Abstract
Introduction:
To improve patient safety, the Centers for Medicare & Medicaid Services (CMS) has promoted systematically measuring and reporting harm due to patient care. The CMS’s Partnership for Patients program identified 9 hospital-acquired conditions (HACs) for reduction, to make care safer, more reliable, and less costly. However, the proportion of inpatient pediatric harm represented by these HACs is unknown.
Methods:
We conducted a retrospective review of 240 harms previously identified using the Pediatric All-Cause Harm Measurement Tool, a trigger tool that is applied to medical records to comprehensively identify harms. The original sample included 600 randomly selected patients from 6 children’s hospitals in February 2012. Patients with rehabilitation, obstetric, newborn nursery, and psychiatric admissions were excluded. The 240 identified harms were classified as a HAC if the event description potentially met the definition of 1 of the 9 CMS-defined HACs. HAC assessment was performed independently by 2 coauthors and compared using Cohen’s Kappa.
Results:
Two hundred forty harms across 6 children’s hospitals were identified in February 2012 using a pediatric global trigger tool. Agreement between the coauthors on HAC classification was high (Kappa = 0.77). After reconciling differences, of the 240 identified harms, 58 (24.2%; 95% confidence interval: 9.1–31.7%) were classified as a CMS-defined HAC.
Conclusions:
One-fourth of all harms detected by a pediatric-specific trigger tool are represented by HACs. Although substantial effort is focused on identifying and minimizing HACs, to better understand and ultimately mitigate harm, more comprehensive harm identification and quantification may be needed to address events unidentified using this approach.
INTRODUCTION
Over the last 2 decades, knowledge of patient safety has advanced substantially. Many hospital acquired harms once thought inevitable are not preventable.1–4 The United States government, largely through the Centers for Medicare & Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, has promoted measuring, reporting, and decreasing patient harm. In an effort to decrease harm, the CMS’s Partnership for Patients program defined a list of 9 hospital-acquired conditions (HACs), and funded Hospital Engagement Networks to decrease occurrence of these events by 40% in 3 years over their baseline rates5 (Table 1). These HACs are useful for comparisons across similar hospitals and are worthy targets for quality improvement efforts, given common definitions and an increasing literature base describing effective prevention strategies. In a separate but similar CMS program, the Hospital Acquired Condition Reduction Program utilizes HACs that are similar to those in the CMS Partnership for Patients program.6
Table 1.
Centers for Medicare & Medicaid Services’ Partnership for Patient’s Hospital-acquired Conditions and Occurrence Count
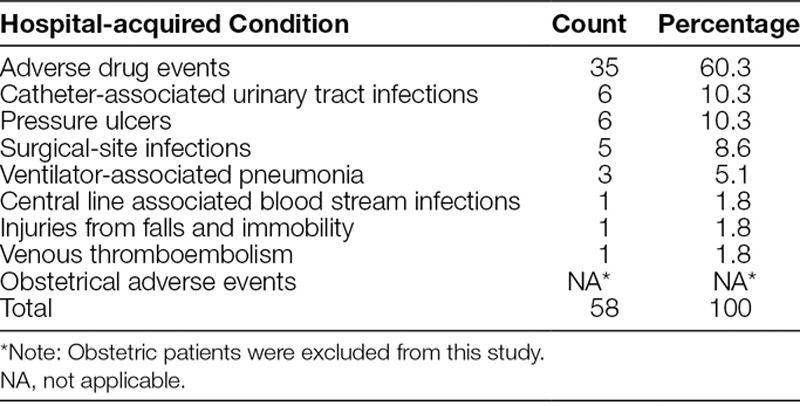
Recognizing that HACs represent only a portion of patient harm (temporary or permanent injury resulting from the patient’s care rather than disease6), CMS notes in its Partnership for Patients program that “the Partnership will target all forms of harm” and produce guidance for reducing “all-cause harm.”6 Other forms of patient harm, such as unplanned extubation, surgical complications, sepsis, clostridium difficile, and delirium, still contribute substantially to harm-related morbidity and resource utilization.7–11 In response, policymakers discuss all-cause harm identification as a desirable and achievable goal, yet there is no requirement for consistent application of all-cause-harm measurement.12
Previous studies have identified harm using a trigger tool to detect specific “triggers,” defined as medical record–based hints, presented in a patient’s medical record that may be associated with harm.13,14 In a multi-site pediatric study, the rate of harm was reported as 40 harms per 100 admissions.15 These results are consistent with findings in pediatrics and adult medicine.16,17 To better understand the proportion of the total harm burden that HACs represent, we compared the number of CMS-defined HACs to the total harms identified using a previously published pediatric trigger tool.15
METHODS
Design/Setting/Patients
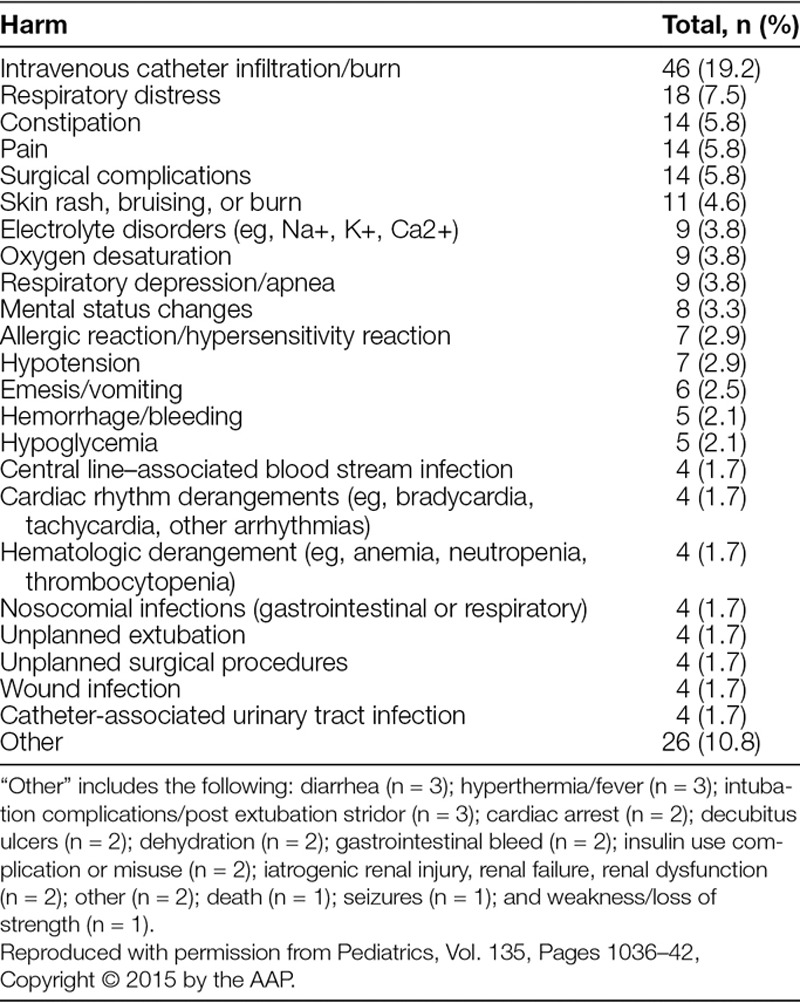
Data were originally collected for a cross-sectional, retrospective study aimed to pilot test a trigger tool that would detect the most common causes of harm in pediatric inpatient settings. The method of data collection and harm identification using the Pediatric All-Cause Harm Measurement Tool (PACHMT) at 6 participating academic children’s hospitals has been recently described.15 Briefly, we applied the pediatric trigger tool to 100 randomly selected patient charts per hospital (600 total) from February 2012. Eligible patient records were inpatients younger than 22 years of age, had a length of stay between 24 hours and 6 months, and were discharged in February 2012. The exclusion criteria in our original article and this article were patients admitted for rehabilitation, to the newborn nursery, to day-treatment areas, or with a primary discharge diagnosis related to psychiatric or obstetric care.15 This approach mirrors that used by other authors.16 The PACHMT was designed to identify the most common causes of pediatric harm, using a trigger list that includes medical triggers, medication administration triggers, health care–associated infections, perioperative triggers, readmission triggers, and resuscitation/death triggers. However, the PACHMT did not have specific triggers for Falls, Surgical Site Infections, and Venous Thromboemboli. Trained reviewers identified triggers in the medical record, assessed them, and documented the presence of harm; a secondary reviewer at each site confirmed the findings. From the 600 patient charts evaluated, 240 harmful events were identified (Table 2). Of the 240 harms, the PACHMT detected 1,093 triggers, resulting in identification of 204 (85.0%) of the total harms identified.15 Institutional review board waiver or approval for this study was granted at each site.
Table 2.
Total Harm Events Identified by the Pediatric All-Cause Harm Measurement Tool (N = 240)
Intervention
For this investigation, the 240 previously identified harms were individually examined by 2 co-authors (D.S., D.K.) for the potential to meet the definition of 8 of the 9 HACs identified in the Partnership for Patients program. An obstetrical HAC was excluded because obstetrical patients were not included in the sample. The reviewers used the detailed description of each confirmed harm to classify the 240 harms either as a HAC or not, comparing the description of the harm to the Partnership for Patients program HAC definitions. For example, if a description of a harm was “deep decubitus pressure injury,” the event was classified as a Pressure Ulcer. Likewise, if a harm description noted a sedation event following the use of opioids, the event was classified as an Adverse Drug Event. The reviewers discussed and resolved all differences in classification.
Outcomes
The primary outcome was the percentage of overall harm identified with the PACHMT that were also identified as HACs.
Statistical Analysis
We summarized harm identified as HACs by both co-authors and compared the level of agreement using Cohen’s Kappa.
RESULTS
Reviewer 1 identified 62 harm events as HACs and reviewer 2 identified 56 harm events as HACs. Agreement between the 2 reviewers was high (Kappa = 0.775). Upon initial review, 49 of these events had agreement between the 2 reviewers. The disagreements were subsequently reviewed, and after reconciling differences, an additional 9 harm events were reclassified as HACs. Of these 9 harm events, 7 were reclassified as adverse drug events, 1 was reclassified as a surgical-site infection, and 1 was reclassified as a ventilator-associated pneumonia. For each event, both reviewers revisited and discussed the original event description together, comparing the event to the definitions of HACs determined by the Partnership for Patients program, and the harm event was resolved when consensus was reached. HACs represented 58 (24.2%; 95% confidence interval: 9.1–31.7%) of the total 240 harms identified by the PACHMT (Table 1). A complete list of the total 240 harms and their frequency is shown in Table 2.
DISCUSSION
To our knowledge, this is the first study that identifies the percentage of all-cause harm due specifically to HACs. At 24.2%, HACs represent only a subset of the total harm detected by a trigger tool that patients experience and therefore the substantial majority of harms are not HACs. The heavy regulatory focus placed on identifying, reporting, and improving HACs is useful, but its impact relative to overall harm may be limited. We recognize each HAC has varying influence on morbidity and resource utilization, but other preventable harms still have a significant impact on the overall harm burden patients experience.7–11 In a recent press release, the Partnership for Patients Hospital Engagement Network outlined a list of additional harms unaddressed by HACs that should be accounted for to pursue safety across the board.18 This may in part explain the lack of significant progress witnessed in multiple studies in reducing overall harm rates in the past 2 decades, even in the face of substantial efforts, many of them successful, to reduce HACs and other discrete harms.2 Also, because the scope of harm appears much broader, working on specific types of harms 1 at a time will likely not be transformational. Rather, establishing a true culture of safety, identifying and addressing root causes within hospital systems that drive multiple types of harms, and focusing on integrating high reliability principles and human-centered design across hospital care processes may have greater impact.
In the original study, for the 240 harms identified, only 22 (9.2%) were also identified within the hospital’s voluntary reporting system.15 Therefore, voluntary reporting of harms appears to underestimate the total burden of harm occurring in hospitals. Unfortunately, many types of harm not captured in the HACs, and infrequently reported in voluntary reporting systems, may go entirely unaddressed.
Although HACs do not capture all events, it is important to recognize their focus has been an improvement over relying solely on voluntary reporting for harm identification. Additionally, HACs have standard definitions and a body of best practices, which help target them. Voluntary reporting enhances understanding of safety vulnerabilities, even though it captures only a small fraction of all harms. These harm identification methods should not be viewed as competitive, but rather as complementary. As trigger tools and other methodologies come into usage, they provide a more systematic, comprehensive measurement of harm rates that complement and enhance these approaches. This will benefit providers, health care systems, and most importantly, patients.
This study has several limitations. Several HACs did not have an associated trigger (eg, falls, surgical-site infections, or venous thromboemboli) and may have been present in a patient record but not identified in our study. Also, because medical record review and HAC reviews were conducted at 2 distinct points in time (medical record reviews done as part of an earlier study), some HACs were not described in enough detail to confirm or deny their existence. Lastly, we recognize that at the time of original data collection, HAC definitions had not been fully developed. Future studies should attempt to review more recent medical records to determine if the percentage of harm burden that HACs represent has changed with the development of succinct definitions of HACs.
Importantly, Solutions for Patient Safety,19,20 a large group of pediatric hospitals whose work began as a part of the Partnership for Patients program, initially used the same HAC list cited herein as the types of harms that should be captured. In later years, Solutions for Patient Safety has broadened their HAC list to include additional harms, including Peripheral Intravenous Infiltration and Extravasations, Unplanned Extubations, and C. Difficile and Antimicrobial Stewardship, that may be more representative of the scope of harm occurring in a typical pediatric inpatient environment.21
CONCLUDING SUMMARY
In a 6-hospital effort to examine safety events, 1 in 4 identified adverse events identified by a pediatric-specific trigger tool met the definition of a HAC. Although substantial effort is focused on identifying and minimizing HACs, there remain many harm events that are unidentified and unmanaged using this approach.
DISCLOSURE
Dr. Stockwell reports partial employment by Pascal Metrics, a federally certified Patient Safety Organization. Dr. Landrigan reports having served as a paid consultant to Virgin Pulse to help develop a Sleep and Health Program. He has been supported in part by the Children’s Hospital Association for his work as an Executive Council member of the Pediatric Research in Inpatient Settings network. Dr. Landrigan has consulted with and holds equity in the I-PASS Institute, which seeks to train institutions in best handoff practices and aid in their implementation. In addition, Dr. Landrigan has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for teaching and consulting on sleep deprivation, physician performance, handoffs, and safety, and has served as an expert witness in cases regarding patient safety and sleep deprivation. Dr. Classen reports employment by Pascal Metrics, a federally certified Patient Safety Organization.
Footnotes
Published online May 25, 2018
Supported by the Children’s Hospital Association. Drs. Landrigan and Schuster and data collection at Boston Children’s Hospital were supported by U18 HS020513. The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality or the Centers for Medicare & Medicaid Services.
To cite: Stockwell DC, Landrigan CP, Schuster MA, Klugman D, Bisarya H, Classen DC, Dizon ZB, Hall M, Wood M, Sharek PJ. Using a Pediatric Trigger Tool to Estimate the Proportion of Total Harm Burden Hospital-acquired Conditions Represent. Pediatr Qual Saf 2018;3:081.
REFERENCES
- 1.Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. 1999Washington, D.C.: National Academies Press. [PubMed] [Google Scholar]
- 2.Landrigan CP, Parry GJ, Bones CB, et al. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363:2124–2134.. [DOI] [PubMed] [Google Scholar]
- 3.Classen DC, Resar R, Griffin F, et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30:581–589.. [DOI] [PubMed] [Google Scholar]
- 4.Levinson DR.Office of the inspector general. Adverse events in hospitals: methods for identifying events. Available at https://oig.hhs.gov/oei/reports/oei-06-08-00221.pdf. Accessed January 26, 2017.
- 5.Centers for Medicare & Medicaid Services. About the partnership for patients. Available at https://partnershipforpatients.cms.gov/about-the-partnership/aboutthepartnershipforpatients.html. Accessed January 26, 2017.
- 6.The Official U.S. Government Site for Medicaid. Hospital-acquired condition reduction program. Available at https://www.medicare.gov/hospitalcompare/HAC-reduction-program.html. Accessed January 26, 2017.
- 7.Roddy DJ, Spaeder MC, Pastor W, et al. Unplanned extubations in children: impact on hospital cost and length of stay. Pediatr Crit Care Med. 2015;16:572–575.. [DOI] [PubMed] [Google Scholar]
- 8.Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310.. [DOI] [PubMed] [Google Scholar]
- 10.Brown CH, 4th, LaFlam A, Max L, et al. Delirium after spine surgery in older adults: incidence, risk factors, and outcomes. J Am Geriatr Soc. 2016;64:2101–2108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGlone SM, Bailey RR, Zimmer SM, et al. The economic burden of Clostridium difficile. Clin Microbiol Infect. 2012;18:282–289.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway PH, Mostashari F, Clancy C. The future of quality measurement for improvement and accountability. JAMA. 2013;309:2215–2216.. [DOI] [PubMed] [Google Scholar]
- 13.Resar RK, Rozich JD, Classen D. Methodology and rationale for the measurement of harm with trigger tools. Qual Saf Health Care. 2003;12:ii39–45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffen FA, Resar RK. IHI global trigger tool for measuring adverse events. IHI Innovation Series White Paper. 2009. 2nd ed Cambridge, Mass.: Institute for Healthcare Improvement; Available at www.IHI.org. Accessed January 26, 2017. [Google Scholar]
- 15.Stockwell DC, Bisarya H, Classen DC, et al. A trigger tool to detect harm in pediatric inpatient settings. Pediatrics. 2015;135:1036–1042.. [DOI] [PubMed] [Google Scholar]
- 16.Kirkendall ES, Kloppenborg E, Papp J, et al. Measuring adverse events and levels of harm in pediatric inpatients with the Global Trigger Tool. Pediatrics. 2012;130:e1206–e1214.. [DOI] [PubMed] [Google Scholar]
- 17.Matlow AG, Cronin CM, Flintoft V, et al. Description of the development and validation of the Canadian Paediatric Trigger Tool. BMJ Qual Saf. 2011;20:416–423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services. Partnership for patients and hospital engagement networks: continuing forward momentum on reducing patient harm. Available at https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-09-25.html. Published September 25. 2015. Accessed February 13, 2018.
- 19.Lyren A, Brilli RJ, Zieker K, et al. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics. 2017;140 Epub: 16 August 2017. [DOI] [PubMed] [Google Scholar]
- 20.Lyren A, Brilli R, Bird M, et al. Ohio children’s hospitals’ solutions for patient safety: a framework for pediatric patient safety improvement. J Healthc Qual. 2016;38:213–222.. [DOI] [PubMed] [Google Scholar]
- 21.Children’s Hospitals’ Solutions for Patient Safety. SPS Network 2017 year in review annual report. Available at http://www.solutionsforpatientsafety.org/wp-content/uploads/2017-Year-in-Review.pdf. Accessed March 6, 2017.


