Abstract
Recent reading research implicates executive control regions as sites of difference in struggling readers. However, as studies often employ only reading or language tasks, the extent of deviation in control engagement in children with reading difficulties is not known. The current study investigated activation in reading and executive control brain regions during both a sentence comprehension task and a nonlexical inhibitory control task in third–fifth grade children with and without reading difficulties. We employed both categorical (group-based) and individual difference approaches to relate reading ability to brain activity. During sentence comprehension, struggling readers had less activation in the left posterior temporal cortex, previously implicated in language, semantic, and reading research. Greater negative activity (relative to fixation) during sentence comprehension in a left inferior parietal region from the executive control literature correlated with poorer reading ability. Greater comprehension scores were associated with less dorsal anterior cingulate activity during the sentence comprehension task. Unlike the sentence task, there were no significant differences between struggling and nonstruggling readers for the nonlexical inhibitory control task. Thus, differences in executive control engagement were largely specific to reading, rather than a general control deficit across tasks in children with reading difficulties, informing future intervention research.
Keywords: brain networks, cognitive control, fluency, reading comprehension, stop-signal task
Introduction
Executive control, including multiple “executive functions” (EFs), broadly refers to cognitive abilities that support goal-directed behaviors, such as reading (Diamond 2013). Executive control abilities may support the manipulation of information necessary for fluent reading by linking the core reading comprehension components of decoding and listening comprehension (as suggested by the Simple View of Reading model; Cutting et al. 2009; Aboud et al. 2016; Hudson et al. 2016; Butterfuss and Kendeou 2017; Cirino et al. Under review). In addition to reading comprehension, executive control uniquely contributes to word accuracy and fluency processes associated with comprehension (Cutting et al. 2009; Aboud et al. 2016; Hudson et al. 2016; Butterfuss and Kendeou 2017; Cirino et al. Under review). Understanding the role of executive control in reading fluency and comprehension is critical because reading skills in elementary school predict future reading ability, overall academic achievement, and occupational success (Cunningham and Stanovich 1997; Kamil et al. 2008). Unfortunately, 31% of fourth graders in US public schools perform below the basic reading level, meaning that they are unable to use relevant information to make inferences or to interpret the meaning of words in a text (National Assessment of Educational Progress 2015). To address this gap in performance, there is a need for greater neurobiological understanding of how related abilities like executive control interact in children who struggle with reading fluency and comprehension.
While the majority of neuroimaging studies of children with reading difficulties have used single-word tasks, some studies have investigated sentence comprehension (SC). In these sentence-level studies, poor readers show underactivation primarily in the left hemisphere dorsal (temporoparietal: inferior parietal, middle/superior temporal cortex) and ventral (occipitotemporal: ventral fusiform) functional pathways (Meyler et al. 2008; Rimrodt et al. 2009; Schulz et al. 2009; Simos et al. 2011a, 2011b; Langer et al. 2015; Aboud et al. 2016). Studies identifying subregions of the left inferior frontal gyrus (IFG) show mixed differences in activation during sentence reading in children with reading difficulties relative to typical readers (reduced activation in BA 44/45/47 (Rimrodt et al. 2009; Aboud et al. 2016); increased activation in left inferior frontal in BA 44; Kronbichler et al. 2006).
Sentence or passage-level studies find activation differences between struggling and nonstruggling readers that are largely consistent with the reading-related brain regions found in single-word studies (though see Cutting et al. 2013). Agreement between the results of word-level and sentence-level fMRI studies may reflect the close link between reading accuracy and fluency, supporting fluency deficits as one source of poor reading comprehension. Additionally, the posterior and anterior patterns of left hemisphere brain activation during sentence and single-word neuroimaging studies map onto the behavioral constructs of the triangle reading model (Harm and Seidenberg 2004; Taylor et al. 2013). These constructs include phonology (e.g., supramarginal gyrus, IFG BA 44), semantics (e.g., IFG BA 45 and 47, anterior/posterior middle temporal gyrus), and orthography (e.g., putative visual word form area, VWFA; Perfetti 2007; Schlaggar and McCandliss 2007; Price 2012; Hudson et al. 2016).
Though these regions in the dorsal, ventral, and frontal pathways are consistently identified in the reading literature, regions in bilateral inferior parietal cortex and inferior frontal cortex are also active for other cognitively demanding tasks in children (Church et al. 2017; McKenna et al. 2017) and adults (Niendam et al. 2012). For example, the bilateral IFG has been implicated in tasks that measure inhibitory control (intentionally overriding a prepared response), working memory (temporarily storing information in mind), and task switching in children (McKenna et al. 2017; Engelhardt et al. 2018). The bilateral inferior parietal cortex is engaged during task switching (Church et al. 2017) and across working memory tasks in children (McKenna et al. 2017). Further, the BOLD signal timecourses of these regions correlate at rest in children and adults, forming frontoparietal and cingulo-opercular control networks (Dosenbach et al. 2007; Petersen and Posner 2012; Power and Petersen 2013; Vogel et al. 2013).
Struggling and nonstruggling readers also differ on behavioral measures of executive control, consistent with activation differences in regions of executive control during reading (e.g., inferior parietal cortex; Meyler et al. 2008; Schulz et al. 2008, 2009; Langer et al. 2015). Children with poor comprehension performance have shown impairments in executive control abilities including planning (Cutting et al. 2009; Sesma et al. 2009; Locascio et al. 2010), response inhibition (Protopapas et al. 2007; Altemeier et al. 2008; Locascio et al. 2010), working memory (Christopher et al. 2012; Arrington et al. 2014), and task switching (Potocki et al. 2017). While executive control behavior and activity during comprehension may be altered in struggling readers, the breadth or specificity of differences in control activation is not well understood, as studies of struggling readers have generally focused only on reading-related tasks rather than those of executive control (Kronbichler et al. 2006; Perfetti 2007; Schlaggar and McCandliss 2007; Meyler et al. 2008; Schulz et al. 2008; Rimrodt et al. 2009; Schulz et al. 2009; Simos et al. 2011a, 2011b; Price 2012; Langer et al. 2015; Aboud et al. 2016; Hudson et al. 2016).
The current study tested executive control engagement in children who struggle with fluency and reading comprehension at an age where classroom instruction shifts to using reading as a learning tool (O’Brien 2008). The aim was to evaluate differences in executive control-related activation between struggling and nonstruggling readers from a school-based sample of third–fifth graders using both dichotomous group comparisons and individual difference approaches for whole brain and executive control literature-based applied ROI analyses. We analyzed control activation during 2 fMRI tasks: a SC (Meyler et al. 2007; Meyler et al. 2008) task and an inhibitory control task (a variant of the classic stop-signal task [SST]; Schall and Godlove 2012). The SST and other inhibitory control tasks have been found to strongly engage putative executive control regions from frontoparietal (e.g., right IFG, right dorsolateral prefrontal cortex [dlPFC]) and cingulo-opercular networks (e.g., dorsal anterior cingulate [dACC], bilateral anterior insula) in children (McKenna et al. 2017; Engelhardt et al. 2018). Further, behavioral evidence from motor response inhibition tasks suggests that struggling readers ages 10–14 may show deficits relative to typical readers (Locascio et al. 2010). We hypothesized that if struggling readers, defined using thresholds on fluency and comprehension measures, had a general control deficit relative to nonstruggling readers, they would have altered activity in executive control regions of interest (ROIs) during both the sentence task and the nonlexical control task (the SST).
For the individual differences approach, we analyzed out-of-scanner continuous measures of standardized word reading and comprehension in relation to BOLD activations during our scanner tasks across all individuals. Activation in putative executive control regions has been shown to positively relate to continuous measures of comprehension and word reading ability measured outside the scanner (Hoeft et al. 2006; Meyler et al. 2007; Horowitz-Kraus et al. 2013). We hypothesized that activity in control ROIs during both the sentence reading and response inhibition control tasks would positively correlate with reading ability measured outside the scanner. Executive control-related results in struggling readers across lexical and nonlexical tasks would indicate that alterations in control-related activation may not be specific to reading comprehension. A widespread task control deficit in struggling readers would inform options for fluency and comprehension skill instruction and would lead to interventions that target abilities beyond the primary focus of reading skills.
Materials and Methods
Participants
Struggling readers were recruited from a larger study of third–fifth grade students (ages 8–11 years) enrolled in an in-school reading comprehension intervention as part of the Texas Center for Learning Disabilities (TCLD) intervention studies in Houston, and Austin, Texas (https://texasldcenter.org). The TCLD intervention studies consisted of 3 collection waves (2012–2014, 2014–2015, and 2015–2016) with pretest and post-test assessments each school year. The intervention was similar across all 3 studies but varied across cohorts due to TCLD intervention-related questions about length (1 or 2 years) and format (during versus after school; Vaughn et al. 2016).
The 2012–2014 cohort of children with reading comprehension and fluency difficulties were identified using the Gates-MacGinitie Reading Test with standard scores below 85 (GMRT-fourth edition; MacGinitie et al. 2000). All children identified with the GMRT also showed reading fluency problems via the Test of Sentence Reading Efficiency and Comprehension (TOSREC; Wagner et al. 2010) or the Test of Word Reading Efficiency Sight Word Efficiency subtest (TOWRE-2; Torgesen et al. 1999; score below 90 on either measure). The 2014–2015 and 2015–2016 cohorts were identified using standardized scores below 90 on the TOSREC, which is a timed sentence measure that loads on a combined fluency and comprehension factor in latent variable studies of reading measures (Cirino et al. 2013). Therefore, the TOSREC allowed more efficient screening of children with comprehension and fluency difficulties; TOSREC scores correlated 0.62 (P < 0.001) with GMRT scores. The struggling reader data reported here includes pretest assessments collected from all three waves after reading ability screening (i.e. pretests for 2012–2015) and neuroimaging data collected between September and the start of the second semester (mid-January) from the same waves, prior to the bulk of intervention administration. The current analysis is cross-sectional, collapsing across all three waves. The struggling reader group that participated in the neuroimaging study included children randomly assigned to either intervention or business as usual (BAU) groups.
Nonstruggling readers were recruited to participate in the neuroimaging study from the same Austin and Houston schools involved in the TCLD studies, as well as from the surrounding area communities. The criterion for nonstruggling readers was a standard score at or above 90 on the TOSREC. One struggling reader initially recruited from the Austin community to be a nonstruggling reader was re-classified into the struggling group because they met criteria for poor reading comprehension on the TOSREC. Both struggling and nonstruggling readers had Verbal IQ subtest scores >70 on the Kaufman Brief Intelligence Test (KBIT-2; Kaufman and Kaufman 2004).
Additional neuroimaging-specific criteria excluded struggling readers from participation in the imaging study based on a history of known neurological disorders other than attention deficit hyperactivity disorder (ADHD) or learning disabilities. We allowed children identified with ADHD in the struggling reader sample because comorbidity of ADHD and reading difficulties is common (Willcutt and Pennington 2000), and we intended for our data to reflect the broader struggling reader population. An additional goal of the study, not reported here, was to examine relations between symptoms of attention difficulty and reading difficulties. Of the 74 struggling readers, 9 presented with ADHD, according to parent/teacher report, and of those with reported ADHD, 2 took medication on the day of the MRI. The nonstruggling readers had no identified disorders. None of the children in either group were treated with medications for any psychiatric disorder other than ADHD and did not have hearing impairments or visual impairments uncorrectable by MRI safe glasses.
Participant characteristics are provided in Table 1 (data coverage of the sample is provided in Supplementary Table S1). For the struggling reader group, we report data for 74 of the 102 children who enrolled in the MRI study: 28 were excluded from the current study for various reasons described in Supplementary Section A. Of the 74, 60 struggling readers completed the SC task (26 females, mean age = 10.12, standard deviation [SD] age = 0.56) and 67 completed the SST (26 females, mean age = 10.15, SD age = 0.64); 53 contributed data for both tasks, with 21 nonoverlapping (7 in SC only, 14 in the SST only).
Table 1.
Participant characteristics, group selection criteria, and correlate measures
| Nonstruggling Readers (total unique N = 34) | Struggling Readers (total unique N = 74) | |||
|---|---|---|---|---|
| SC (N = 32) | SST (N = 32) | SC (N = 60) | SST (N = 67) | |
| N (%) | N (%) | N (%) | N (%) | |
| Gender | ||||
| Female | 15 (46.9) | 15 (46.9) | 26 (43.3) | 26 (38.8) |
| Male | 17 (53.1) | 17 (53.1) | 34 (56.7) | 41 (61.2) |
| Race/ethnicity | ||||
| Hispanic | 9 (28.1) | 10 (31.3) | 30 (50.0) | 34 (50.7) |
| Non-Hispanic white | 19 (59.4) | 21 (56.3) | 17 (28.3) | 21 (31.3) |
| Black | 3 (9.4) | 3 (9.4) | 10 (16.7) | 10 (15.0) |
| Multiracial | 1 (3.1) | 1 (3.1) | 3 (5.0) | 2 (3.0) |
| ADHD Diagnosis | – | – | 7 (11.7) | 9 (13.4) |
| M (SD) | M (SD) | |||
| Age† | 9.93 (.83) | 10.14 (.62) | ||
| KBIT-2†* | 116.00 (11.01) | 97.21 (11.35) | ||
| Verbal* | 111.24 (12.31) | 92.61 (14.65) | ||
| Nonverbal* | 114.58 (13.85) | 102.16 (11.16) | ||
| GMRT* | 107.33 (9.67) | 88.41 (9.46) | ||
| TOSREC* | 110.44 (11.90) | 76.72 (8.52) | ||
| WJ-III PC†* | 105.76 (8.78) | 89.77 (8.79) | ||
| TOWRE-2†* | 103.15 (9.56) | 83.43 (10.88) | ||
Notes. Participant characteristics for each reader group in each fMRI task, and group selection criteria and correlate measures for each group collapsed across tasks. Race, ethnicity, age, and presence of ADHD were reported by parents and/or teachers. SC = sentence comprehension task; SST = stop-signal task; KBIT-2 = Kaufman Brief Intelligence Test, Second Edition; GMRT = Gates-MacGinitie Reading Test GMRT-fourth edition; TOSREC = Test of Sentence Reading Efficiency and Comprehension. WJ-III PC = Woodcock Johnson Diagnostic Reading Battery Passage Comprehension subtest; TOWRE-2 = Test of Word Reading Efficiency Sight Word Efficiency subtest. †Measures used as correlates. * P < 0.05.
For the nonstruggling reader group, we report data from 34 of the 73 nonstruggling readers enrolled in the study: 39 enrolled nonstruggling readers were excluded from the study for reasons reported in Supplementary Section A. Of the 34, 32 nonstruggling readers completed the SC task (15 females, mean age = 9.91, SD age = 0.84) and 32 completed the SST (15 females, mean age = 10.00, SD age = 0.80). Overall, 30 nonstruggling readers contributed data for both tasks, with 4 participants nonoverlapping (2 in SC only, 2 in the SST only).
Correlates Selected for Neuroimaging Analyses
Out of a much larger battery of pretest assessments, we included 2 reading measures in our analyses as correlates, in addition to age and IQ, measured using the KBIT-2 (see Table 1 and Supplementary Table S1). The reading measures were chosen because they were not used as screeners for the larger TCLD study and had the greatest number of participants with scores. They include one comprehension assessment, the Passage Comprehension subtest of the Woodcock–Johnson III (WJ-III PC; Woodcock et al. 2001), and one decoding fluency measure, the TOWRE-2 (Torgesen et al. 1999).
Neuroimaging Acquisition
The Institutional Review Board of the University of Texas Health Science Center at Houston approved the study for both sites. Parents of the children gave consent for their child to participate and children gave informed assent. All participants were compensated for their time.
Participants in Austin and Houston attended 1–2 visits to the neuroimaging lab. The first visit served to inform the family and child of their involvement in the study, to administer any missing neuropsychological assessments, and to become familiar with the scanner experience using a mock scanner, a method to help minimize in-scanner movement (Church et al. 2010; Greene et al. 2016). The second visit was the MRI session, lasting approximately 90 min. Several participants combined the data collections into a single visit, as travel distance made multiple visits prohibitive. At both Austin and Houston imaging sites, each scan session included high-resolution structural (T1 and T2) and DTI scans, resting state and task fMRI scans, including the 2 in-scanner tasks reported here: the SC task (up to 3 runs per participant) and the SST (1–2 runs per participant).
The sites had different Siemens 3 T scanners that necessitated minor variation in image acquisition procedures. The Austin site MRI data were acquired with a Siemens Skyra 3 T MRI scanner with a 32-channel head coil at the University of Texas at Austin Imaging Research Center. We used an MPRAGE sequence to collect T1-weighted structural images (TR = 2530 ms, TE = 3.37 ms, FOV = 256, 1 × 1 × 1 mm3 voxels) and a turbo-spin echo sequence to collect T2-weighted structural images (TR = 3200 ms, TE = 412 ms, FOV = 250, 1 × 1 × 1 mm3 voxels). Functional images for both tasks were acquired using a multiband echo-planar sequence (TR = 2000 ms, TE = 30 ms, flip angle = 60°, multiband factor = 2, 48 axial slices, 2 × 2 × 2 mm3 voxels, base resolution = 128 × 128). Stimuli were presented using PsychoPy software (Peirce 2007) on a PROPixx projector displaying stimuli at a resolution of 1920 × 1080 on a screen located behind the scanner, which participants could see through a mirror mounted on top of the head-coil. Participants were provided with Optoacoustics (OptoACTIVE Optical MRI Communication System with Active Noise Control) headphones and a microphone, providing ear protection, allowing participants to communicate with the researchers throughout the session in-between scans, and ability to hear a movie during the structural sequences.
The Houston site MRI data were acquired with a Siemens TIM Trio Syngo 3 T MRI scanner with a 12-channel head coil at the Baylor Imaging Center. Isotropic 3D T1-weighted structural images were acquired in the sagittal plane (TR = 2170 ms, TE = 3.6 ms, FOV = 256, 1 × 1 × 1 mm3 voxels, flip angle = 7°, NEX = 1, iPAT = 3) and a turbo-spin echo sequence to collect T2-weighted structural images (TR = 3200 ms, TE = 410 ms, FOV = 256, 1 × 1 × 1 mm3 voxels). Isotropic 2D functional images for both tasks were acquired in the axial plane (TR = 2000 ms, TE = 30 ms, flip angle = 79°, 32 axial slices, 3 × 3 × 3 mm3 voxels, base resolution = 96 × 96, NEX = 1, iPAT = 3). Stimuli at the Houston site were presented on a Cambridge Research Systems BOLDscreen 32 LCD monitor projector at the same resolution as the Austin site. Houston participants were provided with the same Optoacoustics headphones.
Sentence Comprehension Task
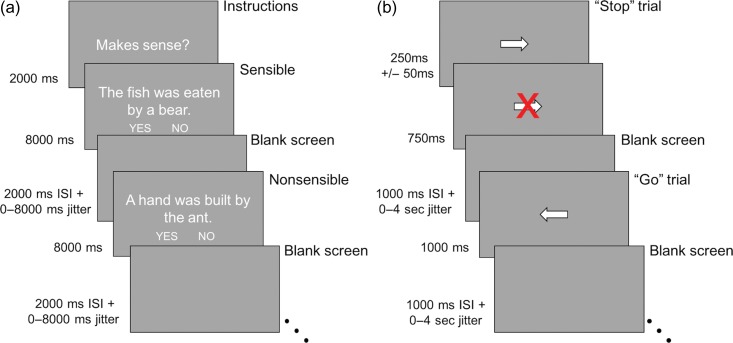
The SC task was closely adapted from Meyler and colleagues (Fig. 1a; Meyler et al. 2007). Participants indicated if short sentences were sensible or nonsensible. Vocabulary was selected from high frequency early reader lists. There were 4 sentence categories: active sensible, passive sensible, active nonsensible, and passive nonsensible. For the purpose of the current analysis, the active and passive conditions were collapsed in order to investigate the general effect of SC processing. Therefore, we analyzed all correct sentence trials relative to baseline (correct versus baseline) and the correct trials within the sensible versus nonsensible contrasts. The task was administered up to 3 times (7′6″ runs, each with 212 frames); each run started with the probe “Makes Sense?” for 2000 ms, followed by 32 sentence trials. The sentences were presented for 8000 ms followed by 2000 ms of blank screen (inter-stimulus interval) and interspersed with a jittered blank screen ranging from 0 to 8000 ms. Participants used their left and right thumbs to press buttons on a FIU-932 Current Designs button box that indicated the sensibility of the sentence. The words “No” and “Yes” appeared in small font below the sentence on either side of the screen to remind the participant of the response mapping onto the buttons (left/right), which was counterbalanced across participants. Before performing the task in the scanner, participants were trained on a set of practice stimuli on a computer and instructed to think carefully about each sentence before making a decision. SC scans with less than 60% accuracy on the task were excluded from analyses.
Figure 1.
(a) Timing of the scanner sentence comprehension (SC) task. Adaptation of Meyler et al.'s task (Meyler et al. 2007). (b) Timing of the scanner stop-signal task (SST). Adaptation of de Jong et al. (2009).
Stop-Signal Task
The SST was adapted from the classic SST to have a visual, rather than auditory stop (Rubia et al. 2003; de Jong et al. 2009) to prevent difficulty in perception due to scanner noise. Participants were instructed to quickly respond to arrows pointing left or right on the screen (“Go” trials), but to not respond to the direction of the arrow if a red letter X appeared (“Stop” trials; Fig. 1b). The participants completed up to 2 scans of the task, each with 96 “Go” trials and 32 “Stop” trials (6′0″ runs, each with 180 frames). A “Go” trial consisted of a left- or right-pointing arrow in the center of the screen presented for 1000 ms then followed by 1000 ms of blank screen (inter-stimulus interval) and interspersed with a jittered blank screen ranging from 0 to 4000 ms. A “Stop” trial started with the presentation of a left- or right-pointing arrow in the center of the screen for 250 ms (the initial stop signal delay [SSD]), then the “Stop” signal (a red X) appeared overlaid on top of the arrow; the “Stop” signal remained on the screen for the duration of that trial. If the participant correctly avoided pressing a button during the first “Stop” trial, the SSD for the next “Stop” trial increased 50 ms (SSD to 300 ms), but if the participant incorrectly made a button response during a stop trial, then the SSD decreased by 50 ms on the next “Stop” trial (SSD to 200 ms); this staircasing continued throughout each scan of the SST. During the practice session before entering the scanner, participants were instructed to try to not respond during a “Stop” trial but also not to wait for a stop-signal to appear before pressing.
Behavioral calculations and exclusionary criteria were based on a study by Congdon and colleagues (Congdon et al. 2012) that tested multiple calculation methods for the most reliable stop signal response time (SSRT) estimates. These criteria exclude stop-signal runs with any of the following: less than 70% accuracy on “Go” trials (with the exception of one run for one participant with 69.79% “Go” accuracy), less than 10% “Go” errors (defined as a button press for the opposite direction of the arrow), less than 25% or greater than 75% accuracy on “Stop” trials, and/or less than 50 ms SSRT. SSRT was calculated by subtracting the mean time between the presentation of the arrow and the red X (SSD) from the mean response time (RT) for the “Go” trials (Congdon et al. 2012). SST-related BOLD results are reported for contrasts using correct trials, correct stop versus correct go and correct stop versus baseline contrasts, to parallel the SC task contrast of all correct sentences relative to baseline.
Neuroimaging Processing and Data Analysis
fMRI data processing was carried out using the FEAT (FMRI Expert Analysis Tool) Version 6.00 part of FSL version 5.0.2 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) separately for each task (Smith et al. 2004). High-resolution T1-weighted structural images were skull stripped and extracted using Freesurfer 5.3.0 (Reuter et al. 2010). We used the Boundary Based Registration (BBR) algorithm to register the functional data to the high-resolution structural image (Greve and Fischl 2009). Registration of the high-resolution structural to standard space (2 mm MNI152) was executed using FMRIB’s Linear Image Registration Tool (FLIRT; Jenkinson and Smith 2001; Jenkinson et al. 2002). The following prestatistics processing was applied: spatial smoothing using a Gaussian kernel of FWHM 5 mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s).
Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al. 2001). We used a double-gamma HRF time-series model for both the SC task and the SST. The models for both tasks also included 6 motion regressors, temporal derivatives for each regressor, and nuisance regressors that modeled out single TR’s identified to have excessive motion according to a framewise displacement (FD) > 0.9 mm (Siegel et al. 2014). At least 60% of frames (127 for SC and 108 for SST) were required for a run to be included after censoring for FD (see Supplementary Section A). Second-level analysis, which averaged contrast estimates over runs within subject, was carried out using a fixed effects model (Beckmann et al. 2003; Woolrich et al. 2004; Woolrich 2008). The third-level group analysis was also executed using FLAME stage 1 (Beckmann et al. 2003; Woolrich et al. 2004; Woolrich 2008). Z statistic images were thresholded with a cluster-forming threshold of z > 3.1 and a cluster probability of P < 0.05, using Gaussian random field theory (Worsley 2001). Brain regions are reported in MNI coordinates and identified using the Harvard-Oxford atlas in the FMRIB software. For visualization of the statistical maps, we projected the data onto an average inflated map using Caret software (Van Essen 2005). Coordinates and voxel size of group analysis results were obtained using the FSL version 5.0.2 Cluster tool.
Third-level models for the effects of time between cohorts and for site comparison were also executed using FLAME stage 1 (Beckmann et al. 2003; Woolrich et al. 2004; Woolrich 2008) with Gaussian random field theory (Worsley 2001) and Z statistic images thresholded at z > 3.1 and P < 0.05. Tests for site differences and cohort differences were conducted in addition to the group comparisons. There were no significant differences between cohorts or sites for all SC and SST contrasts, allowing a collapse across cohorts and sites for analyses.
In order to investigate group differences in brain activation in more detail, we performed literature-derived ROIs analyses using FSL and R version 3.3.3 (R Development Core Team 2017). To confirm previous sentence-level findings in our sample of struggling readers, we evaluated reading-related regions from the word and sentence literature during the SC fMRI task. The SC task ROIs included a set of 25 5 mm radius spheres using MNI coordinates from the reading and cognitive control literature: 10 reading-related regions (Cohen and Dehaene 2004; Richardson et al. 2011; Vogel et al. 2013; Rao and Singh 2015) and 15 cognitive control regions (5 cingulo-opercular and 10 frontoparietal; Dosenbach et al. 2010; Greene et al. 2014; see Supplementary Table S2, Supplementary Fig. S1). The SST ROIs included a set of 20 5 mm radius spheres: the same 15 cognitive control regions and 5 additional regions from the stop-signal inhibition literature (Aron and Poldrack 2006; Aron et al. 2007; Boehler et al. 2010; Supplementary Fig. S1). The 5 mm sphere size has been used in previous reading studies (Van Der Mark et al. 2009; Richlan et al. 2010; Wimmer et al. 2010; Benjamin and Gaab 2012). The spheres were created using the T1 MNI152 2 mm brain mask in FSL with the center of each sphere at the literature-based coordinates (Supplementary Table 2, Supplementary Fig. S1). The mean BOLD percent signal change for each ROI for each individual for the contrast of interest was calculated using FSL. We report corrected results using false discovery rate (FDR) for each ROI set (25 for the SC task and 20 for the SST). To demonstrate that performance on the SC task was not impacted by the participants with ADHD status, we analyzed any regions that showed significant uncorrected group results for the SC task ROI analysis without the 7 struggling readers with ADHD. Uncorrected results, including those without the struggling readers with ADHD, may be found in the Supplementary Materials.
Whole Brain Analyses With WJ-III PC and TOWRE-2 as Correlates
For both SC and SST, across all participants, we ran a whole-brain regression to examine the relation between out-of-scanner measures of reading comprehension and reading fluency, and cortical activation during sentence reading or inhibition. We selected the correct versus baseline contrast from the SC task and the correct stop versus baseline contrast for SST. Mean-centered standardized scores for struggling and nonstruggling participants for the WJ-III PC (SC: N = 89, SST: N = 94) and the TOWRE-2 Word Reading Efficiency Sight Word Efficiency subtest (SC: N = 89, SST: N = 95) were each added separately, given that we did not have all measures on each individual (see Supplementary Table S1). These scores were entered as correlates to a third-level group analysis using FLAME stage 1 (z > 3.1, Ps < 0.05; Beckmann et al. 2003; Woolrich et al. 2004; Woolrich 2008). Age and IQ (KBIT-2) were also added separately as correlates. We had 85 participants for the SC task and 90 for SST with the KBIT-2, however, the whole brain correlation results with the KBIT-2 were not significant, and thus are not reported. For age we were able to include all participants (struggling and nonstruggling) for each task (SC N = 92, SST N = 99; see Supplementary Section B and Supplementary Fig. S2). The results of these models are reported with hot colors (yellow/orange) indicating a positive linear relation (slope) and cool colors (blue) indicating a negative linear relation between task BOLD activation and the behavioral measure. We projected the data onto the same average inflated map (Human PALS-B12 atlas) as the whole-brain main effect and group difference results, using Caret software (Van Essen 2005; Van Essen and Dierker 2007). Brain regions were identified in MNI coordinates using the Harvard-Oxford atlas in the FMRIB software.
ROI Level Analyses Correlating With Reading Ability
In order to look at task-related activation in specific regions as it relates to individual differences in out-of-scanner measures of age, IQ, comprehension, and fluency, we ran Pearson correlations with the extracted mean BOLD percent signal change for each ROI for each individual during the correct versus baseline SC contrast and the correct stop versus baseline SST contrast. We used R version 3.3 (R Development Core Team 2017) to conduct these correlations with the standardized scores on the reading measures (WJ-III PC and the TOWRE-2), age, and IQ (KBIT-2). We found no significant ROI correlation results with age and IQ. To confirm that the fMRI SC task was consistent with standardized reading ability, and test relationships in performance with SST, we also ran additional Pearson correlations between SC and SST accuracy and response times and out-of-scanner WJ-III PC and TOWRE-2 scores across all participants. We correlated our sample’s WJ-III PC and TOWRE-2 scores to confirm a relation between these out-of-scanner reading measures. We report all result sections with FDR-corrected P-values (see uncorrected results in Supplementary Materials). To confirm that the SC task ROI correlations with the WJ-III PC and the TOWRE-2 were not impacted by the participants with ADHD status, we also conducted this analysis without the 7 struggling readers with ADHD (N = 83; see Supplementary Section C).
Results
Behavioral Performance Differences Between Struggling and Nonstruggling Readers
SC Task: Behavioral Performance
On the SC task, struggling readers were significantly slower, t(70.87) = 6.00, FDR-corrected P < 0.001 and less accurate than nonstruggling readers, t(78.21) = −6.60, FDR-corrected P < 0.001 (Table 2). Out of scanner WJ-III PC negatively correlated with fMRI SC task response time, r = −0.58, 95% CI [−0.70, −0.42], FDR-corrected P < 0.001, and positively correlated with task accuracy, r = 0.61, 95% CI [0.46, 0.73], FDR-corrected P < 0.001. TOWRE-2 also negatively related to SC task response time, r = −0.64, 95% CI [−0.75, −0.50], FDR-corrected P < 0.001, and positively related to accuracy, r = 0.47, 95% CI [0.30, 0.62], FDR-corrected P < 0.001. As expected, TOWRE-2 and WJ-III PC were positively correlated with each other, r = 0.56, 95% CI [0.40, 0.69], FDR-corrected P < 0.001.
Table 2.
SC task and SST behavioral performance group and correlational results
| Group differences | |||||
|---|---|---|---|---|---|
| Nonstruggling Readers | Struggling Readers | ||||
| 95% CI | 95% CI | ||||
| SC task | |||||
| RT* | 3.39 s | [3.13, 3.64] | 4.38 s | [4.16, 4.59] | |
| Accuracy* | 92.33% | [90.28, 94.39] | 83.19% | [81.29, 85.10] | |
| SST | |||||
| “Go” RT | 645.75 ms | [622.81, 668.68] | 655.20 ms | [637.98, 672.42] | |
| “Go” accuracy* | 91.36% | [89.63, 93.09] | 86.27% | [84.66, 87.88] | |
| “Stop” accuracy | 55.62% | [54.31, 560.92] | 55.71% | [54.69, 56.74] | |
| SSRT | 218.47 ms | [195.24, 241.70] | 224.27 ms | [206.64, 241.90] | |
| Correlations across all participants | |||||
| WJ-III PC | TOWRE | ||||
| SC task | |||||
| RT | r = −0.58** | r = −0.64** | |||
| Accuracy | r = 0.61** | r = 0.47** | |||
| SST | |||||
| “Go” RT | n.s. | n.s. | |||
| “Go” accuracy | r = 0.26* | r = 0.49** | |||
| “Stop” accuracy | n.s. | n.s. | |||
| SSRT | n.s. | n.s. | |||
Notes. Behavioral performance for the SC task and SST. SC task results show struggling readers were significantly slower, t(70.87) = 5.60, FDR-corrected P < .001, and less accurate, t(78.21) = −6.60, FDR-corrected P < .001, than the nonstruggling readers; SST results show the groups were significantly different on “Go” accuracy, t(81.15) = −4.34, FDR-corrected P < 0.001; SC = sentence comprehension task; SST = stop-signal task; RT = response time; CI = confidence interval; s = seconds; ms = milliseconds; *P < 0.01, **P < 0.001.
SST: Behavioral Performance
The 2 reading groups did not differ on SSRT, t(67.93) = 0.40, uncorrected P = 0.69, “Go” response time, t(67.28) = 0.67, P = 0.51, or “Stop” accuracy, t(70.35) = 0.12, uncorrected P = 0.90 (Table 2). Struggling and nonstruggling readers were significantly different on “Go” accuracy, t(81.15) = −4.34, FDR-corrected P < 0.001, although both groups had high accuracy (86% and 91%). “Go” accuracy was also positively correlated with WJ-III PC, r = 0.26, 95% CI [0.06, 0.44], FDR-corrected P = 0.045, and TOWRE-2, r = 0.49, 95% CI [0.33, 0.63], FDR-corrected P < 0.001.
SC Brain Differences Between Struggling and Nonstruggling Readers
SC Task: Whole Brain Analysis
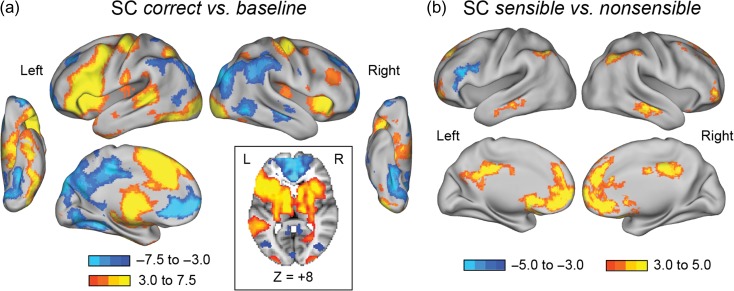
First, we examined significant activity across all participants to confirm that our SC task was evoking activity in established reading-related regions. Indeed, in our correct sentences versus baseline contrast, across the entire sample of 92 children, there was robust activation in left lateralized reading-related areas including along the dorsal route (posterior superior temporal sulcus, supramarginal gyrus [SMG]), in frontal cortex (IFG), and along the ventral route (ventral occipital cortex, including the putative VWFA; Fig. 2a).
Figure 2.
Whole brain sentence comprehension (SC) task results for all participants (N = 92). Z-score thresholds represented by the colored bars. Zs > 3.1, Ps < 0.05, corrected for multiple comparisons. (a) Brain areas showing the main effect of correct sentences versus baseline, with more positive (or less negative) activation for sentence reading in yellow/orange and more positive (or less negative) activation for baseline in blue colors. Horizontal view at z = +8 shows bilateral subcortical activity, including the bilateral caudate. (b) Brain areas showing the sensible versus nonsensible comparison across all participants. Yellow regions are less negative for sensible stimuli and blue regions are more positive for nonsensible stimuli.
For correct sentences versus baseline, there was also widespread statistically significant activation in attentional and executive control regions, including the bilateral anterior insula, dACC, bilateral inferior parietal sulci (IPS), bilateral dlPFC, and bilateral caudate (Fig. 2a). Bilateral inferior parietal cortex, potentially overlapping with frontoparietal cognitive control regions, had negative activity relative to fixation during sentence reading. Further, some areas of negative activation were consistent with the default mode network including bilateral ventromedial PFC, posterior cingulate, and right superior frontal cortex (Dosenbach et al. 2010; Raichle 2015; Fig. 2a).
More positive activity for nonsensible stimuli in the sensible versus nonsensible contrast was observed in left IFG into left middle frontal gyrus, while less negative activity for sensible stimuli relative to nonsensible stimuli was observed in many members of the default-mode network and the inferior parietal cortex (Fig. 2b).
SC Task: Whole Brain Group Comparison
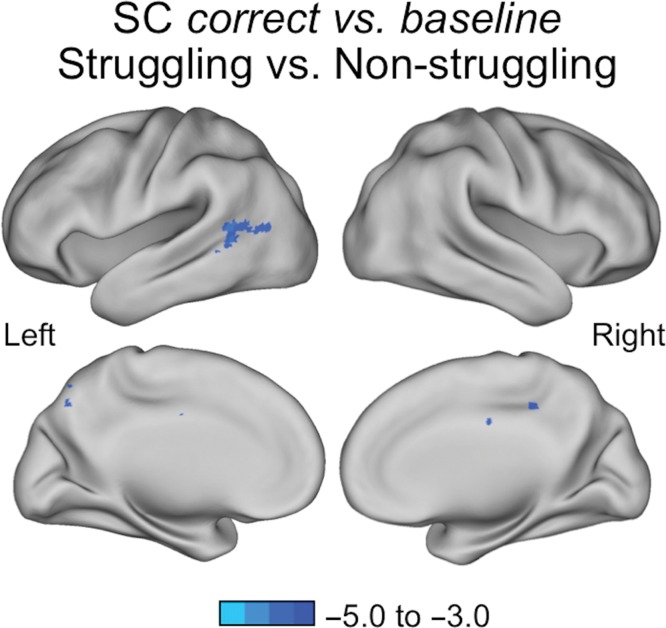
For the correct versus baseline contrast, struggling readers showed less positive BOLD activation relative to nonstruggling readers in a swath of left midposterior temporal and lateral occipital cortex; these results remain significant when controlling for age (MNI coordinates: −60, −58, +6; Fig. 3, Table 3). The sensible versus nonsensible comparison had no group differences that survived multiple comparison correction.
Figure 3.
Group comparison for SC task correct versus baseline. Z-score thresholds represented by the colored bars. Zs > 3.1, Ps < 0.05, corrected for multiple comparisons. Brain areas showing less positive BOLD activation in struggling readers (N = 60) relative to nonstruggling readers (N = 32) in the left posterior temporal cortex (MNI coordinates: −60, −58, +6).
Table 3.
MNI coordinates for the SC task correct versus baseline whole brain results
| Area | Coordinates | No. of voxels | ||
|---|---|---|---|---|
| x | y | z | ||
| Group comparison | ||||
| Left mid/posterior temporal cortex | −60 | −58 | +6 | 422 |
| Left precuneus | −18 | −50 | +24 | 207 |
| Right posterior cingulate | +14 | −26 | +32 | 167 |
| TOWRE-2 correlation | ||||
| Right temporoparietal | +58 | −40 | +52 | 681 |
| Left mid/posterior temporal and inferior parietal cortex | −44 | −60 | +8 | 593 |
| Right subcortical/caudate | +24 | −20 | +20 | 466 |
| Left angular gyrus | −44 | −50 | +40 | 446 |
| Midcingulate | 0 | −18 | +24 | 291 |
| Right posterior precuneus | +24 | −58 | +26 | 174 |
| Left occipital fusiform gyrus | −22 | −72 | −10 | 153 |
Notes. Zs > 3.1, Ps < 0.05, corrected for multiple comparisons.
SC Task: ROIs Group Comparison
The 10 ROIs derived from the reading literature (Supplementary Table S2) yielded no significant group effects (uncorrected P-values ranged from 0.08 to 0.97 for the correct versus baseline contrast and 0.08 to 0.92 for the sensible versus nonsensible contrast).
The 15 ROIs derived from the executive control regions had no differences that survived FDR correction for the correct versus baseline and the sensible versus nonsensible contrasts, but we report uncorrected differences for future research in the Supplementary Section C and Supplementary Fig. S3 (uncorrected P-values ranged from 0.005 to 0.80 for the correct versus baseline contrast and 0.007 to 0.93 for the sensible versus nonsensible contrast).
The ROI analysis for the SC task correct versus baseline and sensible versus nonsensible contrasts of the struggling reader group without the ADHD participants (N = 53) showed the same direction of effects for the executive control regions as the uncorrected results with the ADHD participants (see Supplementary Section C).
Inhibition Brain Differences Between Struggling and Nonstruggling Readers
SST: Whole Brain Analysis
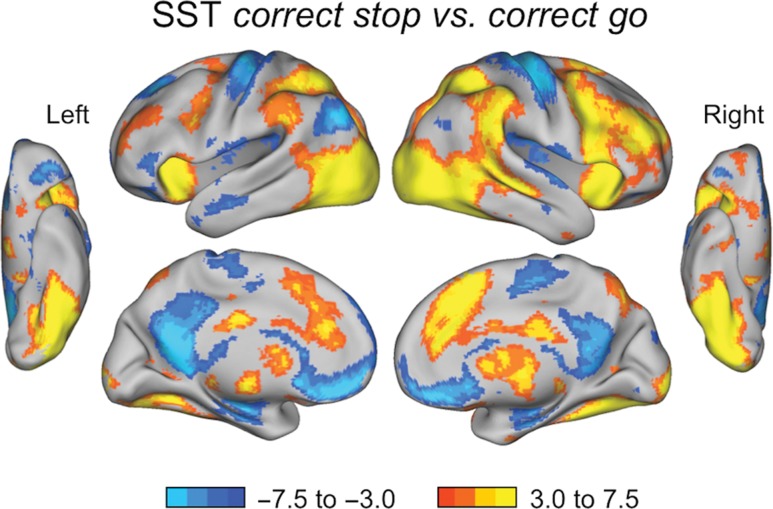
For the correct stop versus correct go contrast across all participants (N = 99), children had higher activity for “Stop” trials relative to “Go” trials in the areas typically found for the SST, including right IFG, right subthalamic nucleus (STN), right temporoparietal junction, and right globus pallidus. Additional attentional and executive control-related regions included bilateral dlPFC, bilateral IPL and IPS, bilateral anterior insula, and dACC (Fig. 4). As might be expected, children had stronger activation for “Go” trials relative to “Stop” trials in bilateral finger sensorimotor cortex, as well as less negative activity for “Go” trials in default mode network regions, including left angular gyrus, posterior cingulate, and ventral medial prefrontal cortex (Fig. 4).
Figure 4.
SST whole brain results for all participants (N = 99). Zs > 3.1, Ps < 0.05, corrected for multiple comparisons. Brain areas showing the correct stop versus correct go contrast, with more positive activation for correct “Stop” trials in yellow/orange and more positive (or less negative) activation for correct “Go” trials in blue.
The correct stop versus baseline contrast had higher activation for “Stop” trials in putative control regions and regions typically found in SST, very similar to those regions found to be greater in “Stops” relative to “Go” trials (see Supplementary Fig. S4).
SST: Whole Brain Analysis and ROIs Group Comparisons
There were no group activation differences at the whole brain level that survived correction for the correct stop versus correct go or correct stop versus baseline contrast.
There were also no significant struggling versus nonstruggling reader group differences in activation during the correct stop versus correct go or correct stop versus baseline contrasts for the 15 ROIs (uncorrected P-values ranged from 0.07 to 0.99).
Correlations Between fMRI Task Activation and Continuous Reading Measures
SC Task: Whole Brain Correlation Analysis
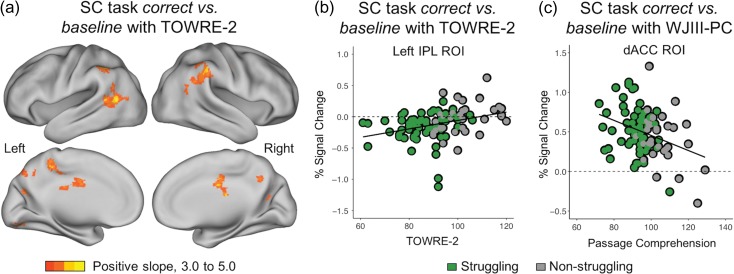
TOWRE-2: Across participants there was a positive correlation, even when controlling for age, between the mean-centered TOWRE-2 values and whole brain BOLD activation during the SC task in the right temporoparietal junction (MNI coordinates: +58, −40, +52), midcingulate (MNI coordinates: 0, −18, +24) and precuneus, and left posterior temporal and inferior parietal cortex (MNI coordinates: −44, −60, +8; Fig. 5a, Table 3).
Figure 5.
(a) Whole-brain correlation analysis for the SC task correct versus baseline (Ns = 89). Zs > 3.1, Ps < 0.05, corrected for multiple comparisons. Yellow/orange indicates a positive linear relationship (slope) and blue indicates a negative linear relationship. A positive correlation was found between BOLD activity during the SC task and the TOWRE-2 reading measure. (b) ROI correlation analysis for SC task with the TOWRE-2. Left IPL percent signal change related to word reading ability (TOWRE-2) across both reader groups, r = 0.33, FDR-corrected P = 0.035. (c) ROI correlation analysis for SC task with the WJ-III PC found a negative correlation between dACC activation and comprehension ability, r = −0.33, FDR-corrected P = 0.038. Not pictured: activation in the left caudate was also negatively correlated with the WJ-III PC, r = −0.31, FDR-corrected P = 0.045.
WJ-III passage comprehension: There were no significant whole brain results between WJ-III PC scores and BOLD activation in the SC correct versus baseline contrast.
SC Task: ROIs Correlation Analysis
TOWRE-2: TOWRE-2 scores had a positive correlation with BOLD activity in the left IPL ROI (MNI coordinates: −53, −50, +39), r = 0.33, 95% CI [0.13, 0.51], FDR-corrected P = 0.035 (Fig. 5b; see additional uncorrected results in Supplementary Section D). Higher TOWRE-2 scores were associated with less negative BOLD activity. The positive correlation between the TOWRE-2 and BOLD activity in the left IPL (MNI coordinates: −53, −50, +39) remained significant when the 7 ADHD participants were removed from the analysis, r = 0.32, 95% CI [0.11, 0.50], FDR-corrected P = 0.046, and when controlling for age, r = 0.32, 95% CI [0.13, 0.50], FDR-corrected P = 0.045.
WJ-III passage comprehension: For the correct versus baseline SC task contrast, across all participants, there was a negative correlation between WJ-III PC and BOLD activity in the dACC control ROI (MNI coordinates: 0, +15, +45), r = −0.33, 95% CI [−0.51, −0.13], FDR-corrected P = 0.038; this correlation remained significant when controlling for age, r = −0.33, 95% CI [−0.51, −0.13], FDR-corrected P = 0.04 (Fig. 5c). When analyzing the SC task ROI correlations without the 7 participants with ADHD, the negative correlation between the WJ-III PC and BOLD activity in the dACC control ROI remained significant: r = −0.36, 95% CI [−0.54, −0.16], FDR-corrected P = 0.02. The WJ-III PC was also negatively related to BOLD activity in the left caudate ROI (MNI coordinates: −10, +11, +8), r = −0.31, 95% CI [−0.48, −0.10], FDR-corrected P = 0.046, though it did not survive correction with the ADHD participants removed or when controlling for age (Supplementary Section D). Higher passage comprehension scores were related to lower engagement of these regions during correct trials of the SC task.
SST: Whole Brain Correlation Analysis
TOWRE-2 and WJ-III passage comprehension: WJ-III PC and TOWRE-2 did not show any significant relations with BOLD activation for correct stop versus baseline.
SST: ROIs Correlation Analysis
TOWRE-2 and WJ-III passage comprehension: There was no relation between BOLD activity in any of the ROIs for the correct stop versus baseline contrast and WJ-III PC or TOWRE-2.
Discussion
We assessed the consistency of executive control engagement in reading and nonreading tasks in children who struggle with reading fluency and comprehension. We predicted that struggling readers would have differential activity relative to nonstruggling readers in executive control regions across both tasks, testing the hypothesis of overall altered control engagement in struggling readers. However, no group differences in executive control regions survived correction for either the comprehension or control task at the whole brain or ROI level. Significant correlations between reading ability and brain activation were found across our entire sample, and these areas of activation were primarily in regions from the executive control literature and not in regions applied from the reading literature (Dosenbach et al. 2010; Supplementary Table S2). Executive control differences were not seen during inhibition trials of the SST, inconsistent with an overall control deficit in struggling readers, and instead suggestive of executive control differences specific to lexical tasks.
Mixed Profile of Executive Control Engagement in Struggling Readers During Reading
We found a positive correlation between out-of-scanner reading ability (TOWRE-2) and SC task activation in the left IPL, often invoked as part of the frontoparietal control network, such that better reading fluency was associated with less negative activity. Indeed, it was one of a number of additional regions in posterior temporal, parietal, and precuneus cortex where brain activity related to TOWRE-2 scores (Fig. 5a,b). Importantly, the inferior parietal cortex is a point of intersection between the reading and executive control literatures. Group differences in the IPL have been found in previous sentence-level (Meyler et al. 2007; Schulz et al. 2008, 2009) and word-level studies, supporting the role of the IPL in reading (Cao et al. 2006; Hoeft et al. 2006, 2007; Van Der Mark et al. 2009). Yet, our applied ROI came from a meta-analysis of cross-task engagement of this region in adults (Dosenbach et al. 2010). Thus, the role of the inferior parietal cortex as a point of intersection for different reading and nonreading tasks in struggling readers should continue to be addressed.
Unlike the negative activity observed in the IPL, other applied executive control regions had higher engagement in struggling readers. We found a negative relation between passage comprehension (WJ-III PC) and activation in the dACC ROI of the cingulo-opercular control network and left caudate ROI from the frontoparietal control network. Better readers activated these control-related regions less, consistent with our prediction. Thus, struggling readers engaged multiple control regions to a greater extent to accomplish correct performance on the SC task, but these differences in control engagement were not seen for inhibiting a response during the nonlexical control task.
Differences in Executive Control Activation may be Specific to the Reading Process
In the nonlexical but control-demanding SST, we did not find group activation differences or relations between activation and individual differences in reading ability. We selected the SST due to evidence that it strongly activates regions of control in typically developing children (McKenna et al. 2017; Engelhardt et al. 2018), allowing us to investigate the specificity of control engagement in struggling readers. While other studies have not directly tested brain activity in struggling readers during inhibition, some behavioral evidence suggested that struggling readers may show differences in SST. Children ages 10–14 with poor word reading ability have shown distinct deficits on motor response inhibition tasks relative to typical readers (Locascio et al. 2010). Additionally, a study conducted with seventh graders found that struggling and nonstruggling readers could be differentiated by inhibitory control ability (Protopapas et al. 2007).
Behaviorally, we did find a group difference in “Go” accuracy on the inhibition task, such that nonstruggling readers were more accurate, and this tracked with passage comprehension scores. These differences for “Go” trials did not extend to group differences in the main behavioral measure of control ability for the inhibition task (SSRT) or in brain activity during the correct stop versus correct go contrast. Other behavioral studies that emerged since our study began have also reported a lack of findings regarding inhibition and reading comprehension in good (Christopher et al. 2012; Arrington et al. 2014) and poor readers (Borella et al. 2010). These results suggest that children with reading difficulties are able to successfully recruit executive control regions for some control-demanding tasks but show control differences when the task also involves reading.
Despite the null results, executive control differences could occur in other types of control-demanding but nonlexical tasks. In large behavioral studies that seek to decompose EFs through factor analysis, including from a larger behavioral sample of our same collection, it is difficult to isolate inhibition from a general EF factor (Friedman et al. 2008; Miyake and Friedman 2012; Cirino et al. 2018). Thus, as inhibition may not have unique neural correlates separate from a common EF neural structure (McKenna et al. 2017), the lack of differences in our struggling readers for inhibition may imply that general EF ability is intact in struggling readers. However, using a bifactor model of EF (where there is no unique inhibition factor), Cirino et al. (Under review) found that general EF does interact with decoding and listening comprehension ability in the Simple View of Reading. Meta-analyses (Jacob and Parkinson 2015), and some single studies (Cutting et al. 2009; Sesma et al. 2009; Locascio et al. 2010), also strongly suggest that executive control ability is important for multiple components of reading ability. Additional research on the consistency and interplay between EF abilities and reading difficulties at both the brain and behavioral level is needed. Our analysis of a nonlexical but control-demanding task is uniquely positioned to suggest that while control engagement is critical for adequate reading performance, control differences observed between struggling and nonstruggling readers may be specific to the reading process.
Differences in Left Posterior Temporal Cortex During Reading
Struggling readers were significantly slower and less accurate on the SC task, and reading ability measured outside the scanner negatively related to response time and positively related to accuracy on the SC task. Consistent with the limited extant literature for sentence processing in children, we found decreased BOLD activation in struggling relative to nonstruggling readers in the left hemisphere midposterior temporal and lateral occipital cortex during the SC task. Posterior middle and superior temporal cortex in the left hemisphere has been implicated in integrating phonological and semantic information at both the word-level (Chou et al. 2006; Booth et al. 2007; Aboud et al. 2016) and the sentence-level (Schulz et al. 2009; Aboud et al. 2016). We also found that TOWRE-2 scores positively related to BOLD activation in the left posterior temporal cortex during the SC task. A previous study using a similar comprehension task (Meyler et al. 2007) in which children were selected for reading fluency difficulties also found a positive correlation between fluency (TOWRE) and BOLD activation in the left middle temporal gyrus, similar in location to our results. Our findings compliment Meyler and colleagues’ (Meyler et al. 2007), and further support the posterior temporal cortex as a site of difference in fluency and reading comprehension between struggling and nonstruggling readers.
Interestingly, we did not find significant differences in the reading regions applied mainly from the single-word reading neuroimaging literature, which were all more anterior than the regions found in our whole brain results (see Table 3 and Supplementary Table S2). The long presentation (8 s) and sentence aspects of the SC task design may have contributed to the null group ROI results in regions known to be important for word reading (e.g., VWFA; Paulesu et al. 2014; Martin et al. 2016).
Advantages and Limitations of the Current Study
This study benefited from a highly diverse and relatively large neuroimaging sample of struggling readers. In-scanner movement was also tightly controlled, using frame censoring motion correction (FD > 0.9 mm; Siegel et al. 2014). To the best of our knowledge, this neuroimaging study is novel in its collection of both reading and inhibition in separate tasks in the same children, as well as to test a priori the role of executive control network regions in struggling readers (for an adult study, see Ihnen et al. 2015).
There were also several limitations. This relatively large school-based study of middle childhood struggling and nonstruggling readers found small group effects, likely because our 2 groups were defined using a firm threshold that resulted in a continuous distribution rather than requiring a significant gap in reading comprehension abilities between groups. Additionally, socioeconomic status (SES) can impact reading-related brain activity (Noble et al. 2006). In the United States, educational outcomes and SES are often highly related. At higher levels of SES, reading comprehension is influenced by shared environments in twin studies (Hart et al. 2013). Other work from our group has found reading scores were more impacted by SES than math scores (Engelhardt et al. In press). Unfortunately, we were unable to control for SES for the current sample because only 7.9% (3 out of 38 total participants) of the nonstruggling readers and 87.8% (65 out of 74 total participants) of the struggling readers completed a free/reduced lunch measure. Our future work will attempt to more closely collect and control for these variables, though in general, fMRI studies of reading difficulties and SES find main effects rather than interactions (Noble et al. 2006; Monzalvo et al. 2012). Additional academic and behavioral measures not reported here but collected as part of the larger TCLD study could allow for further analyses beyond the scope of this study. However, the tests reported here were present in the greatest number of the current sample.
This analysis was also cross-sectional, and we plan to look at reading-related change over time within the same individuals in the future. Prospective longitudinal analysis will be possible given data collected postintervention not reported here. While our SC task had predictably large performance differences between our groups, we saw similar patterns of results when examining continuous measures of reading ability that were less susceptible to performance confounds. Thus, our results are more tightly linked to reading ability itself and are less likely to be driven by uneven contribution of data between groups or by artificial group differences.
Conclusions and Implications
Several findings from the present study have important implications for understanding the neurobiological basis of reading difficulties. First, we used dichotomous and individual difference approaches to test the role of executive control regions in reading comprehension with literature-based ROIs. We found relations between reading ability and activation in executive control-derived regions specific to the comprehension task, including the left IPL from the frontoparietal control network, and the dACC of the cingulo-opercular control network. Second, we replicated and extended previous SC research finding differences in left posterior superior temporal cortex (Meyler et al. 2007) by assessing child groups defined by their reading comprehension abilities.
The current study supports investigation of executive control brain areas beyond the classic reading regions in samples of struggling readers. We also demonstrate executive control neural specificity to a reading task, as our effects were not replicated in a separate nonlexical control task of response inhibition. The specificity of any altered engagement of control regions may be important to consider when designing and implementing targeted reading interventions, as well as when identifying children at risk for reading difficulties.
Supplementary Material
Footnotes
We would like to thank the UT Meadows Center for Prevention of Educational Risk, especially Sharon Vaughn, Greg Roberts, Garrett J. Roberts, and Stephanie Stillman, for their critical roles in the intervention and assessment arms of the TCLD project. We would also like to thank the Texas Institute for Measurement, Evaluation, and Statistics, especially Jeremy Miciak for his role in the TCLD project. We thank Annie Zheng, Leonel Olmedo, Lauren Deschner, Tehila Nugiel, and Laura Engelhardt for help with data collection, helpful advice on the manuscript, and statistical analyses. We would also like to thank Russell Poldrack for help with task design. We acknowledge the Core for Advanced MRI (CAMRI) and MR technologist Lacey Berry, BS, RT(R)(MR) for scanning assistance at the Houston site under direction of Jenifer Juranek. Conflict of Interest: None declared.
Funding
Award P50HD052117 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to the University of Houston, for the Texas Center for Learning Disabilities (PI: Fletcher). The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health (NIH) or NICHD.
References
- Aboud KS, Bailey SK, Petrill SA, Cutting LE. 2016. Comprehending text versus reading words in young readers with varying reading ability: distinct patterns of functional connectivity from common processing hubs. Dev Sci. 19:632–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemeier LE, Abbott RD, Berninger VW. 2008. Executive functions for reading and writing in typical literacy development and dyslexia. J Clin Exp Neuropsychol. 30:588–606. [DOI] [PubMed] [Google Scholar]
- Aron A, Poldrack R. 2006. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TEJ, Smith S, Frank MJ, Poldrack RA. 2007. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 27:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington CN, Kulesz PA, Francis DJ, Fletcher JM, Barnes MA. 2014. The contribution of attentional control and working memory to reading comprehension and decoding. Sci Stud Read. 18:325–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. 2003. General multi-level linear modelling for group analysis in FMRI. Neuroimage. 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Benjamin C, Gaab N. 2012. What’s the story? The tale of reading fluency told at speed. Hum Brain Mapp. 33:2572–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. 2010. Pinning down response inhibition in the brain—conjunction analyses of the Stop-signal task. Neuroimage. 52:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Bebko G, Burman DD, Bitan T. 2007. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 45:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borella E, Carretti B, Pelegrina S. 2010. The specific role of inhibition in reading comprehension in good and poor comprehenders. J Learn Disabil. 43:541–552. [DOI] [PubMed] [Google Scholar]
- Butterfuss R, Kendeou P. 2017. The role of executive functions in reading comprehension. Educ Psychol Rev. 1–26. https://doi.org/10.1007/s10648-017-9422-6 (date last accessed 15 July, 2018). [Google Scholar]
- Cao F, Bitan T, Chou T-L, Burman DD, Booth JR. 2006. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry. 47:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T-L, Booth JR, Bitan T, Burman DD, Bigio JD, Cone NE, Lu D, Cao F. 2006. Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Hum Brain Mapp. 27:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher M, Miyake A, Keenan J, Pennington B, DeFries J, Wadsworth S, Willcutt E, Olson R. 2012. Predicting word reading and comprehension with executive function and speed measures across development: a latent variable analysis. J Exp Psychol Gen. 141:470–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Petersen S, Schlaggar BL. 2010. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 31:852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Bunge SA, Petersen SE, Schlaggar BL. 2017. Preparatory engagement of cognitive control networks increases late in childhood. Cereb Cortex. 27:2139–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino PT, Ahmed Y, Miciak J, Taylor WP, Gerst EH, Barnes MA. 2018. A framework for executive function in the late elementary years. Neuropsychology. 32:176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino PT, Miciak J, Ahmed Y, Barnes MA, Taylor WP, Gerst EH Under review. Executive function: Association with multiple reading skills. [DOI] [PMC free article] [PubMed]
- Cirino PT, Romain MA, Barth AE, Tolar TD, Fletcher JM, Vaughn S. 2013. Reading skill components and impairments in middle school struggling readers. Read Writ. 26:1059–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. 2004. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 22:466–476. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford J, Cohen JR, Galvan A, Canli T, Poldrack RA. 2012. Measurement and reliability of response inhibition. Front Psychol. 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A, Stanovich KE. 1997. Early reading acquisition and its relation to reading experience and ability 10 years later. Dev Psychol. 33:934–945. [DOI] [PubMed] [Google Scholar]
- Cutting LE, Clements-Stephens A, Pugh KR, Burns S, Cao A, Pekar JJ, Davis N, Rimrodt SL. 2013. Not all reading disabilities are dyslexia: distinct neurobiology of specific comprehension deficits. Brain Connect. 3:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting LE, Materek A, Cole CAS, Levine TM, Mahone EM. 2009. Effects of fluency, oral language, and executive function on reading comprehension performance. Ann Dyslexia. 59:34–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong CG, Van De Voorde S, Roeyers H, Raymaekers R, Oosterlaan J, Sergeant JA. 2009. How distinctive are ADHD and RD? Results of a double dissociation study. J Child Psychol Psychiatry. 37:1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. 2013. Executive functions. Annu Rev Psychol. 64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. . 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. . 2010. Prediction of individual brain maturity using fMRI. Science. 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Church JA, Harden KP, Tucker-Drob EM In press. Accounting for the shared environment in cognitive abilities and academic achievement with measured socioecological contexts. Dev Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Harden KP, Tucker-Drob EM, Church JA 2018. The neural architecture of executive functions is established by middle childhood. Retrieved from biorxiv.org. [DOI] [PMC free article] [PubMed]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. 2008. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 137:201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Black KJ, Schlaggar BL. 2016. Considerations for MRI study design and implementation in pediatric and clinical populations. Dev Cogn Neurosci. 18:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Laumann TO, Dubis JW, Ihnen SK, Neta M, Power JD, Pruett JR Jr, Black KJ, Schlaggar BL. 2014. Developmental changes in the organization of functional connections between the basal ganglia and cerebral cortex. J Neurosci. 34:5842–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. 2009. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. 2004. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychol Rev. 111:662–720. [DOI] [PubMed] [Google Scholar]
- Hart SA, Soden B, Johnson W, Schatschneider C, Taylor J. 2013. Expanding the environment: gene × school-level SES interaction on reading comprehension. J Child Psychol Psychiatry. 54:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, et al. . 2006. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 26:10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrieli S, Glover GH, Keller TA, Kobayashi N, Mazaika P, Jo B, et al. . 2007. Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behav Neurosci. 121:602–613. [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Vannest J, Holland SK. 2013. Overlapping neural circuitry for narrative comprehension and proficient reading in children and adolescents. Neuropsychologia. 51:2651–2662. [DOI] [PubMed] [Google Scholar]
- Hudson N, Scheff J, Tarsha M, Cutting L. 2016. Reading comprehension and executive function neurobiological findings. Perspect Lang Lit. 42:23. [Google Scholar]
- Ihnen SKZ, Petersen SE, Schlaggar BL. 2015. Separable roles for attentional control sub-systems in reading tasks: a combined behavioral and fMRI study. Cereb Cortex. 25:1198–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Parkinson J. 2015. The potential for school-based interventions that target executive function to improve academic achievement: a review. Rev Educ Res. 85:512–552. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. 2001. A global optimisation method for robust affine registration of brain images. Med Image Anal. 5:143–156. [DOI] [PubMed] [Google Scholar]
- Kamil M, Borman G, Dole J, Kral C, Salinger T, Torgesen J. 2008. Improving adolescent literacy: effective classroom and intervention practices. IES practice guide. NCEE 2008–4027: National Center for Education Evaluation and Regional Assistance.
- Kaufman AS, Kaufman NL. 2004. Kaufman brief intelligence test—second edition (KBIT-2). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. 2006. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 44:1822–1832. [DOI] [PubMed] [Google Scholar]
- Langer N, Benjamin C, Minas J, Gaab N. 2015. The neural correlates of reading fluency deficits in children. Cereb Cortex. 25:1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio G, Mahone EM, Eason SH, Cutting LE. 2010. Executive dysfunction among children with reading comprehension deficits. J Learn Disabil. 43:441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGinitie WH, MacGinitie RK, Maria K, Dreyer LG. 2000. Gates-MacGinitie Reading Tests. 4th ed Itasca, IL: Riverside. [Google Scholar]
- Martin A, Kronbichler M, Richlan F. 2016. Dyslexic brain activation abnormalities in deep and shallow orthographies: a meta‐analysis of 28 functional neuroimaging studies. Hum Brain Mapp. 37:2676–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna R, Rushe T, Woodcock KA. 2017. Informing the structure of executive function in children: a meta-analysis of functional neuroimaging data. Front Hum Neurosci. 11:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JD, Just MA. 2008. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: a longitudinal study of neuroplasticity. Neuropsychologia. 46:2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, Gabrieli JDE, Just MA. 2007. Brain activation during sentence comprehension among good and poor readers. Cereb Cortex. 17:2780–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. 2012. The nature and organization of individual differences in executive functions: four general conclusions. Curr Dir Psychol Sci. 21:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzalvo K, Fluss J, Billard C, Dehaene S, Dehaene-Lambertz G. 2012. Cortical networks for vision and language in dyslexic and normal children of variable socio-economic status. Neuroimage. 61:258–274. [DOI] [PubMed] [Google Scholar]
- National Assessment of Educational Progress 2015. A report card for the nation and the states. Washington, DC: National Center for Educational Statistics. [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. 2012. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Wolmetz M, Ochs L, Farah M, McCandliss BD. 2006. Brain–behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev Sci. 9:642–654. [DOI] [PubMed] [Google Scholar]
- O’Brien EM. 2008. From beginning to stellar: five tips on developing skillful readers. Alexandria, VA: Center for Public Education. [Google Scholar]
- Paulesu E, Danielli L, Berlingeri M. 2014. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front Hum Neurosci. 8:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. 2007. PsychoPy—psychophysics software in Python. J Neurosci Methods. 162:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti C. 2007. Reading ability: lexical quality to comprehension. Sci Stud Read. 11:357–383. [Google Scholar]
- Petersen S, Posner M. 2012. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki A, Sanchez M, Ecalle J, Magnan A. 2017. Linguistic and cognitive profiles of 8- to 15-year-old children with specific reading comprehension difficulties. J Learn Disabil. 50:128–142. [DOI] [PubMed] [Google Scholar]
- Power JD, Petersen SE. 2013. Control-related systems in the human brain. Curr Opin Neurobiol. 23:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. 2012. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 62:816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopapas A, Archonti A, Skaloumbakas C. 2007. Reading ability is negatively related to stroop interference. Cogn Psychol. 54:251–282. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2017. R: A language and environment for statistical computing. Vienna, Austria.
- Raichle ME. 2015. The brain’s default mode network. Ann Rev Neurosci. 38:433–447. [DOI] [PubMed] [Google Scholar]
- Rao C, Singh NC. 2015. Visuospatial complexity modulates reading in the brain. Brain Lang. 141:50–61. [DOI] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. 2010. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson FM, Seghier ML, Leff AP, Thomas MS, Price CJ. 2011. Multiple routes from occipital to temporal cortices during reading. J Neurosci. 31:8239–8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, Wimmer H. 2010. A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading? PLoS One. 5:e12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Clements-Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE. 2009. Functional MRI of sentence comprehension in children with dyslexia: beyond word recognition. Cereb Cortex. 19:402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. 2003. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 20:351–358. [DOI] [PubMed] [Google Scholar]
- Schall JD, Godlove DC. 2012. Current advances and pressing problems in studies of stopping. Curr Opin Neurobiol. 22:1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B, McCandliss B. 2007. Development of neural systems for reading. Annu Rev Neurosci. 30:475–503. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, Brandeis D. 2008. Impaired semantic processing during sentence reading in children with dyslexia: combined fMRI and ERP evidence. Neuroimage. 41:153–168. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, Van Der Mark S, Bucher K, Brem S, Martin E, Brandeis D. 2009. Reading for meaning in dyslexic and young children: distinct neural pathways but common endpoints. Neuropsychologia. 47:2544–2557. [DOI] [PubMed] [Google Scholar]
- Sesma HW, Mahone EM, Levine T, Eason SH, Cutting LE. 2009. The contribution of executive skills to reading comprehension. Child Neuropsychol. 15:232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE. 2014. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum Brain Mapp. 35:1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Rezaie R, Fletcher JM, Juranek J, Passaro AD, Li Z, Cirino PT, Papanicolaou AC. 2011. a. Functional disruption of the brain mechanism for reading: effects of comorbidity and task difficulty among children with developmental learning problems. Neuropsychology. 25:520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Rezaie R, Fletcher JM, Juranek J, Papanicolaou AC. 2011. b. Neural correlates of sentence reading in children with reading difficulties. Neuroreport. 22:674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. . 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Taylor JS, Rastle K, Davis MH. 2013. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol Bull. 139:766–791. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. 1999. Test of word reading efficiency (TOWRE). Austin, TX: Pro-Ed. [Google Scholar]
- Van Der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, Kronbichler M, Loenneker T, Klaver P, Martin E, et al. . 2009. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 47:1940–1949. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. 2005. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 28:635–662. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker DL. 2007. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 56:209–225. [DOI] [PubMed] [Google Scholar]
- Vaughn S, Solis M, Miciak J, Taylor WP, Fletcher JM. 2016. Effects from a randomized control trial comparing researcher and school-implemented treatments with fourth graders with significant reading difficulties. J Res Educ Eff. 9:23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Church JA, Power JD, Miezin FM, Petersen SE, Schlaggar BL. 2013. Functional network architecture of reading-related regions across development. Brain Lang. 125:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA, Pearson NA. 2010. Test of Silent Reading Efficiency and Comprehension (TOSREC) examiner’s manual. Austin, TX: Pro-Ed. [Google Scholar]
- Willcutt E, Pennington B. 2000. Comorbidity of reading disability and attention-deficit/hyperactivity disorder: differences by gender and subtype. J Learn Disabil. 33:179–191. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Schurz M, Sturm D, Richlan F, Klackl J, Kronbichler M, Ladurner G. 2010. A dual-route perspective on poor reading in a regular orthography: an fMRI study. Cortex. 46:1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. 2001. Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside. [Google Scholar]
- Woolrich MW. 2008. Robust group analysis using outlier inference. Neuroimage. 41:286–301. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. 2001. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. 2001. Statistical analysis of activation images In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an introduction to methods. 1st ed.Oxford (GB): Oxford Medical Publications; p. 251–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.