Abstract
Background:
A combined intravenous and intra-articular regimen is one of the most effective administration routes of tranexamic acid (TXA) to reduce perioperative blood loss in unilateral total knee arthroplasty. However, there have been few reports regarding use of the combined regimen for patients undergoing simultaneous bilateral total knee arthroplasty, in which blood-management strategy is more challenging.
Methods:
We compared perioperative blood loss in 30 consecutive patients undergoing simultaneous bilateral total knee arthroplasty who received both 1,000 mg of TXA intravenously and 1,000 mg of intra-articular TXA in each knee (combined TXA group) with that in a consecutive series of 51 patients who only received 1,000 mg of TXA intravenously (intravenous TXA group). Additional intravenous TXA was administered 6 hours after the initial administration in both groups. Except for the intraoperative TXA administration regimen, an identical perioperative blood-management strategy was applied to both groups; this consisted of transfusion of 800 or 400 mL of predeposited autologous blood except for patients with a preoperative hemoglobin level of <11.0 g/dL, who received 4 units of allogenic blood. All surgical procedures were performed with spinal anesthesia and without use of a pneumatic tourniquet. Perioperative blood loss was calculated using the blood volume and change in hemoglobin level from the preoperative measurement to postoperative day 3.
Results:
There was significantly less perioperative blood loss in the combined TXA group compared with the intravenous TXA group (mean and standard deviation, 1,201 ± 347 versus 1,638 ± 400 mL, respectively; mean difference, 437 mL; 95% confidence interval, 263 to 613 mL; p < 0.0001). No patient in the combined TXA group and 1 patient (2%) in the intravenous TXA group required additional allogenic blood transfusion. No thrombotic events occurred in either group.
Conclusions:
In a nonrandomized comparison, combined intra-articular and intravenous TXA significantly reduced the calculated perioperative blood loss in simultaneous bilateral total knee arthroplasty compared with that found in patients treated only with intravenous TXA.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Recent studies demonstrated early functional recovery following total knee arthroplasties done with a multimodal approach that reduced perioperative blood loss1, used spinal anesthesia2, decreased perioperative pain3, and did not use a pneumonic tourniquet4. Administration of tranexamic acid (TXA) plays a central role in reducing perioperative blood loss in total knee arthroplasty5. Nielsen et al. designated total knee arthroplasty performed with spinal anesthesia, with an intraoperative periarticular injection of an analgesic, and without use of a pneumonic tourniquet as multimodal, modern, fast-track total knee arthroplasty6. They demonstrated that combined intravenous and intra-articular administration of TXA was superior to intravenous TXA alone in patients undergoing such a total knee arthroplasty unilaterally6.
We are aware of only 1 study regarding the effectiveness of combined intravenous and intra-articular TXA for patients undergoing simultaneous bilateral total knee arthroplasty7 and no studies assessing the utility of combined intravenous and intra-articular TXA for simultaneous bilateral total knee arthroplasty performed with a fast-track strategy6. Thus, the current study was performed to test the hypothesis that combined intravenous and intra-articular administration of TXA would result in less perioperative blood loss than intravenous TXA alone in patients undergoing simultaneous bilateral total knee arthroplasty under spinal anesthesia with an intraoperative periarticular injection of an analgesic and without use of a pneumonic tourniquet.
Materials and Methods
This comparative study was performed at a single orthopaedic clinic that specializes in knee and hip surgery. The study protocol was approved by the institutional review board.
The study consisted of 2 consecutive series of patients undergoing simultaneous bilateral total knee arthroplasty. One group was treated with combined intravenous and intra-articular TXA between October 2016 and December 2016 (combined TXA group) and the other was treated with intravenous TXA alone between January 2016 and September 2016 (intravenous TXA group). The exclusion criteria, which we defined prior to review of the medical records, were a total knee arthroplasty performed without use of TXA, use of general anesthesia, refusal of blood products, or enrollment in another interventional clinical trial within 6 months prior to the total knee arthroplasty.
Study Interventions
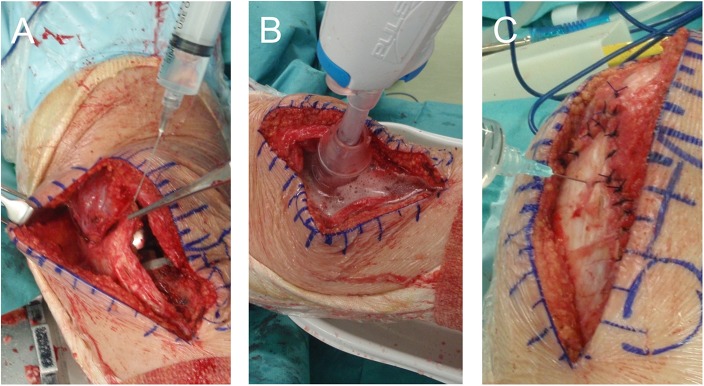
The combined TXA group received 1,000 mg of TXA (Transamin; Daiichi-Sankyo) administered intravenously just before the skin incision in the first knee. After implantation of the prosthesis and a periarticular analgesic injection followed by washing out of the solution (Figs. 1-A and 1-B), we closed the capsule and retinaculum. Then, 1,000 mg of TXA (10 mL of 100 mg/mL TXA) was administered intra-articularly into each knee (Fig. 1-C). Thus, a total of 3,000 mg of TXA was administered in the operating room. Six hours later, another 1,000 mg of TXA was given intravenously.
Fig. 1.
The technique for intra-articular TXA administration began with injection of periarticular analgesic solution (Fig. 1-A), which was washed out using pulse irrigation (Fig. 1-B). Then, after closure of the capsule and retinaculum, TXA was injected through the medial patellar retinaculum (Fig. 1-C).
The intravenous TXA group received 1,000 mg of TXA administered intravenously just before skin incision in the first knee and again 6 hours after the first dose. No intra-articular TXA was given in this group.
Surgery and Perioperative Medications
All of the simultaneous bilateral total knee arthroplasties were performed in sequence, by 1 of 2 surgeons, with the operation on the second knee started after completion of the wound closure on the first side. All were done with spinal anesthesia (0.5% bupivacaine [Marcaine; AstraZeneca]), and neither a pneumatic tourniquet nor a drain was used during the study period. Patients with a systolic blood pressure of >110 mm Hg after receiving spinal anesthesia were given nicardipine hydrochloride (Perdipine; Astellas Pharma) as a bolus and/or by continuous intravenous infusion during the surgery. All of the arthroplasties were done through a subvastus approach and with a cemented, posterior stabilized prosthesis (Scorpio NRG; Stryker Orthopaedics). We used an intramedullary femoral resection guide for femoral bone cutting, and we filled the femoral canal with an autologous bone plug after preparation.
All patients received an intraoperative periarticular injection of an analgesic solution containing 40 mL of ropivacaine (Anapeine, 7.5 mg/mL; AstraZeneca), 1.0 mL of morphine hydrochloride hydrate (10 mg/mL; Takeda), 0.6 mL of epinephrine (Bosmin, 1.0 mg/mL; Daiichi-Sankyo), 80 mg of methylprednisolone (Sol Mercort; Fuji), and 50 mg of ketoprofen (Capisten; Kissei)8. These agents were mixed with normal saline solution to achieve a combined volume of 120 mL, and 60 mL of the mixture was injected into each knee8.
For thromboprophylaxis, we injected 1.5 or 2.5 mg of fondaparinux (Arixtra; GlaxoSmithKline) subcutaneously once every evening for 7 days, starting on the first postoperative day.
Blood-Management Strategies
During the study period, patients scheduled for simultaneous bilateral total knee arthroplasty predeposited 800 mL (70 patients) or 400 mL (7 patients) of blood for autologous transfusion, unless their preoperative hemoglobin level was <11.0 g/dL (4 patients), in which case 4 units of allogenic red blood cells were prepared.
The patients who predeposited 800 mL of blood had 400 mL collected 4 weeks before the total knee arthroplasty and 400 mL collected 2 weeks before the total knee arthroplasty. The 7 patients who deposited only 400 mL did so 2 to 3 weeks before the total knee arthroplasty.
Half (400 mL) of the 800-mL predeposit of autologous blood was routinely returned to the patient on the day of surgery, and the remaining 400 mL was returned on the day after the surgery. Those who predeposited only 400 mL received all 400 mL on the day of the surgery. The patients for whom 4 units of allogenic red blood cells had been prepared had 2 units routinely transfused on the day of the surgery and the remaining 2 units transfused on the day after the surgery. We did not use any intraoperative blood salvage technique.
We planned additional allogenic blood transfusion for patients with a hemoglobin level of <6.5 g/dL who were asymptomatic and those with a hemoglobin level of <10.0 g/dL who had symptoms related to anemia.
Primary Outcome
The primary outcome was the volume of perioperative blood loss measured using the calculated blood volume and change in hemoglobin from preoperatively to postoperative day 39. First, we calculated the blood volume of the patient using the formula reported by Nadler et al.10:
 |
where k1 = 0.3669 for male patients and 0.3561 for female patients, k2 = 0.03219 for male patients and 0.03308 for female patients, and k3 = 0.6041 for male patients and 0.1833 for female patients.
Second, we estimated the loss of hemoglobin according to the following formula:
where Hbloss (g) was the amount of hemoglobin lost up to day 3 after surgery, Hbi (g/L) was the hemoglobin concentration before surgery, Hbe (g/L) was the hemoglobin concentration on day 3 after surgery, and Hbt (g/L) was the amount of hemoglobin transfused.
For the patients who received predeposited autologous blood, we used the hemoglobin level just prior to the donation for this calculation. For the patients who received allogenic blood, we used a hemoglobin level of 19 g/dL and 140 mL for 1 unit according to the data of the Japanese Red Cross Society.
Finally, the total blood loss was calculated as follows9:
Secondary Outcomes
We compared the number of patients requiring allogenic blood transfusion in addition to the predonated autologous blood, or in addition to the 4 units of allogenic blood for those who had not predonated, between the combined and intravenous groups. We also compared the serum D-dimer levels, measured using a latex agglutination turbidimetric immunoassay11 on postoperative day 3, between the combined and intravenous groups. In addition, we assessed the complications with particular emphasis on thrombotic events. D-dimer values of 0.20 to 0.25 μg/mL are usually considered the threshold when screening for deep vein thrombosis or pulmonary embolism12,13, but the threshold after total knee arthroplasty remains unclear because D-dimer values are elevated due to the activated fibrinolytic system14. During the study period, we performed contrast-enhanced computed tomography (CT) for patients with a D-dimer level of >15.0 μg/mL.
Sample Size Calculation
We considered a 400-mL decrease in perioperative blood loss as clinically meaningful when comparing different regimens of TXA administration for total knee arthroplasty15. We calculated that, with a sample of 16 patients per treatment group, the study would have 80% power to detect a 400-mL mean decrease in perioperative blood loss with a type-I error of 5%. For power analysis, we used a standard deviation of 400 mL for perioperative blood loss based on data from a previous series of simultaneous bilateral total knee arthroplasties without use of a pneumatic tourniquet8. Recognizing this minimum required sample size, we collected all of the available data to improve the statistical power for the secondary outcomes as much as possible.
Missing Data and Statistical Analysis
To analyze the primary outcome, we replaced missing data for total blood loss with the mean value for the treatment group in which the patient had been included. The significance of differences in mean perioperative blood loss and the 95% confidence intervals were calculated with the Student t test.
Other comparisons between the study groups were performed using the chi-square test for categorical variables and the Student t test for continuous variables.
All tests were 2-sided, and p < 0.05 was considered significant.
All statistical analyses were performed with R (The R Foundation for Statistical Computing).
Results
Patients
The combined TXA group consisted of 30 consecutive patients, and the intravenous TXA group consisted of 51 consecutive patients (Fig. 2). Patient demographics and baseline clinical characteristics are summarized in Table I. These characteristics were similar in the 2 groups.
Fig. 2.
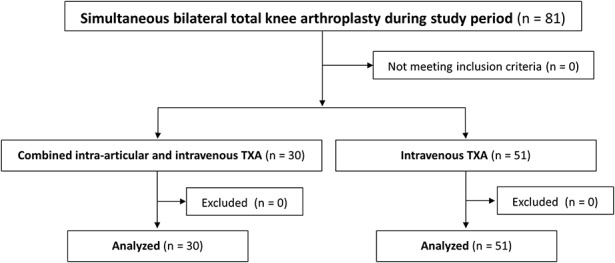
Patient flow diagram.
TABLE I.
Patient Demographic and Baseline Clinical Characteristics
Primary Outcome
The combined TXA group had significantly less perioperative blood loss than the intravenous TXA group (mean and standard deviation, 1,201 ± 347 versus 1,638 ± 400 mL, respectively; mean difference, 437 mL; 95% confidence interval, 263 to 613 mL; p < 0.0001). Perioperative blood-loss data were missing for 1 patient in the intravenous TXA group, as she had been transferred to a different hospital due to third-degree atrioventricular block before postoperative day 3. This missing value was replaced with the mean value for the intravenous TXA group.
Secondary Outcomes
No patient required additional allogenic blood transfusions in the combined TXA group, whereas 1 patient required 4 additional units of allogenic blood in the intravenous TXA group. The rates of additional allogenic blood transfusion (0% and 2%, respectively) did not differ significantly different between the 2 groups (p = 0.44).
The D-dimer levels measured on day 3 after the surgery averaged 7.5 ± 3.3 μg/mL in the combined TXA group and 9.1 ± 4.1 μg/mL in the intravenous TXA group (p = 0.080).
No thrombotic events occurred in either group. One patient in the intravenous TXA group developed a postoperative surgical site infection, and another patient in that group had arrhythmia that required implantation of a pacemaker.
Discussion
Thirty consecutive patients treated with combined intravenous and intra-articular TXA had significantly less perioperative blood loss than 51 consecutive patients treated with only intravenous TXA.
Although the intravenous regimen is the most common route for administration of TXA in total knee arthroplasty16, recent studies have indicated that a combined intravenous and intra-articular regimen would be more effective6,17. To our knowledge, our study is the first to demonstrate that combined intravenous and intra-articular TXA administration is superior to intravenous TXA alone in patients undergoing simultaneous bilateral total knee arthroplasty. One randomized controlled trial indicated that, compared with no TXA administration, combined intravenous and intra-articular TXA significantly reduced the postoperative volume of drained blood in patients undergoing simultaneous bilateral total knee arthroplasty using a pneumatic tourniquet and drain7.
Although the authors of a recent study recommended simultaneous, rather than staged, bilateral total knee arthroplasty because of its cost effectiveness18, perioperative bleeding remains an important issue19. The effectiveness of intravenous TXA in simultaneous bilateral total knee arthroplasty was reported in previous retrospective comparative studies20-22 and randomized controlled trials15,23. The effectiveness of intra-articular TXA was also reported in a retrospective comparative study24. Several randomized controlled trials compared the effectiveness of intravenous TXA with that of intra-articular TXA25,26. Combined intravenous and intra-articular TXA administration represents 1 option for dealing with the issue of perioperative bleeding in simultaneous bilateral total knee arthroplasty, and more rigorous trials should be performed to determine its effectiveness. Although pneumatic tourniquets were applied during surgery in all patients in the above studies7,15,20-26, it is inappropriate for us to discuss the usefulness of pneumatic tourniquets here as we did not focus on that in this study.
Patients in our combined group (intravenous and intra-articular TXA) were given a fixed dose of 4,000 mg of TXA within a 6-hour period regardless of their body weight. The dose was determined according to 3 studies. In 1 of them, a randomized controlled trial of patients who underwent unilateral total knee arthroplasty, 1,000 mg of TXA was administered intravenously and 3,000 mg of TXA was administered intra-articularly without increasing the complication rate compared with that in a group treated with intravenous administration of 1,000 mg of TXA6. In another randomized controlled trial, patients undergoing simultaneous bilateral total knee arthroplasty were given TXA as a bolus dose of 15 mg/kg 10 minutes before inflation of the tourniquet on the first side, 3,000 mg of TXA intra-articularly 10 minutes before deflation of the tourniquet, and then intravenous infusion of 10 mg/kg/hr continued for 3 hours following completion on the second side7. More than 4,000 mg of TXA was generally administered in this protocol7. Finally, in a retrospective cohort study of 872,416 patients who underwent total knee or total hip arthroplasty in a total of 510 hospitals, the complication rate did not differ among patients who had received 0, ≤1,000, 2,000, or ≥3,000 mg of TXA27. However, it should be noted that there is no consensus about the safety of a fixed dose of 4,000 mg TXA for Asian patients, and more data are required to reach definitive conclusions regarding its safety.
The present study had several limitations. First, the allocation was not randomized, and this could have caused selection bias due to unmeasured data, even though we used homogeneous inclusion and exclusion criteria and found no significant differences in the demographic data that we compared between our 2 consecutive series of patients. Second, although this study showed less perioperative blood loss with use of combined intravenous and intra-articular TXA, it was underpowered to investigate the rate of complications due to the combined regimen. It was also underpowered to assess whether combined intravenous and intra-articular TXA could reduce the rate of additional allogenic blood transfusions, which is more clinically relevant than the calculated perioperative blood loss that was the primary outcome in our study. Third, to screen for venous thromboembolism, we measured the D-dimer level at 3 days after surgery instead of routinely performing studies that directly detect venous thromboembolism, such as ultrasound or contrast-enhanced CT.
In conclusion, our study of patients undergoing simultaneous bilateral total knee arthroplasty without the use of a tourniquet showed the calculated total perioperative blood loss to be significantly lower when a combined intravenous and intra-articular regimen was used for TXA administration compared with when only the intravenous regimen was used. Additional studies are required to assess the rate of complications and requirement for additional blood transfusion associated with these regimens.
Disclosure of Potential Conflicts of Interest
Footnotes
Investigation performed at Nekoyama Miyao Hospital, Niigata, Niigata, Japan
Disclosure: This study received no specific grant from any funding agency. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A10).
References
- 1.Diamond PT, Conaway MR, Mody SH, Bhirangi K. Influence of hemoglobin levels on inpatient rehabilitation outcomes after total knee arthroplasty. J Arthroplasty. 2006. August;21(5):636-41. [DOI] [PubMed] [Google Scholar]
- 2.Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009. July;91(7):935-42. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada S, Wakui M, Hoshino A. Postoperative epidural analgesia compared with intraoperative periarticular injection for pain control following total knee arthroplasty under spinal anesthesia: a randomized controlled trial. J Bone Joint Surg Am. 2014. September 03;96(17):1433-8. [DOI] [PubMed] [Google Scholar]
- 4.Ejaz A, Laursen AC, Kappel A, Laursen MB, Jakobsen T, Rasmussen S, Nielsen PT. Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop. 2014. August;85(4):422-6. Epub 2014 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016. January;98-B(1)(Suppl A):98-100. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen CS, Jans Ø Ørsnes T, Foss NB, Troelsen A, Husted H. Combined intra-articular and intravenous tranexamic acid reduces blood loss in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2016. May 18;98(10):835-41. [DOI] [PubMed] [Google Scholar]
- 7.Karaaslan F, Karaoğlu S, Mermerkaya MU, Baktir A. Reducing blood loss in simultaneous bilateral total knee arthroplasty: combined intravenous-intra-articular tranexamic acid administration. A prospective randomized controlled trial. Knee. 2015. March;22(2):131-5. Epub 2014 Dec 13. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada S, Wakui M, Hoshino A. Pain control after simultaneous bilateral total knee arthroplasty: a randomized controlled trial comparing periarticular injection and epidural analgesia. J Bone Joint Surg Am. 2015. March 04;97(5):367-73. [DOI] [PubMed] [Google Scholar]
- 9.Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012. September;470(9):2605-12. Epub 2012 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962. February;51(2):224-32. [PubMed] [Google Scholar]
- 11.Kelly J, Rudd A, Lewis RR, Hunt BJ. Plasma D-dimers in the diagnosis of venous thromboembolism. Arch Intern Med. 2002. April 08;162(7):747-56. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs MJ, MacKinnon KM, Anderson D, O’Rourke K, Keeney M, Kearon C, Ginsberg J, Wells PS. A comparison of three rapid D-dimer methods for the diagnosis of venous thromboembolism. Br J Haematol. 2001. October;115(1):140-4. [DOI] [PubMed] [Google Scholar]
- 13.Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003. September 25;349(13):1227-35. [DOI] [PubMed] [Google Scholar]
- 14.Rafee A, Herlikar D, Gilbert R, Stockwell RC, McLauchlan GJ. D-Dimer in the diagnosis of deep vein thrombosis following total hip and knee replacement: a prospective study. Ann R Coll Surg Engl. 2008. March;90(2):123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Cao X, Yang C, Guo K, Zhu Q, Zhu J. Effectiveness and safety of fixed-dose tranexamic acid in simultaneous bilateral total knee arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2016. November;31(11):2471-5. Epub 2016 Apr 13. [DOI] [PubMed] [Google Scholar]
- 16.Lin ZX, Woolf SK. Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics. 2016. Mar-Apr;39(2):119-30. Epub 2016 Mar 4. [DOI] [PubMed] [Google Scholar]
- 17.Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015. May;30(5):776-80. Epub 2014 Dec 5. [DOI] [PubMed] [Google Scholar]
- 18.Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013. August 21;95(16):1441-9. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J, Rasouli MR. Simultaneous-bilateral TKA: double trouble - affirms. J Bone Joint Surg Br. 2012. November;94(11)(Suppl A):90-2. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon MS, Bali K, Prabhakar S. Tranexamic acid for control of blood loss in bilateral total knee replacement in a single stage. Indian J Orthop. 2011. March;45(2):148-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014. March;29(3):501-3. Epub 2013 Sep 17. [DOI] [PubMed] [Google Scholar]
- 22.Bagsby DT, Samujh CA, Vissing JL, Empson JA, Pomeroy DL, Malkani AL. Tranexamic acid decreases incidence of blood transfusion in simultaneous bilateral total knee arthroplasty. J Arthroplasty. 2015. December;30(12):2106-9. Epub 2015 Jun 22. [DOI] [PubMed] [Google Scholar]
- 23.Kim TK, Chang CB, Kang YG, Seo ES, Lee JH, Yun JH, Lee SH. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014. August;22(8):1870-8. Epub 2013 Apr 17. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Chen JY, Yew AK, Chia SL, Lo NN, Yeo SJ. Intra-articular tranexamic acid wash during bilateral total knee arthroplasty. J Orthop Surg (Hong Kong). 2015. December;23(3):290-3. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016. July;31(7):1442-8. Epub 2015 Dec 21. [DOI] [PubMed] [Google Scholar]
- 26.Maniar RN, Singhi T, Patil A, Kumar G, Maniar P, Singh J. Optimizing effectivity of tranexamic acid in bilateral knee arthroplasty - a prospective randomized controlled study. Knee. 2017. January;24(1):100-6. Epub 2016 Nov 23. [DOI] [PubMed] [Google Scholar]
- 27.Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, Boettner F, Memtsoudis SG. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014. August 12;349:g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.