Abstract
Background and aim
Limited information is currently available about whether carotid intima-media thickness (IMT) correlates with the degree of disease activity in spondyloarthritis. The objective of this study was to evaluate the correlation between articular and carotid ultrasound data and laboratory and clinical variables in patients with spondyloarthritis.
Methods
Twenty-two patients with spondyloarthritis, recruited consecutively via the spondyloarthritis service of the Universidade Pontifícia Católica de Campinas, São Paulo, Brazil, were assessed using carotid artery ultrasound (radiofrequency quality intima-media thickness, RF-QIMT), joint ultrasound, clinical evaluation, and laboratory tests.
Results
Mean (standard deviation, SD) carotid RF-QIMT was 0.643 (0.16) mm. Mean (SD) resistive index (RI) values for the right and left carotid arteries were 0.67 (0.12) and 0.82 (0.38), respectively. Mean (SD) RI values for the right and left sacroiliac joints were 1.10 (0.97) and 0.94 (0.13), respectively. Several significant correlations were detected between ultrasound, clinical, and laboratory variables. Notably, there were correlations between sacroiliac RI and erythrocyte sedimentation rate (p=0.027) and RF-QIMT (p=0.037); between RF-QIMT and Framingham score (p=0.012) and metabolic parameters, including abdominal waist measurement, body mass index (BMI) (p=0.032 to p=0.044).
Conclusions
In patients with spondyloarthritis, RF-QIMT detected atherosclerotic changes in the carotid artery wall, and spectral Doppler detected inflammatory activity in sacroiliac joints. Positive correlations were observed between these ultrasound findings and parameters reflecting patients’ metabolic profile and alterations in inflammatory markers.
Keywords: atherosclerosis, carotid artery, inflammation, sacroiliac joint, spectral Doppler, spondyloarthritis, ultrasonography
Introduction
The spondyloarthritides are rheumatologic inflammatory diseases of the axial skeleton. A key feature of these disorders is sacroiliitis, which may be one-sided or bilateral. Patients with seronegative ankylosing spondylitis show an abnormal increase in vascularization within the sacroiliac joint, which can be observed using ultrasound, with a decrease in the internal resistive index (RI) of the blood vessels, providing a quantitative indication that the disease is in its active phase.1 A study involving patients with ankylosing spondylitis demonstrated blood flow with an average RI of 0.56 among 205 sacroiliac joints, which corresponded to 90.7% of sacroiliitis detected by spectral Doppler and 37 (38.5%) sacroiliac joints with subclinical inflammatory activity.2 In another study, the average RI was 0.97 in a control group of healthy individuals, compared to 0.53 in patients with ankylosing spondylitis,3 illustrating the correlation between reduced RI and increased inflammatory activity in the disease, which has also been demonstrated in other studies.1,2,4–6
Nowadays, nuclear magnetic resonance imaging (MRI) is used frequently for the radiologic diagnosis of this disease and to detect early regressive lesions, with X-ray used for later lesions. However, ultrasound is used widely within the rheumatology field to detect articular inflammatory alterations.7,8
In addition to joint inflammation, patients with spondyloarthritis experience systemic inflammation, including atherosclerosis.9 They have an increased risk of cardiovascular events10 and can develop inflammation of large blood vessels, including the aorta.11 Increased carotid intima-media thickness (IMT) is a common marker of atherosclerotic involvement of the vascular structure, thereby indicating coronary artery disease, cerebrovascular disease, and peripheral arterial disease. Carotid IMT has received increasing attention due to its role as an independent prognostic factor for chronic kidney disease, diabetes, hypertension, and systemic inflammatory diseases such as systemic lupus erythematosus, Behçet’s disease, and psoriasis.12,13 Indeed, several studies have found that carotid IMT is increased in patients with spondyloarthritis even in the absence of classical cardiovascular risk factors.9,14,15 However, little information is currently available about whether carotid IMT correlates with the degree of disease activity in spondyloarthritis, though one small study did find a positive correlation.14 The objective of the current study was to evaluate correlations between articular and carotid ultrasound data and laboratory and clinical variables in patients with spondyloarthritis. To the best of our knowledge, the current study is the first to evaluate new ultrasonographic variables including an evaluation of automated measurement of the medial and intimal layer by radiofrequency and the correlation with other variables such as sacroiliac and carotid RI.
Methods
Patients and study design
The study enrolled consecutive patients from the rheumatology outpatient clinic who had spondyloarthritis, which was classified using the Assessment of SpondyloArthritis international Society (ASAS) criteria for axial spondyloarthritis. In addition to meeting ASAS criteria, patients had to have low density lipoprotein cholesterol (LDL) levels below 130 mg/dL, in line with the Modified National Cholesterol Education Program (2004) guidelines.16,17 Patients with other inflammatory pathologies or hypothyroidism were excluded from the study.
Patients were evaluated at the PUC-Campinas Ambulatory Rheumatology Center. Ethical approval was obtained for the study protocol, and all patients provided written informed consent.
Patients underwent clinical assessment of their spondyloarthritis, laboratory parameters reflecting their metabolic profile, and their cardiovascular risk. They also underwent ultrasound evaluation of the sacroiliac joints, common carotid artery, and calcaneus (Achilles tendon).
Clinical assessments
Disease functionality was evaluated using the Bath Ankylosing Spondylitis Functional Index (BASFI) and disease activity using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the ankylosing spondylitis activity score (ASDAS).17,18
Laboratory tests included measurement of the erythrocyte sedimentation rate (ESR), C-reactive protein level, lipid profile (including total cholesterol, LDL, high density lipoprotein cholesterol [HDL] and triglycerides), fasting glucose level, and uric acid level. In addition, homocysteine was measured in some patients. Homocysteine is a sulfur-containing amino acid formed from demethylation of the essential amino acid methionine.19 It is considered a marker of blood vessel injury, which can lead to atherogenesis and thrombosis. The relationship between hyperhomocysteinemia and blood vessel damage was originally proposed in 1969, and since then several studies have shown that it is an independent risk factor for the development of coronary disease.20–22 Due to budget constraints, HLA-B27 testing was not performed.
Patients’ cardiovascular risk was assessed using anthropometric data including abdominal waist measurement and body mass index (BMI) and the Framingham score.
Ultrasound assessments
Ultrasonography assessments of the common carotid artery, sacral and internal iliac arteries, sacroiliac joint, and Achilles tendon were performed in blinded fashion by a rheumatologist with 9 years of experience in ultrasonography.
The ultrasound machine used was an Esaote – MyLab 50 model. The calcaneus and sacroiliac joint assessments used mode B linear probes of frequency 6.0–18 MHz and power Doppler frequency of 6.6–8 MHz with Pulse Repetition Frequency that varied from 0.5 Hz to 1.0 MHz, and low filter. For the carotid assessments, a linear probe with 3.5–10 MHz frequency, Doppler 3.3–5.0 MHz, and average filter were used. The parameters used for carotid and articular spectral RI were based on Doppler frequencies of 3.3 and 8.0 MHz, respectively. The angulation to calculate the common carotid artery RI was less than 60 degrees. IMT and the carotid average IMT were determined using automatic software that assesses radiofrequency quality intima-media thickness (RF-QIMT). This software provides, in real time, a measurement of the average of six mean values obtained during six consecutive cardiac cycles. IMT was measured in the wall of the most distal 15 mm length of the common carotid artery, 10 mm from the distal extremity, just proximal to the bifurcation. According to age-categorized values (based on the standard deviation for a sample population aged ≥25 years), mean RF-QIMT values of between 0.4 and 0.65 mm are considered normal (expected QIMT), between 0.65 and 0.75 mm are abnormal, and between 0.75 and 1.5 mm are very abnormal.23
Bilateral assessments of the sacroiliac joints, Achilles tendons, and carotid arteries were performed. The probe position used to assess the sacroiliac joints was cross-sectional, from the superior iliac crest to the inferior iliac crest (Figure 1). To assess the Achilles tendon, the probe position was longitudinal, in the calcaneal posterior recess. For the evaluation of the common carotid artery, the probe began transversely in the direction of the thyroid gland and was set longitudinally (Figure 2). Measurements in the sacroiliac gray-scale scan were accomplished by tracing a line between the recess of the cortical bones from the iliac and sacral crest and a line to the sacrum area in order to calculate the polygon area. The Achilles tendon assessment was included because structural alterations such as thickening are common in patients with enthesopathy due to spondyloarthritis.24
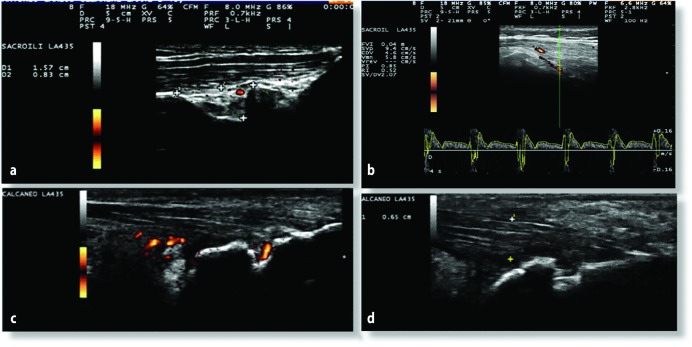
Figure 1.
(a) Sacroiliac: D1 (measurement between the cortical bones of iliac crest and sacrum)/D2 (measurement in inferior iliac crest from D1, to evaluate the bottom of sacrum recess). Area = 1.2 cm2; (b) Sacroiliac: RI = 0.52; (c) and (d) Achilles tendon: PD+, enthesopathy and active erosion, 0.65 cm (normal: 0.43 cm).
PD, power Doppler; RI, resistive index.
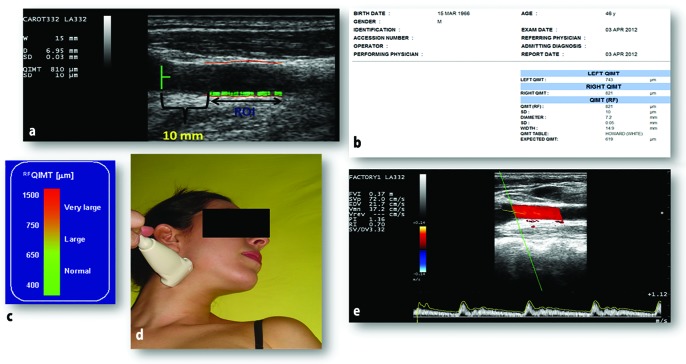
Figure 2.
(a) Evaluation of altered RF-QIMT of left carotid equal to 0.810 mm; (b) RF-QIMT Report; (c) QIMT alteration values in μm; (d) Probe position to evaluate the longitudinal incision; (e) Carotid from a patient without stenosis and with preserved hemodynamics, with RI = 0.70.
RF-QIMT, radiofrequency quality intima-media thickness; RI, resistive index
Ultrasound parameters obtained for this study included carotid RI and sacroiliac RI, carotid RF-QIMT, carotid diameter, sacral area measurement, and Achilles tendon measurement. The presence of carotid plaques was also evaluated.
Statistical methods
A descriptive evaluation of the study sample and analysis of parametric and nonparametric variables was performed, with data presented as mean (standard deviation, SD) or percentages. Spearman’s and Pearson’s correlation analyses were performed to identify significant relationships between ultrasound, clinical, and laboratory parameters. Statistical significance was indicated by p<0.05. Statistical analysis was performed using SPSS17 software.
Results
A total of 22 patients were evaluated, of whom 27.27% had antero-posterior sacroiliac X-ray evidence of spondyloarthritis, of either degree 3 (articular space reduction and sclerosis) or degree 4 (ankylosis), while 72.72% had sacroiliac MRI evidence (based on weighted sequences in T1 and T2 or short-time inversion recovery), with subchondral bone marrow edema. Three patients had psoriatic arthritis as well as axial involvement.
Patients’ demographic and disease characteristics are summarized in Table 1. Most were male (63.6%) and Caucasian (86.4%). The average age of the patients was just over 38 years, and the mean duration of inflammatory back pain was 8 years. Mean (SD) scores for the BASFI, BASDAI and ASDAS were 4.69 (2.30), 4.41 (2.00), and 2.51 (0.68), respectively. The most common treatment being received for spondyloarthritis was anti-tumor necrosis factor therapy (45.45%). Mean values for parameters reflecting patients’ metabolic/cardiovascular profile were generally within the normal range (Table 2).
Table 1.
Demographics and spondyloarthritis disease characteristics (n=22).
| Parameter | Value, mean (SD) unless indicated otherwise |
|---|---|
|
| |
| Sex (male/female), n (%) | 14 (63.6)/8 (36.4) |
|
| |
| Race, n (%) | |
| Caucasian | 19 (86.36) |
| African | 3 (13.63) |
|
| |
| Age (years) | 38.36 (9.48) |
|
| |
| Duration of inflammatory back pain (years) | 8 (7.18) |
|
| |
| BASFI score | 4.69 (2.30) |
|
| |
| BASDAI score | 4.41 (2.00) |
|
| |
| ASDAS score | 2.51 (0.68) |
|
| |
| C-reactive protein (mg/dL) | 1.59 (2.78) |
|
| |
| Erythrocyte sedimentation rate (mm/h) | 20.16 (21.90) |
|
| |
| Treatment for spondyloarthritis, n (%) | |
| Anti-tumor necrosis factor therapy | 10 (45.45) |
| Methotrexate | 4 (18.18) |
| Non-hormonal anti-inflammatory agents | 4 (18.18) |
| No treatment | 4 (18.18) |
ASDAS, ankylosing spondylitis activity score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; SD, standard deviation.
Table 2.
Metabolic and cardiovascular parameters (n=22).
| Parameter | Value, mean (SD) |
|---|---|
|
| |
| Abdominal waist measurement (cm) | 84.28 (12.12) |
|
| |
| Body mass index (kg/m2) | 24.82 (4.70) |
|
| |
| Systolic/diastolic blood pressure (mmHg) | 119.77 (14.26)/77.72 (9.84) |
|
| |
| Homocysteine (μmol/L) | 10.44 (4.15) |
|
| |
| Lipids (mg/dL) | |
| Total cholesterol | 178.60 (28.18) |
| High density lipoprotein cholesterol | 51.10 (16.18) |
| Low density lipoprotein cholesterol | 108.60 (22.25) |
| Triglycerides | 92.85 (43.64) |
|
| |
| Fasting glucose (mg/dL) | 94.47 (16.33) |
|
| |
| Uric acid (mg/dL) | 5.08 (1.48) |
|
| |
| Framingham score | 4.97 (6.25) |
SD, standard deviation.
Parameters derived from ultrasound measurements of the carotid artery, sacroilium, and Achilles tendon are shown in Table 3. Overall, mean RI values for both sacroiliac joints were above 1.0, whereas mean RI values for the carotid arteries were below 1.0. Only 14 of 44 sacroiliac joints had a low RI (mean RI 0.71). Mean (SD) carotid RF-QIMT, 0.643 (0.16) mm, was higher than the expected age-standardized QIMT, 0.529 (0.10) mm. There was no evidence of carotid plaques.
Table 3.
Carotid artery, sacroilium, and Achilles tendon ultrasound parameters (n=22).
| Parameter | Value, mean (SD) |
|---|---|
|
| |
| Carotid artery | |
|
| |
| RF-QIMT (mm) | 0.643 (0.16) |
|
| |
| Expected QIMTa (mm) | 0.529 (0.10) |
|
| |
| Carotid diameter (mm) | 6.98 (0.82) |
|
| |
| cRI (R) | 0.67 (0.12) |
| cRI (L) | 0.82 (0.38) |
|
| |
| Sacroilium | |
|
| |
| sRI (R) | 1.10 (0.97) |
| sRI (L) | 0.94 (0.13) |
|
| |
| AsGs (R) (cm2) | 1.01 (0.60) |
| AsGs (L) | 1.14 (0.48) |
|
| |
| Achilles tendon | |
|
| |
| TGS (R) (cm) | 0.42 (0.11) |
| TGS (L) | 0.46 (0.12) |
Expected QIMT according to age-categorized values (based on the standard deviation for a sample population aged ≥25 years): mean values of 0.4–0.65 mm is normal.
AsGs, sacral area measurement; cRI, carotid RI; L, left; QIMT, quality intima-media thickness; R, right; RF, radiofrequency; RI, resistive index; SD, standard deviation; sRI, sacroiliac RI; TGS, Achilles tendon measurement.
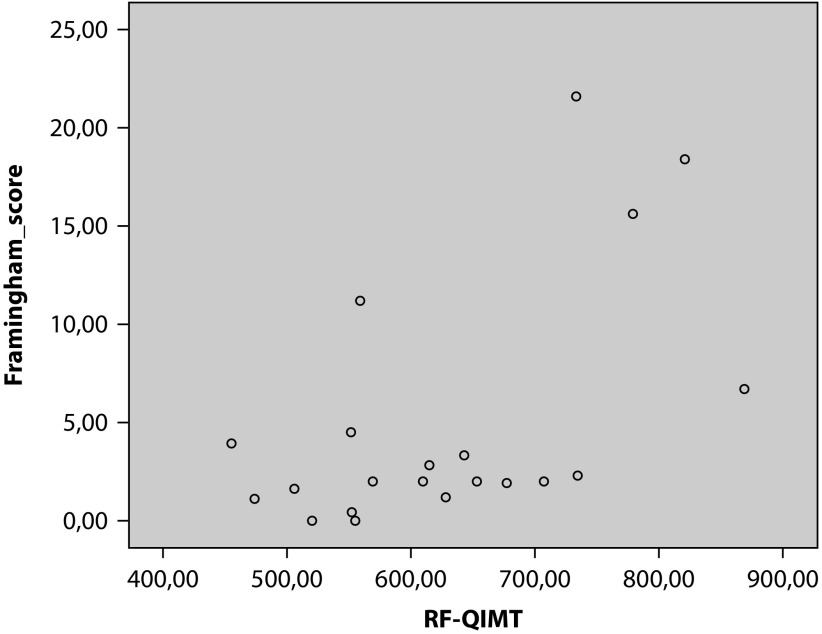
Significant correlations between parameters are summarized in Table 4. There was no statistically significant correlation between ultrasound parameters and disease activity scores (BASDAI/ASDAS). Correlations were found between sacroiliac RI and ESR and between sacroiliac RI and Framingham score. Expected QIMT (but not RF-QIMT) correlated with Framingham score (Figure 3). There were correlations between sacral area and both sacroiliac RI and carotid RI, and between sacral area and various metabolic parameters (abdominal waist measurement, BMI, total cholesterol, and triglycerides). In addition, as expected, there was a statistically significant correlation between QIMT and age (r=0.580; p=0.005); there was no statistically significant correlation between QIMT and disease duration or triglycerides.
Table 4.
Significant correlations found between parameters using Spearman’s and Pearson’s Correlation analysis.
| Parameters | r (p-value) |
|---|---|
| BASDAI and ASDAS | 0.635 (p=0.01) |
| BASFI and homocysteine | 0.738 (p=0.037) |
| BASFI and BMI | 0.556 (p<0.01) |
| RF-QIMT and sRI (L): | 0.482 (p=0.037) |
| RF-QIMT and Framingham score | 0.537 (p=0.012) |
| Expected QIMT and Framingham score | 0.915 (p<0.01) |
| sRI (R) and ESR | 0.506 (p=0.027) |
| sRI (R) and cRI (R) | 0.816 (p<0.01) |
| cRI (R) and cRI (L) | 0.847 (p<0.01) |
| AsGs (L) and AsGs (R) | 0.733 (p<0.01) |
| AsGs (L) and abdominal waist measurement | 0.381 (p=0.044) |
| AsGs (L) and BMI | 0.459 (p=0.032) |
| TGS (L) and TGS (R) | 0.518 (p=0.016) |
ASDAS, ankylosing spondylitis activity score; AsGs, sacral area measurement; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BMI, body mass index; cRI, carotid RI; ESR, erythrocyte sedimentation rate; L, left; QIMT, quality intima-media thickness; R, right; RF, radiofrequency; RI, resistive index (decrease); sRI, sacroiliac RI; TGS, Achilles tendon measurement.
Figure 3.
Correlation between radiofrequency quality intima-media thickness (RF-QIMT) and Framingham score.
Discussion
Spectral Doppler ultrasound can demonstrate the active phase of the inflammatory process in sacroiliitis.1 A reduced RI value indicates a reduction in the resistance to blood flow within vessels, reflecting inflammation.25,26 The RI is high outside of the period of active inflammation and in healthy people.1,2 Spectral Doppler ultrasound can also help in evaluating the response to therapy; along with an improvement in clinical symptoms, an increase in RI indicates a successful response.27–29
To the best of our knowledge, the current study is the first to present new ultrasonographic variables including an evaluation of automated measurement of the medial and intimal layer by radiofrequency and the correlation with other variables such as sacroiliac and carotid resistance. Unfortunately, in the current study, sacroiliac joint inflammation was not marked, because only 14 sacroiliac joints had a low RI, with a mean value of 0.71 (below the value of 0.97 reported for healthy volunteers in the literature1). Nonetheless, a significant correlation was found between sacroiliac RI and ESR. In the future, we hope to extend our investigations to a larger sample of patients with higher activity or in patients with/without sacroiliac bone edema.
Patients with chronic inflammatory rheumatologic diseases such as spondyloarthritis are exposed to systemic inflammatory reactions as well as articular inflammation. Consequently, this exposes them to the effects of chronic inflammation, including macrovascular and microvascular changes.30–33 Chronic inflammation is a key factor involved in the early stages of the development of cardiovascular diseases, and its detection could help with prevention. Ultrasonography is not only capable of detecting inflammatory reactions in joints but also of detecting alterations in large blood vessels, such as the carotid artery, caused by chronic systemic inflammation. While an evaluation by gray scale cannot detect the real articular space because it is too narrow and, therefore, difficult to obtain an acoustic window, it is important to assess the vascular flow of the iliac and sacral arteria at the sacroiliac articular space, characterizing a hemodynamic response toward the presence of an inflammatory process.
Carotid ultrasonography measuring IMT is a noninvasive method of detecting subclinical atherosclerosis. Increased IMT values reflect local abnormalities that correlate with widespread histologically proven atherosclerosis.34–37 Measurement of IMT has predictive value in terms of cardiovascular events, independent of traditional risk factors.38–42 Common carotid artery IMT shows positive correlations with the duration of inflammatory articular disease, laboratory parameters reflecting inflammation, age, and traditional risk factors for cardiovascular disease.43–46 Cardiology societies suggest that evaluation of common carotid artery IMT should form part of the medical assessment of patients at increased cardiovascular risk,40 and rheumatology guidelines recommend that patients with inflammatory arthritis should undergo ultrasound evaluation of the carotid arteries.47 Evaluation of these criteria in arthritis was established based on histologic evidence.48–55
In the current study, though the study population had, on average, a normal lipid profile, subclinical alterations consistent with atherosclerosis were seen based on an increased mean RF-QIMT. A previous study in patients with chronic inflammatory articular disease, specifically psoriatic arthritis, demonstrated the relationship between such disorders and an increased risk of subclinical atherosclerosis and cardiovascular events using the QIMT method.56
The use of ultrasonography in the field of rheumatology is increasingly common because it is noninvasive, low cost, and widely accessible. Techniques are evolving. Ultrasound with spectral Doppler using the QIMT technique is an appropriate imaging method for inflammatory reactions; it enables visualization of atherosclerotic changes in the vessel wall caused by the chronic inflammatory activity that occurs in rheumatologic diseases such as spondyloarthritis.
Limitations of the current study include the heterogeneity of the patients’ results and the small number of participants. The latter is largely due to the fact that this patient group included individuals with mobility problems and other disabilities due to chronic disease, which make participation difficult for patients. All ultrasound measurements, including the carotid artery assessments, were performed by a rheumatologist. However, the rheumatologist had extensive experience in ultrasonography, and it has been shown that rheumatologists who are not expert in vascular ultrasound can perform reliable carotid RF-QIMT assessments.57 Furthermore, while differences in RI values between right and left could possibly reflect poor reproducibility, it must be borne in mind that bilateral sacroiliac inflammation does not always exist; thus, right/left differences are likely to reflect the latter observation. Finally, while the findings from our study provide supportive evidence, it is important to note that cross-sectional analysis does not provide information on causality.
In conclusion, our study provides evidence supporting the use of ultrasound for the detection of inflammatory activity in joints and in blood vessels, and provides evidence of the lesions caused by widespread inflammation in these patients. The study also shows that there were positive correlations between these ultrasound findings and parameters reflecting patients’ metabolic profile and alterations in inflammatory markers.
Acknowledgments
Editorial assistance was provided by Content Ed Net (Brazil) with funding from UCB Biopharma S/A, São Paulo, Brazil.
Footnotes
Contributions: All the medical team of the Pontifical Catholic University of Campinas contributed with the methodological and clinical aspects. Guilherme B Damian was accountable for medical writing and is a full-time employee of UCB, Sao Paulo, Brazil.
Disclosure and potential conflicts of interest: The authors declare no conflict of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2018/08/dic.212538-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2018 Mendonça JA, Bisetto de Andrade B, Braga de Aquino JL, Leandro-Merhi VA, Damian GB. https://doi.org/10.7573/dic.212538. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 25 June 2018;
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Arslan H, Sakarya ME, Adak B, Unal O, Sayarlioglu M. Duplex and color Doppler sonographic findings in active sacroiliitis. AJR Am J Roentgenol. 1999;173(3):677–680. doi: 10.2214/ajr.173.3.10470902. [DOI] [PubMed] [Google Scholar]
- 2.Hu Y, Zhu J, Xue Q, Wang N, Hu B. Scanning of the sacroiliac joint and entheses by color Doppler ultrasonography in patients with ankylosing spondylitis. J Rheumatol. 2011;38(8):1651–1655. doi: 10.3899/jrheum.101366. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Hu B, Wang N, Zhang X, Kuang S, Li J. Preliminary evaluation of color power doppler ultrasonography in diagnosis of sacroiliitis in patients with ankylosing spondylitis [Chinese] J Shanghai Jiaotong University. 2008;28:1146–1148. [Google Scholar]
- 4.Jiang Y, Chen L, Zhu J, et al. Power Doppler ultrasonography in the evaluation of infliximab treatment for sacroiliitis in patients with ankylosing spondylitis. Rheumatol Int. 2013;33(8):2025–2029. doi: 10.1007/s00296-013-2682-7. [DOI] [PubMed] [Google Scholar]
- 5.Kabasakal Y, Kitapcioglu G, Yargucu F, Taylan A, Argin M, Gumusdis G. Efficacy of SLZ and MTX (alone or combination) on the treatment of active sacroiliitis in early AS. Rheumatol Int. 2009;29(12):1523–1527. doi: 10.1007/s00296-009-1057-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Xing C, Jiang Y, Hu Y, Hu B, Wang N. Evaluation of complex appearance in vascularity of sacroiliac joint in ankylosing spondylitis by color Doppler ultrasonography. Rheumatol Int. 2012;32(1):69–72. doi: 10.1007/s00296-010-1543-x. [DOI] [PubMed] [Google Scholar]
- 7.Ryan LM, Carrera GF, Lightfoot RW, Jr, Hoffman RG, Kozin F. The radiographic diagnosis of sacroiliitis. A comparison of different views with computed tomograms of the sacroiliac joint. Arthritis Rheum. 1983;26(6):760–763. doi: 10.1002/art.1780260609. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi A, Ghasemi-rad M, Aghdashi M, Mladkova N, Baradaransafa P. Evaluation of disease activity in ankylosing spondylitis; diagnostic value of color Doppler ultrasonography. Skeletal Radiol. 2012;42(2):219–224. doi: 10.1007/s00256-012-1412-7. [DOI] [PubMed] [Google Scholar]
- 9.Stanek A, Cholewka A, Wielkoszyński T, Romuk E, Sieroń K, Sieroń A. Increased levels of oxidative stress markers, soluble cd40 ligand, and carotid intima-media thickness reflect acceleration of atherosclerosis in male patients with ankylosing spondylitis in active phase and without the classical cardiovascular risk factors. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9712536. 9712536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu S, Pereira B, Soubrier M. Cardiovascular events in ankylosing spondylitis: an updated meta-analysis. Semin Arthritis Rheum. 2015;44(5):551–555. doi: 10.1016/j.semarthrit.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Eder L, Sadek M, McDonald-Blumer H, Gladman DD. Aortitis and spondyloarthritis--an unusual presentation: case report and review of the literature. Semin Arthritis Rheum. 2010;39(6):510–514. doi: 10.1016/j.semarthrit.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Uslu AU, Kucuk A, Balta S, et al. The relation between ischemia modified albumin levels and carotid intima media thickness in patients with rheumatoid arthritis. Int J Rheum Dis. 2016 Mar 30; doi: 10.1111/1756-185X.12851. [DOI] [PubMed] [Google Scholar]
- 13.Balta S, Aparci M, Ozturk C, Yildirim AO, Demir M, Celik T. Carotid intima media thickness and subclinical early atherosclerosis. Int J Cardiol. 2016;203:1146. doi: 10.1016/j.ijcard.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Verma I, Krishan P, Syngle A. Predictors of atherosclerosis in ankylosing spondylitis. Rheumatol Ther. 2015;2(2):173–182. doi: 10.1007/s40744-015-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skare TL, Verceze GC, Oliveira AA, Perreto S. Carotid intima-media thickness in spondyloarthritis patients. Sao Paulo Med J. 2013;131(2):100–105. doi: 10.1590/S1516-31802013000100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Merz CN, et al. National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira A, Alvarenga C, Barcelos G, Polito E. Espondilite anquilosante. Rev Bras Reumatol. 2008;48(4):243–247. doi: 10.1590/S0482-50042008000400008. [DOI] [Google Scholar]
- 18.Sampaio-Barros P, Keiserman M, Souza Meirelles E, et al. Spondyloarthritis Commision of the Brazilian Society of Rheumatology. Recommendations for the management and treatment of ankylosing spondylitis. Rev Bras Reumatol. 2013;53(3):242–257. doi: 10.1590/S0482-50042013000300003. [DOI] [PubMed] [Google Scholar]
- 19.Bydlowski SP, Magnanelli AC, Chamone D. Hiper-homocisteinemia e doenças vaso-oclusivas [Hyperhomocysteinemia and vaso-occlusive diseases] Arq Bras Cardiol. 1998;71(1):69–76. doi: 10.1590/S0066-782X1998000700013. [DOI] [PubMed] [Google Scholar]
- 20.McCully KS, Wilson RB. Homocysteine theory of arteriosclerosis. Atherosclerosis. 1975;22(2):215–227. doi: 10.1016/0021-9150(75)90004-0. [DOI] [PubMed] [Google Scholar]
- 21.Wilcken DE, Wilcken B. The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J Clin Invest. 1976;57(4):1079–1082. doi: 10.1172/JCI108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcken DE, Reddy SG, Gupta VJ. Homocysteinemia, ischemic heart disease, and the carrier state for homocystinuria. Metabolism. 1983;32(4):363–370. doi: 10.1016/0026-0495(83)90045-8. [DOI] [PubMed] [Google Scholar]
- 23.Di Geso L, Zardi E, Afeltra A, et al. Comparison between conventional and automated software-guided ultrasound assessment of bilateral common carotids intima-media thickness in patients with rheumatic diseases. Clin Rheumatol. 2012;31(5):881–884. doi: 10.1007/s10067-011-1915-y. [DOI] [PubMed] [Google Scholar]
- 24.Iagnocco A, Riente L, Delle Sedie A, et al. Ultrasound imaging for the rheumatologist. XXII. Achilles tendon involvement in spondyloarthritis. A multi-centre study using high frequency volumetric probe. Clin Exp Rheumatol. 2009;27(4):547–551. [PubMed] [Google Scholar]
- 25.Tinkanen H, Kujansuu E. Doppler ultrasound findings in tubo-ovarian infectious complex. J Clin Ultrasound. 1993;21(3):175–178. doi: 10.1002/jcu.1870210305. [DOI] [PubMed] [Google Scholar]
- 26.Tepper R, Aviram R, Cohen N, Cohen I, Holtzinger M, Beyth Y. Doppler flow characteristics in patients with pelvic inflammatory disease: responders versus nonresponders to therapy. J Clin Ultrasound. 1998;26(5):247–249. doi: 10.1002/(SICI)1097-0096(199806)26:5<247::AID-JCU3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh A, Mondal S, Sinha D, Nag A, Chakraborty S. Ultrasonography as a useful modality for documenting sacroiliitis in radiographically negative inflammatory back pain: a comparative evaluation with MRI. Rheumatology (Oxford) 2014;53(11):2030–2034. doi: 10.1093/rheumatology/keu220. [DOI] [PubMed] [Google Scholar]
- 28.Terslev L, Torp-Pedersen S, Qvistgaard E, Danneskiold-Samsoe B, Bliddal H. Estimation of inflammation by Doppler ultrasound: quantitative changes after intra-articular treatment in rheumatoid arthritis. Ann Rheum Dis. 2003;62(11):1049–1053. doi: 10.1136/ard.62.11.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Midiri M, Iovane A, Finazzo M, Brancatelli G, Gallo C, Lagalla R. Color Doppler-echo in rheumatoid arthritis with extra-articular location. Preliminary experience. Radiol Med. 1999;98(3):123–126. [PubMed] [Google Scholar]
- 30.Shoenfeld Y, Gerli R, Doria A, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112(21):3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Gay MA, Gonzalez-Juanatey C, Lopez-Diaz MJ, et al. HLADRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57(1):125–132. doi: 10.1002/art.22482. [DOI] [PubMed] [Google Scholar]
- 32.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 33.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 35.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87(3 Suppl):II56–65. [PubMed] [Google Scholar]
- 36.Bots ML, Hofman A, De Jong PT, Grobbee DE. Common carotid intima-media thickness as an indicator of atherosclerosis at other sites of the carotid artery. The Rotterdam Study. Ann Epidemiol. 1996;6(2):147–153. doi: 10.1016/1047-2797(96)00001-4. [DOI] [PubMed] [Google Scholar]
- 37.Naredo E, Möller I, Corrales A, et al. Automated radiofrequency-based US measurement of common carotid intima-media thickness in RA patients treated with synthetic vs synthetic and biologic DMARDs. Rheumatology (Oxford) 2012;52(2):376–381. doi: 10.1093/rheumatology/kes260. [DOI] [PubMed] [Google Scholar]
- 38.Touboul PJ, Labreuche J, Vicaut E, Amarenco P GENIC Investigators. Carotid intima-media thickness, plaques, and Framingham risk score as independent determinants of stroke risk. Stroke. 2005;36(8):1741–1745. doi: 10.1161/01.STR.0000174490.23495.57. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide range. Prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37(1):87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 40.Stein JH, Korcarz CE, Hurst RT, et al. American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Polak JF, Person SD, Wei GS, et al. Segment-specific association of carotid intima-media thickness with cardiovascular risk factors. The Coronary Artery Risk development in Young Adult (CARDIA) Study. Stroke. 2010;41(1):9–15. doi: 10.1161/STROKEAHA.109.566596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyrrell PN, Beyene J, Feldman BM, McCrindle BW, Silverman ED, Bradley TJ. Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2010;30(5):1014–1026. doi: 10.1161/ATVBAHA.109.198424. [DOI] [PubMed] [Google Scholar]
- 43.van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum. 2011;40(5):389–397. doi: 10.1016/j.semarthrit.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Pahor A, Hojs R, Gorenjak M, Rozman B. Accelerated atherosclerosis in pre-menopausal female patients with rheumatoid arthritis. Rheumatol Int. 2006;27(2):119–123. doi: 10.1007/s00296-006-0176-6. [DOI] [PubMed] [Google Scholar]
- 45.Hannawi S, Haluska B, Marwick TH, Thomas R. Atherosclerotic disease is increased in recent-onset rheumatoid arthritis: a critical role for inflammation. Arthritis Res Ther. 2007;9(6):R116. doi: 10.1186/ar2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ristić GG, Subota V, Lepić T, et al. subclinical atherosclerosis in patients with rheumatoid arthritis and low cardiovascular risk: the role of von Willebrand factor activity. PLoS One. 2015;10(8):e0130462. doi: 10.1371/journal.pone.0130462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee Adhikari M, Guin A, Chakraborty S, Sinhamahapatra P, Ghosh A. Subclinical atherosclerosis and endothelial dysfunction in patients with early rheumatoid arthritis as evidenced by measurement of carotid intima-media thickness and flow-mediated vasodilatation: an observational study. Semin Arthritis Rheum. 2012;41(5):669–675. doi: 10.1016/j.semarthrit.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Ciftci O, Yilmaz S, Topcu S, et al. Impaired coronary microvascular function and increased intima-media thickness in rheumatoid arthritis. Atherosclerosis. 2008;198(2):332–337. doi: 10.1016/j.atherosclerosis.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated to accelerated atherogenesis. Semin Arthritis Rheum. 2005;35(1):8–17. doi: 10.1016/j.semarthrit.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Boechat Nde O, Ogusku MM, Boechat AL, Sadahiro A. Interaction between smoking and HLA-DRB1*04 gene is associated with a high cardiovascular risk in Brazilian Amazon patients with rheumatoid arthritis. PLoS One. 2012;7(8):e41588. doi: 10.1371/journal.pone.0041588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.López-Longo FJ, Oliver-Miñarro D, de la Torre I, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(4):419–424. doi: 10.1002/art.24390. [DOI] [PubMed] [Google Scholar]
- 53.Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspective. Ann Rheum Dis. 2011;70(1):8–14. doi: 10.1136/ard.2010.142133. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, Gonzalez-Gay MA. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine (Baltimore) 2003;82(6):407–413. doi: 10.1097/01.md.0000101572.76273.60. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Juanatey C, Llorca J, Martin J, Gonzalez-Gay MA. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38(5):366–371. doi: 10.1016/j.semarthrit.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Azevedo Dias M, Maria Silva de Siqueira L, Nascimento de Carvalho B, Pinheiro M, Mendonça JA, Luz K. The automated software-guided ultrasound assessment of bilateral common carotids intima-media thickness for investigation of cardiovascular risk in psoriasis: comparison between patients with or without arthritis. Arthritis Rheumatol. 2015;67(Suppl 10):173. doi: 10.1002/art.39448. [DOI] [Google Scholar]
- 57.Naredo E, Möller I, Gutiérrez M, et al. Multi-examiner reliability of automated radio frequency-based ultrasound measurements of common carotid intima-media thickness in rheumatoid arthritis. Rheumatology (Oxford) 2011;50(10):1860–1864. doi: 10.1093/rheumatology/ker206. [DOI] [PubMed] [Google Scholar]