Abstract
Recently, aberrantly high levels of d-ribose have been discovered in type II diabetic patients. d-ribose glycates proteins more rapidly than d-glucose, resulting in the production of advanced glycation end products (AGEs). Accumulations of these products can be found in impaired renal function, but the mechanisms are poorly understood. The present study tested whether d-ribose induces renal dysfunction via the RAGE-dependent NF-κB signaling pathway. In vivo, administration of d-ribose was found to lower blood glucose and regulate insulin tolerance. Compared to controls, urine nitrogen and creatinine excretion were increased in mice receiving d-ribose and were accompanied by severe pathological renal damage. Furthermore, immunohistochemistry showed that NF-κB, AGEs, and receptor of AGEs (RAGE) increased in the kidneys of the mice with d-ribose treatment. In vitro, by western blot and immunofluorescent staining, we confirmed that d-ribose induced NF-κB activation and accumulation of AGEs and RAGE in mesangial cells. By co-immunoprecipitation, we found that the pull-down of RAGE remarkably increased the expression of NF-κB. Silencing the RAGE gene blocked the phosphorylation of NF-κB induced by d-ribose. These results strongly suggest that d-ribose induced NF-κB inflammation in a RAGE-dependent manner, which may be a triggering mechanism leading to nephropathy.
Keywords: Diabetes, AGEs, RAGE, Kidney
Introduction
In 1970, d-ribose was found to be an energy enhancer that decreases blood glucose (Steinberg et al. 1970). In its role as a sugar moiety of adenosine triphosphate (ATP), d-ribose is widely used as a metabolic therapy supplement for chronic fatigue syndrome or cardiac energy metabolism (Segal et al. 1957). However, d-ribose is more active than glucose in protein glycation (Lu and He 2014; Wei et al. 2012; Chen et al. 2010). It glycates proteins and produces advanced glycation end products (AGEs) that have severe cytotoxicity, which can lead to cell dysfunction and death. Administration of d-ribose leads to high yields of AGEs in the mouse brain and subsequently impairs mouse spatial cognition (Han et al. 2011). Evidence has demonstrated a potential role of AGEs in the progression of renal dysfunction (Chilelli et al. 2013). Cellular proteolysis of AGEs-modified proteins forms AGEs-free adducts, which accumulate markedly in plasma with a decline in the glomerular filtration rate (Agalou et al. 2005). It has been proposed that AGEs bind specifically to cell surface receptors to activate cell dysfunction (Fehrenbach et al. 1998). The receptor for advanced glycation end products (RAGE) is one of the receptors closely correlated with nephropathy (Fehrenbach et al. 1998; Hou et al. 2004; Tanji et al. 2000). Mice that overexpressed RAGE showed severe renal dysfunction compared to their littermates or to RAGE-gene knockout mice (Sourris et al. 2010). These findings suggested that the blockage of AGEs and RAGE accumulations improves renal dysfunction (Wendt et al. 2003). However, whether d-ribose induces AGEs and RAGE accumulation in the kidney with consequential renal dysfunction is still unknown.
Accumulating evidence about the pathogenesis of nephropathy indicates that activation of the nuclear factor-kappa B (NF-κB) signaling pathway is involved in the progression of renal dysfunction, including minimal change disease and diabetic nephropathy (Mezzano et al. 2001, 2004). NF-κB helps to control the expression of numerous genes encoding proteins involved in immune and inflammatory responses. Specifically, it is activated in nephropathy (Navarro and Mora 2005; Costa et al. 2006), inhibiting it attenuates the activation of interstitial fibroblasts (Tamada et al. 2003), and it is induced by various cellular stress-associated stimuli such as reactive oxygen species (ROS) (Karin and Greten 2005). d-ribose reacts with β2-microglobulin and induced ribosylated protein via a ROS-mediated pathway (Kong et al. 2011). However, to our knowledge, until now no study has linked the detrimental effect of d-ribose to the activation of NF-κB inflammation in the development of nephropathy.
The present study was designed to test the hypothesis that d-ribose induces NF-κB inflammation in a RAGE-dependent manner, which may be a triggering mechanism leading to nephropathy. We first tested whether d-ribose would induce renal damage in mice. Next, we tested whether the mechanism of nephropathy induced by d-ribose is associated with the AGEs, RAGE, and NF-κB signaling pathways. Then, we performed in vitro experiments to test whether RAGE is required for the NF-κB activation induced by d-ribose.
Materials and methods
Animal grouping and treatment
Male BABL/c mice (n = 45, 8–10 weeks, 20 ± 2 g) were purchased from Vital River Laboratory Animal Technology (Beijing, China) [License No. SCXK (Jing) 2016-0011]. After 7 days of acclimatization to the laboratory environment, the BABL/c mice were randomly divided into three groups (n = 15), control, 1.6 g/kg d-ribose and 3.2 g/kg d-ribose groups, which respectively received daily intraperitoneal (i.p.) injections with 0.9% saline, 1.6 g/kg, or 3.2 g/kg d-ribose for 30 days. All mice were maintained in animal facilities under SPF conditions. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Peking University First Hospital (Approval Number: J201534).
Blood glucose and renal function analysis
Blood glucose was assessed using the glucose oxidase method; the OGTT (oral glucose tolerance test) and ITT (insulin tolerance test) were performed as described previously (Hong et al. 2017; Wu et al. 2018). Urea nitrogen (UN) and creatinine (Cr) in serum were measured with urease methods and sarcosine oxidase methods, respectively, both using commercial detecting kits according to the manufacturer’s protocol.
Ultrastructural analysis, histopathological analysis, and immunohistochemistry analysis
After treatment for 30 days, the mice were sacrificed, and the kidneys were removed. Ultrastructural analysis, hematoxylin and eosin (HE), periodic acid-Schiff (PAS) staining, AGEs (Abcam, UK), RAGE (Abcam, UK) and NF-κB (Cell Signaling Technology, USA) staining were performed and quantified as described (Hong et al. 2017).
Cell culture and treatment
The SV40 MES 13 cell line (mouse renal glomerular mesangial cells, MSCs) was purchased from the China Center for Type Culture Collection cell bank (China) and cultured in low-glucose DMEM (Gibco, Rockville, MD, USA) and F-12 medium (Gibco, Rockville, MD, USA) (low-glucose DMEM:F-12 medium = 3:1; final concentration, 6.7 mM glucose) supplemented with 5% FBS (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The cells were incubated with high glucose (high-glucose DMEM:F-12 medium = 3:1; final concentration, 25 mM glucose) or d-ribose (25 mM and 50 mM) for 48 h after an initial 24 h incubation in non-serum 6.7 mM glucose.
Western blot
After the kidneys were collected from the mice, an equivalent of protein was resolved on dodecyl sulfate (SDS)-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. The antibodies used are shown below: AGEs and RAGE antibodies were from Abcam, UK; p-IKKα/β, IKKβ, IKKα, p-IκBα, IκBα and NF-κB p65 antibodies were from Cell Signaling Technology, USA; p-NF-κB p65 antibody was from Santa Cruz Biotechnology, Glostrup, Denmark and GAPDH antibody was from Beijing Zhong Shan Golden Bridge Biotechnology Co., Ltd., China, with a dilution of 1:1000. Horseradish peroxidase-labeled secondary antibody was from Beijing Zhong Shan Golden Bridge Biotechnology Co., Ltd., with a dilution of 1:10,000. The membranes were developed with enhanced chemiluminescence (Thermo Scientific, USA) and visualized using a digital imaging system (BIO-RAD Laboratories, Inc., USA).
ELISA
Sera were collected after treatment for 10, 20, and 30 days. Insulin (ALPCO, USA), AGEs (Abbexa Ltd., UK), and RAGE (Bio-Techne, USA) in serum were measured using commercial ELISA kits according to the manufacturer’s protocol.
Immunofluorescence staining
After treatment, the cells were fixed, blocked, and stained with NF-κB antibody overnight at 4 °C. After incubation with a secondary antibody (1:400) conjugated to fluorescein isothiocyanate (FITC, Zhongshan Golden Bridge Biotechnology) for 1 h at room temperature, the cells were mounted with diamidinophenylindole (DAPI) and then observed and photographed with fluorescent microscopy (ECLIPSE TE2000-S, Nikon, Japan).
Co-immunoprecipitation (Co-IP)
Co-IP was performed to confirm the interaction between RAGE and NF-κB. Cells were seeded in 10 cm dishes and grown to 80% confluence. After 24 h of non-serum starvation, the cells were incubated with or without 50 mM d-ribose for 48 h for co-IP. After washing three times with PBS, 1 mg cellular protein was lysed in 500 ul of cold cell lysis buffer and then incubated with RAGE antibody (Santa Cruz Biotechnology, Glostrup, Denmark) for 6 h. The immunocomplex was captured by adding 30 μl of protein G (Miltenyi Biotec, Germany) and subjected to western blot analysis (anti-NF-κB antibody).
RNA interference of RAGE
RAGE small interference RNAs (siRAGE) were purchased from Santa Cruz Biotechnology, Glostrup, Denmark. SiRAGE was performed with silent Lipid Reagent (Bio-Rad, USA) according to the manufacturer’s instructions. The final concentration of siRAGE was 10 nM.
Data analysis
All the data were analyzed using SPSS 19.0 statistical software and reported as the mean ± standard deviation (SD). Different groups were analyzed using a one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison post-test. P < 0.05 was considered statistically significant.
Results
d-ribose down-regulated blood glucose and induced renal damage in mice
We first investigated whether the d-ribose treatment affected glucose and insulin tolerance in the mice. BABL/c mice were i.p. injected with 1.6 g/kg or 3.2 g/kg d-ribose for 30 days. As shown in Fig. 1a, the fasting blood glucose in the d-ribose-treated mice significantly decreased after treatment for 10 and 20 days in comparison with the control group, confirming that d-ribose down-regulated blood glucose in mice (Segal et al. 1957). Furthermore, an OGTT analysis was performed to determine whether d-ribose affects glucose tolerance. After the d-ribose treatment for 10 or 20 days, the area under the curve of the OGTT was decreased but returned to the normal level after 30 days of treatment (Fig. 1b). These changes indicated that d-ribose ameliorated glucose tolerance in the shorter time periods (10 and 20 days). To further detect whether d-ribose played a role in insulin tolerance, the insulin level in serum and ITT analysis was measured. As shown in Fig. 1c, the insulin level in serum was up-regulated after treatment for 10 and 20 days and returned to a normal level after that. The area under the curve of the ITT decreased in a dose-dependent manner compared to the controls (Fig. 1d). All these suggested that d-ribose might be beneficial for insulin tolerance.
Fig. 1.
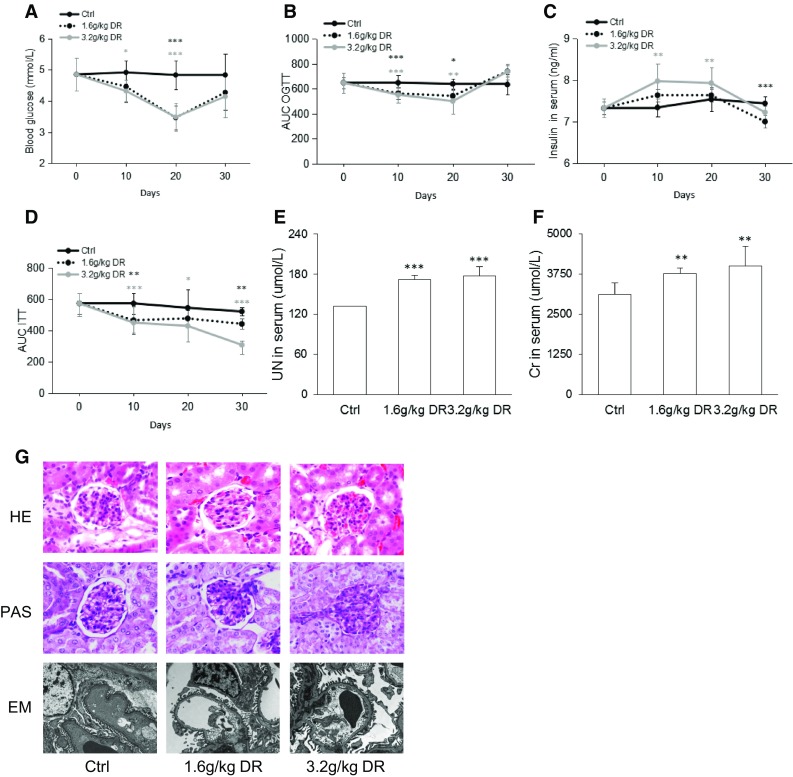
d-ribose down-regulated blood glucose and induced renal damage in mice. a Fasting blood glucose of mice (n = 5). b Area under the OGTT curve of mice (n = 5). c Insulin level in serum of mice (n = 5). d Area of ITT of mice (n = 5). e Urine nitrogen level in the serum of mice after 30 days of treatment (n = 5). f Creatinine level in the serum of mice after 30 days of treatment (n = 5). g Representative images of hematoxylin and eosin (HE) staining (original magnification ×400), periodic acid-Schiff (PAS) staining (original magnification ×400) and electron microscopy (EM) (original magnification ×400) analysis of mouse kidney after 30 days of treatment (n = 5). Ctrl control, DR d-ribose. Data are expressed as mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group
To investigate whether d-ribose induces renal dysfunction, UN and Cr in serum were measured. As shown in Fig. 1e and f, d-ribose induced severe levels of UN and Cr in serum in a dose-dependent manner. We further observed morphological changes microscopically (Fig. 1g). The HE staining showed that plasma proteins had been extravagated from the renal capsules and that the glomerular capillaries were dilated and filled with red blood cells, indicating more severe pathological changes in the kidneys of the mice who had undergone the d-ribose treatment than in the controls. The PAS staining showed a variety of typical glomerular damage, including severe basement membrane thickening, glomerular hypertrophy, and increased mesangial matrix area in the mice receiving d-ribose. Furthermore, electron microscopy of the renal cortex showed mesangial matrix deposition, mesangial expansion, podocyte fusion, and glomerular basement membrane thickening in the mice treated with d-ribose. All these changes were less severe in the control group, indicating that d-ribose accelerated renal dysfunction.
d-ribose activated NF-κB signaling pathway in mice
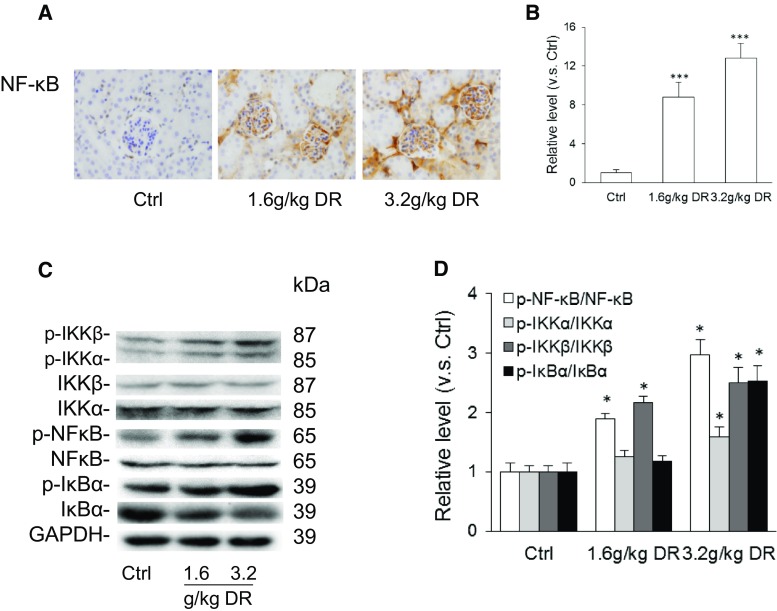
To test whether d-ribose initiates a cellular inflammatory response through the NF-κB pathway, we evaluated the NF-κB signaling pathway activity in mice. The results of immunohistochemistry staining showed elevated NF-κB accumulation in the kidneys of the mice receiving d-ribose in a dose-dependent manner (Fig. 2a, b). As depicted in Fig. 2c and d, a Western blot showed up-regulated expression of phosphorylated IKKβ, IKKα, NF-κB p65, and IκBα with d-ribose treatment in comparison with the controls, indicating that the NF-κB signaling pathway was activated with d-ribose treatment.
Fig. 2.
d-ribose activated NF-κB signaling pathway in mice. a and b Representative images (original magnification ×400) and summarized data of immunohistochemical stained kidney for NF-κB production in mice (n = 5). c and d Representative western blot gel document and summarized data from AGEs, RAGE, p-IKKβ, p-IKKα, IKKβ, IKKα, p-NF-κB, NF-κB, p-IκBα, and IκBα in kidney (n = 5). Ctrl control, DR d-ribose. Data are expressed as mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group
d-ribose enhanced AGEs and RAGE accumulation in mice
Based on a previous study, AGEs and RAGE played a vital role in the progression and aggravation of chronic kidney disease (CKD) and end-stage renal disease (ESRD) (Gugliucci and Menini 2014). The present study examined the effect of d-ribose on AGEs and RAGE accumulation. By immunohistochemistry, AGEs and RAGE accumulations were found remarkably elevated in the kidneys of the mice with d-ribose treatment in a dose dependent manner in comparison with the controls (Fig. 3a, b). Consistently, AGEs and RAGE levels in serum (Fig. 3c) as well as AGEs and RAGE protein expression (Fig. 3d, e) in the kidneys were up-regulated in the mice receiving d-ribose treatment as compared to the controls. All these findings confirmed that d-ribose induced AGEs and RAGE accumulations (Han et al. 2011).
Fig. 3.
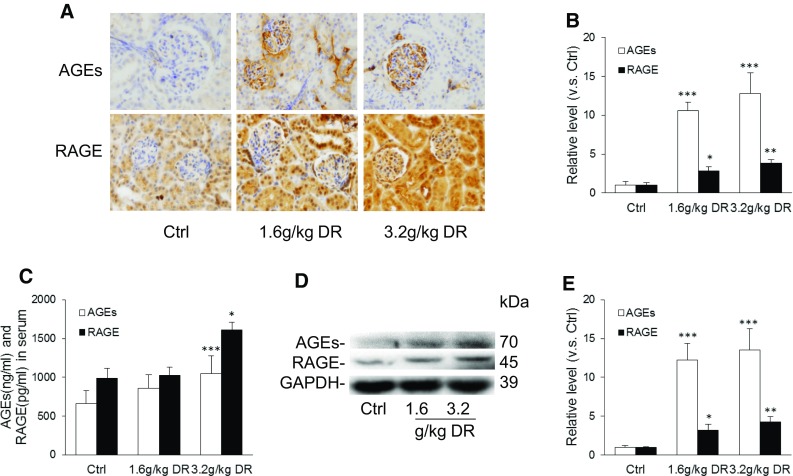
d-ribose enhanced AGEs and RAGE accumulation in mice. a and b Representative images (original magnification ×400) and summarized data of immunohistochemical stained kidney for AGEs and RAGE production in (n = 5). c AGEs and RAGE levels in the serum of mice after 30 days’ treatment (n = 5). d and e Representative western blot gel document and summarized data of AGEs and RAGE in kidney (n = 5). Ctrl control, DR d-ribose. Data are expressed as mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group
d-ribose induced AGEs and RAGE enhancement and NF-κB activation in mesangial cells
To further elucidate the effect of d-ribose on AGEs, RAGE, and NF-κB, we performed experiments in vitro with MSCs. The activity of NF-κB, phosphorylation of total NF-κB, and NF-κB in the nucleus and cytoplasm, were each detected, and immunofluorescence staining of NF-κB was performed. As depicted in Fig. 4a, both d-glucose and d-ribose up-regulated the expression of phosphorylated NF-κB and IκBα. NF-κB was expressed more prominently in the nucleus than the cytoplasm after d-glucose or d-ribose incubation in comparison with the controls (Fig. 4b). Consistently, co-localization of NF-κB and DAPI showed that in MSCs, NF-κB expressed more in the nucleus than the cytoplasm after incubation with d-glucose or d-ribose (Fig. 4c). All these results indicated that d-ribose activated NF-κB signaling pathways in MSCs.
Fig. 4.
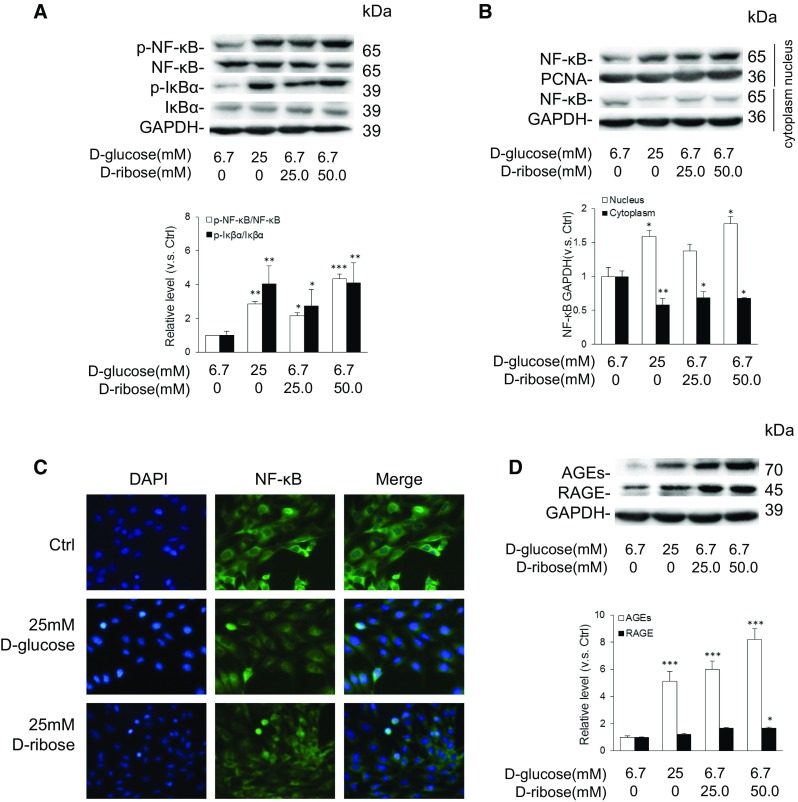
d-ribose induced AGEs and RAGE enhancement and NF-κB activation in mesangial cells. a Representative western blot gel document and summarized data of p-NF-κB, NF-κB, p-IκBα, and IκBα in mesangial cells (n = 5). b Representative western blot gel document and summarized data of NF-κB in nucleus and cytoplasm of mesangial cell (n = 5). c Representative images of immunostained mesangial cells for NF-κB (original magnification ×200) (n = 5). d Representative western blot gel document and summarized data of AGEs and RAGE in mesangial cells (n = 5). Data are expressed as mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group
As shown in Fig. 4d, the western blot gel document showed that both d-glucose and d-ribose up-regulated AGEs and RAGE expression. MSCs incubated with d-ribose showed a higher expression of AGEs and RAGE compared to d-glucose; this is consistent with studies that showed that d-ribose gives rise to AGEs more quickly than d-glucose (Wei et al. 2012; Han et al. 2011).
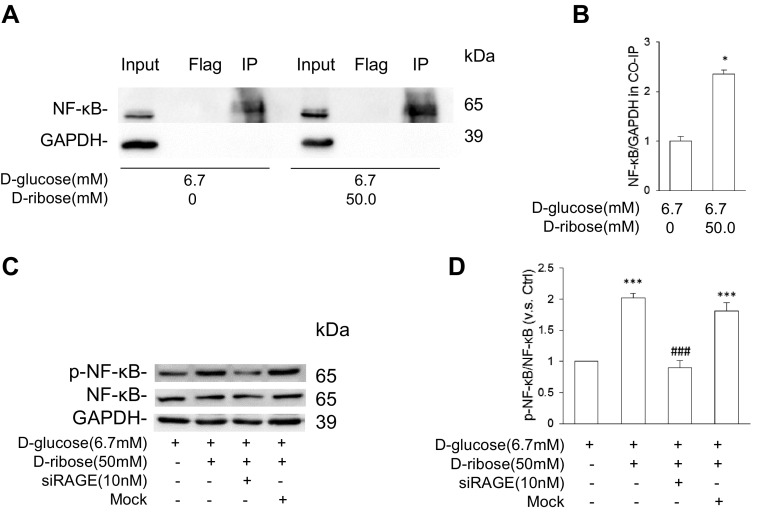
RAGE played a vital role in d-ribose-induced NF-κB activation
To determine whether RAGE is correlated to NF-κB activation, co-IP with RAGE antibody was performed, and the NF-κB expression was evaluated. MSCs were treated with or without 50 mM d-ribose for 48 h and then co-IPed with RAGE antibody. As shown in Fig. 5a and b, the expression of NF-κB was higher in MSC treated with d-ribose than in the controls, indicating that d-ribose up-regulated the expression of NF-κB and that RAGE is required in the process. To further confirm the role of RAGE in d-ribose-induced NF-κB activation, a siRNA against RAGE was transfected to MSCs prior to d-ribose incubation. As shown in Fig. 5c and d, siRAGE effectively down-regulated the expression of phosphorylated NF-κB induced by d-ribose according to the western blot analysis. All these results confirmed that d-ribose activated NF-κB in a RAGE-dependent way.
Fig. 5.
RAGE played a vital role in d-ribose-induced NF-κB activation. a and b Representative western blot gel document and summarized data of co-IP demonstrating a direct interaction of RAGE and NF-κB in mesangial cell (n = 5). c and d Representative western blot gel document and summarized data of p-NF-κB and NF-κB in mesangial cell with siRAGE pre-treatment (n = 4). Data are expressed as mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group
Discussion
The present study was designed to determine whether d-ribose induced nephropathy through NF-κB-mediated inflammation and whether RAGE played a role in this NF-κB activation. In vivo, d-ribose was found to induce renal dysfunction and morphological changes in mice, with NF-κB activation, AGEs and RAGE accumulation in the kidneys. Furthermore, in vitro studies with mesangial cells showed that d-ribose indeed elevated AGEs and RAGE accumulation and NF-κB activation. The pull-down of RAGE remarkably up-regulated the expression of NF-κB, and the silencing of RAGE attenuated the accumulation of NF-κB induced by d-ribose. These findings suggested that RAGE-dependent NF-κB inflammation may play a vital role in nephropathy induced by d-ribose.
Previous studies have demonstrated that d-ribose elevated tau hyper-phosphorylation and Aβ-like deposits and caused memory loss and anxiety-like behavior in mice (Wu et al. 2015). However, whether d-ribose induces renal damage remains poorly studied. In the present study, we first demonstrated that d-ribose injections in mice induced severe urine nitrogen and creatinine excretion compared to the controls, accompanied by a series of characteristic pathological changes in the kidneys. To our knowledge, these results provided the first experimental evidence that d-ribose may induce renal damage.
It has been reported that inflammation plays a crucial role in a variety of inflammatory diseases including diabetic nephropathy (DN) (Park et al. 2011). As one of the important cellular mediators (Patel and Santani 2009; Kolati et al. 2015), NF-κB was found to be up-regulated in the glomeruli and tubules (Vallon 2011; Imig and Ryan 2013), suggesting a close correlation between NF-κB and nephropathy. Normally, NF-κB is inactive and present in cytoplasm, but once it is phosphorylated, free and active p-NF-κB migrates to the nucleus and regulates pro-inflammatory cytokines (DiDonato et al. 1997; Mercurio et al. 1997). NF-κB was observed to be transferred to the nucleus from the cytoplasm in mesangial cells treated with d-ribose using both immunofluorescent analysis and western blot analysis, indicating NF-κB signaling pathway activation. Phosphorylated-NF-κB serves as an important parameter of nucleus translocation. We also found an increased level of active p65-NF-κB in mesangial cells and in the mice treated with d-ribose. These results are consistent with previous reports that ribosylation-induced by d-ribose activates inflammation and astrocyte activation in the mouse brain (Han et al. 2014). Therefore, we hypothesized that NF-κB plays a crucial role in d-ribose-induced mesangial cell damage and renal damage.
d-ribose was first found to down-regulate blood glucose (Segal et al. 1957) and up-regulate insulin levels (Sloviter and Petkovic 1969), and thus “oral administration of d-ribose in diabetes mellitus” was described in 1957. However, when d-ribose is glycated, it gives rise to the formation of advanced glycation end products (AGEs) and RAGE in astrocytoma cells, resulting in direct damage to the nervous system (Patel and Santani 2009). However, there are almost no studies about the involvement of AGEs and RAGE in d-ribose-induced renal damage. The present study confirmed there is lower fasting blood glucose and higher insulin levels in the serum of mice treated with d-ribose. We also found significantly higher levels of AGEs and RAGE accumulations in the kidneys and mesangial cells treated with d-ribose in comparison with controls. d-glucose produced less AGEs and RAGE compared to d-ribose, confirming that d-ribose is a more reducing monosaccharide than d-glucose (Wei et al. 2009; Chen et al. 2009). The binding of RAGE by AGEs evokes a vicious cycle of increased oxidative stress and inflammatory reaction, leading to subsequent cell and tissue injury (Vlassara 2001), such as has been found in chronic renal diseases (Tanji et al. 2000). This led us to hypothesize that d-ribose activates NF-κB and induces mesangial cell damage and renal dysfunction, in which AGEs and RAGE play a role.
To test this hypothesis, a co-immunoprecipitation assay with RAGE and silencing RNA of RAGE were performed to test whether RAGE plays a role in the activation of NF-κB induced by d-ribose. Elevated NF-κB expression was found after a pull-down with RAGE, and NF-κB expressed more in MSCs treated with d-ribose in comparison with the controls, indicating that RAGE plays a role in NF-κB activation. Furthermore, prior treatment with siRAGE was confirmed to block the phosphorylation of NF-κB. In previous studies, the AGEs-RAGE pathway is widely recognized as a pro-inflammatory mechanism in nephropathy, and the therapeutic blockade of RAGE ameliorated renal and endothelial functions under a high AGEs burden (Yeh et al. 2017). Based on these results, it appears that RAGE is essential for the activation of NF-κB induced by d-ribose. However, in vivo experiments are needed to further confirm the role of RAGE in d-ribose-induced NF-κB activation in nephropathy.
In summary, the present study demonstrated d-ribose-induced mesangial cell damage and renal dysfunction in mice, a process which was mediated by the RAGE-dependent NF-κB signaling pathway. These findings imply a new mechanism for mediated d-ribose-induced nephropathy, providing glycation of d-ribose as a new target for the treatment of renal damage, especially nephropathy.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant Nos. 81573763 and 81530099), Natural Science Foundation of Beijing Municipality (Grant No. 7172221), and State’s Key Project of Research and Development Plan (Grant No. 2016YFE0116200). We are grateful to the center of Ultra-structural Pathology, Lab of Electron Microscopy, and Laboratory Animal Facility of Peking University First Hospital, Beijing, P. R. China, for their assistance.
Compliance with ethical standards
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Agalou S, Ahmed N, Babaei-Jadidi R, Dawnay A, Thornalley PJ. Profound mishandling of protein glycation degradation products in uremia and dialysis. J Am Soc Nephrol. 2005;16:1471–1485. doi: 10.1681/ASN.2004080635. [DOI] [PubMed] [Google Scholar]
- Chen L, Wei Y, Wang XQ, He RQ. D-ribosylated Tau forms globular aggregates with high cytotoxicity. Cell Mol Life Sci. 2009;66:2559–2571. doi: 10.1007/s00018-009-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wei Y, Wang XQ, He RQ. Ribosylation rapidly induces alpha-synuclein to form highly cytotoxic molten globules of advanced glycation end products. PLoS ONE. 2010;5:e9052. doi: 10.1371/journal.pone.0009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilelli NC, Burlina S, Lapolla A. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: a “glycoxidation-centric” point of view. Nutr Metab Cardiovasc Dis. 2013;23:913–919. doi: 10.1016/j.numecd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Costa JCSR, Costa RS, Silva CGA, Coimbra TM. Enalapril reduces the expression of nuclear factor-kappa B and c-Jun N-terminal kinase in the renal cortices of five-sixths-nephrectomized rats. Am J Nephrol. 2006;26:281–286. doi: 10.1159/000093960. [DOI] [PubMed] [Google Scholar]
- Didonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive I kappa B kinase that activates the transcription factor NF-kappa B. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998;44:1147–1157. [PubMed] [Google Scholar]
- Gugliucci A, Menini T (2014) The axis AGE-RAGE-soluble RAGE and oxidative stress in chronic kidney disease. In: Oxidative stress and inflammation in non-communicable diseases—molecular mechanisms and perspectives in therapeutics, vol 824, pp 191–208. Springer, Cham [DOI] [PubMed]
- Han C, Lu Y, Wei Y, Liu Y, He R. D-ribose induces cellular protein glycation and impairs mouse spatial cognition. PLoS ONE. 2011;6:e24623. doi: 10.1371/journal.pone.0024623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Lu Y, Wei Y, Wu B, Liu Y, He R. D-ribosylation induces cognitive impairment through RAGE-dependent astrocytic inflammation. Cell Death Dis. 2014;5:e1117. doi: 10.1038/cddis.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JN, Li WW, Wang LL, Guo H, Jiang Y, Gao YJ, Tu PF, Wang XM. Jiangtang decoction ameliorate diabetic nephropathy through the regulation of PI3K/Akt-mediated NF-kappa B pathways in KK-Ay mice. Chin Med. 2017;12:13. doi: 10.1186/s13020-017-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou FF, Ren H, Owen WF, Guo ZH, Chen PY, Schmidt AM, Miyata T, Zhang X. Enhanced expression of receptor for advanced glycation end products in chronic kidney disease. J Am Soc Nephrol. 2004;15:1889–1896. doi: 10.1097/01.ASN.0000131526.99506.F7. [DOI] [PubMed] [Google Scholar]
- Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol. 2013;3:957–976. doi: 10.1002/cphy.c120028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF kappa B: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kolati SR, Kasala ER, Bodduluru LN, Mahareddy JR, Uppulapu SK, Gogoi R, Barua CC, Lahkar M. BAY 11-7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-kappa B pathway. Environ Toxicol Pharmacol. 2015;39:690–699. doi: 10.1016/j.etap.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Kong FL, Cheng W, Chen J, Liang Y. D-Ribose glycates beta(2)-microglobulin to form aggregates with high cytotoxicity through a ROS-mediated pathway. Chem Biol Interact. 2011;194:69–78. doi: 10.1016/j.cbi.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Lu Y, He RQ. GRP75 of CHO cells responds to ribosylation. Prog Biochem Biophys. 2014;41:1191–1192. [Google Scholar]
- Mercurio F, Zhu HY, Murray BW, Shevchenko A, Bennett BL, Li JW, Young DB, Barbosa M, Mann M. IKK-1 and IKK-2: cytokine-activated I kappa B kinases essential for NF-kappa B activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Mezzano SA, Barria M, Droguett MA, Burgos ME, Ardiles LG, Flores C, Egido J. Tubular NF-kappa B and AP-1 activation in human proteinuric renal disease. Kidney Int. 2001;60:1366–1377. doi: 10.1046/j.1523-1755.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, Ruiz-Ortega M, Egido J. NF-kappa B activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transpl. 2004;19:2505–2512. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transpl. 2005;20:2601–2604. doi: 10.1093/ndt/gfi155. [DOI] [PubMed] [Google Scholar]
- Park NY, Park SK, Lim Y. Long-term dietary antioxidant cocktail supplementation effectively reduces renal inflammation in diabetic mice. Br J Nutr. 2011;106:1514–1521. doi: 10.1017/S0007114511001929. [DOI] [PubMed] [Google Scholar]
- Patel S, Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61:595–603. doi: 10.1016/S1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- Segal S, Foley J, Wyngaarden JB. Hypoglycemic effect of D-ribose in man. Proc Soc Exp Biol Med. 1957;95:551–555. doi: 10.3181/00379727-95-23286. [DOI] [PubMed] [Google Scholar]
- Sloviter HA, Petkovic MR. Stimulation of insulin secretion in the rabbit by D-ribose. Nature. 1969;221:371–372. doi: 10.1038/221371a0. [DOI] [PubMed] [Google Scholar]
- Sourris KC, Morley AL, Koitka A, Samuel P, Coughlan MT, Penfold SA, Thomas MC, Bierhaus A, Nawroth PP, Yamamoto H, Allen TJ, Walther T, Hussain T, Cooper ME, Forbes JM. Receptor for AGEs (RAGE) blockade may exert its renoprotective effects in patients with diabetic nephropathy via induction of the angiotensin II type 2 (AT2) receptor. Diabetologia. 2010;53:2442–2451. doi: 10.1007/s00125-010-1837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg T, Poucher RL, Sarin RK, Rees RB, Gwinup G. Oral administration of D-ribose in diabetes mellitus. Diabetes. 1970;19:11–16. doi: 10.2337/diab.19.1.11. [DOI] [PubMed] [Google Scholar]
- Tamada S, Nakatani T, Asai T, Tashiro K, Komiya T, Sumi T, Okamura M, Kim S, Iwao H, Kishimoto T, Yamanaka S, Miura K. Inhibition of nuclear factor-kappa B activation by pyrrolidine dithiocarbamate prevents chronic FK506 nephropathy. Kidney Int. 2003;63:306–314. doi: 10.1046/j.1523-1755.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- Tanji N, Markowitz GS, Fu CF, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D’agati VD. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656–1666. doi: 10.1681/ASN.V1191656. [DOI] [PubMed] [Google Scholar]
- Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436–443. doi: 10.1002/dmrr.233. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen L, Chen J, Ge L, He RQ. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biology. 2009;10:10. doi: 10.1186/1471-2121-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Han CS, Zhou J, Liu Y, Chen L, He RQ. D-ribose in glycation and protein aggregation. Biochim Biophys Acta Gen Subj. 2012;1820:488–494. doi: 10.1016/j.bbagen.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Wendt TM, Tanji N, Guo JC, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’agati VD, Schmidt AM. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BB, Wei Y, Wang YJ, Su T, Zhou L, Liu Y, He RQ. Gavage of D-ribose induces Aβ-like deposits, Tau hyperphosphorylation as well as memory loss and anxiety-like behavior in mice. Oncotarget. 2015;6:34128–34142. doi: 10.18632/oncotarget.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Liu XC, Yin JY, Wu HJ, Cai XL, Wang NS, Qian YC, Wang F. IL-6 receptor blockade ameliorates diabetic nephropathy via inhibiting inflammasome in mice. Metabolism. 2018;83:18–24. doi: 10.1016/j.metabol.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Yeh WJ, Yang HY, Pai MH, Wu CH, Chen JR. Long-term administration of advanced glycation end-product stimulates the activation of NLRP3 inflammasome and sparking the development of renal injury. J Nutr Biochem. 2017;39:68–76. doi: 10.1016/j.jnutbio.2016.09.014. [DOI] [PubMed] [Google Scholar]