Abstract
Pain is common among older adults and negatively impacts functioning. Sleep disturbances and mood disorders, specifically depression and anxiety, are closely associated with pain in older individuals, but the directionality of these associations remains unclear. In this study, we deconstruct long-term temporal effects of two key insomnia symptoms on incident pain into direct and indirect pathways, with focus on depression and anxiety symptoms, within a nationally representative sample. We utilized 2011–2013 data from the National Health and Aging Trends Study, a longitudinal survey of 2239 community-dwelling Medicare beneficiaries. Participants completed annual in-person interviews with assessments of sleep initiation and maintenance; depression, and anxiety (using the Patient Health Questionnaire-2 [PHQ-2] and the Generalized Anxiety Disorder Scale-2 [GAD-2] respectively); and bothersome pain. Causal mediation analysis was applied to examine direct effects of the two insomnia symptoms at baseline on incident pain, and their indirect effects through depression and anxiety symptoms. Almost one-third of the study participants were 69 years old or younger. A similar proportion reported bothersome pain in 2013. The two baseline insomnia symptoms predicted the development of pain. Adjusted analyses suggested that compared to older adults without the two baseline insomnia symptoms, participants with sleep initiation or maintenance difficulties had 24% (95% confidence interval [CI] 2%,51%) and 28% (95% CI 4%,55%) higher odds of incident pain, respectively. Anxiety symptoms partially mediated the relationship between the insomnia symptoms and incident pain, accounting for up to 17% of the total effect, but depressive symptoms did not. These results suggest that improved sleep or anxiety could reduce the risk for future pain.
Keywords: sleep initiation, sleep maintenance, insomnia, depression, anxiety, pain, mediation, causal mediation
Statement of Significance
A majority of older adults experience pain, a significant contributor to physical disability, falls, and mortality. Identification of long-term modifiable predictors of pain is critical to guide strategies to reduce the impact of pain and its consequences in older adults. We used longitudinal data from a nationally representative sample of older adults to examine the role of insomnia symptoms in the development of pain, and whether this relationship was mediated by depression or anxiety. Difficulties with sleep initiation or maintenance were common and increased the risk for the development of pain. Anxiety, but not depression, mediated the relationship between sleep problems and pain. Treating sleep disturbances and anxiety in older adults may reduce the risk of developing pain.
Introduction
Pain is exceptionally common among older adults (≥65 years of age), affecting more than half of this population [1] with a far-reaching impact. In older adults, physical disability, including inability to independently complete physical tasks of daily living, and slower walking speed, are strongly associated with pain [1]. Older adults who experience pain are prone to falls [2], and those with pain that interferes with their daily life have an increased risk of all-cause mortality [3]. As the number of older Americans grows exponentially, to an estimated 83.7 million individuals by 2050 [4], the development of strategies to reduce the impact of pain and its consequences in this population is critical.
Chronic sleep disturbances are also reported by a majority of older adults and may contribute to the development of pain [5]. The proportion of older adults who experience pain and at least one concomitant sleep complaint ranges from 26% to 77% [6, 7]. Further, older adults with chronic pain, in comparison to those without pain, are more likely to report clinically significant insomnia symptoms [8]. Although insomnia and pain are related, the nature of their relationship is complex. Recent studies suggest that sleep disturbances may ultimately predict pain more robustly than pain predicts sleep disturbances [9–14]. However, prior studies have often involved younger populations [15], without sufficient generalizability to older adults who have sleep disturbances, pain, and other age-related comorbidities.
Sleep disturbances are also significant prospective predictors of new-onset depression and anxiety in the general population [16, 17], and anxiety and depression predict pain [18]. The few studies that examined the relationship of depression and anxiety in the context of sleep-pain associations [15] suggested that psychiatric conditions such as depression may mediate the relationship between sleep and pain [19, 20]. Although the exact mechanisms that link sleep, mood, and pain are unknown, the mesolimbic dopamine system is implicated as a possible common pathway [21]. A model outlined by Finan and Smith in a recent review proposes complex, bidirectional relationships between the dopaminergic system and sleep disturbance, pain, and depression [21]. An imbalance between tonic and phasic dopamine (i.e. homeostatic dysregulation) is hypothesized to promote arousal during sleep, inhibit analgesia, and increase symptoms of depression such as anhedonia. In turn, insomnia, stress (a core feature of depression), and pain may further maladaptively remodel the dopaminergic system through feedback [21]. As many aspects of this bidirectional relationship remain unclear, studies that include assessment of mood disorders to elucidate their role in the relationship between sleep and pain are needed [21]. To date, only one prospective study has examined sleep disturbances and mood (i.e. depression and anxiety symptoms) as independent predictors of new onset pain in older adults, but did not investigate the potential role of mood disorders as mediators in the sleep and pain association [12].
The purpose of the current study was to identify longer-term temporal relationships between sleep disturbances, depression and anxiety symptoms, and pain in older adults. We applied a causal mediation analysis within a large, nationally representative sample of older Americans who were studied across a 3-year period and hypothesized that two key insomnia symptoms reported at baseline (2011) would predict incident pain 2 years later (2013). We also hypothesized that incident depressive and anxiety symptoms, assessed in 2012, would independently mediate the relationship between these insomnia symptoms and incident pain.
Methods
Study population and procedure
All study procedures were approved by the University of Michigan Institutional Review Board (IRBMED).
Data were obtained from the National Health and Aging Trends Study (NHATS), a nationally representative, longitudinal survey of Medicare beneficiaries designed to assess the impact of aging on long-term health, functional, and social outcomes (http://www.nhats.org/). The authors previously used this dataset to study the frequency of obstructive sleep apnea risk, assessment, and treatment in older adults [22]. In the present study, we utilized NHATS data on sleep, pain, mood, and health that were collected between 2011 and 2013. In 2013, NHATS respondents completed 5097 full interviews, corresponding to a response rate of 88%. After exclusion of participants with baseline pain in 2011, the final analytic sample included 2239 respondents, representative through survey weights of 14988380 older Americans.
Measures
Exposure: difficulties with sleep initiation and maintenance
Two questions about insomnia were included in the NHATS “health conditions” section. These questions were: “In the last month, (1) how often did it take you more than 30 minutes to fall asleep?” and (2) “on nights when you woke up before you wanted to get up, how often did you have trouble falling back to sleep?”. Possible responses for both questions were: “every night (7 nights/week)”, “most nights (5–6 nights/week”, “some nights (2–4 nights/week”, “rarely (<1 night/week”), and “never”. Respondents were classified as having difficulty in sleep initiation if they did not fall asleep within 30 minutes at least two to four times a week in the last month. Similarly, respondents were classified as having difficulty in sleep maintenance if they had trouble falling back to sleep, after waking up earlier than planned, at least two to four times per week in the last month. We analyzed sleep data that were collected at baseline (2011).
Mediators: depression and anxiety
The NHATS “health conditions” section included two reliable and validated instruments to screen for depression and anxiety: (1) The Patient Health Questionnaire-2 (PHQ-2), a validated two-item scale with good sensitivity and specificity for depression screening [23]; and (2) The Generalized Anxiety Disorder Scale-2 (GAD-2) instrument to screen for anxiety [24]. In the PHQ-2 instrument, NHATS respondents were asked: “over the last month, how often have you: (1) had little interest or pleasure in doing things; and (2) felt down, depressed, or hopeless”. The GAD-2 tool asked: “over the last month, how often have you: (1) felt nervous, anxious, or on edge; (2) been unable to stop or control worrying?” Possible Likert scale responses were: 0 = “not at all”, 1 = “several days”, 2 = “more than half the days”, 3 = “nearly every day”. We used 2012 data in accordance with the PHQ-2 criteria, to classify respondents to have depressive symptoms if their cumulative score for both items was 3 or more [23]. Similarly, NHATS 2012 respondents with a cumulative score of 3 or more for the two GAD-2 items were classified as positive for anxiety symptoms [25].
Outcome measure: incident pain
The “sensory impairments and symptoms” section of NHATS contained questions about bothersome pain. Respondents were asked, on an annual basis: “in the last month, have you been bothered by pain?” Incident bothersome pain was assigned to NHATS respondents who answered “yes” to this item in 2013. Participants with reported pain at baseline (2011) which could confound future temporal relationships between sleep and incident pain were excluded from the analytic sample.
Potential confounders
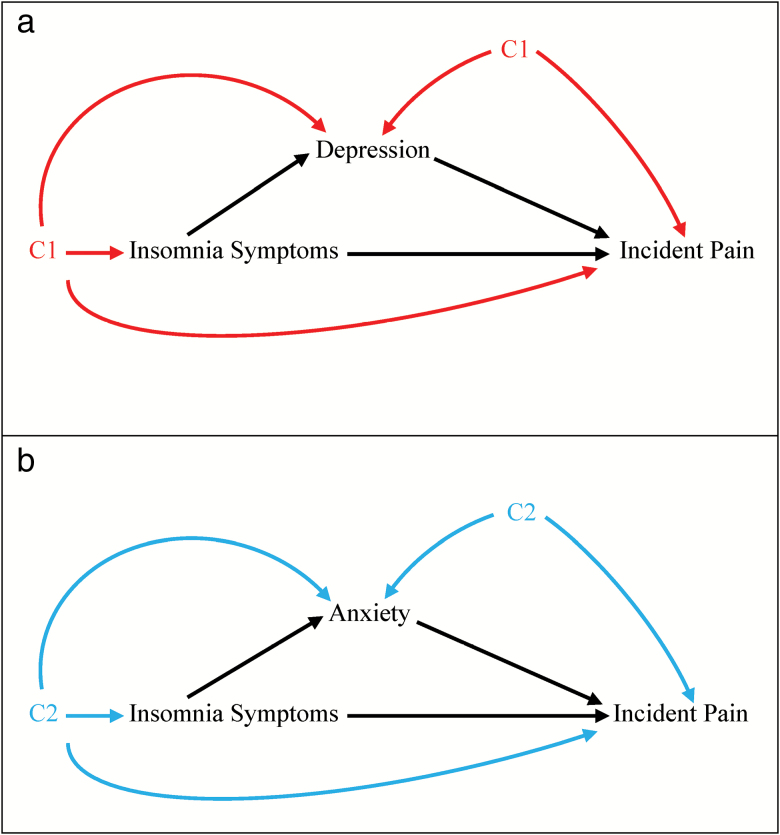
Baseline information on sociodemographic and prevalent health conditions that may be associated with symptoms of insomnia, depression, and anxiety, and predict incident pain in NHATS respondents were selected a priori as potential confounders using directed acyclic graphs (Figure 1, a and b) and previous literature [26, 27]. Potential confounders included sex, age, race, education, use of a mobility aid (cane, walker or wheelchair), marital or co-habitation status, cardiovascular disease, and diabetes.
Figure 1.
(a) Causal diagram representing potential mediation by depression symptoms of the association between key insomnia symptoms and incident pain. (b) Causal diagram representing potential mediation by anxiety symptoms of the association between key insomnia symptoms and incident pain. C2 = baseline confounders: age, race, education, sex, use of mobility device, marital status, and cardiovascular disease.
Statistical analysis
We conducted bivariate analysis to evaluate baseline predictors of incident pain, captured by NHATS in 2013. In this bivariate analysis, we estimated the proportion of older adults with incident pain, according to sociodemographic and health characteristics and used a modified Poisson regression approach to compute the risk ratios and 95% confidence intervals (CIs) of incident pain by these characteristics. Similar bivariate analyses were carried out between potential confounders and depression or anxiety symptoms data (mediators of interest), collected in 2012. In addition, we examined potential interaction between depression and anxiety with respect to incident pain.
We then performed causal mediation analysis using the “PARAMED” module in Stata 9.4 [28]. This allowed computation of total and direct effects of two insomnia symptoms and incident pain, in addition to their indirect effects through the pathways of depression or anxiety symptoms. We specified logistic regression models to account for the dichotomous outcome (incident pain), and dichotomous mediators (depression or anxiety symptoms, in separate models). In adjusted analyses, we included all potential confounders on the pathways between exposure-outcome, exposure-mediator, and mediator-outcome (Figure 1, a and b). These adjusted models did not include anxiety or depression at baseline (2011) because the insomnia symptoms and mood disorders were measured concurrently and as insomnia symptoms have been shown to precede mood disorders. For each model, we calculated the total, direct, and indirect effects and the bootstrapped 95% CI for those effects. If the indirect effects were statistically significant at p < 0.05, we also computed the proportion of the total effect that was mediated by depression or anxiety symptoms [29, 30]. Finally, we tested for the presence of an interaction between the two insomnia symptoms and anxiety symptoms, or between the two insomnia symptoms and depression symptoms, in relation to incident pain. As there was no evidence of significant interaction we used the module’s “nointeraction” option throughout our analyses. In all analyses, we incorporated survey weights to account for differential selection probabilities and adjust for potential non-response bias.
Finally, to explore potential confounding effects of baseline anxiety and depression on associations between sleep and subsequent mental health markers, we conducted sensitivity analyses in which we adjusted for anxiety and depression at baseline. We did not use this as our primary adjustment strategy as temporality could not be established (baseline sleep, anxiety, and depression were measured simultaneously).
Results
Approximately half of the sample was between the ages of 65 to 74 years, the majority were of white race/ethnicity, and half were female (Table 1). Approximately one-third of NHATS respondents, representing 4.7 million older Americans, reported incident pain in 2013. Bivariate models suggested that women, and those with cardiovascular disease, diabetes, hypertension, and limited mobility at baseline, were more likely to develop incident bothersome pain in 2013 (Table 1). With relation to pain, we did not find a significant interaction between depression and anxiety (p = 0.3). In 2011, the insomnia symptoms of difficulty with sleep initiation or maintenance were reported by one-third of NHATS respondents. In 2012, depressive symptoms and anxiety symptoms were prevalent among 8% and 14% of NHATS participants, accounting for 1.1 and 2 million older Americans, respectively. Women, African-Americans, participants who were single, older and without college education, and those with cardiovascular morbidity and limited mobility were more likely to report depression and anxiety symptoms (Table 2).
Table 1.
Risk of incident pain according to NHATS participants characteristics (n = 2239)
| Demographic and health characteristics | Overall sample | Bothered by pain | |||
|---|---|---|---|---|---|
| Unweighted frequencies (N) | Weighted proportion (%) | Unweighted frequencies (N) | Weighted proportion (%) | RR (95% CI) | |
| Pain (2013 data) | 717 | 32 | — | — | — |
| Sex | |||||
| Male | 1049 | 49 | 300 | 28 | 0.82 (0.73, 0.92) |
| Female | 1190 | 51 | 417 | 35 | Reference |
| Age | |||||
| 65–69 | 436 | 30 | 140 | 31 | Reference |
| 70–74 | 489 | 26 | 150 | 30 | 0.96 (0.78, 1.17) |
| 75–79 | 446 | 19 | 153 | 35 | 1.07 (0.88, 1.31) |
| 80–84 | 457 | 14 | 127 | 28 | 0.87 (0.69, 1.09) |
| 85–89 | 266 | 8 | 92 | 34 | 1.09 (0.89, 1.34) |
| 90+ | 145 | 3 | 55 | 37 | 1.18 (0.94, 1.49) |
| Race/ethnicity | |||||
| White | 1602 | 81 | 510 | 31 | Reference |
| African-American | 437 | 7 | 136 | 30 | 0.98 (0.81, 1.18) |
| Hispanic | 123 | 7 | 44 | 33 | 1.12 (0.73, 1.73) |
| Othera | 59 | 4 | 18 | 30 | 0.96 (0.67, 1.36) |
| Education | |||||
| Less than high school | 510 | 19 | 191 | 40 | 1.26 (1.05, 1.53) |
| High school | 584 | 26 | 183 | 29 | 1.05 (0.88,1.26) |
| Some college | 516 | 24 | 159 | 30 | 1.00 (0.81, 1.23) |
| College or higher | 608 | 30 | 181 | 32 | Reference |
| Married | |||||
| Yes | 1198 | 60 | 399 | 32 | 1.09 (0.96, 1.23) |
| No | 1037 | 39 | 317 | 30 | Reference |
| Body mass index | |||||
| Underweight | 45 | 2 | 13 | 28 | 0.93(0.56, 1.54) |
| Normal weight | 812 | 34 | 252 | 31 | Reference |
| Overweight | 885 | 41 | 260 | 29 | 0.95 (0.80,1.11) |
| Obese | 485 | 23 | 188 | 38 | 1.25 (1.05, 1.48) |
| Smokers | |||||
| Yes | 1098 | 51 | 338 | 30 | 0.93 (0.81, 1.07) |
| No | 1139 | 49 | 377 | 33 | Reference |
| Cardiovascular disease | |||||
| Yes | 607 | 25 | 220 | 37 | 1.19 (1.08, 1.32) |
| No | 1631 | 75 | 496 | 30 | Reference |
| Diabetes | |||||
| Yes | 454 | 19 | 165 | 37 | 1.18 (1.03, 1.35) |
| No | 1785 | 81 | 552 | 30 | Reference |
| Hypertension | |||||
| Yes | 1355 | 57 | 471 | 35 | 1.25 (1.11, 1.41) |
| No | 884 | 43 | 246 | 27 | Reference |
| Use mobility device | |||||
| Yes | 350 | 11 | 141 | 40 | 1.33 (1.16, 1.51) |
| No | 1888 | 89 | 576 | 30 | Reference |
aAsian, American Indian or Pacific Islander.
Table 2.
Risk of depressive and anxiety symptoms in 2012 according to NHATS participant characteristics (n = 2239)
| Demographic and health characteristics | Depressive symptoms n = 196; 8% | Anxiety symptoms n = 319; 14% | ||||
|---|---|---|---|---|---|---|
| Unweighted frequencies (N) | Weighted proportion (%) | RR (95% CI) | Unweighted frequencies (N) | Weighted Proportion (%) | RR (95% CI) | |
| Sex | ||||||
| Male | 73 | 7 | 0.66 (0.50, 0.88) | 101 | 9 | 0.53 (0.42, 0.66) |
| Female | 123 | 9 | Reference | 218 | 18 | Reference |
| Age | ||||||
| 65–69 | 26 | 6 | Reference | 47 | 11 | Reference |
| 70–74 | 42 | 8 | 1.44 (0.89, 2.32) | 63 | 13 | 1.19 (0.85, 1.67) |
| 75–79 | 31 | 6 | 1.19 (0.70, 2.05) | 65 | 14 | 1.35 (0.98, 1.85) |
| 80–84 | 48 | 10 | 1.76 (1.03, 2.99) | 70 | 16 | 1.41 (0.99, 2.01) |
| 85–89 | 29 | 12 | 1.88 (1.03, 3.43) | 45 | 18 | 1.63 (1.09, 2.43) |
| 90+ | 20 | 14 | 2.33 (1.24, 4.37) | 29 | 22 | 1.85 (1.20, 2.85) |
| Race/ethnicity | ||||||
| White | 113 | 7 | Reference | 220 | 13 | Reference |
| African-American | 56 | 12 | 1.76 (1.22, 2.54) | 58 | 14 | 0.94 (0.72, 1.24) |
| Hispanic | 19 | 16 | 2.17 (1.31, 3.57) | 31 | 26 | 1.83 (1.29, 2.57) |
| Othera | 6 | 8 | 1.40 (0.65, 3.04) | 8 | 13 | 0.97 (0.45, 2.13) |
| Education | ||||||
| Less than high school | 70 | 14 | 2.14 (1.39, 3.30) | 103 | 22 | 2.04 (1.57, 2.64) |
| High school | 45 | 7 | 1.18 (0.77, 1.80) | 87 | 15 | 1.50 (1.11, 2.02) |
| Some college | 38 | 7 | 1.16 (0.75, 1.80) | 67 | 13 | 1.32 (0.96, 1.82) |
| College or higher | 39 | 5 | Reference | 60 | 9 | Reference |
| Married | ||||||
| Yes | 83 | 7 | 0.64 (0.49, 0.83) | 151 | 12 | 0.77 (0.62, 0.95) |
| No | 113 | 10 | Reference | 168 | 16 | Reference |
| Body mass index | ||||||
| Underweight | 5 | 7 | 1.24 (0.64, 2.42) | 9 | 23 | 1.32 (0.69, 2.54) |
| Normal weight | 74 | 8 | Reference | 122 | 15 | Reference |
| Overweight | 72 | 7 | 0.92 (0.66, 1.27) | 126 | 14 | 0.95 (0.76, 1.17) |
| Obese | 43 | 9 | 0.97 (0.64, 1.48) | 59 | 12 | 0.81 (0.61, 1.08) |
| Smokers | ||||||
| Yes | 88 | 7 | 0.82 (0.66, 1.04) | 144 | 13 | 0.85 (0.71, 1.02) |
| No | 108 | 9 | Reference | 174 | 14 | Reference |
| Cardiovascular disease | ||||||
| Yes | 66 | 10 | Reference | 118 | 20 | 1.57 (1.29, 1.91) |
| No | 130 | 7 | 1.36 (0.99, 1.86) | 201 | 12 | Reference |
| Diabetes | ||||||
| Yes | 54 | 11 | 1.46 (1.14, 1.96) | 68 | 15 | 1.04 (0.82, 1.33) |
| No | 142 | 7 | Reference | 251 | 14 | Reference |
| Hypertension | ||||||
| Yes | 131 | 9 | 1.31 (0.96, 1.79) | 222 | 11 | 1.49 (1.20, 1.85) |
| No | 65 | 6 | Reference | 97 | 16 | Reference |
| Use mobility device | ||||||
| Yes | 53 | 15 | 2.02 (1.45, 2.81) | 73 | 22 | 1.57 (1.19, 2.07) |
| No | 142 | 7 | Reference | 246 | 13 | Reference |
aAsian, American Indian or Pacific Islander.
After controlling for sociodemographic characteristics and comorbidities, the direct effects at baseline of the two insomnia symptoms on incident pain were attenuated, although remained significant (Table 3). These results remained unchanged after additional adjustment for self-report health status. In adjusted analysis with depressive symptoms as a mediator, difficulty with sleep initiation and sleep maintenance at baseline predicted a 24% and a 28% increased odds of incident pain, respectively, but depressive symptoms did not mediate these effects. Similarly, in adjusted analysis with anxiety symptoms as a mediator, those who experienced difficulties with sleep initiation or sleep maintenance at baseline had an increased likelihood of 22% and 25% to develop pain, respectively. However, anxiety symptoms significantly mediated the pathway between sleep initiation and incident pain, and the pathway between sleep maintenance and incident pain, accounting respectively for 17% and 15% of the total effects (Table 3).
Table 3.
Mediation by depression or anxiety of the association between sleep difficulties in 2011 and incident pain in 2013
| Odds ratio (bootstrapped 95% CI) | Percentage of sleep association mediateda | |||
|---|---|---|---|---|
| Total sleep association | Direct sleep association | Indirect sleep association through mediator | ||
| Mediator: Depression | ||||
| >30 minutes to fall asleep | ||||
| Unadjustedb | 1.35 (1.12, 1.62) | 1.34 (1.11, 1.60) | 1.01 (1.00, 1.04) | 0% |
| Adjustedc | 1.25 (1.03, 1.51) | 1.24 (1.02, 1.51) | 1.00 (0.99, 1.51) | 0% |
| Difficulty falling back to sleep | ||||
| Unadjustedb | 1.33 (1.10, 1.60) | 1.33 (1.10, 1.60) | 1.01 (1.00, 1.03) | 0% |
| Adjustedc | 1.28 (1.04, 1.55) | 1.28 (1.04, 1.55) | 1.00 (1.00, 1.02) | 0% |
| Mediator: Anxiety | ||||
| >30 minutes to fall asleep | ||||
| Unadjustedb | 1.36 (1.12, 1.64) | 1.28 (1.05, 1.54) | 1.06 (1.03, 1.11) | 19% |
| Adjustedd | 1.26 (1.03, 1.52) | 1.22 (0.99, 1.48) | 1.04 (1.01, 1.08) | 17% |
| Difficulty falling back to sleep | ||||
| Unadjustedb | 1.36 (1.13, 1.64) | 1.28 (1.05, 1.56) | 1.06 (1.03, 1.12) | 19% |
| Adjustedd | 1.30 (1.07, 1.58) | 1.25 (1.03, 1.51) | 1.04 (1.01, 1.08) | 15% |
aCalculated as the natural logarithm of the indirect effect divided by the natural logarithm of the total effect.
bIncludes adjustment for survey weights.
cAdjusted for the following baseline variables: age, race, education, sex, use of mobility device, marital status, cardiovascular disease, diabetes, and survey weights.
dAdjusted for the following baseline variables: age, race, education, sex, use of mobility device, marital status, cardiovascular disease, and survey weights.
Discussion
This longitudinal, prospective, and nationally representative study shows that about one-third of older Americans experience at least one of two key insomnia symptoms, that in turn confer increased risk for new bothersome pain 2 years later. Depression was not found to mediate this relationship. However, a significant, indirect effect—through anxiety symptoms—accounted for 17% of the total effect of sleep initiation difficulty on incident bothersome pain, and 15% of the total effect of sleep maintenance difficulty on incident bothersome pain. These findings raise the possibility that two common insomnia symptoms, which are highly prevalent in older age, could contribute to the future experience of pain both directly and indirectly, through the pathway of anxiety.
The prevalence of pain and its impact on physical performance and function have previously been investigated on a cross-sectional basis within the NHATS population [31]. However, neither sleep disturbances nor anxiety were considered in the context of pain. Longitudinal studies that examined associations between sleep and pain have focused primarily on samples of younger to middle-aged adults. Two recent longitudinal studies showed that sleep problems at baseline were predictive of pain: insomnia predicted incident diagnosis of headache 11 years later [9]; and women who experienced sleep problems “often or always” vs. “never” at baseline were at a greater risk of incident fibromyalgia 10 years later [10]. In contrast, a 1-year follow-up study found that insomnia at baseline predicted the persistence of pain, but not incident pain [11].
Depression and anxiety may be particularly relevant to older adults, as these conditions are commonly comorbid with insomnia [32, 33] and pain [34, 35] in this population. Interestingly, our hypothesis that both depression and anxiety would mediate the relationship between sleep disturbances and pain was partially supported, as anxiety but not depression was shown to mediate this relationship. Associations between sleep, anxiety, and depression, outside of the context of pain, may be different among younger adults than in older adults. A recent population-based study of more than 2000 older adults found that anxiety was associated with many self-reported sleep complaints, whereas depression was associated only with the reported use of sleep medication. Thus, the sleep problems may be less salient for depression in older adults [36]. Our findings are largely consistent with previous reports about the influence of depression or anxiety on the sleep-pain association. Findings of studies that have examined the influence of depression alone have been mixed. A 6-year longitudinal study of 18- to 65-year-old adults showed that depression symptoms partially mediated the relationship between insomnia, short sleep duration and the development of chronic musculoskeletal pain [19]. Notably, several other studies suggest that sleep and pain are directly related, independent of depression. For example, a study of adult psychiatric patients with depression found that the sleep-pain relationship was not diminished by treatment of depression [37], and several studies showed a persistent relationship between sleep disturbances and pain after controlling for depression [38, 39]. Our finding that anxiety, but not depression, is more closely linked in the sleep-pain relationship is also further supported by the few studies that have considered the roles of anxiety and depression separately in the context of the sleep-pain relationship, wherein anxiety emerged a stronger contributor than depression, although these studies were not focused on older adults [37, 40]. Moreover, to our knowledge, only one other longitudinal study has examined depression, anxiety, and sleep as independent predictors of new-onset pain in a community-based sample of 4000 older adults in the United Kingdom. Consistent with our results, anxiety and non-restorative sleep predicted the new-onset of widespread pain, but depression did not [12].
Mechanisms by which anxiety, but not depression, could mediate the relationship between sleep disturbances and the development of new-onset pain in older adults are speculative. The pathophysiological underpinnings of chronic insomnia offer one possible explanation for why anxiety may be more critical to the sleep-pain relationship than depression. An initial acute period of sleep disturbance may lead to the development of hyperarousal (defined as increased somatic, cognitive, and cortical activation) [41, 42]. This activation can manifest in several ways, including, but not limited to, physiological hyperarousal (e.g. increased high-frequency electroencephalographic activity during sleep, enhanced processing of sensory information, increased muscle tension) and cognitive hyperarousal (e.g. increased worry, rumination, catastrophic thinking) [41]. Many of these manifestations (e.g. increased muscle tension, vigilance, worry, ruminative and catastrophic thinking) are core features of anxiety. In turn, aspects of physiological hyperarousal, such as heightened processing of somatosensory information, and increased muscle tension, could cause, or increase sensitivity to, pain. Indeed, within the theoretical framework proposed by Finan and Smith [21], dopamine serves to both initiate and maintain arousal, which is inherent in both insomnia and anxiety. Thus, dysregulation of the dopaminergic system could trigger insomnia and anxiety; over time, feedback to the system may lead to remodeling of dopaminergic receptor function such that vulnerability to pain is increased via diminished analgesia. More research, particularly using longitudinal designs, is necessary to fully disentangle relationships between sleep, anxiety, and pain in older adults, and to confirm anxiety as a more robust contributor relative to depression in the sleep-pain relationship in this population.
As insomnia is a treatable condition, efforts to understand its relationship to pain—through direct or indirect pathways—could offer new avenues to treat or prevent pain in older adults using sleep-based approaches. Indeed, cognitive-behavioral therapy for insomnia improves both sleep and pain in older adults with co-morbid chronic pain (osteoarthritis) and insomnia [43, 44].
This study has several strengths. First, we used a nationally representative sample of older Americans who were followed longitudinally with a high response rate (88%). These comprehensive data include demographic characteristics, daily activities, morbidities and health conditions of older Americans. Second, the analytic strategy employed contemporary causal mediation analysis to examine and quantify: (1) direct effects of two key insomnia symptoms on incident pain; and (2) indirect effects between those insomnia symptoms and incident pain through anxiety and depression symptoms. These analyses allow identification of pathways to incident pain, a prevalent morbidity in older adults. Third, to ensure temporality, we first excluded study participants with baseline pain, then identified positive responses for exposure, mediator, and outcome variables over consecutive years: 2011, 2012, and 2013, respectively. Fourth, we used validated scales for screening of anxiety and depression. These scales have demonstrated good validity and sensitivity in identifying depression [45] and generalized anxiety disorder [24] in older adults.
We also acknowledge some limitations. By design, NHATS focuses on community-dwelling adults; those in residential care were excluded. However, census data from 2014 showed that the majority of older Americans (87.6%) remained living in their communities [46]. The self-reported reporting of bothersome pain using a single question may be an additional limitation, but a previous investigation using these data provided evidence of criterion and face validity for this particular question [1]. Similarly, sleep was assessed using two self-report questions about sleep onset and sleep maintenance, as data regarding objective sleep measures captured with polysomnography were not available. Nonetheless, sleep restriction and sleep disruption have been associated with pain in experimental studies [15]. A further concern was the potential bi-directional associations between insomnia and mood disorders. To address this concern, we conducted sensitivity analysis by adding mood disorders at baseline to the adjusted models. While the effect estimates were slightly attenuated, the mediated anxiety pathway persisted. Finally, in some populations, sleep, mood, and pain may fluctuate or exhibit tighter temporal relationships over shorter time intervals, and the annual collection of these data—per the study design—may have resulted in more conservative estimates between these exposures and the outcome. However, we believe that any measurement error is likely random and therefore may attenuate the associations and produce conservative effect estimates.
In summary, these data, which deconstruct the total effect of two key insomnia symptoms on incident pain into direct and indirect pathways within a causal framework, provide new evidence that insomnia could contribute to incident pain in older Americans, and that anxiety symptoms may in part mediate this relationship. Our findings raise the possibility that efforts to improve sleep, reduce anxiety, or accomplish both could serve as strategies to manage pain in older adults.
Funding
G.L.D. reports grants from National Institute of Neurological Disorders and Stroke (NIH/NINDS T32 NS007222), and grants from National Institute of Child Health and Human Development (NIH/NICHD F32 HD091938), during the conduct of the study. L.M.S. reports grants from National Heart, Lung, and Blood Institute (K23 HL122461), during the conduct of the study. E.C.J. reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases (5T32 DK071212-12), during the conduct of the study. R.D.C. reports grants from NIH during the conduct of the study; his institution and collaborators have received grant support from the American Sleep Medicine Foundation; grant support from the National Multiple Sclerosis Society; non-financial support and other from Michigan Medicine, other from International Pediatric Sleep Association, personal fees and non-financial support from American Academy of Sleep Medicine, personal fees and non-financial support from American Academy of Dental Sleep Medicine, personal fees from UpToDate, personal fees from Cambridge University Press, non-financial support from Association of Professional Sleep Societies, outside the submitted work.
Conflict of interest statement. In addition, R.D.C. developed questionnaires for childhood sleep problems (PSQ, PSQ-SRBD Scale), copyright owned by University of Michigan with royalties paid to Zansors, and patents or patents pending, for technology, tools, or agents relevant to diagnosis and treatment of sleep disorders. L.M.O. has nothing to disclose. L.D.L. has nothing to disclose. T.J.B.’s research is supported by the American Sleep Medicine Foundation (115-SR-15), National Multiple Sclerosis Society, the Patient Centered Outcomes Research Institute (PCORI), Sanofi-Genzyme, and Genentech-Roche. In addition, T.J.B. has a patent concerning a treatment for sleep apnea pending.
References
- 1. Patel KV, et al. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154(12):2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel KV, et al. High prevalence of falls, fear of falling, and impaired balance in older adults with pain in the United States: findings from the 2011 National Health and Aging Trends Study. J Am Geriatr Soc. 2014;62(10):1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith D, et al. Pain and mortality in older adults: the influence of pain phenotype. Arthritis Care Res (Hoboken). 2018;70(2):236–243. [DOI] [PubMed] [Google Scholar]
- 4. Ortman JM, et al. An aging nation: the older population in the United States. In: Bureau USC, ed. Current Population Reports. Washington, DC: 2014. [Google Scholar]
- 5. Foley DJ, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. [DOI] [PubMed] [Google Scholar]
- 6. Lindstrom V, et al. Prevalence of sleep problems and pain among the elderly in Sweden. J Nutr Health Aging. 2012;16(2):180–183. [DOI] [PubMed] [Google Scholar]
- 7. Blay SL, et al. Chronic painful physical conditions, disturbed sleep and psychiatric morbidity: results from an elderly survey. Ann Clin Psychiatry. 2007;19(3):169–174. [DOI] [PubMed] [Google Scholar]
- 8. Dragioti E, et al. Insomnia severity and its relationship with demographics, pain features, anxiety, and depression in older adults with and without pain: cross-sectional population-based results from the PainS65+ cohort. Ann Gen Psychiatry. 2017;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Odegård SS, et al. The long-term effect of insomnia on primary headaches: a prospective population-based cohort study (HUNT-2 and HUNT-3). Headache. 2011;51(4):570–580. [DOI] [PubMed] [Google Scholar]
- 10. Mork PJ, et al. Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis Rheum. 2012;64(1):281–284. [DOI] [PubMed] [Google Scholar]
- 11. Jansson-Fröjmark M, et al. Bidirectionality between pain and insomnia symptoms: a prospective study. Br J Health Psychol. 2012;17(2):420–431. [DOI] [PubMed] [Google Scholar]
- 12. McBeth J, et al. Predictors of new-onset widespread pain in older adults: results from a population-based prospective cohort study in the UK. Arthritis Rheumatol. 2014;66(3):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edwards RR, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137(1):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dzierzewski JM, et al. Daily variations in objective nighttime sleep and subjective morning pain in older adults with insomnia: evidence of covariation over time. J Am Geriatr Soc. 2010;58(5):925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finan PH, et al. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baglioni C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1-3):10–19. [DOI] [PubMed] [Google Scholar]
- 17. Pigeon WR, et al. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44. [DOI] [PubMed] [Google Scholar]
- 18. Gerrits MM, et al. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64–70. [DOI] [PubMed] [Google Scholar]
- 19. Generaal E, et al. Insomnia, sleep duration, depressive symptoms, and the onset of chronic multisite musculoskeletal pain. Sleep. 2017;40. [DOI] [PubMed] [Google Scholar]
- 20. O’Brien EM, et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010;26(4):310–319. [DOI] [PubMed] [Google Scholar]
- 21. Finan PH, et al. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17(3):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braley TJ, et al. Recognition and diagnosis of obstructive sleep Apnea in older Americans. J Am Geriatr Soc. 2018. doi: 10.1111/jgs.15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kroenke K, et al. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. [DOI] [PubMed] [Google Scholar]
- 24. Wild B, et al. Assessing generalized anxiety disorder in elderly people using the GAD-7 and GAD-2 scales: results of a validation study. Am J Geriatr Psychiatry. 2014;22(10):1029–1038. [DOI] [PubMed] [Google Scholar]
- 25. Kroenke K, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–325. [DOI] [PubMed] [Google Scholar]
- 26. Rashedi V, et al. Mental health and pain in older adults: findings from Urban HEART-2. Community Ment Health J. 2017;53(6):719–724. [DOI] [PubMed] [Google Scholar]
- 27. Leggett A, et al. The effect of sleep disturbance on the association between chronic medical conditions and depressive symptoms over time. Longit Life Course Stud. 2017;8(2):138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emsley R. PARAMED: Stata Module to Perform Causal Mediation Analysis Using Parametric Regression Models. Statistical Software Components 2013. [Google Scholar]
- 29. Baglioni C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 30. Pigeon WR, et al. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44. [DOI] [PubMed] [Google Scholar]
- 31. Patel KV, et al. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154(12):2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Almeida OP, et al. Sleep complaints among older general practice patients: association with depression. Br J Gen Pract. 2005;55(520):864–866. [PMC free article] [PubMed] [Google Scholar]
- 33. Brenes GA, et al. Insomnia in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2009;17(6):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharpe L, et al. Pain severity predicts depressive symptoms over and above individual illnesses and multimorbidity in older adults. BMC Psychiatry. 2017;17(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feeney SL. The relationship between pain and negative affect in older adults: anxiety as a predictor of pain. J Anxiety Disord. 2004;18(6):733–744. [DOI] [PubMed] [Google Scholar]
- 36. Potvin O, et al. Subjective sleep characteristics associated with anxiety and depression in older adults: a population-based study. Int J Geriatr Psychiatry. 2014;29(12): 1262–1270. [DOI] [PubMed] [Google Scholar]
- 37. Chung KF, et al. Relationship between insomnia and pain in major depressive disorder: a sleep diary and actigraphy study. Sleep Med. 2010;11(8):752–758. [DOI] [PubMed] [Google Scholar]
- 38. Wilson KG, et al. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18(2):77–83. [DOI] [PubMed] [Google Scholar]
- 39. Vgontzas A, et al. Are sleep difficulties associated with migraine attributable to anxiety and depression?Headache. 2008;48(10):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boardman HF, et al. Psychological, sleep, lifestyle, and comorbid associations with headache. Headache. 2005;45(6):657–669. [DOI] [PubMed] [Google Scholar]
- 41. Levenson JC, et al. The pathophysiology of insomnia. Chest. 2015;147(4):1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riemann D, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. [DOI] [PubMed] [Google Scholar]
- 43. Vitiello MV, et al. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- 44. Vitiello MV, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014;155(8): 1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li C, et al. Validity of the patient health questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596–602. [DOI] [PubMed] [Google Scholar]
- 46. Freedman VA, et al. Making National Estimates with the National Health and Aging Trends Study. NHATS Technical Paper #17. Johns Hopkins University School of Public Health, 2016. [Google Scholar]