Abstract
Study Objectives
(1) Examine performance on an objective measure of reward-related cognitive impulsivity (delay discounting) among self-reported habitual short sleepers and medium (i.e. recommended 7–9 hours) length sleepers either reporting or not reporting daytime dysfunction; (2) Inform the debate regarding what type and duration of short sleep (e.g. 21 to 24 hours of total sleep deprivation, self-reported habitual short sleep duration) meaningfully influences cognitive impulsivity; (3) Compare the predictive utility of sleep duration and perceived dysfunction to other factors previously shown to influence cognitive impulsivity via delay discounting performance (age, income, education, and fluid intelligence).
Methods
We analyzed data from 1190 adults from the Human Connectome Project database. Participants were grouped on whether they reported habitual short (≤6 hours) vs. medium length (7–9 hours) sleep duration and whether they perceived daytime dysfunction using the Pittsburgh Sleep Quality Index.
Results
All short sleepers exhibited increased delay discounting compared to all medium length sleepers, regardless of perceived dysfunction. Of the variables examined, self-reported sleep duration was the strongest predictor of delay discounting behavior between groups and across all 1190 participants.
Conclusions
Individuals who report habitual short sleep are likely to exhibit increased reward-related cognitive impulsivity regardless of perceived sleep-related daytime impairment. Therefore, there is a reason to suspect that these individuals exhibit more daytime dysfunction, in the form of reward-related cognitive impulsivity, than they may assume. Current findings suggest that assessment of sleep duration over the prior month has meaningful predictive utility for human reward-related impulsivity.
Keywords: short sleep, daytime dysfunction, delay discounting, reward sensitivity, cognitive impulsivity
Statement of Significance
Do humans who claim to thrive on little sleep function as well as they feel that they do? Prior evidence suggests that individuals who report habitual short sleep may be at increased risk of drowsiness in situations characterized by low environmental stimulation, regardless of whether they perceive sleep-related daytime dysfunction. The current study finds that habitual short sleepers are also likely to exhibit increased reward-related cognitive impulsivity, regardless of whether they perceive sleep-related daytime impairment. As 30% of working U.S. adults report habitual short sleep duration and approximately 10% of U.S. adults report habitual short sleep duration and do not report daytime impairment, continued objective validation of these claims appears warranted.
Introduction
Humans who claim to thrive on little sleep represent an important area for biological and psychological investigation. These individuals do not report the low behavioral drive, negative affect, and cognitive impairment typically associated with experimental sleep deprivation [1, 2] and endorsed by habitual short sleepers who report daytime dysfunction (e.g. individuals with insomnia [3, 4] or insufficient sleep syndrome [5]). Rather, habitual short sleepers not reporting daytime dysfunction have been described as active, vigorous, restless, and over-meticulous [6], efficient, energetic, ambitious, decisive, extroverted, and non-worriers [7], sub-clinically hypomanic [8], and behaviorally driven [9, 10].
The claim of normative, or even superior daytime functioning despite habitual short sleep duration (≤6 hours/night [11]) raises a fundamental question: Do these individuals function as well as they feel that they do? For the past 50 years, empirical examination of this question has been limited by relatively small sample sizes (N = 2 [6, 9]; N = 12 [8]; N = 37 [10]; N = 46 [7]) and by primarily relying on clinical judgments [6] or self-report questionnaires and interviews [7–10] to characterize outcomes of interest. Self-reports of functionality in habitual short sleepers may be problematic, as evidence suggests that we tend to underestimate our objective levels of daytime impairment as sleep deprivation [12] or sleep restriction [13] progress over time to a habitual/chronic state. Therefore, objective measures of daytime functioning in larger samples of habitual short sleepers are needed.
Prior research suggests that habitual short sleepers may have difficulty maintaining daytime alertness in the absence of environmental stimulation, regardless of perceived daytime dysfunction [14]. This tentative conclusion was reached on the basis of resting functional brain connectivity patterns using the Human Connectome Project (HCP) database. In the present study, we continue this line of investigation by examining an objective measure of reward-related cognitive impulsivity—monetary delay discounting performance—in habitual short and medium (i.e. recommended 7–9 hours) length sleepers with and without perceived daytime dysfunction using the HCP database.
Delay discounting refers to the decrease in subjective value of a desirable outcome as the time to obtain the outcome increases [15]. In humans, delay discounting taps the construct of cognitive impulsivity [16], with greater discounting of delayed rewards (i.e. preferring smaller, immediate rewards over larger, delayed rewards) indicating greater impulsivity. Although delay discounting appears ubiquitous across species and situations, suggesting evolutionary adaptability [15], excessive delay discounting appears to be a non-adaptive hallmark across a range of mental health disorders: hypomania [17], bipolar disorder and schizophrenia [18], major depressive disorder [19], addictive behaviors [20], and attention-deficit/hyperactivity disorder [21] (in particular, hyperactive-impulsive symptoms [22]).
Although experimental total sleep deprivation has been shown to have a negative effect on cognition across multiple domains (particularly simple attention and vigilance tasks [1]), tests of the effects on delay discounting performance have produced conflicting results. Twenty-one hours of total sleep deprivation was sufficient to enhance discounting of delayed monetary rewards in 12 healthy, young adult undergraduate students [16]. In contrast, delay discounting was unaffected by 24 hours of total sleep deprivation in 20 healthy adults [23] and 30 healthy young adults [24]. Attempts to model more real-world/ecologically valid instances of partial sleep deprivation (i.e. four consecutive nights of 6 hours/night; “short sleep”) in 37 medium length-sleeping healthy adults found evidence for short-sleep-induced diminished behavioral inhibition via a Go/No-Go task, but no difference in impulsive decision-making via a computerized delay discounting task [25]. These findings are similar to evidence of lowered behavioral inhibition on an emotional Go/No-Go task following 36 hours of total sleep deprivation in 32 medium length-sleeping healthy adults [26]. Given the relatively small sample sizes of these studies, examining the effects of a different form of short sleep (self-reported habitual short sleep duration) on delay discounting performance in a larger sample of adult participants may help inform the debate regarding what type and duration of short sleep is associated with cognitive impulsivity via delay discounting. However, examination of whether self-reported habitual short sleep duration is related to delay discounting behavior has not been reported to our knowledge.
Recently, the basic utility of asking about self-reported sleep duration without coincident objective data on sleep duration and quality has been questioned [27]. To examine the predictive utility of self-reported sleep duration (and perceived daytime dysfunction) on delay discounting performance, we compared these measures to other factors previously shown to have a negative effect on delay discounting, including age [28], income [29], education [30], and objective measures of fluid intelligence [31, 32] across all participants with complete delay discounting data in the HCP database 1200 participant release.
Approximately 30% of employed U.S. adults in a large nationally-representative sample reported sleeping 6 hours or less each day [33]. A recent report using the HCP database [34] supports this prevalence estimate [14]. Furthermore, these data also indicated that approximately 12% of participants reporting short sleep did not report daytime dysfunction, providing a tentative prevalence estimate [14]. Therefore, as approximately 10% of the adult U.S. population may claim to thrive on little sleep, objective validation of these claims appears warranted.
Materials and Methods
We analyzed data from 1190 participants with full delay discounting data from the HCP database [34] 1200 Participants Release. Participants were grouped based on whether they reported habitual short (≤6 hours) vs. medium length (7 to 9 hours) sleep duration over the past month, consistent with current National Sleep Foundation (NSF) sleep duration recommendations [35]. These data were derived from self-report answers to the Pittsburgh Sleep Quality Index (PSQI), a 24-item questionnaire comprising seven component scores, including sleep duration (component 3) and daytime dysfunction (component 7) [36]. Sleep duration was obtained from question #4 of the PSQI: “During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spend in bed.)” [36]. Participants not reporting daytime dysfunction reported scores of zero on PSQI component 7: daytime dysfunction. This corresponds to answering “Not during the past month” to PSQI question #8: “During the past month, how often have you had trouble staying awake while driving, eating meals, or engaging in social activity?” and answering “No problem at all” to PSQI question #9: “During the past month, how much of a problem has it been for you to keep up enough enthusiasm to get things done?” [36]. Participants reporting daytime dysfunction were conservatively characterized as having PSQI Component 7 scores greater than zero [14].
This strategy resulted in the following groups: (1) all habitual short sleepers (All HSS; n = 362); (2) all medium length sleepers (All MLS; n = 708); (3) habitual short sleepers not reporting daytime dysfunction (HSS-NRD; n = 142); (4) habitual short sleepers reporting daytime dysfunction (HSS-RD; n = 220), (5) medium length sleepers not reporting daytime dysfunction (MLS-NRD; n = 381), and (6) medium length sleepers reporting daytime dysfunction (MLS-RD; n = 327). This grouping strategy led to the exclusion of 118 participants reporting more than 6 and less than 7 hours of sleep at night over the prior month and to the exclusion of 12 participants reporting more than 9 hours of sleep at night over the prior month. To examine delay discounting in these individuals, multiple regression analysis with sleep duration as a continuous variable was performed across all 1190 participants with full delay discounting data from the HCP database 1200 participants release.
Delay discounting task
The HCP assessed cognitive impulsivity (in the “self regulation/impulsivity” category of HCP measures) using a version of a monetary delay discounting task to identify indifference points at which an individual is equally likely to choose a smaller reward sooner (e.g. $100 today) vs. a larger reward later (e.g. $200 in 1 month) (p. 173 [37]). Reward amounts are adjusted on a trial-by-trial basis to efficiently determine indifference points [37–39]. An area under the curve (AUC) summary measure is provided based on small monetary amount ($200) and high monetary amount ($40000) conditions to yield a non-theoretical, valid, and reliable index of how quickly an individual discounts delayed rewards [37, 40]. As described [41], participants are presented with two choices on each trial: a smaller monetary amount today or a larger amount at a later time point. Participants choose amounts at each of six delays: 1 month, 6 months, 1 year, 3 years, 5 years, and 10 years based on two delayed amounts: $200 and $40000. For each choice of delay and amount of delayed reward, participants make 5 choices. The indifference point for each condition is the value for a “sixth” choice, which is never presented to the participant but is based on an increment or decrement from the immediate value of their fifth choice. Participants make all five delay choices based on $200 before moving on to the next combination of delay choices based on $40000. The order of delayed amounts based on $200 was fixed in order as follows: (1) today vs. 6 months; (2) today vs. 3 years; (3) today vs. 1 month; (4) today vs. 5 years; (5) today vs. 10 years; (6) today vs. 1 year. Once these choices based on $200 are made, participants are presented with the same order of delay decisions based on $40000.
The first choice at each delay is between the delayed amount (i.e. $200 or $40000) and an immediate amount equal to 50% of the delayed amount (i.e. $100 today vs. $200 in 1 month; $20000 today vs. $40000 in 1 month). If participants choose the immediate amount, the immediate amount is reduced by 50% on the next choice (e.g. $50 today vs. $200 in 1 month; $10000 today vs. $40000 in 1 month). If participants choose the delayed amount, the immediate amount is increased by 50% on the next choice (e.g. $150 today vs. $200 in 1 month; $30000 today vs. $40000 in 1 month). On the third trial, the immediate value will always increase or decrease by 50% of the prior change (i.e. by $25 for the $200 condition and $5000 for the $40000 condition), regardless of whether the participant chooses immediate or delayed amounts. Similarly, the fourth choice will always increase or decrease immediate values by $12.50 ($200 condition) or $2500 ($40000 condition) and the fifth choice will always increase or decrease immediate values by $6.25 ($200 condition) or $1250 ($40000 condition). The “sixth” choice value, which is never presented to participants but entered into the HCP database, is always an increase or decrease of the immediate value by $3.125 ($200 condition) or $625 ($40000 condition). This process was adopted to rapidly determine indifference points where immediate gains are close to subjective values for delayed gains for each participant.
Delay discounting data analyses
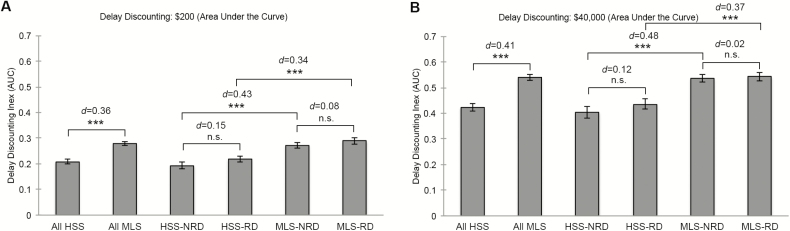
Theoretically neutral AUC estimates of delay discounting behavior [40] were examined to quantify global differences in delay discounting between groups (Figure 1, A and B). AUC was selected to overcome limitations of positive skew in parameter estimates for discounting functions [40], to remain consistent with the HCP database selecting AUC as their discounting summary measure (p. 173 [37];), and to enable direct comparison with prior experimental total sleep deprivation delay discounting studies [24]. Two-tailed independent-samples t-tests with false discovery rate correction for multiple comparisons (i.e. five AUC comparisons per monetary condition ($200 or $40000); p < 0.01 significance threshold) and Cohen’s d effect size estimates were run to examine these global differences. Lower AUC values indicate greater discounting of delayed rewards (i.e. increased cognitive impulsivity) [24]. AUC measures were calculated as described [41].
Figure 1.
Global differences in monetary delay discounting between self-reported short sleepers, medium length sleepers, and their subtypes. All HSS = all habitual short sleepers; All MLS = all medium length sleepers; HSS-NRD = habitual short sleepers not reporting daytime dysfunction; HSS-RD = habitual short sleepers reporting daytime dysfunction; MLS-NRD = medium length sleepers not reporting daytime dysfunction; MLS-RD = medium length sleepers reporting daytime dysfunction; Error bars = standard error of the mean; n.s. = not significant. **p < 0.01; ***p < 0.001. d = Cohen’s d.
Predictors of delay discounting behavior
Two-tailed independent-samples t-tests with false discovery rate correction were run to examine between-group differences in factors previously implicated in delay discounting behavior: age [28], income [29], education [30], and fluid intelligence [31, 32]. Sex was included as a covariate, although prior research indicates that sex does not meaningfully impact delay discounting behavior [42] (Table 1; five variables examined between groups, p < 0.05/5 = p < 0.01 significance threshold).
Table 1.
Differences in age, sex, income, education, and fluid intelligence between groups
| All HSS | All MLS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | M | SD | n | M | SD | t | P |
| Age | 362 | 28.86 | 3.61 | 708 | 28.71 | 3.73 | 0.63 | 0.53 |
| Female | 180 | 394 | −1.84 | 0.07 | ||||
| Male | 182 | 314 | ||||||
| Income | 360 | 4.73** | 2.09 | 705 | 5.16 | 2.19 | −3.08 | 0.002 |
| Education | 362 | 14.43*** | 1.87 | 707 | 15.09 | 1.73 | −5.78 | <0.001 |
| Fluid intelligence CR | 362 | 15.73*** | 4.97 | 707 | 17.09 | 4.78 | −4.35 | <0.001 |
| HSS-NRD | MLS-NRD | |||||||
| n | M | SD | n | M | SD | |||
| Age | 142 | 28.70 | 3.75 | 381 | 28.78 | 3.75 | −0.20 | 0.84 |
| Female | 64* | 220 | −2.60 | 0.01 | ||||
| Male | 78* | 161 | ||||||
| Income | 142 | 4.92 | 2.09 | 380 | 5.31 | 2.13 | −1.86 | 0.06 |
| Education | 142 | 14.32*** | 1.95 | 380 | 15.05 | 1.73 | −4.17 | <0.001 |
| Fluid intelligence CR | 142 | 14.88*** | 4.87 | 380 | 16.75 | 4.89 | −3.89 | <0.001 |
| HSS-RD | MLS-RD | |||||||
| n | M | SD | n | M | SD | |||
| Age | 220 | 28.97 | 3.53 | 327 | 28.64 | 3.71 | 1.04 | 0.30 |
| Female | 116 | 174 | −0.11 | 0.91 | ||||
| Male | 104 | 153 | ||||||
| Income | 218 | 4.60 | 2.09 | 325 | 4.98 | 2.24 | −1.98 | 0.05 |
| Education | 220 | 14.50*** | 1.82 | 327 | 15.13 | 1.74 | −4.14 | <0.001 |
| Fluid intelligence CR | 220 | 16.28** | 4.97 | 327 | 17.49 | 4.62 | −2.91 | 0.004 |
HSS, habitual short sleepers; MLS, medium length sleepers; NRD, not reporting daytime dysfunction; RD, reporting daytime dysfunction; n, subsample size; M, mean; CR, correct responses. Bolded comparisons were significant after p < 0.01 false discovery rate correction.
**p < 0.01, ***p < 0.001.
Given the known curvilinear relationship between self-reported sleep duration and a host of adverse outcomes [11, 27, 43], including risky decision-making [44], multiple regression analyses were performed to examine the relative contribution of the following factors in predicting delay discounting behavior between groups (Table 2) and across the entire HCP 1200 database (Table 3): age, sex, income, education, fluid intelligence, sleep duration, and daytime dysfunction. These seven predictors were entered into each model, with a Bonferroni-corrected significance threshold of p < 0.007 (i.e. p < 0.05/7 = p < 0.007).
Table 2.
Predictors of delay discounting behavior between groups
| All HSS and all MLS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Area under the curve ($200) | Area under the curve ($40000) | |||||||
| Variable | B | SE B | β | % Var | B | SE B | β | % Var |
| Constant | .256*** | .009 | .500*** | .008 | ||||
| Income | .003 | .027 | .027 | 0.06 | .007 | .004 | .049 | 0.18 |
| Education | .014*** | .004 | .120 | 1.08 | .024*** | .005 | .151 | 1.74 |
| Fluid intelligence CR | .005*** | .001 | .118 | 1.14 | .007*** | .002 | .115 | 1.21 |
| Age | −0.001 | .002 | −.025 | 0.05 | −.001 | .002 | −.011 | 0.01 |
| Sex | .000 | .013 | .001 | 0.00 | .004 | .017 | .007 | 0.00 |
| Sleep duration | .027*** | .005 | .152 | 2.13 | .045*** | .008 | .179 | 3.00 |
| Daytime dysfunction | .010 | .006 | .050 | 0.24 | .010 | .008 | .036 | 0.12 |
| R 2 | 0.077 | 0.105 | ||||||
| F | 12.51*** | 17.607*** | ||||||
| HSS-NRD and MLS-NRD | ||||||||
| Area under the curve ($200) | Area under the curve ($40000) | |||||||
| B | SE B | β | % Var | B | SE B | β | % Var | |
| Constant | .250*** | .008 | .492*** | .018 | ||||
| Income | −.001 | .004 | −.016 | 0.02 | .001 | .006 | .009 | 0.01 |
| Education | .016** | .005 | .147 | 1.66 | .027*** | .007 | .169 | 2.19 |
| Fluid intelligence CR | .006** | .002 | .142 | 1.63 | .008** | .003 | .136 | 1.49 |
| Age | .003 | .002 | .056 | 0.27 | .003 | .003 | .039 | 0.13 |
| Sex | −.007 | .018 | −.018 | 0.03 | .013 | .026 | .022 | 0.04 |
| Sleep duration | .025** | .008 | .137 | 1.80 | .039** | .011 | .145 | 1.99 |
| Daytime dysfunction | - | - | - | - | - | - | - | - |
| R 2 | 0.088 | 0.103 | ||||||
| F | 8.249*** | 9.819*** | ||||||
| HSS-RD and MLS-RD | ||||||||
| Area under the curve ($200) | Area under the curve ($40000) | |||||||
| B | SE B | β | % Var | B | SE B | β | % Var | |
| Constant | .256*** | .012 | .496*** | .021 | ||||
| Income | .006 | .005 | .067 | 0.35 | .012 | .006 | .090 | 0.62 |
| Education | .011* | .006 | .096 | 0.69 | .021** | .008 | .131 | 1.30 |
| Fluid intelligence CR | .004 | .002 | .089 | 0.66 | .006* | .003 | .097 | 0.77 |
| Age | −.006* | .003 | −.100 | 0.86 | −.005 | .003 | −.061 | 0.32 |
| Sex | .005 | .018 | .013 | 0.02 | −.005 | .024 | −.008 | 0.01 |
| Sleep duration | .029*** | .008 | .165 | 2.53 | .050*** | .010 | .209 | 4.04 |
| Daytime dysfunction | .011 | .010 | .049 | 0.22 | .018 | .013 | .057 | 0.31 |
| R 2 | 0.077 | 0.114 | ||||||
| F | 6.401*** | 9.871*** | ||||||
Sex: male = 0, female = 1. CR = correct responses. B = unstandardized. % Var = percent of unique variance (squared semi-partial correlations). Predictors are centered about their means. Predictors satisfying Bonferroni-corrected p < 0.007 are bolded.
*p < 0.05, **p < 0.01, ***p < 0.001.
Table 3.
Predictors of delay discounting behavior across the HCP 1200 database
| Area under the curve ($200) | Area under the curve ($40000) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | % Var | B | SE B | β | % Var |
| Constant | .263*** | .009 | .507*** | .012 | ||||
| Income | .003 | .003 | .036 | 0.10 | .010* | .004 | .073 | 0.41 |
| Education | .013*** | .004 | .119 | 1.08 | .022*** | .005 | .142 | 1.54 |
| Fluid intelligence CR | .005*** | .001 | .108 | 0.98 | .006*** | .002 | .109 | 0.98 |
| Age | −.001 | .002 | −.011 | 0.01 | −.002 | .002 | −.025 | 0.05 |
| Sex | −.009 | .012 | −.023 | 0.05 | −.004 | .016 | −.008 | 0.00 |
| Sleep duration | .027*** | .005 | .151 | 2.16 | .040*** | .007 | .161 | 2.43 |
| Daytime dysfunction | .009 | .006 | .043 | 0.18 | .012 | .008 | .042 | 0.17 |
| R 2 | 0.073 | 0.095 | ||||||
| F | 13.33*** | 17.77*** | ||||||
N = 1190. Sex: male = 0, female = 1. CR = correct responses. B = unstandardized. % Var = percent of unique variance (squared semi partial correlations). Predictors are centered about their means. Predictors satisfying Bonferroni-corrected p < 0.007 are bolded.
*p < 0.05, ***p < 0.001.
Age
Age in years was obtained from participants and included in the HCP 1200 Restricted Access database [34].
Sex
Sex (male or female) was obtained from participants and included in the HCP 1200 Open Access database.
Income
Total annual household income was obtained from participant responses to the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA [45]) and included in the HCP 1200 Restricted Access database. Total household income was scored and entered as follows: <$10000 = 1; $10000–19999 = 2; $20000–29999 = 3; $30000–39999 = 4; $40000–49999 = 5; $50000–74999 = 6; $75000–99999 = 7; ≥ $100000 = 8.
Education
Total years of completed education was obtained from participant responses to the SSAGA [45] and included in the HCP 1200 Restricted Access database. Years of education was scored and entered as follows: <11 years = 11; 12 years = 12; 13 years = 13; 14 years = 14; 15 years = 15; 16 years = 16; ≥17 years = 17.
Fluid intelligence
Non-verbal fluid intelligence was measured using Form A of an abbreviated version of Raven’s Progressive Matrices [46]. Participants were presented with 2 × 2, 3 × 3, or 1 × 5 arrangements of square patterns, with one square missing per pattern [47]. Participants selected one of five choices that best completed the missing square on the pattern. Form A has 24 items and three bonus items in order of increasing difficulty. The task is discontinued after five consecutive incorrect responses. The total number of correct responses was entered into the HCP Open Access database.
Results
Global AUC measures of delay discounting behavior at $200 and $40000 conditions are shown in Figure 1, A and B. Following false discovery rate correction for multiple comparisons (i.e. five comparisons per monetary condition; p < 0.05/5 = p < 0.01 threshold), all habitual short sleepers exhibited significantly greater discounting of delayed monetary rewards (i.e. decreased AUC values; increased cognitive impulsivity) compared to all medium length sleepers at $200 and $40000 conditions. Habitual short sleepers, regardless of perceived dysfunction, evidenced greater delay discounting compared to medium length sleepers.
To examine non-sleep-related factors previously shown to be associated with delay discounting performance that may contribute to current findings, we examined differences in participant age, sex, income, education, and fluid intelligence between groups (Table 1). These findings indicate that habitual short sleepers report fewer years of education (Cohen’s d range = 0.35–0.40) and may exhibit decreased fluid intelligence (Cohen’s d range = 0.28–0.38) compared to medium length sleepers, regardless of their perception of sleep-related daytime dysfunction.
To compare the predictive utility of self-reported habitual sleep duration and perceived daytime dysfunction to these non-sleep-related factors in predicting global delay discounting performance between groups, we ran a series of multiple regressions (Table 2). Seven predictors were entered into each model, resulting in a Bonferroni-corrected significance threshold of p < 0.007. Using these criteria, income, age, sex, and perceived daytime dysfunction did not meaningfully predict delay discounting performance between groups. Years of education, fluid intelligence, and self-reported sleep duration were consistently the strongest predictors of delay discounting behavior between groups. Increased years of education, higher fluid intelligence, and longer self-reported sleep duration predicted greater AUC values (i.e. less global cognitive impulsivity/delay discounting). Notably, with one exception, self-reported sleep duration accounted for the largest amount of unique variance in delay discounting performance between all habitual short sleepers and all medium length sleepers, between short sleepers and medium length sleepers not reporting dysfunction, and between short sleepers and medium length sleepers reporting dysfunction.
Finally, to examine whether these variables similarly predicted delay discounting behavior across the entire HCP database, we applied the above regression model to all 1190 participants who completed the delay discounting task (Table 3). This entire sample includes 120 additional participants with self-reported sleep durations falling outside of our short (≤6 hours; n = 362) and medium length (7–9 hours; n = 708) sleep duration cutoffs in prior analyses. Similar to findings between groups, years of education, fluid intelligence, and self-reported habitual sleep duration remained the strongest predictors of delay discounting behavior. Notably, self-reported habitual sleep duration was the strongest predictor of delay discounting behavior within $200 and $40000 conditions across all 1190 participants.
Discussion
In this study, we examined an objective measure of reward-related cognitive impulsivity among self-reported habitual short sleepers. The findings suggest that self-reported short sleepers, regardless of their perceived level of dysfunction, exhibit significant and meaningfully greater reward-related impulsivity compared to self-reported medium length sleepers (Figure 1, A and B; Table 2). In other words, there is a reason to suspect that habitual short sleepers who do not report daytime dysfunction may exhibit more functional difficulties than they assume. The current study also found that reported short sleep duration was a more meaningful predictor of delay discounting behavior compared to age [28], income [29], education [30], and fluid intelligence [31, 32].
To our knowledge, the present findings provide the first reported answer to the question of whether self-reported habitual short sleep duration is meaningfully associated with delay discounting performance. Our findings indicate that the answer to this question is yes—habitual short sleep duration is associated with greater reward-related cognitive impulsivity (Figure 1). These findings are consistent with a related and conceptually overlapping literature on the effects of sleep loss on risk-taking behavior [44, 48] and may help to clarify prior conflicting results regarding what type and duration of short sleep meaningfully influences cognitive impulsivity via delay discounting performance. Twenty-one hours of total sleep deprivation was shown to significantly increase delayed discounting in one study [16], whereas 24 hours of total sleep deprivation failed to replicate these results in two subsequent investigations [23, 24]. Partial sleep deprivation to 6 hours/night over four consecutive nights did not meaningfully impact delay discounting behavior [25]. Three differences between these prior studies and the present findings appear particularly useful for discussion: (1) monetary amounts and time delays used in delay discounting tasks; (2) sample sizes; and (3) the nature of short sleep duration.
The range of monetary amounts ($0.30 to $40000), time delays (60 seconds to 120 months), and sample sizes (12 to 1070) vary dramatically between studies. Although Reynolds and colleagues reported increased delay discounting following 21 hours of sleep deprivation based on a standard amount of $0.30 and rapid decision delays of 0–60 seconds in 12 within-subjects participants [16], Acheson and colleagues failed to replicate these findings using the same discounting task, 24 hours of total sleep deprivation, and an increased sample size of 20 within-subjects participants [23]. Accordingly, it would appear that only the present findings using standard monetary amounts based on considerably larger rewards ($200 and $40000) over much longer time intervals (1–120 months) may be sufficient to reveal the effects of short sleep duration on cognitive impulsivity. Future research examining whether acute total sleep deprivation and partial sleep restriction replicate the present findings based on these monetary amounts and time delays are needed.
A fundamental difference among these studies is the nature of short sleep duration. It seems reasonable to expect different outcomes based on different short sleep scenarios, such as staying up all night (e.g. 21–24 hours of total sleep deprivation [16, 23, 24]), obtaining less sleep than normal during a particularly stressful week (e.g. partial sleep restriction to 6 hours/night over four consecutive nights [25]), or habitually sleeping 6 hours/night or less, on average, during the past month (present study). These different scenarios and possible outcomes highlight a known obstacle inherent in this type of research on the effects of short sleep duration [11]. Whereas the self-report nature of sleep duration and daytime dysfunction represent fundamental limitations in the present study, the perception of being a short sleeper and the perception of thriving or experiencing daytime dysfunction as a result of one’s short sleep schedule are of primary interest in our ongoing line of research.
In addition to recommendations for bridging the gap between experimental and survey studies of short sleep duration [11], the basic utility of asking about self-reported sleep duration without coincident objective data on sleep duration and quality has been questioned [27]. The present findings that self-reported sleep duration was consistently the strongest predictor of delay discounting behavior compared to other predictive factors (age [28], income [29], education [30], and similar objective measures of fluid intelligence as the present study [31, 32]), suggests that even without objective verification, subjective reports have meaningful predictive utility, at least in the domain of reward-related impulsivity. Future research comparing the predictive utility of reported and objective habitual sleep duration to similar cognitive outcomes would help to clarify and extend the present findings.
Limitations, Future Directions, and Conclusions
The current study demonstrated that self-reported short sleep duration, regardless of perceived daytime dysfunction, was associated with greater cognitive impulsivity. The use of a large, nationally representative sample and an objective cognitive assessment are notable strengths. Findings are qualified by several limitations, however. Categorization of habitual short sleepers was limited as daytime dysfunction was derived from two items on the PSQI that ask about subjective trouble staying awake and keeping up enthusiasm to gets things done during the past month. Direct questioning of subjective functioning in the same domain as objective testing (e.g. reward-related cognitive impulsivity in the present study) would represent a more rigorous test of subjective/objective discrepancies in future research. The study was also limited by the cross-sectional nature of data available in the HCP database. Accordingly, we cannot explore questions of causation or mechanisms underlying observed differences in delay discounting performance between habitual short and medium length sleepers.
With these limitations in mind, viewing the present findings using the lens of the past 50 years of research on habitual short sleepers raises hypotheses to explore in future investigations. Are there individuals who objectively thrive on short sleep that are not represented in the current study? For example, there may be genetic short sleepers [9] who are quite rare; estimated by experts in sleep genetics to range from 1% even among short sleepers [49] to 1% of the general population [50]. To what extent do habitual short sleepers reporting or not reporting daytime dysfunction exhibit objective symptoms of (hypo)mania and/or attention-deficit/hyperactivity disorder (ADHD)? Sub-clinical hypomanic symptoms [8], increased energy and ambition [7], increased activity and restlessness [6], and increased behavioral drive [9, 10] have been suggested to characterize habitual short sleepers who do not report daytime dysfunction and are consistent with symptoms of (hypo)mania and ADHD (in particular, hyperactive-impulsive symptoms). Accordingly, it may be notable that symptoms of hypomania [17], bipolar disorder [18], and ADHD [21] (in particular, hyperactive-impulsive symptoms [22]) have all been associated with increased delay discounting performance similar to habitual short sleepers in the present study. Our prior findings that habitual short sleepers may require environmental stimulation to maintain wakefulness [14] is consistent with a vigilance regulation model of mania and ADHD, whereby an increased drive for seeking environmental stimulation may be a behavioral strategy to override underlying daytime sleepiness [51]. Therefore, future efforts to objectively explore symptoms of (hypo)mania and ADHD between habitual short sleepers reporting or not reporting daytime dysfunction (e.g. Conners’ Continuous Performance Test [CPT] for symptoms of ADHD [52], actigraphic assessment of motor activity for (hypo)mania [53, 54]) appear warranted.
Claims by some habitual short sleepers of adequate or even superior daytime functioning raises a fundamental question: Do these individuals function as well as they feel that they do? Our prior findings suggest that regardless of whether individuals who report habitual short sleep perceive sleep-related daytime dysfunction, they may be at increased risk of drowsiness in situations characterized by low environmental stimulation [14]. The present findings suggest that habitual short sleepers are also likely to exhibit increased reward-related cognitive impulsivity, regardless of whether they perceive sleep-related daytime impairment. As 30% of working U.S. adults report habitual short sleep duration [14, 33], and approximately 10% of U.S. adults report sleeping 6 hours or less each night without perceived daytime dysfunction [14], continued objective validation of these claims appears warranted.
Funding
Data were provided (in part) by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Preparation of this manuscript was supported by the University of Utah Neuroscience Initiative and support from the National Institute of Mental Health (K08 MH092697).
Notes
Conflict of interest statement. None declared.
References
- 1. Lim J, et al. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waters F, et al. Neuropsychological effects of sleep loss: implication for neuropsychologists. J Int Neuropsychol Soc. 2011;17(4):571–586. [DOI] [PubMed] [Google Scholar]
- 3. van de Laar M, et al. The role of personality traits in insomnia. Sleep Med Rev. 2010;14(1):61–68. [DOI] [PubMed] [Google Scholar]
- 4. Fernandez-Mendoza J, et al. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015;24(4):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 3rd ed Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 6. Jones HS, et al. Two cases of healthy insomnia. Electroencephalogr Clin Neurophysiol. 1968;24(4):378–380. [DOI] [PubMed] [Google Scholar]
- 7. Hartmann E, et al. Psychological differences between long and short sleepers. Arch Gen Psychiatry. 1972;26(5):463–468. [DOI] [PubMed] [Google Scholar]
- 8. Monk TH, et al. A sleep diary and questionnaire study of naturally short sleepers. J Sleep Res. 2001;10(3):173–179. [DOI] [PubMed] [Google Scholar]
- 9. He Y, et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325(5942):866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curtis BJ, et al. Short sleeper syndrome (SSS): a possible sleep-duration, circadian, metabolic, affective, pain-tolerance, normal variant in humans. Sleep. 2011;34:A259–A260. [Google Scholar]
- 11. Grandner MA, et al. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14(4):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Dongen HPA, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 13. Cohen DA, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2(14):14ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curtis BJ, et al. Sleep duration and resting fMRI functional connectivity: examination of short sleepers with and without perceived daytime dysfunction. Brain Behav. 2016;6(12):e00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vanderveldt A, et al. Delay discounting: pigeon, rat, human-does it matter?J Exp Psychol Anim Learn Cogn. 2016;42(2):141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reynolds B, et al. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 2004;67(3):343–356. [DOI] [PubMed] [Google Scholar]
- 17. Mason L, et al. I want it now! Neural correlates of hypersensitivity to immediate reward in hypomania. Biol Psychiatry. 2012;71(6):530–537. [DOI] [PubMed] [Google Scholar]
- 18. Ahn WY, et al. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120(4):911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pulcu E, et al. Temporal discounting in major depressive disorder. Psychol Med. 2014;44(9):1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacKillop J, et al. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl). 2011;216(3):305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson JN, et al. Attention-deficit/hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(4):316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beauchaine TP, et al. Attention-deficit/hyperactivity disorder, delay discounting, and risky financial behaviors: a preliminary analysis of self-report data. PLoS One. 2017;12(5):e0176933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acheson A, et al. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91(5):579–587. [DOI] [PubMed] [Google Scholar]
- 24. Libedinsky C, et al. Sleep deprivation alters effort discounting but not delay discounting of monetary rewards. Sleep. 2013;36(6):899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demos KE, et al. Partial sleep deprivation impacts impulsive action but not impulsive decision-making. Physiol Behav. 2016;164(Pt A):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson C, et al. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res. 2011;217(2):463–466. [DOI] [PubMed] [Google Scholar]
- 27. Bianchi MT, et al. An open request to epidemiologists: please stop querying self-reported sleep duration. Sleep Med. 2017;35:92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinberg L, et al. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. [DOI] [PubMed] [Google Scholar]
- 29. Ishii K. Subjective socioeconomic status and cigarette smoking interact to delay discounting. Springerplus. 2015;4(1):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaroni JL, et al. Relationship between education and delay discounting in smokers. Addict Behav. 2004;29(6):1171–1175. [DOI] [PubMed] [Google Scholar]
- 31. Osinski JT, et al. Social discounting rate is negatively correlated with fluid intelligence. Pers Individ Dif. 2014;59:44–49. [Google Scholar]
- 32. Shamosh NA, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19(9):904–911. [DOI] [PubMed] [Google Scholar]
- 33. Luckhaupt SE, et al. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Essen DC, et al. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirshkowitz M, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Heal. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 36. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 37. Barch DM, et al. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Estle SJ, et al. Differential effects of amount on temporal and probability discounting of gains and losses. Mem Cognit. 2006;34(4):914–928. [DOI] [PubMed] [Google Scholar]
- 39. Green L, et al. Do adjusting-amount and adjusting-delay procedures produce equivalent estimates of subjective value in pigeons?J Exp Anal Behav. 2007;87(3):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Myerson J, et al. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Project THC. The Human Connectome Project: WU-Minn HCP Quarter 1 (Q1) Data Release: Reference Manual 2013. https://www.humanconnectome.org/storage/app/media/documentation/s900/HCP_S900_Release_Reference_Manual.pdf. Accessed June 6, 2018.
- 42. Cross CP, et al. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137(1):97–130. [DOI] [PubMed] [Google Scholar]
- 43. Bliwise DL, et al. The parable of parabola: what the U-shaped curve can and cannot tell us about sleep. Sleep. 2007;30(12):1614–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hisler G, et al. Sleepiness and behavioral risk-taking: do sleepy people take more or less risk?Behav Sleep Med. 2017:1–14. doi:10.1080/15402002.2017.1357122 [DOI] [PubMed] [Google Scholar]
- 45. Bucholz KK, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. [DOI] [PubMed] [Google Scholar]
- 46. Bilker WB, et al. Development of abbreviated nine-item forms of the Raven’s standard progressive matrices test. Assessment. 2012;19(3):354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elam J. HCP Data Dictionary Public - 500 Subject Release https://wiki.humanconnectome.org/display/PublicData/HCP+Wiki+-+Public+Data. Accessed June 6, 2018.
- 48. Womack SD, et al. Sleep loss and risk-taking behavior: a review of the literature. Behav Sleep Med. 2013;11(5):343–359. [DOI] [PubMed] [Google Scholar]
- 49. Harmon K. Rare genetic mutation lets some people function with less sleep. Sci Am. 2009. https://www.scientificamerican.com/article/genetic-mutation-sleep-less/. Accessed June 7, 2018. [Google Scholar]
- 50. Ramsey L. A Tiny Percentage of the Population Needs Only 4 Hours of Sleep per Night Business Insider; 2015. [Google Scholar]
- 51. Hegerl U, et al. The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev. 2014;44: 45–57. [DOI] [PubMed] [Google Scholar]
- 52. Epstein JN, et al. Relations between continuous performance test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31(5):543–554. [DOI] [PubMed] [Google Scholar]
- 53. Krane-Gartiser K, et al. Actigraphic assessment of motor activity in acutely admitted inpatients with bipolar disorder. PLoS One. 2014;9(2):e89574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rock P, et al. Daily rest-activity patterns in the bipolar phenotype: a controlled actigraphy study. Chronobiol Int. 2014;31(2):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]