue Scale | |||||||||||
| 6. Digit-Symbol Substitution | |||||||||||
| 7. Letter-Number Sequencing | |||||||||||
| 8. Stroop Task | |||||||||||
| 9. Plus-Minus Task | |||||||||||
| 10. SBP-R intercept | |||||||||||
| 11. SBP-R linear | |||||||||||
| 12. SBP-R quadratic | 1 | ||||||||||
| 13. ERA Mental | 0.001 | 1 | |||||||||
| 14. ERA Physical | 0.063 | 0.543 | 1 | ||||||||
| 15. ERA Cognitive | 0.019 | 0.474 | 0.568 | 1 | |||||||
| 16. Need for Cognition | −0.088 | 0.368 | 0.341 | 0.327 | 1 | ||||||
| 17. Technical Activity (R)a | −0.020 | 0.263 | 0.091 | 0.120 | 0.393 | 1 | |||||
| 18. Physical Activity (R) | −0.074 | 0.144 | 0.095 | 0.136 | 0.345 | 0.272 | 1 | ||||
| 19. Experiential Activity (R) | −0.003 | 0.102 | 0.055 | 0.135 | 0.208 | 0.277 | 0.198 | 1 | |||
| 20, Developmental Activity (R) | 0.023 | 0.197 | 0.332 | 0.283 | 0.306 | 0.149 | 0.117 | 0.252 | 1 | ||
| 21. Game Activity (R) | 0.056 | 0.117 | 0.154 | 0.053 | 0.085 | 0.156 | 0.019 | 0.063 | 0.074 | 1 | |
| 22. Social Activity (R) | −0.112 | 0.207 | 0.057 | 0.105 | 0.119 | 0.208 | 0.343 | 0.298 | 0.236 | 0.138 | 1 |
| 23. TV Activity (R) | −0.020 | −0.124 | −0.046 | −0.071 | −0.068 | 0.007 | −0.071 | 0.096 | −0.158 | 0.250 | 0.058 |
| 24. Technical Activity (W)a | −0.063 | 0.252 | 0.050 | 0.126 | 0.251 | 0.767 | 0.232 | 0.279 | 0.136 | 0.208 | 0.212 |
| 25. Physical Activity (W) | −0.081 | 0.118 | 0.057 | 0.092 | 0.406 | 0.357 | 0.800 | 0.191 | 0.160 | 0.170 | 0.331 |
| 26. Experiential Activity (W) | −0.023 | 0.133 | 0.161 | 0.280 | 0.127 | 0.270 | 0.206 | 0.457 | 0.032 | 0.125 | 0.126 |
| 27, Decvelopmental Activity (W) | 0.022 | 0.220 | 0.318 | 0.272 | 0.349 | 0.273 | 0.211 | 0.237 | 0.746 | 0.245 | 0.285 |
| 28. Game Activity (W) | 0.074 | 0.097 | 0.165 | 0.061 | 0.153 | 0.294 | 0.159 | 0.036 | 0.131 | 0.737 | 0.243 |
| 29. Social Activity (W) | 0.030 | −0.110 | −0.131 | −0.093 | 0.096 | 0.106 | 0.180 | 0.191 | 0.089 | 0.035 | 0.405 |
| 30. TV Activity (W) | 0.067 | −0.126 | −0.064 | −0.016 | −0.008 | 0.022 | −0.037 | 0.039 | −0.191 | 0.308 | 0.057 |
| 23. | 24. | 25. | 26. | 27. | 28. | 29. | 30. | |
|---|---|---|---|---|---|---|---|---|
| 1. Chronic conditions | ||||||||
| 2. SF36 Physical Health | ||||||||
| 3. SF36 Mental Health | ||||||||
| 4. Geriatric Depression Scale | ||||||||
| 5. Mental Fatigue Scale | ||||||||
| 6. Digit-Symbol Substitution | ||||||||
| 7. Letter-Number Sequencing | ||||||||
| 8. Stroop Task | ||||||||
| 9. Plus-Minus Task | ||||||||
| 10. SBP-R intercept | ||||||||
| 11. SBP-R linear | ||||||||
| 12. SBP-R quadratic | ||||||||
| 13. ERA Mental | ||||||||
| 14. ERA Physical | ||||||||
| 15. ERA Cognitive | ||||||||
| 16. Need for Cognition | ||||||||
| 17. Technical Activity (R)a | ||||||||
| 18. Physical Activity (R) | ||||||||
| 19. Experiential Activity (R) | ||||||||
| 20, Developmental Activity (R) | ||||||||
| 21. Game Activity (R) | ||||||||
| 22. Social Activity (R) | ||||||||
| 23. TV Activity (R) | 1 | |||||||
| 24. Technical Activity (W)a | 0.01 | 1 | ||||||
| 25. Physical Activity (W) | −0.027 | 0.314 | 1 | |||||
| 26. Experiential Activity (W) | 0.088 | 0.283 | 0.231 | 1 | ||||
| 27, Decvelopmental Activity (W) | −0.084 | 0.312 | 0.358 | 0.177 | 1 | |||
| 28. Game Activity (W) | 0.219 | 0.35 | 0.343 | 0.245 | 0.353 | 1 | ||
| 29. Social Activity (W) | 0.185 | 0.079 | 0.242 | 0.086 | 0.102 | 0.21 | 1 | |
| 30. TV Activity (W) | 0.82 | 0.069 | 0.075 | 0.08 | −0.019 | 0.337 | 0.216 | 1 |
(R) = retrospective activity assessments. (W) = weekly activity assessments.
Footnotes
Some results reported here have also been presented at the US – Hong Kong 2018 Conference: Aging across Time and Contexts, May, 2018.
Due to equipment problems during testing, we do not have data from the cold-pressor task for four participants.
We also tried several other potential indices of reactivity—intercept, peak response, and mean response—with minimal change in outcome.
Correlations between all variables included in the model are presented in the Appendix.
Contributor Information
Thomas M. Hess, Department of Psychology, North Carolina State University.
Claire M. Growney, Department of Psychology, North Carolina State University.
Erica L. O’Brien, Department of Psychology, North Carolina State University.
Shevaun D. Neupert, Department of Psychology, North Carolina State University.
Andrew Sherwood, Department of Psychiatry and Behavioral Sciences, Duke University..
References
- Baltes MM, & Lang FR (1997). Everyday functioning and successful aging: the impact of resources. Psychology and Aging, 12, 433–443. doi: 10.1037/0882-7974.12.3.433 [DOI] [PubMed] [Google Scholar]
- Bielak AAM (2017). Different perspectives on measuring lifestyle engagement: a comparison of activity measures and their relation with cognitive performance in older adults. Aging, Neuropsychology, and Cognition, 24, 435–452, doi: 10.1080/13825585.2016.1221378 [DOI] [PubMed] [Google Scholar]
- Bryant C, Bei B, Gilson K, Komiti A, Jackson H, & Judd F (2012). The relationship between attitudes to aging and physical and mental health in older adults. International Psychogeriatrics, 24, 1674–1683. doi: 10.1017/S1041610212000774 [DOI] [PubMed] [Google Scholar]
- Bryant C, Bei B, Gilson K, Komiti A, Jackson H, & Judd F (2016). Antecedents of attitudes to aging: A study of the roles of personality and well-being. The Gerontologist, 56, 256–265. doi: 10.1093/geront/gnu041 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Petty RE, Feinstein JA, & Jarvis WBG (1996). Dispositional differences in cognitive motivation: The life and times of individuals varying in need for cognition. Psychological Bulletin, 119, 197–253. [Google Scholar]
- Cacioppo JT, Petty RE, & Kao CF (1984). The efficient assessment of need for cognition, Journal of Personality Assessment, 48, 306–307. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, & Reuter-Lorenz PA (2010). Age differences in prefrontal recruitment during verbal working memory maintenance depend on memory load. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 46, 462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, & Dermen D (1976). Manual for the Kit of Factor-Referenced Cognitive Tests Educational Testing Service. Princeton. [Google Scholar]
- Emile M, Chalabaev A, Stephan Y, Corrion K, & d’Arripe-Longueville F (2014). Aging stereotypes and active lifestyle: Personal correlates of stereotype internalization and relationships with level of physical activity among older adults. Psychology of Sport and Exercise, 15, 198–204. doi: 10.1016/j.psychsport.2013.11.002 [DOI] [Google Scholar]
- Ennis GE, Hess TM, & Smith BT (2013). The impact of age and motivation on cognitive effort: Implications for cognitive engagement in older adulthood. Psychology and Aging, 28, 495–504. doi: 10.1037/a0031255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SG & Staveland LE (1988). Development of NASA-TLX (task load index): Results of empirical and theoretical research In Hancock PA and Meshkati N (Eds.) Human Mental Workload. Amsterdam: North Holland Press. [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, & Lindenberger U (2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, 9, 1–65. [DOI] [PubMed] [Google Scholar]
- Hess TM (2014). Selective engagement of cognitive resources: Motivational influences on older adults’ cognitive functioning. Perspectives on Psychological Sciences, 9, 388–407. doi: 10.1177/1745691614527465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM, Auman C, Colcomb SJ, & Rahhal TA (2003). The impact of stereotype threat on age differences in memory performance. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences, 58B, P3–P11. doi: 10.1093/geronb/58.1.P3 [DOI] [PubMed] [Google Scholar]
- Hess TM, Emery L, & Neupert SD (2012). Longitudinal relationships between resources, motivation, and functioning. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 67, 299–308. doi: 10.1093/geronb/gbr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM, & Ennis GE (2012). Age differences in the effort and cost associated with cognitive activity. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 67, 447–455. doi: 10.1093/geronb/gbr129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM, & Ennis GE (2014). Assessment of adult age differences in task engagement: The utility of systolic blood pressure. Motivation and Emotion, 38, 844–854. doi: 10.1007/s11031-014-9433-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM, Smith BT, & Sharifian N (2016). Aging and effort expenditure: the impact of subjective perceptions of difficulty, motivation, and performance. Psychology and Aging, 31, 653–660. doi: 10.1037/pag0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SA, & Siedlecki KL (2017). Leisure Activity Engagement and Positive Affect Partially Mediate the Relationship Between Positive Views on Aging and Physical Health, The Journals of Gerontology: Series B, 72, 259–267. doi: 10.1093/geronb/gbw049 [DOI] [PubMed] [Google Scholar]
- Hughes ML, Geraci L, & De Forrest RL (2013). Aging 5 years in 5 minutes: The effect of taking a memory test on older adults’ subjective age. Psychological Science, 24, 2481–2488. doi: 10.1177/0956797613494853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman HW, Van Rooyen JM, Malan NT, Eloff FC, Laubscher PJ, Steyn HS, & Pretorius PJ (2002). Cardiovascular reactivity patterns elicited by the cold pressor test as a function of aging. Aging Clinical and Experimental Research, 14, 202. doi: 10.1007/BF03324437 [DOI] [PubMed] [Google Scholar]
- Jeleazcov C, Krajinovic L, Munster T, Birkholz T, Fried R, Schuttler J, Fechner J (2010). Precision and accuracy of a new device (CNAP™) for continuous non-invasive arterial pressure monitoring: assessment during general anaesthesia. British Journal of Anaesthesia, 105, 264–72. doi: 10.1093/bja/aeq143 [DOI] [PubMed] [Google Scholar]
- Johansson B, Starmark A, Berglund P, Rödholm M, & Rönnbäck L (2010). A self-assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Injury, 24, 2–12. doi: 10.3109/02699050903452961 [DOI] [PubMed] [Google Scholar]
- Jopp D, & Hertzog C (2007). Activities, self-referent memory beliefs, and cognitive performance: Evidence for direct and mediated relations. Psychology and Aging, 22, 811–825. doi: 10.1037/0882-7974.22.4.811 [DOI] [PubMed] [Google Scholar]
- Jopp D, & Hertzog C (2010). Assessing adult leisure activities: An extension of a self-report activity questionnaire. Psychological Assessment, 22, 108–120. doi: 10.1037/a00176622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schecter R, & Shimmel H (1983). Validation of a short orientation-memory-concentration test of cognitive impairment. American Journal of Psychiatry, 140, 734–739. [DOI] [PubMed] [Google Scholar]
- Kornadt AE, & Rothermund K (2012). Internalization of age stereotypes into the self-concept via future self-views: A general model and domain-specific differences. Psychology and Aging, 27, 164–172. doi: 10.1037/a0025110 [DOI] [PubMed] [Google Scholar]
- Levy B (2009). Stereotype embodiment a psychosocial approach to aging. Current Directions in Psychological Science, 18, 332–336. doi: 10.1111/j.1467-8721.2009.01662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Zonderman AB, Slade MD, & Ferrucci L (2009). Age stereotypes held earlier in life predict cardiovascular events in later life. Psychological Science, 20, 296–298. doi: 10.1111/j.1467-9280.2009.02298.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Zonderman AB, Slade MD, & Ferrucci L (2012). Memory shaped by age stereotypes over time. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences, 67, 432–436. doi: 10.1093/geronb/gbr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, & Tammen VV (1989). The effects of subjective and objective competitive outcomes on intrinsic motivation. Journal of Sport and Exercise Psychology, 11, 84–93. [Google Scholar]
- Obrist PA (1981). Cardiovascular psychophysiology: A perspective. New York: Plenum. [Google Scholar]
- Palacios CS, Torres MVT, & Mena MJB (2009). Negative aging stereotypes and their relation with psychosocial variables in the elderly population. Archives of Gerontology and Geriatrics, 48, 385–390. doi: 10.1016/j.archger.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Queen TL, & Hess TM (2018). Linkages between resources, motivation, and engagement in everyday activities. Motivation Science, 4, 26–38. doi: 10.1037/mot0000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian CA, Steers WN, Hays RD, & Mangione CM (2005). Development of the 12-items expectation regarding aging survey. The Gerontologist, 45, 240–248. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, & Yesavage JA (1986). Geriatric depression scale (GDS): Recent evidence and development of a shorter version In Clinical gerontology: A guide to assessment and intervention (pp. 165–173). New York: The Hawthorne Press. [Google Scholar]
- Smith BT, & Hess TM (2015). The impact of motivation and task difficulty on resource engagement: differential influences on cardiovascular responses of young and older adults. Motivation Science. 1, 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Birmingham W, & Berg CA (2010). Are older adults less or more physiologically reactive? A meta-analysis of age-related differences in cardiovascular reactivity to laboratory tasks. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65B, 154–162. doi: 10.1093/geronb/gbp127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE (1993). SF-36 health survey: Manual and interpretation Guide. Boston: The Health Institute, New England Medical Center. [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale (3rd ed.). New York, NY: Psychological Corporation. [Google Scholar]
- Wright RA (1996). Brehm’s theory of motivation as a model of effort and cardiovascular response In Gollwitzer PM and Bargh JA (Eds.), The psychology of action: Linking cognition and motivation to behavior (pp. 424–453). New York: Guilford. [Google Scholar]
- Wurm S, Tomasik MJ, & Tesch-Römer C (2009). On the importance of a positive view on ageing for physical exercise among middle-aged and older adults: Cross-sectional and longitudinal findings. Psychology and Health, 25, 25–42. doi: 10.1080/08870440802311314 [DOI] [PubMed] [Google Scholar]
- Zafeiriou A, & Gendolla GHE (2017). Implicit activation of the aging stereotype influences effort-related cardiovascular response: The role of incentive. International Journal of Psychophysiology, 119, 79–86. doi: 10.1016/j.ijpsycho.2017.01.011 [DOI] [PubMed] [Google Scholar]
- Zhang X, Fung HH, Stanley JT, Isaacowitz DM, & Ho MY (2013) Perspective taking in older age revisited: A motivational perspective. Developmental Psychology, 49, 1848–1858. doi: 10.1037/a0031211 [DOI] [PubMed] [Google Scholar]