Abstract
Background:
The visual analog scale (VAS) is a validated, subjective measure for acute and chronic pain. Scores are recorded by making a handwritten mark on a 10-cm line that represents a continuum between “no pain” and “worst pain.”
Methods:
One hundred consecutive patients aged ≥18 years who presented with a chief complaint of pain were asked to record pain scores via a paper VAS and digitally via both the laptop computer and mobile phone. Ninety-eight subjects, 51 men (age, 44 ± 16 years) and 47 women (age, 46 ± 15 years), were included. A mixed-model analysis of covariance with the Bonferroni post hoc test was used to detect differences between the paper and digital VAS scores. A Bland–Altman analysis was used to test for instrument agreement between the platforms. The minimal clinically important difference was set at 1.4 cm (14% of total scale length) for detecting clinical relevance between the three VAS platforms. A paired one-tailed Student t-test was used to determine whether differences between the digital and paper measurement platforms exceeded 14% (P < 0.05).
Results:
A significant difference in scores was found between the mobile phone–based (32.9% ± 0.4%) and both the laptop computer– and paper-based platforms (31.0% ± 0.4%, P < 0.01 for both). These differences were not clinically relevant (minimal clinically important difference <1.4 cm). No statistically significant difference was observed between the paper and laptop computer platforms. Measurement agreement was found between the paper- and laptop computer–based platforms (mean difference, 0.0% ± 0.5%; no proportional bias detected) but not between the paper- and mobile phone–based platforms (mean difference, 1.9% ± 0.5%; proportional bias detected).
Conclusion:
No clinically relevant difference exists between the traditional paper-based VAS assessment and VAS scores obtained from laptop computer– and mobile phone–based platforms.
The visual analog scale (VAS) is a pain rating scale1,2,3,4,5,6,7,8,9 first used by Hayes and Patterson in 1921.2 Scores are based on self-reported measures of symptoms that are recorded with a single handwritten mark placed at one point along the length of a 10-cm line that represents a continuum between the two ends of the scale—“no pain” on the left end (0 cm) of the scale and the “worst pain” on the right end of the scale (10 cm).10 Measurements from the starting point (left end) of the scale to the patients' marks are recorded in centimeters and are interpreted as their pain. The values can be used to track pain progression for a patient or to compare pain between patients with similar conditions. In addition to pain, the scale has also been used to evaluate mood, appetite, asthma, dyspepsia, and ambulation.4 Although there is conflicting evidence with regard to the advantage of the VAS compared with other methods for recording pain,4 it is still commonly used in clinical and home settings.11
The increasing use of electronic medical records makes switching from a paper-based format to a digital format for VAS testing more convenient for tracking and analyzing patient data.12 A digital-based VAS platform could be easily integrated into the electronic medical record, obviating the need to scan individual paper VAS scores into the system for each patient and allowing for more rapid universal access to the results of such tests to improve patient care and pain management. However, paper and digital formats may not be equivalent in terms of reporting VAS scores mainly because of the size of the scale used on each platform. The purpose of this study was to evaluate differences between the traditional paper-based VAS assessment and VAS scores obtained from each of two different digital VAS platforms (laptop computer and mobile phone) and to determine whether there are (1) statistically significant differences between the platforms; (2) clinically relevant differences between the platforms; and (3) instrument agreement between the platforms. It was hypothesized that there would be no statistically significant differences in measurements obtained from the platforms, there would be no clinically relevant differences between the three platforms, and both digital VAS platforms would be in agreement with the traditional paper-based VAS assessment.
Methods
We obtained institutional review board approval for this prospective randomized controlled trial. One hundred consecutive orthopaedic sports medicine patients aged ≥18 years from the practices of two participating sports medicine fellowship-trained, American Board of Orthopaedic Surgery–certified orthopaedic surgeons were enrolled in the study between May 23, 2016, and June 28, 2016. Patient consent was obtained by the same investigator in the clinic. Patients aged < younger than age < 18 years were excluded from the study. Patients with a chief complaint of shoulder, elbow, hip, or knee pain were eligible for inclusion. Two patients were excluded because of a score entry error from the phone touch screen. Ninety-eight subjects (51 men; age, 44 ± 16 years; 47 women; age, 46 ± 15 years) were analyzed. After consent was obtained, patients were asked to record perceived pain on a scale of 0% to 100% (no pain to worst pain) via three separate scales: a traditional paper-based VAS (11″ × 8.5″; 10-cm scale) and digitally via either laptop-based (Thinkpad, 14″ screen; 28.9 cm scale) or mobile phone–based (iPhone 6, 4.7″ screen; 9.6 cm scale) online platforms (Figure 1). The online platform is proprietary web-based software that was developed by a software engineer on the research team. For the digital platforms, the first spot the patient clicked with the mouse on the laptop computer or touched on the mobile phone screen was not the final VAS value. Patients had the opportunity to continue clicking or tapping to adjust their response on the screen until they were satisfied with the location of placement on the scale. Research personnel scored the paper-based VAS using a ruler to measure the distance (in centimeters) from the left end of the VAS scale to the patients' marks.
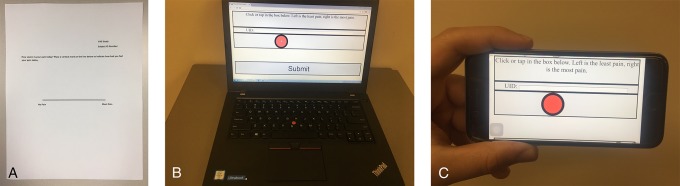
Figure 1.
Photographs showing a traditional paper-based visual analog scale (VAS [A]), a digital laptop-based VAS (B), and a mobile phone–based VAS (C).
Subjects were randomized into six different groups based on order of presentation to the orthopedic clinic: Group 1: paper, mobile phone, and laptop computer; group 2: paper, laptop computer, and mobile phone; group 3: laptop computer, mobile phone, and paper; group 4: laptop computer, paper, and mobile phone; group 5: mobile phone, paper, and laptop computer; and group 6: mobile phone, laptop computer, and paper. On all the VAS platforms, there were no qualitative indicators of pain such as happy or sad faces, qualifiers such as mild, moderate, or severe, or color schemes that are found in conjunction with other VAS recording tests. The platforms included only a single complete line and the phrases “no pain” at the left terminus and “worst pain” at the right terminus. These scores were then recorded in a MySQL database (Oracle) online, along with each patient's unique identifier, date, and device screen size.
We analyzed data using SPSS (version 20; IBM Statistics). To determine whether there was a difference between the VAS platforms, a mixed-model analysis of covariance (covarying on paper VAS ratings as the current standard of measurement) was performed, followed by the Bonferroni post hoc test (α = 0.05). The threshold for minimal clinically important difference (MCID) was set at 1.4 cm (on a 10-cm scale; 14% relative to the size of each scale; paper, laptop computer, mobile phone) for detecting clinical relevance between the digital and paper VAS platforms, consistent with previous platforms.15 Therefore, a paired one-tailed Student t-test was used to determine whether differences between the digital and paper measurement platforms exceeded 14% (P < 0.05). A Bland-Altman analysis was used to test for instrument agreement through detection of proportional bias among the three measurement platforms.
Results
No interaction effect was found between sex and age. Therefore, both were excluded from the final statistical model. Significant differences between the mobile phone and both the laptop computer and paper scores (P < 0.05 for both) (Figure 2) were observed. However, these differences were not clinically relevant (MCID <1.4 cm) (Figure 3). No difference was observed between the paper and laptop computer platforms (P > 0.05). The Bland-Altman analysis revealed instrument disagreement for measurements between the paper- and mobile phone–based platforms (mean difference, 1.9% ± 0.5%; proportional bias detected) (Figure 4A); however, instrument agreement was found between the paper- and laptop computer–based platforms (mean difference, 0.0% ± 0.5%; no proportional bias detected) (Figure 4B).
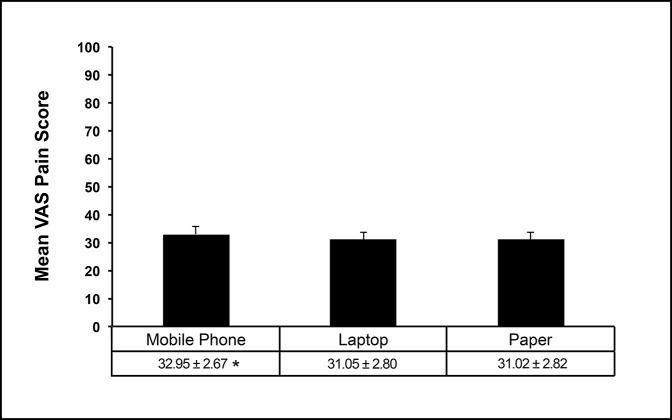
Figure 2.
Graph showing a comparison between mean VAS scores for mobile phone–, laptop-, and paper-based VAS platforms. Data are presented as mean ± SEM. *Significantly different from laptop- and paper-based VAS scores. Type I error was set at α = 0.05. VAS = visual analog scale.
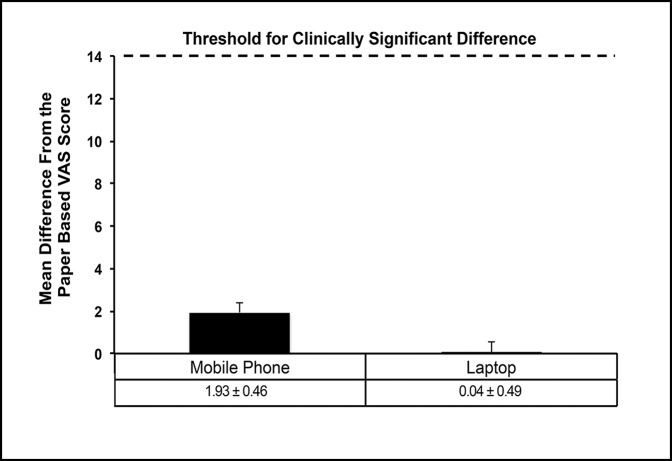
Figure 3.
Graph showing that the threshold for clinically significant difference (minimal clinically important difference) was set at 14 relative to each VAS scale of 0 to 100. As shown, neither the mobile phone– nor the laptop-based platform VAS scores approached or exceeded that threshold. Data are presented as mean difference scores (±SEM) calculated as difference from the paper-based VAS platform. VAS = visual analog scale.
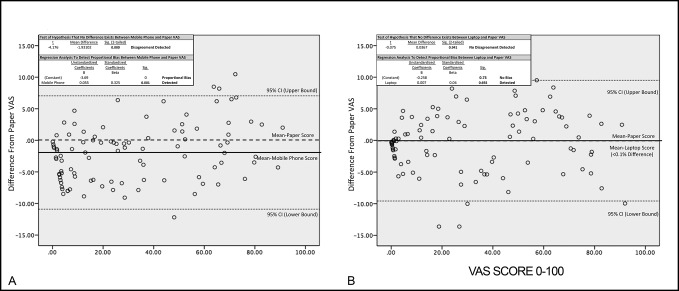
Figure 4.
Data plots representing the results of a Bland-Altman analysis for instrument agreement between mobile phone– and paper-based VAS recording (A) and laptop- and paper-based VAS recording (B). Significance for the initial detection of disagreement and post hoc regression to detect proportional bias was set at α = 0.05. VAS = visual analog scale.
Discussion
Our goals were to (1) determine whether differences were present between the traditional paper-based VAS assessment and VAS scores obtained from two different digital VAS platforms (laptop computer and mobile phone), (2) determine whether any observed differences between the digital and paper-based VAS scores were clinically relevant, and (3) evaluate instrument agreement between the platforms. Although statistically significant differences were observed between the mobile phone– and paper-based platforms, the differences were not clinically relevant. No difference was observed between the laptop computer– and paper-based VAS platforms. Instrument agreement was observed between the paper and laptop computer but not between the paper and mobile phone. These findings provide strong support for the use of the digital VAS in pain assessment in orthopaedic surgery patients.
The reason for statistically significant differences observed between the paper and mobile phone is likely secondary to the size of measurement entity relative to the platform (finger on a mobile phone screen [coarse] versus a pen ticking a mark on a line [fine]). The specificity is greater with the pen or pencil. However, the current investigation attempted to mitigate this by permitting adjustment on the phone scale until the patient was satisfied with the placement of the pain mark designation. The decision to allow subjects to adjust on the phone scale until the patient was satisfied with the placement was similar to the other scales. In each instance, the subjects had the option to modify if they accidentally made a mark that they felt was not accurate for each scale. In this study, the size of the laptop computer screen was similar to that of a sheet of paper. Thus, no statistically significant or clinically relevant difference between the laptop computer and paper was observed.
Similar investigations have attempted to determine whether a relationship exists between the paper and digital devices. However, these studies were not conducted in orthopaedic surgery patients, did not use a fingertip-touch device (eg, stylus for a handheld PalmPilot device), and had smaller sample sizes.4,13 A digital-based VAS could provide enhanced patient communication with physicians.14 Data could be electronically logged via web-based software that could be downloaded by the patient and used in concert with an electronic medical record messaging system. Patients could complete the VAS assessment and have it automatically sent to the electronic medical record with a time and date stamp. Healthcare providers could then access the results of each test and evaluate the pain of their patients. This would not only provide real-time tracking of pain but would also avoid potential recall bias. This data could provide more realistic expectations of pain for patients who are scheduled to undergo the same procedure.
Pain is a highly subjective entity. Despite the fact that two individuals may have the same clinical diagnosis, their quantification of the severity of pain may be markedly different. Some patients may tolerate higher degrees of pain and continue with their daily lives. Other patients may find certain degrees of pain unacceptable, seek care, and undergo treatment with the intent of decreasing the severity of pain. The MCID represents a clinically meaningful change in which a patient should be able to perceive the difference between two points (usually preintervention and postintervention, such as surgery). This minimum amount of change has been shown to be as little as 0.5 cm to as much as 2.0 cm on a VAS.15,16,17 The most commonly used minimum value in musculoskeletal medicine is 1.4 cm (on a 10-cm VAS) and was therefore used in the current investigation.14 The patient-acceptable symptom state for pain has been shown to be 2.0 to 3.0 cm on a 10-cm VAS.14,15 Although not analyzed in the current study, assessment of pain as a patient-acceptable symptom state via digital means is worthy of future investigation.
Strengths of this study include the sample size, the comparison of two different digital platforms with the benchmark paper-based assessment, and the statistical analyses performed.
Limitations include the possible variability resulting from the difference in the screen size of the mobile phone and laptop computer, which may have introduced an unforeseen error not examined in this study. In addition, we did not evaluate the use of this scale on a tablet device. Previous investigations have already demonstrated the efficacy of tablets for a number of clinical monitoring uses.18 Additional research is required to evaluate patients with chronic pain via a digital VAS scale to determine whether it is an adequate way for medical staff to track patients' pain over substantial periods of time.
Conclusion
No clinically relevant difference exists between the traditional paper-based VAS assessment and VAS scores obtained from laptop computer– and mobile phone–based platforms.
Footnotes
Dr. Boutris or an immediate family member is an employee of Zimmer Biomet. Dr. McCulloch or an immediate family member has received research or institutional support from Arthrex and DePuy Synthes; serves as a board member, owner, officer, or committee member of the Journal of Knee Surgery and Orthobullets.com; and is a member of a speakers' bureau or has made paid presentations on behalf of Vericel. Dr. Moreno or an immediate family member has received research or institutional support from 4WEB Medical. Dr. Harris or an immediate family member serves as a board member, owner, officer, or committee member of the American Academy of Orthopaedic Surgeons, the American Orthopaedic Society for Sports Medicine, Arthroscopy, the Arthroscopy Association of North America, and Frontiers In Surgery; has received research or institutional support from DePuy Synthes and Smith & Nephew; serves as a paid consultant to NIA Magellan, Össur, and Smith & Nephew; is a member of a speakers' bureau or has made paid presentations on behalf of Össur and Smith & Nephew; and has received nonincome support (such as equipment or services), commercially derived honoraria, or other non–research-related funding (such as paid travel) from SLACK. None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Ms. Delgado, Dr. Lambert, and Mr. Robbins.
References
- 1.Boonstra AM, Schiphorst Preuper HR, Reneman M, Posthumus JB, Stewart RE: Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 2008;31:165-169. [DOI] [PubMed] [Google Scholar]
- 2.Couper M, Tourangeau R, Conrad F, et al. : Evaluating the effectiveness of visual analog scales: A web experiment. Soc Sci Comput Rev 2006;24:227-245. [Google Scholar]
- 3.Downie WW, Leatham PA, Rhind VW, Wright V, Branco JA, Anderson JA: Studies with pain rating scales. Ann Rheum Dis 1978;37:378-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamison R, Gracely R, Raymond S, et al. : Comparative study of electronic vs. paper VAS ratings: A randomized, crossover trial using healthy volunteers. Pain 2002;99:341-347. [DOI] [PubMed] [Google Scholar]
- 5.Scott J, Huskisson EC: Vertical or horizontal visual analogue scales. Ann Rheum Dis 1979;38:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack HM, Horne DJL, Sheather S: Clinical applications of visual analogue scales: A critical review. Psychol Med 1988;18:1007-1019. [DOI] [PubMed] [Google Scholar]
- 7.Gaston-Johansson F: Measurement of pain: The psychometric properties of the Pain-O-Meter, a simple, inexpensive pain assessment tool that could change health care practices. J Pain Symptom Manage 1996;12:172-181. [DOI] [PubMed] [Google Scholar]
- 8.Todd KH, Funk KG, Funk JP, Bonacci R: Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;4:485-489. [DOI] [PubMed] [Google Scholar]
- 9.Kelly AM: Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med 1998;11:1086-1090. [DOI] [PubMed] [Google Scholar]
- 10.Alexander I: Electronic medical records for the orthopaedic practice. Clin Orthop Relat Res 2007;457:114-119. [DOI] [PubMed] [Google Scholar]
- 11.Younger J, McCue R, Mackey S: Pain outcomes: A brief review of instruments and techniques. Curr Pain Headache Rep 2009;13:39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breivik H, Borchgrevink PC, Allen SM, et al. : Assessment of pain. Br J Anaesth 2008;101:17-24. [DOI] [PubMed] [Google Scholar]
- 13.Kreindler D, Levitt A, Woolridge N, Lumsden CJ: Portable mood mapping: The validity and reliability of analog scale displays for mood assessment via hand-held computer. Psychiatry Res 2003;120:165-177. [DOI] [PubMed] [Google Scholar]
- 14.Tashjian RZ, Deloach J, Porucznik CA, Powell AP: Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg 2009;18:927-932. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Michaud K: Assessment of pain in rheumatoid arthritis: Minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol 2007;34:1674-1683. [PubMed] [Google Scholar]
- 16.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL: Defining the clinically important difference in pain outcome measures. Pain 2000;88:287-294. [DOI] [PubMed] [Google Scholar]
- 17.Farrar JT, Berlin JA, Strom BL: Clinically important changes in acute pain outcome measures: A validation study. J Pain Symptom Manag 2003;25:406-411. [DOI] [PubMed] [Google Scholar]
- 18.Bird ML, Callisaya ML, Cannell J, et al. : Accuracy, validity, and reliability of an electronic visual analog scale for pain on a touch screen tablet in healthy older adults: A clinical trial. Interactive J Med Res 2016;5:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]