Abstract
Background:
Current treatments for focal chondral and osteochondral lesions of the femoral condyle have been associated with variable outcomes. We conducted a clinical trial of the BioPoly RS Partial Resurfacing Knee Implant to address this unmet need.
Methods:
We performed a single-arm, prospective study in which 33 patients with focal cartilage lesions affecting the femoral condyle were managed with the BioPoly RS Partial Resurfacing Knee Implant. Knee injury and Osteoarthritis Outcome Score (KOOS) scores, a visual analog scale (VAS) for pain, the Short Form-36 (SF-36) physical component score , and the Tegner activity score were used to assess outcomes preoperatively and at 6 months, 1 year, and 2 years postoperatively. The KOOS outcomes at 2 years were compared with historical outcomes following microfracture treatment.
Results:
We found significant and clinically meaningful improvements in the KOOS scores, VAS pain score, and SF-36 physical component score (p < 0.025) when the values at all 3 postoperative time points were compared with the preoperative scores, and we also found significant improvements when the Tegner activity score at 2 years was compared with the preoperative score (p < 0.025). More than half of the cohort of patients had had a previous failure of cartilage-repair procedures. No significant differences were detected between younger patients (≤40 years) and older patients (>40 years). When compared with historical microfracture data, the BioPoly RS Implant demonstrated significantly superior KOOS scores for quality of life and sports.
Conclusions:
The present study indicated that the BioPoly RS Partial Resurfacing Knee Implant is safe, that it resulted in significantly improved knee function by 6 months, and that this improvement was sustained for 2 years regardless of patient age. The BioPoly RS Knee Implant allows return to a higher level of sporting activity than microfracture. Additional long-term follow-up is needed to determine the long-term effects of the device.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Focal chondral and osteochondral defects of the knee present a common, challenging clinical problem that can negatively impact quality of life to the same degree as severe osteoarthritis1. Knee arthroscopy has shown cartilage lesions to be common, with the medial femoral condyle and the patella being the most frequently affected locations2-4. Cartilage defects have limited self-healing capacity, and the natural history commonly results in osteoarthritis5. Osteoarthritis of the knee can be disabling6 and can result in a large economic burden due to both direct costs (e.g., hospital stay and insurance expenditure) and indirect costs (e.g., lost productivity and early retirement)7.
While current late-stage treatments for cartilage defects in the knee (i.e., total or partial knee arthroplasty) have provided consistent, positive outcomes for elderly patients8, their use in patients <55 years of age has become controversial because of concerns regarding implant longevity9-12. As a result, biological treatments such as microfracture, the osteochondral autograft transfer system (OATS), autologous chondrocyte implantation (ACI), and matrix-induced ACI (MACI) are used in active younger and middle-aged patients13. Most of these treatments have not demonstrated sustainable, consistent results14-16, and some require long postoperative rehabilitation17. In addition, the magnitude of the positive impact of biological treatments appears to significantly decrease with increasing patient age and when used for revision after the failure of previous biological treatments18-23.
The BioPoly RS Partial Resurfacing Knee Implant was developed as an early intervention and treatment for patients with cartilage lesions who want to delay or eliminate the need for total or partial arthroplasty and quickly return to full activity. BioPoly is a biosynthetic implant that is manufactured from a combination of ultra-high molecular weight polyethylene and a hydrophilic lubricating molecule (hyaluronic acid) that is designed to be less dependent on patient biology and to require less rehabilitation time, with less bone resection in comparison with partial or total arthroplasty.
The objectives of the present study were (1) to assess clinical outcomes and complications after treatment with the BioPoly Knee Implant and (2) to compare clinical outcomes after such treatment with those after treatment with microfracture as reported in the historical literature.
Materials and Methods
We designed a multi-center, single-arm, historically controlled clinical investigation for the purposes of (1) comparing clinical outcomes between the BioPoly RS Knee Implant and microfracture (based on historical literature) and (2) comparing the postoperative clinical outcomes with preoperative findings. The primary end points were Knee injury and Osteoarthritis Outcome Score (KOOS)24 overall score and subscores, a visual analog scale (VAS)25 score for pain, the Short Form-36 (SF-36)26 physical component score, and the Tegner activity27 score at 2 years. While the focus of this interim report is current patient outcomes at 2-year follow-up, patients will eventually be followed to 5 years.
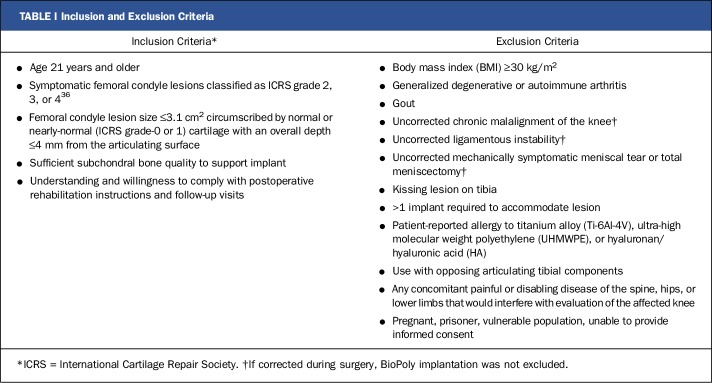
The study was conducted at 5 centers in the United Kingdom, and Good Clinical Practice guidelines were followed. With the level of significance established at p < 0.025 and power set at 0.90, the number of patients required for study enrollment was determined to be 35 in order to demonstrate noninferiority with the mean 18-month KOOS quality-of-life score of a historical microfracture control with an anticipated similar patient population28. In order to make valid comparisons with the results for historical microfracture controls, a literature review was conducted with use of specific keywords and the identified articles were evaluated for suitability with use of a methodological grading system. Forty-five articles were identified on the basis of the literature review, and these articles were graded according to the level of evidence, comparability of KOOS data, and similarity to the current study in terms of follow-up and inclusion/exclusion criteria. With use of this system, 4 sources of microfracture data were identified as appropriate for use as historical controls21,28-30. Informed consent was obtained for all patients, and the clinical protocol and informed consent were approved by the Cambridge Central Research Ethics Committee (REC11/EE/0256). Patients with symptomatic focal cartilage defects on the weight-bearing region of the medial or lateral femoral condyle were enrolled according to specific inclusion and exclusion criteria (Table I).
TABLE I.
Inclusion and Exclusion Criteria
Prior to the preoperative visit, an arthroscopic examination and magnetic resonance imaging (MRI) were conducted for each patient to evaluate and measure the defect. At the preoperative visit, the medical history was recorded and the patient was evaluated with use of the KOOS, VAS pain score, SF-36, and Tegner activity score. In addition, radiographs were taken. At the time of surgery, International Knee Documentation Committee (IKDC) surgical documentation forms were completed. Follow-up visits were conducted at 6 months, 1 year, and 2 years, at which times patient outcomes and radiographs were obtained.
The approved surgical technique for the BioPoly RS Partial Resurfacing Knee Implant was used in order to ensure that each implant was properly prepared and placed (Fig. 1). The implantation site was prepared with use of a simple, bone-sparing technique that establishes the correct implant orientation and depth relative to surrounding anatomy. Once the implantation site was deemed appropriate, the BioPoly implant was press-fit into the site. The BioPoly RS Partial Resurfacing Knee Implant is a microcomposite of ultra-high molecular weight polyethylene and hyaluronic acid that is overmolded onto a grit-blasted titanium-alloy stem. Three sizes (15-mm diameter, 20-mm diameter, and 15 × 24-mm racetrack-shaped) were used in the present study (Fig. 1), and the device was intended to articulate with tibial cartilage and the meniscus.
Fig. 1.

Fig 1-A Intraoperative photograph made during the implantation of a BioPoly RS Partial Resurfacing Knee Implant in the femoral condyle. Fig. 1-B Photograph showing the different sizes of the BioPoly RS Partial Resurfacing Knee Implant.
A 4-phase rehabilitation protocol that was designed to return patients to full activity quickly was used (see Appendix). The protocol allowed immediate weight-bearing and unrestricted range of motion as tolerated. This protocol differed from the suggested rehabilitation protocols for microfracture or other biological treatments, which often recommend return to full activity 6 to 8 months postoperatively16.
Each clinical outcome score at 6 months, 1 year, and 2 years was compared with its preoperative value, and 2-sample, 1-tailed t tests (α = 0.025) were used to examine for significant differences. Age-related differences were also investigated with 2-sample, 2-tailed t tests for the overall KOOS score, the SF-36 physical component score, and the VAS pain score (α = 0.05). KOOS clinical outcome scores were compared with those in 4 recent microfracture studies21,28-30 with use of 2-sample, 1-tailed t tests (α = 0.025), and KOOS quality-of-life outcome scores were compared with those in 1 of the studies28 with use of a noninferiority test (margin = 10, α = 0.025) as established in the protocol. Any missing data were queried and resolved.
Patient Population
A total of 40 patients were enrolled and screened over 3 years (Fig. 2). Five patients were withdrawn before surgery because of failed screening or patient withdrawal. Two patients were withdrawn during surgery but before implantation of the BioPoly device. The first patient was withdrawn because of a tibial kissing lesion, and the second was withdrawn because of a larger-than-expected cartilage lesion. Within the treated group, 2 patients were withdrawn before 6 months because of protocol deviations. The first patient was withdrawn because of an existing spinal tumor that was not disclosed when the patient answered exclusion criteria screening questions before surgery, and the second patient was withdrawn because 2 implantations were performed in the same knee (in the medial and lateral condyles) within a period of 3 months.
Fig. 2.
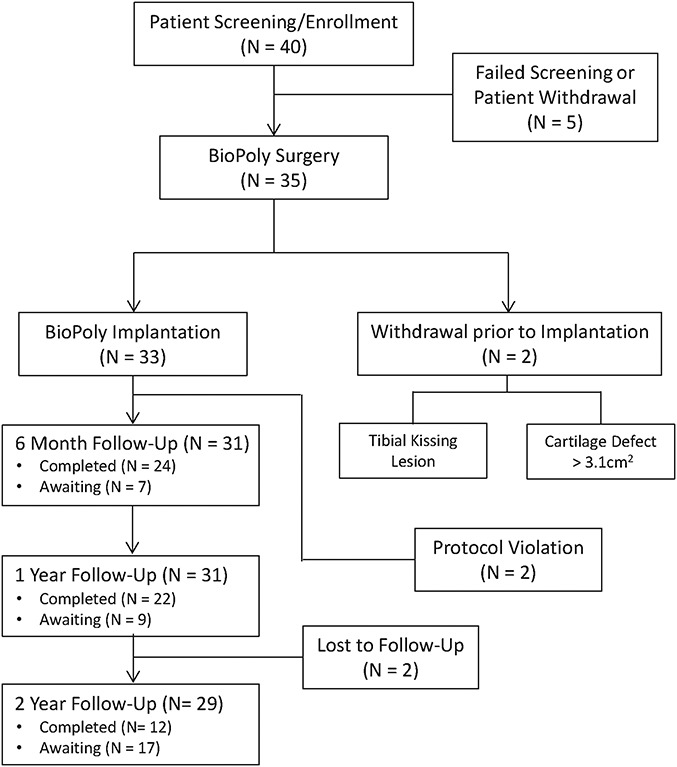
Flowchart detailing patient enrollment and follow-up.
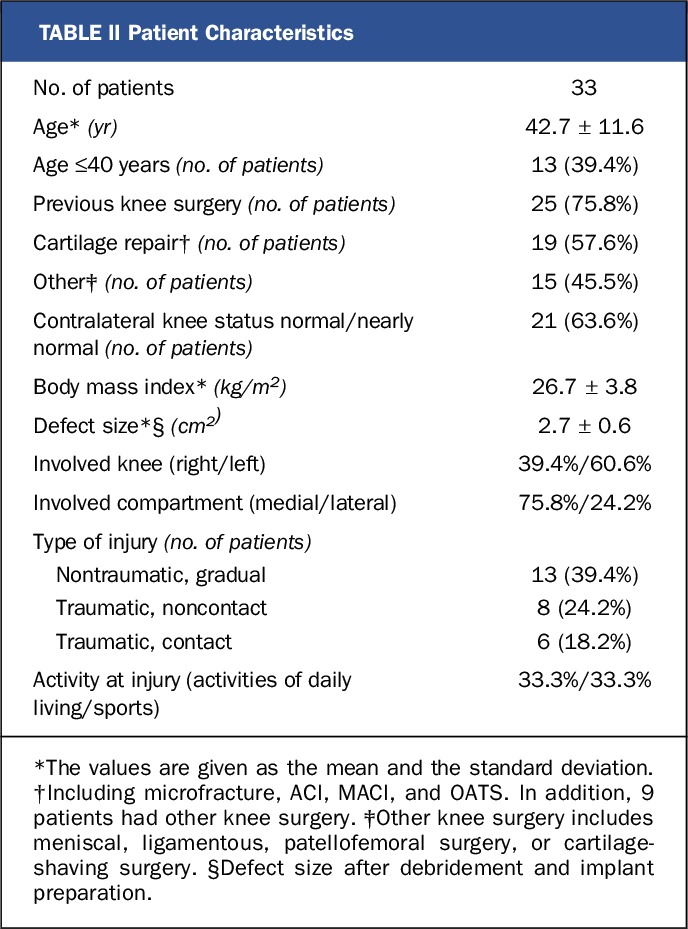
In the treated population, approximately 30% required the 15-mm implant, 35% required the 20-mm implant, and 35% required the 15 × 24-mm implant. The mean age of the patients was 42.7 years (range, 22 to 65 years), and the intraoperative defect sizes ranged from 0.6 to 3.1 cm2. Interestingly, the majority (58%) of the cohort had had previous cartilage-repair surgery. No patient required corrective procedures during implantation of the BioPoly device. Additional information regarding the patient characteristics is provided in Table II.
TABLE II.
Patient Characteristics
The investigators made exhaustive attempts to contact all patients with use of multiple communication modes. Two patients (6.0%) could not be contacted and were considered to have been lost to follow-up.
Implant Safety
No device-related adverse events were reported. The majority (86%) of reported adverse events were of mild or moderate severity, and all severe or serious adverse events improved or resolved. The most common adverse event was knee pain (arthralgia) (9 patients, with the pain being localized to the contralateral compartment in 4 of these patients). Additional adverse events included wound infection (1 patient), stiffness (1 patient), swelling (2 patients), crepitation (3 patients), and a loose cartilage body (1 patient). The loose cartilage body, which was identified in the operatively treated knee 4 months postoperatively, necessitated arthroscopic surgery but was not related to the implant. In fact, at that time, the implant was assessed and was deemed to be functioning and well fixed. One patient underwent revision after the 2-year follow-up because of the failure of osseointegration. This patient had had failures of previous microfracture and ACI operations, which may have resulted in inadequate subchondral bone metabolism. However, other patients who had had failures of previous cartilage-repair procedures did not have failure of osseointegration. The patient who underwent revision was managed with an alternative biological treatment.
Results
Clinical Outcomes
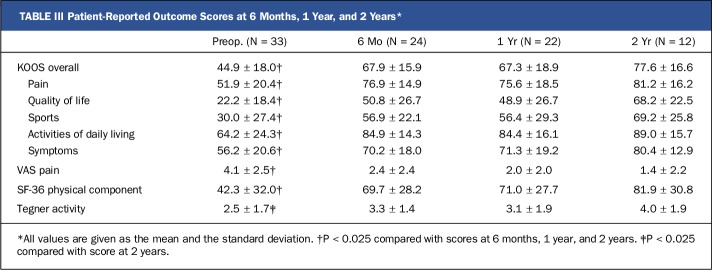
At 6 months, 1 year, and 2 years after surgery, knee function (assessed with the KOOS) demonstrated significant (p < 0.025) and clinically meaningful improvement (difference, >8 to 10 points)31 in comparison with preoperative function. At all 3 of these time points, there was also significant improvement (p < 0.025) in the VAS pain score and the SF-36 physical component score in comparison with the preoperative scores (Table III). At 2 years after surgery, there was significant improvement (p < 0.025) in the Tegner activity score. At the time of this progress report, 24, 22, and 12 patients had reached the 6-month, 1-year, and 2-year time points, respectively.
TABLE III.
Patient-Reported Outcome Scores at 6 Months, 1 Year, and 2 Years*
In order to evaluate the potential effects of age, the outcomes for younger patients (≤40 years old) were compared with those for older patients (>40 years old). Interestingly, no significant differences were detected between these 2 groups in terms of the overall KOOS (Fig. 3), VAS pain score, or SF-36 physical component score (p > 0.05); however, the sample size was limited at the later time points.
Fig. 3.
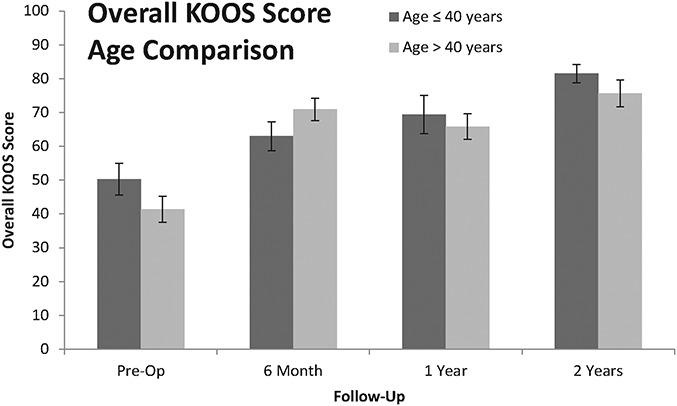
Bar graph showing the mean overall KOOS scores for younger patients (≤40 years old) and older patients (>40 years old). No significant differences were found between these age groups (p > 0.05). The I-bars indicate the standard error.
The BioPoly Knee Implant demonstrated noninferiority (p < 0.025) in terms of the KOOS quality-of-life score when compared with microfracture data28.
The BioPoly RS Partial Resurfacing Knee Implant demonstrated improved clinical outcomes in comparison with historical outcomes following microfracture treatment as reported in multiple studies21,28-30. When the 2-year mean KOOS subscores were compared with microfracture data from the literature, the BioPoly implant demonstrated superior clinical outcomes in terms of quality of life and sports and demonstrated similar clinical outcomes in terms of activities of daily living, pain, and symptoms (Fig. 4). Quality of life and sports are recognized as the most discerning KOOS domains for the assessment of treatment impact. This observation was verified by statistical testing, which demonstrated that the BioPoly implant yielded significantly superior (p < 0.025) outcomes in terms of quality of life when compared with all of the microfracture studies and demonstrated significantly superior outcomes (p < 0.025) in terms of sports and activities of daily living when compared with some of the microfracture studies.
Fig. 4.
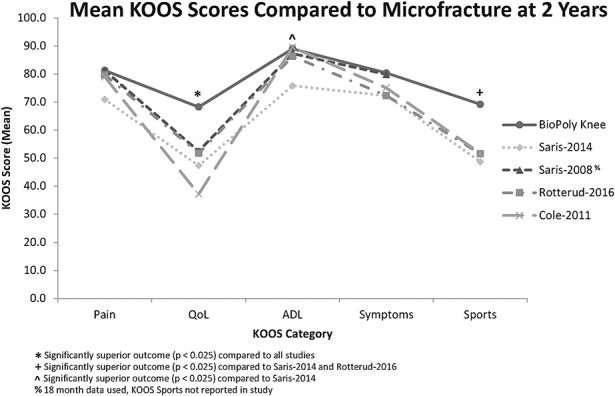
Line graph showing the individual KOOS subscores at 2 years for the patients in the present study as compared with those reported in recent microfracture studies21,28-30. All values are presented as means. QoL = quality of life, ADL = activities of daily living.
Further examination of the KOOS quality-of-life data showed that the preoperative scores for the patients managed with the BioPoly implant were similar to those for the patients in the microfracture studies whereas the 2-year scores associated with the BioPoly implant were significantly superior (p < 0.025) to those in all of the microfracture studies (Fig. 5). The average age of the patients managed with the BioPoly implant in the present study was 7 to 9 years greater than that of the patients in the microfracture studies.
Fig. 5.
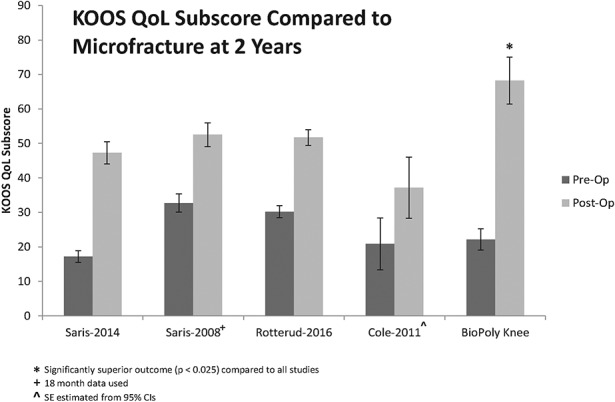
Bar graph showing the mean KOOS quality-of-life (QoL) score at 2 years for the patients in the present study as compared with those reported in recent microfracture studies21,28-30. The I-bars indicate the standard error. SE = standard error, CI = confidence interval.
Radiographic Observations
Radiographically, it was observed that implants were stable after 6 months, 1 year, and 2 years. Integration with surrounding bone was observed, with no evidence of radiolucency or implant migration (Fig. 6).
Fig. 6.
Radiograph showing the BioPoly RS Partial Resurfacing Knee Implant in the femoral condyle.
Discussion
We observed that the BioPoly RS Partial Resurfacing Knee Implant is safe, that it resulted in significantly improved patient outcomes by 6 months, and that this improvement was sustained for 2 years, regardless of patient age (range, 22 to 65 years). Significant improvement was seen at 6 months for the overall KOOS (and all individual subscores), the VAS pain score, and the SF-36 physical component score. This improvement was maintained through 2 years, and the Tegner activity score demonstrated significant improvement at 2 years. Over half of the patients had had a failure of previous cartilage-repair procedures, and no significant differences in outcome scores were observed between younger and older patients. Radiographic evaluation demonstrated adequate device fixation and integration with surrounding bone. There were no serious, device-related adverse events. There was 1 revision, which occurred after the 2-year follow-up. The BioPoly Knee Implant demonstrated significantly superior KOOS quality-of-life scores at 2 years when compared with the historical outcomes of microfracture treatment as reported in the literature21,28-30. The patients who were managed with the BioPoly implant were an average of 7 to 9 years older than those who were managed with microfracture.
Total knee arthroplasty has been extensively studied and has been shown to be consistently effective in older patients, with 20-year survival rates of as high as 97.8%8. Patients ≤55 years old, however, present a challenge because they often desire to return to strenuous physical activity postoperatively, which is not recommended as part of the rehabilitation process and could increase implant wear and decrease implant longevity. Studies have shown decreased short and long-term implant-survival rates for younger patients9,10, and 1 study demonstrated a 10-year revision rate of 16% in association with wear and/or osteolysis in younger patients11. In a series of total knee arthroplasty patients <50 years of age, a 2-year revision rate of 9% was reported12.
Biological treatments, meanwhile, have been examined for use in active younger and middle-aged patients, but these treatments have not demonstrated sustainable, consistent results and require long postoperative rehabilitation. Microfracture is currently the most common biological treatment for the repair of early-stage focal defects, but tissue quality and outcomes consistently have been shown to deteriorate over time (typically beginning at 18 to 36 months) along with increases in the failure rate14-16. ACI, MACI, and OATS also have been examined; however, studies have shown more positive outcomes for younger patients compared with middle-aged patients19-21. The outcomes of those biological procedures are similar to those of microfracture20,32. Those treatments also have been shown to be prone to failure in patients who have already had previous microfracture surgery23, but that finding has not been reported in all studies33.
The hydrophilic, low-wear properties of the BioPoly implant material have been shown in long-term, large-animal studies34. This hydrophilic capability is illustrated by comparing the appearance of a water droplet on ultra-high molecular weight polyethylene with that on the BioPoly surface (Fig. 7)35.
Fig. 7.
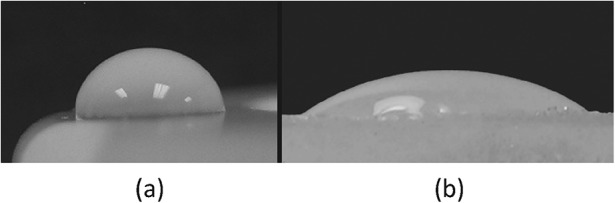
Photographs showing a water droplet on ultra-high molecular weight polyethylene (Fig. 7-A) and BioPoly material (Fig. 7-B). (Reprinted, with permission from: James S, Oldinski R, Zhang M, Schwartz H. UHMWPE/hyaluronan microcomposite biomaterials. In: UHMWPE biomaterials handbook. 2nd ed. New York: Academic Press; 2009. http://www.elsevier.com.)
It is notable that the BioPoly RS Knee Implant did not demonstrate outcome differences between younger and older patients. Mithoefer et al. examined 48 patients who were managed with microfracture and reported that a number of outcomes showed significant improvements after the microfracture treatment22. It was noted, however, that there was a trend toward better outcomes in patients who were ≤30 years of age. It is also interesting to note that while the BioPoly implant demonstrated similar KOOS outcomes in terms of pain, activities of daily living, and symptoms when compared with microfracture studies, it demonstrated superior outcomes in terms of KOOS quality-of-life and sports scores. A possible explanation for this effect is that patients who are managed with microfracture are forced to accept a less-active lifestyle in order to mitigate knee pain and symptoms, whereas those who are managed with the BioPoly implant are able to regain their previous active lifestyle, as evidenced by increasing Tegner scores (Table III). Another possible factor could be the rehabilitation protocol for the BioPoly implant, which allows patients to immediately bear weight and return to activity more quickly than does a standard microfracture rehabilitation protocol.
Of the recent microfracture studies that were analyzed in the present study, the 2008 study by Saris et al.28 had the most similar patient population to our cohort, except that the average age in that study was 8 years lower than that in the present study. In that study, 61 patients with an average age of 33.9 years were managed with microfracture (average lesion size, 2.4 cm2). The authors found no significant differences between the outcomes of cultured chondrocyte implantation and those of microfracture. The average KOOS quality-of-life score in the present study was significantly superior to that for the microfracture arm in the study by Saris et al.28 (68.2 compared with 52.54; p = 0.022).
The limitations of the present study include the lack of long-term clinical outcomes, the use of patient-reported outcome measures, and a comparatively small sample size. The lack of long-term outcomes can primarily be attributed to the recent release of the device.
While there is a need for longer-term clinical studies of the BioPoly RS Partial Resurfacing Knee Implant, the present short-term study demonstrated significant improvement in patient-reported outcomes and an exceptional safety profile for the device out to 2 years.
Appendix
A table showing the 4-phase rehabilitation protocol is available with the online version of this article at jbjs.org (http://links.lww.com/JBJSOA/A6).
Disclosure of Potential Conflicts of Interest
Footnotes
Investigation performed at Imperial College Healthcare NHS Trust – Charing Cross Hospital, The London Clinic, London; Aintree University Hospital, Liverpool; Royal National Orthopaedic Hospital, Stanmore; and Nuffield Health, The Grosvenor Hospital Chester, Chester, United Kingdom
Disclosure: The study was funded by BioPoly LLC. Investigators and patients received no compensation for their participation in the study other than reimbursement for study-related travel from a long distance. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A5).
References
- 1.Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, Arøen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010. February;38(2):231-7. Epub 2009 Dec 30. [DOI] [PubMed] [Google Scholar]
- 2.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997. August;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 3.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007. June;14(3):177-82. Epub 2007 Apr 10. [DOI] [PubMed] [Google Scholar]
- 4.Solheim E, Krokeide AM, Melteig P, Larsen A, Strand T, Brittberg M. Symptoms and function in patients with articular cartilage lesions in 1,000 knee arthroscopies. Knee Surg Sports Traumatol Arthrosc. 2016. May;24(5):1610-6. Epub 2014 Dec 13. [DOI] [PubMed] [Google Scholar]
- 5.Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand. 1996. April;67(2):165-8. [DOI] [PubMed] [Google Scholar]
- 6.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE, Kannel WB. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994. March;84(3):351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014. July;10(7):437-41. Epub 2014 Mar 25. [DOI] [PubMed] [Google Scholar]
- 8.Ritter MA. The Anatomical graduated component total knee replacement: a long-term evaluation with 20-year survival analysis. J Bone Joint Surg Br. 2009. June;91(6):745-9. [DOI] [PubMed] [Google Scholar]
- 9.Julin J, Jämsen E, Puolakka T, Konttinen YT, Moilanen T. Younger age increases the risk of early prosthesis failure following primary total knee replacement for osteoarthritis. A follow-up study of 32,019 total knee replacements in the Finnish Arthroplasty Register. Acta Orthop. 2010. August;81(4):413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS. Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg Am. 2003. February;85(2):259-65. [DOI] [PubMed] [Google Scholar]
- 11.Odland AN, Callaghan JJ, Liu SS, Wells CW. Wear and lysis is the problem in modular TKA in the young OA patient at 10 years. Clin Orthop Relat Res. 2011. January;469(1):41-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meehan JP, Danielsen B, Kim SH, Jamali AA, White RH. Younger age is associated with a higher risk of early periprosthetic joint infection and aseptic mechanical failure after total knee arthroplasty. J Bone Joint Surg Am. 2014. April 2;96(7):529-35. [DOI] [PubMed] [Google Scholar]
- 13.Layton AJ, Arnold RJG, Graham J, Frasco MA, Cote E, Lynch NM. Long-term failure rates associated with knee microfracture surgery. Presented as a poster exhibit at the ISPOR 20th Annual International Meeting; 2015. May 16-20; Philadelphia, PA. [Google Scholar]
- 14.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Südkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006. November;14(11):1119-25. Epub 2006 Jul 11. [DOI] [PubMed] [Google Scholar]
- 15.Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005. April;13(3):213-21. Epub 2004 May 14. [DOI] [PubMed] [Google Scholar]
- 16.Mithoefer K, Williams RJ, 3rd, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006. September;34(9):1413-8. Epub 2006 May 30. [DOI] [PubMed] [Google Scholar]
- 17.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001. October;391(Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 18.Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, Ghanem N, Uhl M, Südkamp N. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006. November;22(11):1180-6. [DOI] [PubMed] [Google Scholar]
- 19.Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004. March;86(3):455-64. [DOI] [PubMed] [Google Scholar]
- 20.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007. October;89(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 21.Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, Emans P, Podskubka A, Tsuchida A, Kili S, Levine D, Brittberg M; SUMMIT Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014. June;42(6):1384-94. Epub 2014 Apr 8. [DOI] [PubMed] [Google Scholar]
- 22.Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005. September;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 23.Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009. May;37(5):902-8. Epub 2009 Mar 4. [DOI] [PubMed] [Google Scholar]
- 24.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998. August;28(2):88-96. [DOI] [PubMed] [Google Scholar]
- 25.Wallerstein SL. Scaling clinical pain and pain relief. In: Bromm B, editor. Pain measurement in man: neurophysiological correlates of pain. New York: Elsevier; 1984. [Google Scholar]
- 26.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992. June;30(6):473-83. [PubMed] [Google Scholar]
- 27.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthrop Relat Res. 1985;198:43-9. [PubMed] [Google Scholar]
- 28.Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008. February;36(2):235-46. [DOI] [PubMed] [Google Scholar]
- 29.Røtterud JH, Sivertsen EA, Forssblad M, Engebretsen L, Årøen A. Effect on patient-reported outcomes of debridement or microfracture of concomitant full-thickness cartilage lesions in anterior cruciate ligament-reconstructed knees: a nationwide cohort study from Norway and Sweden of 357 patients with 2-year follow-up. Am J Sports Med. 2016. February;44(2):337-44. Epub 2015 Dec 11. [DOI] [PubMed] [Google Scholar]
- 30.Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, De Deyne PG. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011. June;39(6):1170-9. Epub 2011 Apr 1. [DOI] [PubMed] [Google Scholar]
- 31.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003. November 3;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Assche D, Van Caspel D, Vanlauwe J, Bellemans J, Saris DB, Luyten FP, Staes F. Physical activity levels after characterized chondrocyte implantation versus microfracture in the knee and the relationship to objective functional outcome with 2-year follow-up. Am J Sports Med. 2009. November;37(Suppl 1):42S-9S. Epub 2009 Oct 27. [DOI] [PubMed] [Google Scholar]
- 33.Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, Nissen C; STAR Study Principal Investigators. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009. January;37(1):42-55. Epub 2008 Oct 16. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JO, Lescun TB, Conner NK, Proch FP, Schwartz HE. An UHMWPE-hyaluronan microcomposite material for partial joint resurfacing in a goat model. Poster 1286. Presented at the Orthopaedic Research Society Annual Meeting, Long Beach, CA, Jan 13-16, 2011. [Google Scholar]
- 35.James S, Oldinski R, Zhang M, Schwartz H. UHMWPE/hyaluronan microcomposite biomaterials. In: UHMWPE biomaterials handbook. 2nd ed. New York: Academic Press; 2009. [Google Scholar]
- 36.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E; International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85(Suppl 2):45-57. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.