Abstract
Study Objectives
To examine the bidirectional association between physical activity (PA) and polysomnographically (PSG)-assessed sleep parameters characterized by total sleep time (TST), sleep onset latency (SOL), wake time after sleep onset (WASO), sleep efficiency and percentage of TST in N1, N2, and N3 stages and rapid eye movement (REM) sleep in middle-aged and older adults.
Methods
Longitudinal study based on a subsample of the Wisconsin Sleep Cohort. Self-reported PA information was used to estimate the metabolic equivalents of task (MET-minutes/week) activity and in-laboratory PSG exams provided information on sleep parameters at baseline and after 3–11 years of follow-up between 2004 and 2015. Poisson and linear regression models controlling for confounders estimated associations of sleep outcomes with changes in PA.
Results
A total of 424 participants (45.8% female; mean ± SD age 60.1 ± 7.5 years) were followed over an average of 5.0 ± 1.6 years. Compared to baseline PA of <500 MET-minutes/week (reference category), 500 to 1500 MET-minutes/week of PA was associated with lower incidences of TST <6 hours (relative risk, RR: 0.49; 95% confidence interval, CI: 0.27; 0.88), WASO >60 minutes (RR: 0.58; 95% CI: 0.41; 0.82) and sleep efficiency <80% (RR: 0.61; 95% CI: 0.39; 0.94), adjusting for sociodemographic, health behaviors and medical conditions. No significant associations were observed between baseline sleep characteristics and changes in PA through the follow-up.
Conclusion
In this prospective study, an intermediate level of PA at baseline predicted lower risk of incident short sleep time, higher WASO and lower sleep efficiency measured with PSG.
Keywords: physical activity, sleep, polysomnography, cohort study
Statement of Significance
Most epidemiological evidence regarding the association between physical activity (PA) and sleep in adults has been based on cross-sectional studies or self-reported sleep parameters. In this longitudinal study based on polysomnography, PA was associated with a lower risk of short sleep duration, wake time after sleep onset and lower sleep efficiency. In addition to the already-established beneficial effects of PA for health, this behavior might be considered as a protective factor for sleep disturbances in middle-aged and older adults.
Introduction
Low levels of physical activity (PA) and poor sleep are known risk factors for cardiovascular and all-cause mortality [1–3]. Systematic reviews based on intervention studies observed that PA is associated with improved subjective sleep quality and objective sleep parameters including sleep onset latency (SOL) and sleep efficiency [4–6]. Several observational studies also examined the association between PA and sleep in adults, but most were cross-sectional [7–9] or based on subjective sleep information [10–14]. Although self-reported sleep data are useful, in middle-aged and older adults they are only poorly-to-moderately correlated with objective measurements from accelerometry [15] and polysomnography (PSG) [16] and, thus, could lead to inaccurate results.
Among the cross-sectional studies that have addressed the relationship between PA and objective sleep, findings are inconsistent. In one study, Mitchell et al. [7] evaluated sleep parameters with wrist accelerometers over seven consecutive days and found no associations between moderate to vigorous PA and nighttime total sleep time (TST) or sleep efficiency in adult women. PSG has also been used in some cross-sectional studies, with conflicting results. For example, in midlife women, greater amounts of sports/exercise activity were associated with higher in-home PSG-assessed sleep efficiency [8]. In contrast, in another study of community-dwelling adults, the authors did not find associations between PSG-assessed sleep measures and sedentary time or moderate to vigorous PA [9].
Although PSG is the gold standard measure to evaluate sleep, we could not find prospective studies which evaluated PA as a predictor of PSG-assessed sleep outcomes. However, several cohort studies have investigated changes in self-reported sleep as outcomes with varied findings. While some found that the higher levels of PA are related to low incidence of insomnia [10–12], others found that PA did not predict sleep quality [13] or changes in sleep duration [14].
Thus, this study aims to examine whether PA at baseline predicts sleep disturbances including TST, SOL, wake time after sleep onset (WASO), sleep efficiency and percentage of TST in N3 (stages 3 and 4) and rapid eye movement (REM) sleep, evaluated with PSG at baseline and an average of 5 years of follow-up. Based on previous evidence derived from intervention studies, we hypothesized that higher levels of PA, independent of confounding factors, are associated with lower risks of short TST, longer SOL and WASO, lower sleep efficiency and lower percentages of TST in N3 and in REM sleep. In addition, as some evidence suggests an inverse relationship in which sleep conditions could be associated with reduced PA [13, 17, 18], we also explored associations of baseline sleep conditions with change in PA over the follow-up period.
Methods
Design and participants
The present study examined longitudinal data from the Wisconsin Sleep Cohort (WSC) study, a prospective population-based study designed to investigate the natural history, risk factors for and outcomes of common sleep disorders and behaviors in adults. The initial population was comprised of 1521 randomly-selected adults who were 30- to 60-year-old employees of state agencies in 1988. The WSC started recruiting subjects for baseline sleep studies in 1989. Subjects were invited for repeat studies at approximately 4-year intervals over up to 25 years of follow-up. Participation rates for 4-year follow-up waves have been an average of ~79%. Collection of detailed data on PA was initiated in October 2004 and is thus not available on all study participants. For approximately one-quarter of the sample, PA assessment and PSG occurred on different occasions (average difference = 1.9 ± 1.1 year). For the other three-quarters of observations, PA was assessed by technician-attended questionnaire administration during the overnight study visits. More detailed descriptions of the design and data collection of the WSC can be found elsewhere [19, 20]. For the present study, we identified the subsample of WSC participants with both PSG and complete information for PA from at least two study visits. This comprised individuals followed between 2004 and 2015. Study protocols and informed consent documents were approved by the Health Sciences Institutional Review Board of the University of Wisconsin-Madison.
Study variables
Sleep
Participants completed study visits at the University of Wisconsin-Madison Clinical Research Unit where they were monitored by PSG overnight during which participants were encouraged to sleep from their usual bedtime to usual waketime, if possible. A detailed description of PSG procedures can be found elsewhere [21]. Briefly, an 18-channel polysomnographic recording system (model 78, Grass Instruments, Quincy, MA) was used to evaluate sleep stage, respiration, body movements, and additional physiologic parameters. The recording montage included electroencephalography, electrooculography and submental electromyography to determine sleep state; continuous pulse oximetry (model 3740, Ohmeda, Englewood, CO) to assess arterial oxygen saturation; nasal-oral thermocouple (ProTec, Hendersonville, TN) to assess nasal and oral airflow; nasal pressure transducer (Validyne Engineering, Northridge, CA) for nasal airflow; respiratory inductance plethysmography (Respitrace, Ambulatory Monitoring, Ardsley, NY) to detect body abdominal and chest wall excursion. Sleep studies were staged, and respiratory events scored by trained sleep technicians and then reviewed by an expert polysomnographer.
For the present investigation, the following variables were obtained from the PSG: TST (hours), SOL (time from lights out to the first epoch of EEG-evidenced sleep in minutes), WASO (time awake between first and last epochs of EEG-assessed sleep in minutes), sleep efficiency (percent of time in bed spent asleep) and percentage of TST in stage N3 and REM sleep. The average number of apnea plus hypopnea events per hour of sleep defined the apnea-hypopnea index (AHI), used as a covariate in analyses as it can plausibly act as a confounding factor by suppressing PA via mechanisms such as general fatigue and depressed mood, and can also directly impact outcomes (examined sleep characteristics) [20, 22].
Short sleep was defined as TST < 6 hours, which is associated with increased mortality [2]. For each of the other sleep parameters, as we could not find a consensus regarding the cutoff to discriminate those with better and worse sleep indicators, we opted to use the medians at baseline, and rounded the values to simplify the interpretation of the results. For example, the baseline median of WASO was 60.25 minutes and we analyzed the risk of WASO > 60 minutes; similarly, sleep efficiency had a baseline median of 82.8% and the cutoff used to define incident lower sleep efficiency was 80%. To evaluate the robustness of our results, we repeated all analyses using the exact value as the cutoff for the outcomes and the results remained essentially identical.
Physical activity
A modified version of the Paffenbarger Physical Activity Questionnaire [23] was administered by trained personnel to assess the participants’ usual frequency, intensity, duration, and type of regular and recreational PA and walking. Responses were used to estimate weekly energy expenditure in these activities in metabolic equivalents of task (MET-minutes/week). PA was divided into three groups according to approximate tertiles of MET-minutes/week: <500 (reference category, 500 MET-minutes/week is approximately equivalent to 25 minutes per day walking at a slow-moderate pace of ~2.5 miles per hour), 500 to 1500 MET-minutes/week, and >1500 MET-minutes/week (1500 MET-minutes/week is approximately equivalent to 40–45 minutes per day of brisk walking at ~4 miles per hour).
Covariates
Sociodemographic variables were obtained to control for potential confounding factors including age (years), sex, educational level (up to high-school vs. higher level) and marital status. Information about smoking (current smoker vs. former or never smoker) and alcohol consumption (number of drinks/week) were also self-reported. Laboratory-assessed weight (kg) and height (m) were used to calculate body mass index in kg/m2. Participants also were queried “In general, would you say your health is” and chose one of the following options: excellent or very good (categorized as optimal self-rated health, the reference category), or good, fair or poor (suboptimal self-rated health). Depression, defined by symptoms or antidepressant medication use at baseline, was also obtained.
Statistical analysis
Sociodemographic and lifestyle characteristics, self-reported general health, PA and sleep parameters of the original WSC, baseline and followed study populations were described with frequencies, medians and interquartile ranges.
To calculate the risk of developing each sleep disturbance, we considered the number of new cases among those without each specific sleep problem at baseline (Note that sample sizes for those followed for new onset of specific sleep problems — i.e. that had baseline levels that were “healthier”— are indicated in the top row of Table 2 for each sleep parameter).
Table 2.
Number and percentage of incident sleep outcomes and relative risksa (95% confidence interval) of sleep outcomes by physical activity level at baseline
| PA at baseline (MET-minutes/week) | TST < 6 hours | SOL > 10 minutes | WASO > 60 minutes | Sleep efficiency < 80% | Percentage of TST in N1 < 10% | Percentage of TST in N2 < 65% | Percentage of TST in N3 < 10% | Percentage of TST in REM < 15% |
|---|---|---|---|---|---|---|---|---|
| Total, n. of cases/ total (%) | 64/232 (27.6) | 122/285 (42.8) | 107/212 (50.5) | 97/258 (37.6) | 60/184 (32.6) | 47/170 (27.6) | 81/186 (43.5) | 97/266 (36.5) |
| PA level at baseline, n (%) | ||||||||
| <500 | 23 (33.8) | 36 (47.4) | 35 (57.4) | 29 (42.0) | 23 (36.5) | 11 (23.9) | 24 (41.4) | 28 (38.9) |
| 500 to 1500 | 15 (19.7) | 40 (41.2) | 31 (42.5) | 25 (28.4) | 21 (35.0) | 16 (29.1) | 29 (45.3) | 34 (39.5) |
| >1500 | 26 (29.6) | 46 (41.1) | 41 (52.6) | 43 (42.6) | 16 (26.2) | 20 (29.0) | 28 (43.8) | 35 (32.4) |
| Unadjusted model | ||||||||
| <500 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 500 to 1500 | 0.58 (0.33, 1.02) | 0.87 (0.62, 1.21) | 0.74 (0.52, 1.04) | 0.67 (0.44, 1.04) | 0.95 (0.60, 1.54) | 1.21 (0.62, 2.35) | 1.09 (0.73, 1.65) | 1.02 (0.69, 1.50) |
| >1500 | 0.87 (0.55, 1.38) | 0.87 (0.63, 1.20) | 0.92 (0.68, 1.24) | 1.01 (0.71, 1.45) | 0.72 (0.42, 1.22) | 1.21 (0.64, 2.28) | 1.06 (0.70, 1.65) | 0.83 (0.56, 1.24) |
| Model 1 | ||||||||
| <500 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 500 to 1500 | 0.60 (0.34, 1.05) | 0.93 (0.66, 1.32) | 0.70 (0.50, 0.99)* | 0.70 (0.45, 1.09) | 1.22 (0.75, 2.01) | 1.17 (0.58, 2.35) | 1.05 (0.69, 1.59) | 1.14 (0.78, 1.67) |
| >1500 | 0.80 (0.48, 1.32) | 0.89 (0.63, 1.26) | 0.82 (0.60, 1.12) | 1.00 (0.68, 1.47) | 0.88 (0.51, 1.53) | 1.24 (0.61, 2.53) | 0.99 (0.64, 1.54) | 1.03 (0.69, 1.54) |
| Model 2 | ||||||||
| <500 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 500 to 1500 | 0.49 (0.27, 0.88)* | 0.93 (0.65, 1.33) | 0.58 (0.41, 0.82)** | 0.61 (0.39, 0.94)* | 1.18 (0.72, 1.92) | 1.42 (0.68, 2.98) | 1.21 (0.77, 1.89) | 1.23 (0.83, 1.83) |
| >1500 | 0.63 (0.37, 1.07) | 0.91 (0.62, 1.33) | 0.66 (0.47, 0.91)* | 0.83 (0.55, 1.26) | 0.83 (0.47, 1.64) | 1.32 (0.61, 2.86) | 1.12 (0.71, 1.74) | 1.09 (0.72, 1.68) |
N1, N2, N3: sleep stages. Model 1: Adjusted by age (years), sex (male vs. female), educational level (through high-school vs. some college), marital status (married vs. not married), current smoker (no vs. yes), alcohol intake (drinks/week), follow-up time (years) and by the difference in time between PA and sleep information (years) at baseline. Model 2: Adjusted by the same variables in Model 1 and by baseline BMI (kg/m2), change in BMI over the follow-up (kg/m2), self-rated health (excellent or very good vs. good, fair or poor), depression (no depression symptoms vs. depression symptoms or current use of antidepressants), baseline apnea-hypopnea index and continuous positive airway pressure use at baseline PSG.
aRelative risks (95% confidence interval) obtained with Poisson regression models adjusted by the covariates indicated for each model.
*p < 0.05; **p < 0.01.
To analyze the relationship between PA levels and the risk of developing sleep disturbances, we used Poisson regression models with robust variance estimates, as described in Zou [24], to estimate relative risks in each category of PA (main predictor) for each sleep outcome. We first examined unadjusted models and then added covariates to adjust the analyses for the potentially confounding effects of age, sex, educational level, marital status, smoking, alcohol intake, follow-up time, and the difference in time between PA and sleep information at baseline (model 1). Finally, we examined a fully adjusted model that additionally included baseline body mass index (BMI), change in BMI over the follow-up, self-rated health, depression, baseline apnea-hypopnea index, and continuous positive airway pressure (CPAP) use at baseline PSG (model 2). Additional sensitivity analyses repeated these analyses including only those observations for which PA and PSG were assessed at the same study visit.
We also explored whether the PA category at baseline predicted continuous changes in each of the sleep parameters. For this purpose, linear mixed-models evaluated the continuous change in the sleep parameter as the outcome and the PA category as the exposure. These models were adjusted for the same covariates as the fully-adjusted models described above. In addition, in supplemental analyses we tested whether continuous PA at baseline and continuous changes in PA over the follow-up period predicted either the discrete sleep outcomes or changes in each of the sleep parameters.
Lastly, “reverse associations” were examined, i.e. whether baseline categorical sleep parameters predict continuous and percent changes in PA (outcomes). In addition to the already-mentioned covariates, these analyses were also adjusted for change in the corresponding sleep parameter over the follow-up period. Additional supplemental analyses examined continuous baseline sleep parameters as well as continuous change in sleep parameters as the predictors in separate models.
The statistical significance level was set at p < 0.05. All analyses were performed with SAS statistical software (version 9.4, SAS Institute Inc., Cary, NC).
Results
Among the 1538 WSC participants, 834 had at least one sleep study since October 2004 (when PA assessment was initiated), and 14 were excluded due to missing information for education (n = 7), marital status (n = 1), depression (n = 3) and CPAP use (n = 3). Of these, 424 (52% of 820) were followed and provided information for all key study variables over a follow-up interval of a mean (±SD) 5.0 ± 1.6 years (minimum 3.3, maximum 11.2 years). Note that many of the 820 did not have an opportunity to be invited for follow-up sleep assessments before sleep studies ceased in 2015. Table 1 presents the characteristics of the baseline and the followed participants. Among followed individuals, the age range varied from 42 to 77 years. PSG data showed a baseline median of 6.2 hours of TST, 7 minutes of SOL, 60 minutes of WASO, 83% of sleep efficiency, 9% of TST in N1, 63% in N2, 9% in N3 sleep, and 17% in REM sleep (Table 1).
Table 1.
Characteristics of participants included in the original Wisconsin Sleep Cohort (original sample), of those considered as the baseline for this study and of those finally followed
| Baseline characteristics | Original WSC | Baseline for the present study | Followed |
|---|---|---|---|
| Total, n | 1538 | 820 | 424 |
| Sociodemographic, lifestyle and health | |||
| Age (years), median (IQR) | 60 (53, 67) | 62 (56, 67) | 60 (54, 65) |
| Female, n (%) | 701 (44.8) | 369 (45.0) | 194 (45.8) |
| Lower educational levela, n (%) | 386 (25.1) | 198 (24.1) | 103 (24.3) |
| Married, n (%) | 1062 (69.1) | 576 (70.2) | 300 (70.8) |
| Current smoker, n (%) | 187 (12.2) | 65 (7.9) | 36 (8.5) |
| Alcohol intake (drinks/week), median (IQR) | 2 (0, 6) | 2 (0, 5) | 2 (0, 5) |
| BMI (kg/m2), median (IQR) | 30 (26, 35) | 30 (26, 35) | 30 (26, 35) |
| Excellent or very good self-rated health, n (%) | 521 (57.1)b | 482 (58.8) | 269 (63.4) |
| Depression or antidepressant use, n (%) | 425 (27.6) | 220 (26.8) | 111 (26.2) |
| CPAP use, n (%) | 93 (6.0) | 61 (7.4) | 14 (3.3) |
| Physical activity (MET-minutes/week), n | 915 | 820 | 424 |
| <500 | 285 (31.1) | 255 (31.1) | 124 (29.2) |
| 500 to 1500 | 287 (31.4) | 257 (31.3) | 144 (34.0) |
| >1500 | 343 (37.5) | 308 (37.6) | 156 (36.8) |
| Median (IQR) | 996 (320, 2165) | 1008 (336, 2091) | 1041 (420, 1997) |
| Sleep measures (PSG), n | 1564 | 820 | 424 |
| Apnea-hypopnea index, median (IQR) | 7.8 (3.7, 18.0) | 8.9 (3.7, 18.2) | 9.5 (3.1, 18.9) |
| TST (hours/night), median (IQR) | 6.0 (5.3, 6.8) | 6.0 (5.4, 6.7) | 6.2 (5.6, 6.8) |
| SOL (minutes), median (IQR) | 10 (5, 20) | 8 (4, 17) | 7 (3.5, 13) |
| WASO (minutes), median (IQR) | 68 (48, 103) | 66 (40, 98) | 60 (37, 91) |
| Sleep efficiency (%), median (IQR) | 81 (72, 88) | 81 (74, 88) | 83 (76, 89) |
| % of TST in N1, median (IQR) | 9 (6, 14) | 9 (6, 14) | 9 (6, 14) |
| % of TST in N2, median (IQR) | 65 (58, 71) | 63 (57, 70) | 63 (56, 69) |
| % of TST in N3 (phases 3 and 4), median (IQR) | 7 (2, 14) | 9 (4, 15) | 9 (4, 16) |
| % of TST in REM, median (IQR) | 16 (11, 20) | 16 (13, 20) | 17 (13, 20) |
| TST < 6 hours, n (%) | 728 (47.3) | 400 (48.8) | 192 (45.3) |
| SOL >10 minutes, n (%) | 756 (49.2) | 339 (41.3) | 139 (32.8) |
| WASO > 60 minutes, n (%) | 880 (57.2) | 455 (55.5) | 212 (50.0) |
| Sleep efficiency <80%, n (%) | 730 (47.5) | 368 (44.9) | 166 (39.2) |
| Percentage of TST in N1 <10%, n (%) | 837 (54.4) | 446 (54.4) | 240 (56.6) |
| Percentage of TST in N2 <65%, n (%) | 750 (48.8) | 464 (46.6) | 254 (59.9) |
| Percentage of TST in N3 <10%, n (%) | 971 (63.1) | 467 (57.0) | 238 (56.1) |
| Percentage of TST in REM <15%, n (%) | 702 (45.6) | 347 (42.3) | 158 (37.3) |
IQR, interquartile range; MET, metabolic equivalent; SF-36, Short-form quality of life instrument.
aUp to high school.
bAvailable for 913 participants.
PA categories predicting binary sleep outcomes
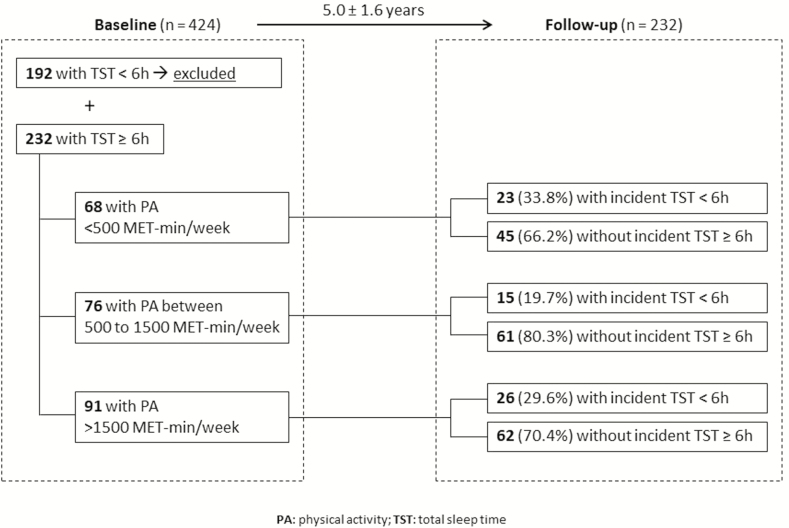
A schematic representation of the study design and the number of participants followed in each group of PA level is presented in Figure 1. Relative risks for developing polysomnographically-assessed sleep problems according to baseline PA level are presented in Table 2. The results for unadjusted Poisson regression models showed no associations between baseline PA category and binary sleep outcomes. However, after the adjustment by sociodemographic, lifestyle and health conditions (model 2), we observed lower risks for short sleep time, higher WASO and lower sleep efficiency in the group with the middle category of PA (500 to 1500 MET-minutes/week at baseline) compared to the reference group (<500 MET-minutes/week). The additional analyses that included only those with PA and PSG assessed at the same study visit showed similar coefficients.
Figure 1.
Diagram of the cohort study design used to explore the association between PA level at baseline and incident short sleep time in the follow-up (observation: this same template was applied for the other sleep outcomes).
PA categories predicting continuous changes in sleep parameters
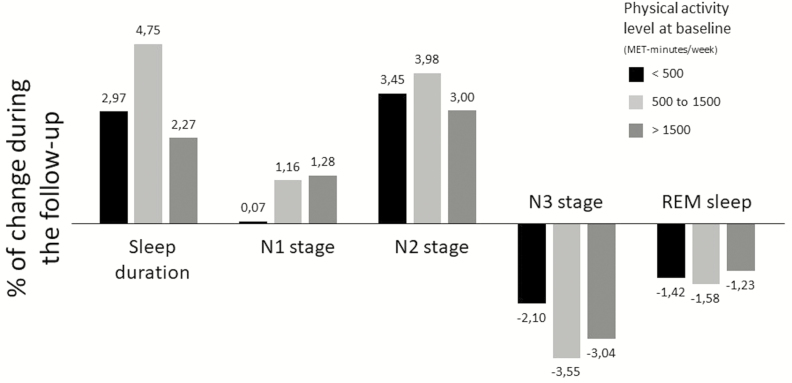
Regarding the relationship between baseline level of PA and changes in sleep during the follow-up, a greater decrease in the percent of TST in N3 was observed for those with intermediate or high PA at baseline when compared to the reference group (Table 3). Figure 2 illustrates the percent change in sleep duration and in each sleep stage between the baseline and the follow-up and according to the level of PA at baseline. Compared with the <500 MET-minutes/week participants, those with an intermediate PA (500 to 1500 MET-minutes/week) at baseline had a greater increase in sleep duration and decrease in N3.
Table 3.
Median and interquartile range of sleep changes and beta coefficientsa (standard error) of continuous changes in sleep parameters by PA level at baseline
| PA at baseline (MET-minutes/ week) | TST change | SOL change | WASO change | Sleep efficiency change | Percentage of TST in N1 change | Percentage of TST in N2 change | Percentage of TST in N3 change | Percentage of TST in REM change |
|---|---|---|---|---|---|---|---|---|
| Total, median (IQR) | 2.3 (−33.5, 46.0) | 2.0 (−3.0, 11.3) | 11.5 (−11.0, 40.7) | −1.9 (−8.5, 4.3) | 1.1 (−2.6, 4.3) | 2.6 (−2.1, 9.6) | −2.5 (−6.6, 0.2) | −1.6 (−5.5, 3.2) |
| PA at baseline, median (IQR) | ||||||||
| <500 | −1.5 (−39.3, 43.0) | 2.5 (−3.5, 14.5) | 8.5 (−14.5, 42.8) | −2.2 (−9.0, 5.3) | 0.3 (−3.4, 3.4) | 2.6 (−3.0, 10.7) | −2.3 (−6.6, 1.6) | −2.2 (−6.4, 3.5) |
| 500 to 1500 | 9.8 (−25.9, 53.5) | 1.5 (−3.3, 8.3) | 13.5 (−9.6 (37.5) | −0.5 (−8.2, 4.5) | 2 (−2.0, 5.0) | 2.5 (−1.5, 10.7) | −2.6 (−6.9, −0.1) | −1.4 (−4.9, 2.6) |
| >1500 | 0.5 (−36.3, 41.3) | 1.8 (−2.5, 9.3) | 16.0 (−9.3, 41.2) | −2.5 (−8.5, 3.2) | 1.2 (−2.3, 4.4) | 2.9 (−2.1, 9.2) | −2.4 (−6.1, 0.1) | −1.2 (−5.1, 3.5) |
| Unadjusted models | ||||||||
| <500 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 500 to 1500 | 0.14 (0.12) | −3.86 (2.51) | 1.40 (5.27) | 0.39 (1.29) | 1.26 (0.78) | 0.41 (1.16) | −1.49 (0.82) | −0.15 (0.82) |
| >1500 | −0.01 (0.12) | −3.22 (2.46) | 6.34 (5.18) | −1.08 (1.26) | 1.18 (0.76) | −0.28 (1.14) | −0.99 (0.81) | 0.07 (0.81) |
| Model 1 | ||||||||
| <500 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 500 to 1500 | 0.16 (0.13) | −3.03 (2.56) | 1.11 (5.39) | 0.41 (1.31) | 1.17 (0.79) | 0.94 (1.14) | −1.76 (0.78)* | −0.34 (0.83) |
| >1500 | 0.01 (0.13) | −2.63 (2.60) | 4.45 (5.48) | −0.66 (1.33) | 1.14 (0.81) | 0.93 (1.15) | −1.66 (0.79)* | −0.42 (0.84) |
| Model 2 | ||||||||
| <500 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 500 to 1500 | 0.24 (0.13) | −1.99 (2.62) | −2.71 (5.49) | 1.26 (1.33) | 1.05 (0.81) | 0.89 (1.17) | −1.83 (0.80)* | −0.11 (0.85) |
| >1500 | 0.12 (0.13) | −0.56 (2.72) | −0.69 (5.71) | 0.29 (1.39) | 0.75 (0.84) | 0.93 (1.21) | −1.82 (0.83)* | 0.13 (0.88) |
N1, N2, N3: sleep stages. Model 1: Adjusted by age (years), sex (male vs. female), educational level (through high school vs. some college), marital status (married vs. not married), current smoker (no vs. yes), alcohol intake (drinks/week), follow-up time (years) and by the difference in time between PA and sleep information (years) at baseline. Model 2: Adjusted by the same variables in Model 1 and by baseline BMI (kg/m2), change in BMI over the follow-up (kg/m2), self-rated health (excellent or very good vs. good, fair or poor), depression (no depression symptoms vs. depression symptoms or current use of antidepressants), baseline apnea-hypopnea index and continuous positive airway pressure use at baseline PSG.
aBeta coefficients (standard error) obtained with mixed-effects models adjusted by the covariates indicated for each model.
*p < 0.05.
Figure 2.
Percentage of change in sleep duration and in sleep stages by PA at baseline.
Continuous PA and change in PA predicting binary sleep outcomes and continuous changes in sleep parameters
No association was seen in additional analyses with continuous PA variables as exposure (Supplementary Table S1).
Sleep conditions at baseline predicting continuous and percent changes in PA
Additionally, in general, sleep conditions did not predict changes in PA over the follow-up (Table 4 and Supplementary Table S2).
Table 4.
Beta-coefficientsa (standard error) of continuous and percent changes in PA by sleep conditions at baseline
| Sleep condition (exposure) | Continuous change in PA (MET-minutes/ week) | Percent change in PA (%) |
|---|---|---|
| Beta-coefficient (standard error) | Beta-coefficient (standard error) | |
| Total | ||
| TST (hours) | ||
| ≥6 | Reference | Reference |
| <6 | −0.6 (2.5) | 27.0 (63.1) |
| SOL (minutes) | ||
| ≤10 | Reference | Reference |
| >10 | −2.4 (2.5) | −34.3 (63.9) |
| WASO (minutes) | ||
| ≤60 | Reference | Reference |
| >60 | −2.8 (2.5) | 46.8 (63.3) |
| Sleep efficiency (%) | ||
| ≥80 | Reference | Reference |
| <80 | −1.2 (2.6) | 52.6 (65.5) |
| TST in N1 sleep (%) | ||
| ≥10 | Reference | Reference |
| <10 | 2.5 (2.5) | 3.3 (62.4) |
| TST in N2 sleep (%) | ||
| ≥65 | Reference | Reference |
| <65 | 1.6 (2.5) | 6.6 (64.0) |
| TST in N3 sleep (%) | ||
| ≥10 | Reference | Reference |
| <10 | −6.1 (2.6)* | −48.0 (66.0) |
| TST in REM (%) | ||
| ≥15 | Reference | Reference |
| <15 | 0.9 (2.5) | 4.4 (63.6) |
N1, N2, N3: sleep stages.
aBeta-coefficients obtained with mixed-effect models adjusted by age (years), sex (male vs. female), educational level (through high school vs. some college), marital status (married vs. not married), current smoker (no vs. yes), alcohol intake (drinks/week), follow-up time (years), difference in time between PA and sleep information (years) at baseline, baseline BMI (kg/m2), change in BMI over the follow-up (kg/m2), self-rated health (excellent or very good vs. good, fair or poor), depression (no depression symptoms vs. depression symptoms or current use of antidepressants), baseline apnea-hypopnea index and continuous positive airway pressure use at baseline PSG.
*p < 0.05.
Discussion
In this prospective cohort of middle-aged and older adults based on in-laboratory PSG-assessed data, compared to lower levels of PA, having PA between 500 and 1500 MET-minutes/week predicted lower risk of new onset of TST < 6 hours/night, WASO > 60 minutes and sleep efficiency <80%. No significant associations were found between PA and either SOL or the percentage of TST in N1, N2, N3, or REM sleep, after adjustment for confounders.
The present findings are consistent with most available evidence regarding potential positive effects of PA on sleep. Previous systematic reviews [4–6, 25] have found that interventions based on PA can improve sleep; the degree of influence of PA varied according to individuals’ characteristics, exercise programs, and sleep parameters analyzed. For instance, King et al. [26] showed that, compared to controls, older adults who participated in a 12-month exercise program had fewer awakenings during the first third of the sleep period, but no differences were found for other PSG parameters examined. In a study by Passos et al. [27], adults with chronic insomnia with a mean age of 45 years who completed a 6-month exercise training protocol had a significant decrease in SOL and WASO, and a significant increase in sleep efficiency following exercise. In another trial with PSG measurements of adults between 57 and 70 years of age, Melancon et al. [28] observed a decrease in WASO and no change in SOL, TST, and SE after 16 weeks of an exercise program. These findings suggest that sleep parameters are variably influenced by PA. These results are consistent with our study, where we found that PA was significantly associated with lower risk of some but not all sleep disturbances.
To the best of our knowledge, ours is the first study to identify that higher levels of PA predict lower risk of PSG-assessed sleep disturbances in middle-aged and older adults. This is consistent with prospective studies based on subjective sleep information. PA was longitudinally associated with lower risk of subjective indicators of better sleep, including REM disturbance [29] and sleep onset and sleep maintenance problems [30]. Morgan et al. [10] followed older adults for 4–8 years and observed that lower PA levels were associated with a significantly elevated risk of both insomnia persistence and insomnia incidence. Likewise, Chen et al. [12] found that adults aged 65 years and over with a high level of PA at baseline who kept this high level after 10 years of follow-up had lower risk of insomnia than other groups. Similar results were found in another study of adults; after 3 years of follow-up, frequent PA was associated with reduced incidence of insomnia, especially difficulty maintaining sleep [11]. On the other hand, Tsunoda et al. [14] followed older adults for 3–4 years and found a lower incidence of subjective insufficient sleep in those with moderate-to-low PA at baseline, but no relationship was found between PA and incidence of self-reported short TST. In another prospective study, Holfeld and Ruthig [13] followed community-dwelling older adults for 2 years and showed that initial PA did not predict later sleep quality after accounting for prior sleep quality. The divergence between the results of these two last studies in relation to ours and other longitudinal studies might be explained, at least partially, by the limited agreement between subjective and objective sleep duration and quality [15, 16].
In our study, we found that an intermediate but not the highest PA level was significantly associated with lower risk of sleep disturbances, in contrast to some short-term clinical trials that found a dose-response association between exercise and sleep [31]. Indeed, in our study the relative risks were very similar in the two categories of higher PA, although only for the WASO > 60 minutes risk did they reach statistical significance in the group with the highest PA level. This could be due to the low statistical power to detect differences, as the frequency of most sleep disturbances was lower in the highest PA category in relation to the reference category. Moreover, baseline PA level did not predict changes in sleep parameters over ~5 years of follow-up for most of the sleep parameters, except for the percentage of TST in N3, where higher PA levels at baseline were associated with lower percentage of change in percentage of TST in N3, though with a small magnitude of effect. Changes in sleep were quite small and tended to be in the negative direction (e.g. increased SOL and decreased sleep efficiency). All results taken together, our data suggest that the relationship between PA and sleep is nonlinear, although these conclusions should be confirmed in future studies.
Regarding the temporality of the relationship between PA and sleep, in this cohort study we did not find a consistent association of baseline sleep with subsequent changes in PA. However, in the study of Holfeld and Ruthig [13], the authors found that better initial sleep quality predicted higher levels of later PA whereas the inverse association was no longer significant when accounting for prior sleep quality. Their results are also consistent with some evidence of a stronger association between sleep predicting next-day PA rather than PA predicting that night’s sleep [17, 18]. Future prospective studies based on objective sleep measurements are still needed to replicate the present findings and to address whether the association between PA and sleep is, in fact, bidirectional over a longer follow-up period.
Several mechanisms may underlie positive associations, if causal, of PA on sleep. These non-exhaustively include: thermoregulatory effects of exercise; sunlight exposure during outdoor PA; exercise-mitigated sleep apnea severity [32]; and, positive effects of PA on mood and stress [33–35]. During PA, body core temperature increases and peripheral temperature decreases [36]; the drop in core temperature subsequent to PA has been hypothesized to promote sleep propensity [37]. Furthermore, when PA occurs in external environments and during the daylight, sunlight exposure could increase melatonin secretion [38], a hormone directly linked to sleep-promoting effects. Also, exercise has been associated with improved mood [39], and depressed mood has been associated with worse future sleep quality in 10 of 13 studies detailed in a systematic review [40].
There are a few important limitations of the present study. First, although in-laboratory PSG is the gold-standard method for sleep evaluation, the laboratory context and PSG equipment can influence some sleep parameters so that they are systematically different from the habitual home setting. Also, we used only one night of sleep data at each study visit; this may be a study limitation because of potential night-to-night variability in sleep parameters. However, because we used as baseline sleep studies (for this analysis) PSG evaluations from 2004 and later, all included participants had had previous in-laboratory PSG evaluations (i.e. from WSC inception in 1988 up to 2003), and were thus at least partially habituated to the sleep laboratory environment. Furthermore, we have no reason to believe there would be substantial systematic variations in the difference in sleep parameters assessed in-laboratory vs. naturalistic environments among participants with higher and lower levels of PA. Second, although we used a validated questionnaire for estimation of energy expenditure in MET-minutes/week, self-reported PA is not a gold-standard assessment method and generally overestimates objectively-assessed PA [41]; if the degree of measurement error in PA at baseline was unrelated to risk of developing sleep problems, this would likely result in underestimates of true associations—i.e. a bias to the null between the highest level of baseline PA and sleep problems. Lastly, although residual confounding is possible, the present analyses controlled for several covariates, including sociodemographic, lifestyle and health conditions, as well as changes in PA and in BMI during the follow-up.
This study provides evidence consistent with a beneficial effect of PA on the risk of short sleep time, higher WASO, and lower sleep efficiency. These findings are in accordance with previously published results, supporting the promotion of PA to improve sleep quality and prevent sleep disturbances. In addition to other well-known health-related benefits of PA, PA might also be considered as a potential protective factor for sleep disturbances in middle-aged and older adults.
Supplementary material
Supplementary material is available at SLEEP online.
Funding
A.E.M. received a postdoctoral scholarship from CAPES – Brazilian Federal Agency for Support and Evaluation of Graduate Education within the Ministry of Education of Brazil (grant number 88881.119033/2016-01). This work was supported by US National Institutes of Health (NIH) grants R01HL62252, R01HL0750350, 1R01AG036838, and 1UL1RR02501.
Notes
Conflict of interest statement. None declared.
Acknowledgments
We thank the following people for their valuable support: Jodi Barnet, Laurel Finn, Amanda Rasmuson, Dr. F. Javier Nieto, Dr. Mari Palta, and Dr. Terry Young.
References
- 1. Hupin D, et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med. 2015;49(19):1262–1267. [DOI] [PubMed] [Google Scholar]
- 2. da Silva AA, et al. Sleep duration and mortality in the elderly: a systematic review with meta-analysis. BMJ Open. 2016;6(2):e008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Javaheri S, et al. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang PY, et al. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–163. [DOI] [PubMed] [Google Scholar]
- 5. Kredlow MA, et al. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–449. [DOI] [PubMed] [Google Scholar]
- 6. Dolezal BA, et al. Interrelationship between sleep and exercise: a systematic review. Adv Prev Med. 2017;2017:1364387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell JA, et al. No evidence of reciprocal associations between daily sleep and physical activity. Med Sci Sports Exerc. 2016;48(10):1950–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kline CE, et al. Consistently high sports/exercise activity is associated with better sleep quality, continuity and depth in midlife women: the SWAN sleep study. Sleep. 2013;36(9):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kline CE, et al. Associations of sedentary time and moderate-vigorous physical activity with sleep-disordered breathing and polysomnographic sleep in community-dwelling adults. Sleep Breath. 2017;21(2):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan K. Daytime activity and risk factors for late-life insomnia. J Sleep Res. 2003;12(3):231–238. [DOI] [PubMed] [Google Scholar]
- 11. Inoue S, et al. Does habitual physical activity prevent insomnia? A cross-sectional and longitudinal study of elderly Japanese. J Aging Phys Act. 2013;21(2):119–139. [DOI] [PubMed] [Google Scholar]
- 12. Chen LJ, et al. Prospective associations between different categories of physical activity and insomnia in older adults. Int J Sport Psychol. 2014;45(3):13. [Google Scholar]
- 13. Holfeld B, et al. A longitudinal examination of sleep quality and physical activity in older adults. J Appl Gerontol. 2014;33(7):791–807. [DOI] [PubMed] [Google Scholar]
- 14. Tsunoda K, et al. Prospective study of physical activity and sleep in middle-aged and older adults. Am J Prev Med. 2015;48(6):662–673. [DOI] [PubMed] [Google Scholar]
- 15. Landry GJ, et al. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. 2015;7:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva GE, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS). J Clin Sleep Med. 2007;3(6):622–630. [PMC free article] [PubMed] [Google Scholar]
- 17. Baron KG, et al. Exercise to improve sleep in insomnia: exploration of the bidirectional effects. J Clin Sleep Med. 2013;9(8):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambiase MJ, et al. Temporal relationships between physical activity and sleep in older women. Med Sci Sports Exerc. 2013;45(12):2362–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young T. Rationale, design and findings from the Wisconsin sleep cohort study: toward understanding the total societal burden of sleep disordered breathing. Sleep Med Clin. 2009;4(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Awad KM, et al. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125(5):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. [DOI] [PubMed] [Google Scholar]
- 22. Peppard PE, et al. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27(3):480–484. [DOI] [PubMed] [Google Scholar]
- 23. Paffenbarger RS, Jr, et al. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. [DOI] [PubMed] [Google Scholar]
- 24. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 25. Driver HS, et al. Exercise and sleep. Sleep Med Rev. 2000;4(4):387–402. [DOI] [PubMed] [Google Scholar]
- 26. King AC, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. 2008;63(9):997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Passos GS, et al. Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep Med. 2011;12(10):1018–1027. [DOI] [PubMed] [Google Scholar]
- 28. Melancon MO, et al. Sleep depth and continuity before and after chronic exercise in older men: electrophysiological evidence. Physiol Behav. 2015;140:203–208. [DOI] [PubMed] [Google Scholar]
- 29. Wong JC, et al. Risk factors for probable REM sleep behavior disorder: a community-based study. Neurology. 2016;86(14):1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hartescu I, et al. Sleep quality and recommended levels of physical activity in older people. J Aging Phys Act. 2016;24(2):201–206. [DOI] [PubMed] [Google Scholar]
- 31. Buman MP, et al. Exercise as a treatment to enhance sleep. Am J Lifestyle Med. 2010;4(6):500–514. [Google Scholar]
- 32. Iftikhar IH, et al. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Atkinson G, et al. Relationships between sleep, physical activity and human health. Physiol Behav. 2007;90(2–3):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dzierzewski JM, et al. Exercise and sleep in community-dwelling older adults: evidence for a reciprocal relationship. J Sleep Res. 2014;23(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chennaoui M, et al. Sleep and exercise: a reciprocal issue?Sleep Med Rev. 2015;20:59–72. [DOI] [PubMed] [Google Scholar]
- 36. Waterhouse J, et al. The circadian rhythm of core temperature: origin and some implications for exercise performance. Chronobiol Int. 2005;22(2):207–225. [DOI] [PubMed] [Google Scholar]
- 37. Gilbert SS, et al. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8(2):81–93. [DOI] [PubMed] [Google Scholar]
- 38. Obayashi K, et al. Positive effect of daylight exposure on nocturnal urinary melatonin excretion in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2012;97(11):4166–4173. [DOI] [PubMed] [Google Scholar]
- 39. Arent SM, et al. The effects of exercise on mood in older adults: a meta-analytic review. J Aging Phys Act. 2000;8:407–430. [Google Scholar]
- 40. Smagula SF, et al. Chronic disease and lifestyle factors associated with change in sleep duration among older adults in the Singapore Chinese Health Study. J Sleep Res. 2016;25(1):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Troiano RP, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.