Supplemental Digital Content is available in the text.
Abstract
Introduction:
Feeding difficulties and malnutrition are important challenges when caring for newborns with critical congenital heart disease (CCHD) without clear available guidelines for providers. This study describes the utilization of a feeding protocol with the focus on standardization, feeding modality, and total parenteral nutrition (TPN) utilization postoperatively.
Methods:
Patients included neonates with CCHD undergoing complex biventricular repair using cardiopulmonary bypass. Data were collected in 2013 preintervention and from 2015 to 2017 postintervention. The feeding protocol outlined guidelines for and postoperative use of TPN. Adverse outcomes data included rates of central line–associated bloodstream infections, necrotizing enterocolitis, chylothorax, and vocal cord dysfunction. Balance outcomes measured were weight for age Z-score at discharge, number of abdominal radiographs obtained, readmission within 90 days, and central venous line utilization.
Results:
We included a total of 121 neonates: 49 in the preintervention group and 72 in the postintervention group. The protocol standardized feeding practices in CCHD neonates undergoing surgery with improved compliance from 70% early in the study period to 90% at the end of the study. Infants were fed enterally more preoperatively (86% versus 67%; P = 0.023), reached a fluid goal sooner (63 hours versus 72 hours; P = 0.035), and postoperative duration of TPN usage was significantly shorter in the postintervention period (48 hours versus 62 hours; P = 0.041) with no increase in adverse outcome events or unintended consequences.
Conclusions:
By implementing a feeding protocol, we reduced practice variation among providers, increased the number of patients fed enterally preoperatively and reduced postoperative use of TPN without increased complications.
INTRODUCTION
Infants undergoing cardiac surgery using cardiopulmonary bypass (CPB) have nutritional challenges resulting from the profound metabolic response they experience, which can result in negative outcomes like suboptimal wound healing and growth failure.1 This is true of most infants with critical congenital heart disease (CCHD), including those with biventricular physiology who are often relatively understudied.2 Physiological reasons why these infants struggle to achieve adequate nutrition include high-energy demands postoperatively, decreased energy stores, complex neuroendocrine responses and the high prevalence of comorbidities including genetic disorders, heart failure, gastrointestinal abnormalities, swallowing and vocal cord dysfunction, and fluid volume overload.3,4 Significant variability in pre- and postoperative feeding practices may also play a large role. Decisions regarding nutrition such as timing of feeds, choice of nutrition delivery, and rate of feeding advancement are often marred by subjectivity and personal biases.
Malnutrition has been described in many studies as linked to increased length of stay (LOS), increased mortality, and worse neurodevelopmental outcomes in neonates after cardiac surgery.3,4 The importance of nutrition in this population is well established; however, translating data into standardized practice and determining the superiority of early enteral nutrition (EN) and total parenteral nutrition (TPN) in hemodynamically stable patients remains challenging. Prior literature supports that adherence to a feeding protocol may promote earlier enteral feeding and shorter time to reach full calories and decrease the use of TPN and central lines.5,6 Studies have also shown patients receiving TPN have an increased risk of a central line–associated blood stram infections (CLABSI) and a reduction of time on TPN is beneficial to patients.7 Adherence to a feeding protocol may decrease these risks and improve quality of care by reducing practice variation among providers..1,4,8,9
The goal of this quality improvement (QI) project was to study the impact of a feeding protocol designed for infants with complex biventricular congenital heart disease on enteral feeding practices before and after congenital heart surgery and the use of TPN postoperatively. We hypothesized that the use of a standardized feeding protocol would improve pre- and postoperative enteral feeding milestones and decrease parenteral nutrition usage postoperatively.
METHODS
Setting and Context
This project is a retrospective, single-center study conducted at Primary Children’s Hospital (PCH) in Salt Lake City, Utah. PCH is a 289-bed, freestanding children’s hospital, serving 6 states in the western region of the United States with approximately 400 open-heart surgeries annually.
Preintervention
In 2014, we established a multidisciplinary QI team consisting of physicians, nurses, nurse practitioners, a speech therapist, a dietitian, a cardiothoracic surgeon, and a representative from the systems improvement department. The team determined that neonates undergoing biventricular repair were fed at different rates and with significant practice variability based on individual provider’s preference. Furthermore, the practitioners applied the single ventricle high-risk feeding protocol sporadically. This variability served as the impetus for pursuing standardization. The QI team investigated several protocols available in the literature and created the moderate risk feeding protocol with adaptations from the University of Alabama and Stanford (Supplemental Digital Content Figure 1, available at http://links.lww.com/PQ9/A24).10,11
The QI team identified factors that influenced the decision-making of physicians for initiation and advancement of nutrition. These factors included patient’s hemodynamic stability, whether the patient had an aortic arch reconstruction and/or delayed sternal closure. A key driver diagram delineated primary drivers for feeding practices as (1) Implementation of “moderate risk patient” feeding protocol with clear inclusion criteria; (2) Need for clear definitions of hemodynamic stability with indications for advancement and holding of feeds; and (3) Prompt involvement of speech and nutrition therapy before and after surgery (Fig. 1).
Fig. 1.
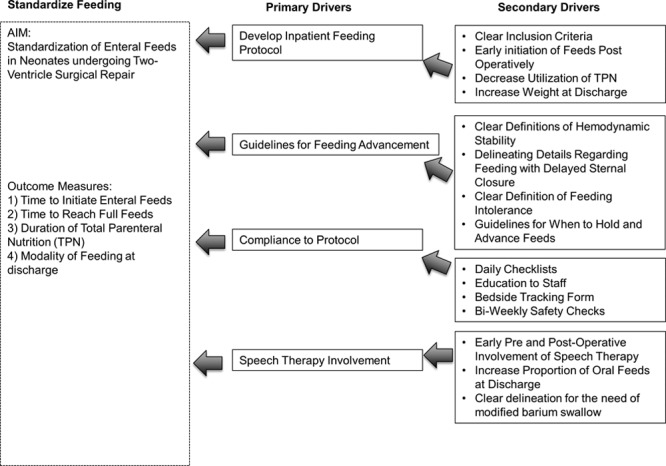
Key driver diagram showing framework for implementation of the moderate risk feeding protocol with presenting aims, outcome measures, and the theories for improvement (key drivers).
Planning and Implementing the Study Interventions
Interventions.
We implemented the moderate risk feeding protocol in all patients undergoing biventricular repair. The protocol focused on early involvement of a speech therapist (ST) and a dietitian. The ST used infant behavioral cues to introduce early oral feeding pre- and postoperatively. They also provided education to staff during the preoperative period. The protocol specified when to initiate EN and the criteria for advancement pre and postoperatively. We set the initial feeding volume goal at 135 ml/kg/d, which is the institutional full volume goal for a mechanically ventilated neonate. This volume goal was selected as a marker of movement through the protocol as it was a simple data point to identify in the medical record and provides > 75% of full extubated goal nutrition.12 The protocol defines criteria for feeding advancement based on hemodynamic stability and feeding tolerance. TPN use was limited to preoperative patients dependent on prostaglandin (PGE) to maintain systemic blood flow, or in those postoperative patients with delayed chest closure, high inotropic support or inability to tolerate enteral feeds. Intravenous lipids were used to supplement calories while feeds were advanced. The preoperative component of the feeding protocol was formalized several months after the initiation of the postoperative feeding protocol. It specified the amount and mode of feeding dependent on whether the patient had systemic or pulmonary ductal dependent circulation.
The QI team educated personnel with a focus on inclusion criteria and the individual components of the feeding protocol. The education program involved live presentations and online education modules. Reinforcement continued using a daily rounding checklist, clinical review meetings, newsletters, and bulletin boards. We conducted several multidisciplinary conferences to receive feedback and to promote discussion regarding potential concerns. These conferences served to reinforce buy-in among individual providers.
Evolution of the Interventions
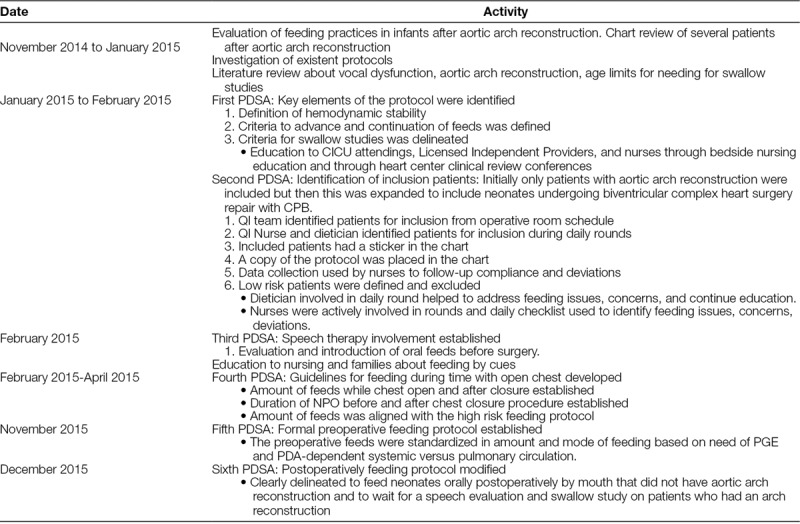
To monitor compliance, we used a checklist during rounds to identify enrollment candidates, protocol deviations, or adverse feeding related events. Bi-weekly safety rounds helped to answer questions and provide education. A bedside tracking form was utilized to identify complications and reasons for not advancing feeds. In addition, the QI team performed weekly audits of patients enrolled in the protocol. A timeline and description of the Plan-Do-Study-Act cycles (PDSA) and evolution of the protocol are described in detail in Table 1.
Table 1.
Timeline and Description of Mini PDSA Cycles
Cohort Identification
We identified preintervention patients who underwent cardiac surgery from January 2013 to December 2013 retrospectively. We selected patients younger than 30 days of age who underwent a biventricular surgical repair. Postintervention patients were identified from February 2015 to February 2017 prospectively from the operative room schedule, cardiothoracic conferences or at the time of preoperative admission.
Inclusion Criteria
Patients included neonates admitted preoperatively to the cardiac intensive care unit (CICU) with pre- or postnatal diagnosis of CCHD undergoing biventricular repair via median sternotomy using CPB, < 30 days of age, older than 36 weeks gestational age at birth and more than 2.2 kg birth weight. Patients admitted to CICU and transferred to the ward were still included as they remained on the feeding protocol once initiated.
Exclusion Criteria
We excluded neonates with a surgical plan of single ventricle palliation, pulmonary artery band, and central shunts, neonates who received preoperative care in the neonatal intensive care unit and those who were home or who underwent urgent surgery the day of admission due to the inability for preoperative evaluation and education by speech therapy. Patients with necrotizing enterocolitis preoperatively and those who had cardiac surgery via thoracotomy were also excluded.
Measures
Collected data included general demographics, patient characteristics such as the presence of genetic abnormalities, primary cardiac lesion, use of PGE, utilization of umbilical arterial catheter (UAC), operative data, surgical complexity measured by The Society of Thoracic Surgeons- European Association for Cardio-Thoracic Surgery categories (STAT) and postoperative clinical features.
Outcome Measures.
Outcome measures include the modality of feeding before surgery and at hospital discharge and the duration of postoperative TPN. We also included the time to initiate oral or tube fed enteral feeds and time to reach 135 ml/kg of enteral feeds postoperatively.
Process Measures.
We measured compliance with initiation and advancement of feeds. Compliance was measured with biweekly audits and the use of a daily rounding checklist. A patient was defined as compliant if they were advanced along the protocol without deviation. Process measures include speech therapy involvement pre- and postoperatively in both pre- and postintervention groups and number of swallow studies per patient for up to 90 days after discharge.
Balance Measures
Balance measures included rates of complications after surgery such as NEC, central venous line (CVL) days, CLABSI, chylothorax, and vocal cord dysfunction. In addition, weight for age Z-score at discharge, rates of rehospitalization within 90 days of discharge, number of abdominal radiographs per patient obtained after surgery to discharge were included.
Statistical Analysis
Demographic and clinical characteristics are expressed as mean ± SD or median/interquartile range (IQR) as appropriate. For comparison of continuous variables between 2 groups, we used a Student’s t test for normally distributed data and a Wilcoxon rank sum test for skewed data. Categorical variables were compared using contingency tables or Fisher’s exact test. A P value of < 0.05 was considered significant. All data were analyzed using SPSS 24.0 for Windows (SPSS, Chicago, Ill.). We used Minitab18 (Minitab, Inc., State College, Pa.) to produce statistical process control charts.
Ethical Considerations
The study was approved and a waiver of informed consent granted by the University of Utah Institutional Review Board and PCH Privacy Board. We followed the Standards for Quality Improvement reporting Excellence Guidelines (SQUIRE 2.0) in this report.13
RESULTS
A total of 129 neonates were initially included in the study. We excluded 4 neonates initially admitted to the neonatal intensive care unit and 2 neonates that had urgent surgery the day of birth. We excluded 2 patients for NEC. One patient required laparotomy secondary to a bowel perforation due to pacing wires at the time of surgery. The other patient presented on the 11th day of life with coarctation of the aorta and was diagnosed with NEC preoperatively. Thus, the final analysis included 121 patients: 49 in the preintervention and 72 in the postintervention groups.
Preintervention Versus Postintervention Groups
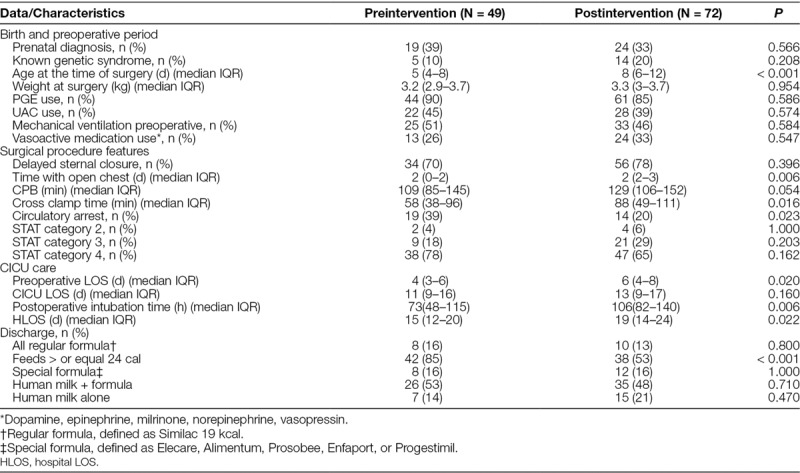
Table 2 summarizes the demographic and clinical characteristics. Baseline characteristics including weight at surgery, the proportion of genetic abnormalities and prenatal diagnosis, CICU LOS, and the surgical STAT category were similar between the 2 groups. The patients in the postintervention group were older at the time of surgery (8 versus 5 days; P < 0.001). They had longer presurgery LOS (6 versus 4 days; P = 0.02) and had open chest longer (2 days [IQR, 2–3] versus 2 days [IQR, 0–2]; P = 0.006). Patients with transposition of the great arteries had the longest presurgery LOS postintervention (4.5 days versus 8 days; P ≤ 0.001). Patients in the postintervention group also had a longer hospital LOS (19 versus 15 days; P = 0.022), longer cross-clamp time (88 versus 58 minutes; P = 0.016), and longer postoperative intubation time (106 versus 73 hours; P = 0.006). The post- and preintervention groups had similar cardiac lesions. The most common included hypoplastic aortic arch with and without other lesions (36% versus 47%; P = 0.26) and transposition of the great vessels with and without intact ventricular septum ± other extracardiac lesions (35% versus 26%; P = 0.42 Supplemental Digital Content 2, Table 1, available at http://links.lww.com/PQ9/A25).
Table 2.
Demographic and Clinical Characteristics of Neonates with Biventricular Repair Pre- and Postimplementation of Feeding Protocol
Measures
Primary Outcome Measures.
In the postintervention group, a higher proportion of infants were fed enterally before surgery (86% versus 67%; P = 0.023) despite similar proportions of PGE and UAC use pre- and postintervention (Table 3). The proportion of patients requiring TPN postoperatively was similar, but the duration of TPN was significantly shorter (48 versus 62 hours; P = 0.041; Fig. 2). Time to postoperative enteral feeds was not different (Figure 2, Supplemental Digital Content available at http://links.lww.com/PQ9/A26). However, time to reach 135 ml/kg was significantly less in the postoperative period (63 hours versus 72 hours; P = 0.035; Figure 3, Supplemental Digital Content available at http://links.lww.com/PQ9/A27). The majority of neonates in both periods were discharged on a combination of oral and nasogastric (NG) feeds, or NG feeds exclusively with a small proportion of patients discharged home on oral feeds or exclusive breastfeeding.
Table 3.
Primary, Process, and Balance Outcome Measures
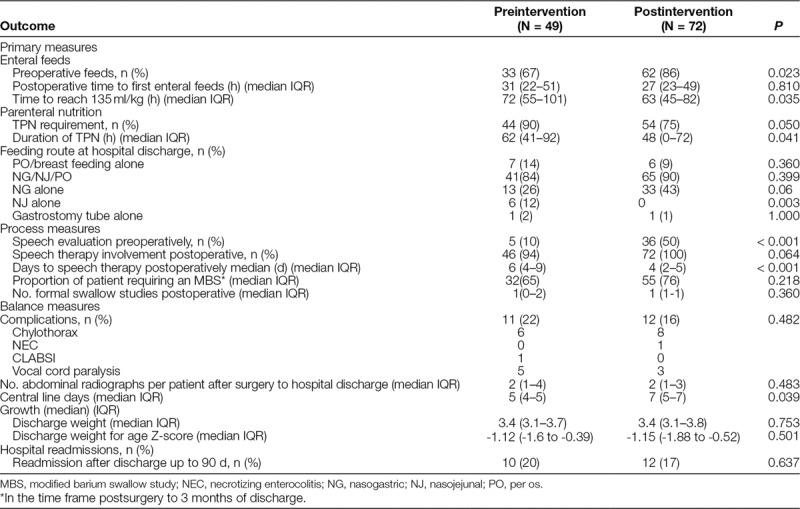
Fig. 2.
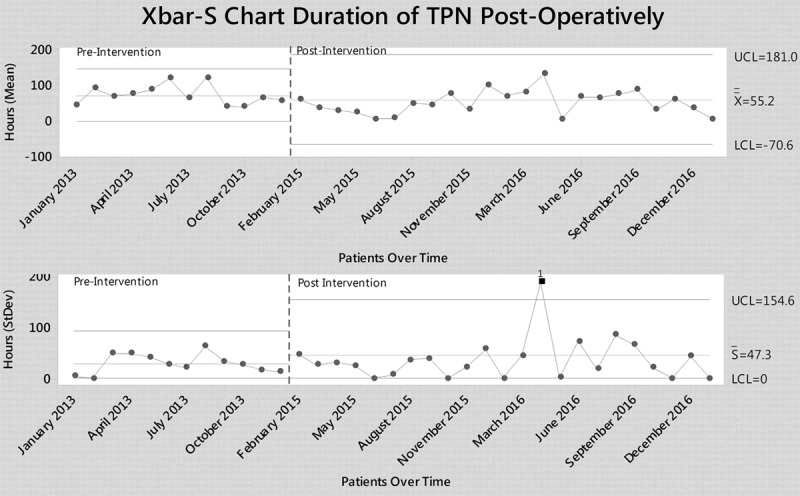
Xbar S control chart showing reduction in total duration of TPN. LCL, lower control limit; UCL, upper control limit;  , mean;
, mean;  , average SD 1: point more than 3 SD from center line. Figure made with Minitab18.
, average SD 1: point more than 3 SD from center line. Figure made with Minitab18.
Process Measures.
Sixty-nine charts were reviewed to evaluate compliance at the initiation and for the advancement of feeds. The compliance at the initiation of the protocol was 70% and improved over time to 90% facilitated by the use of a single order to achieve full volume goal feeds of 135 ml/kg/d (Fig. 3). Infants received significantly more ST consults preoperatively. Furthermore, the timing of speech interventions postoperatively was shortened by 2 days (Table 3).
Fig. 3.
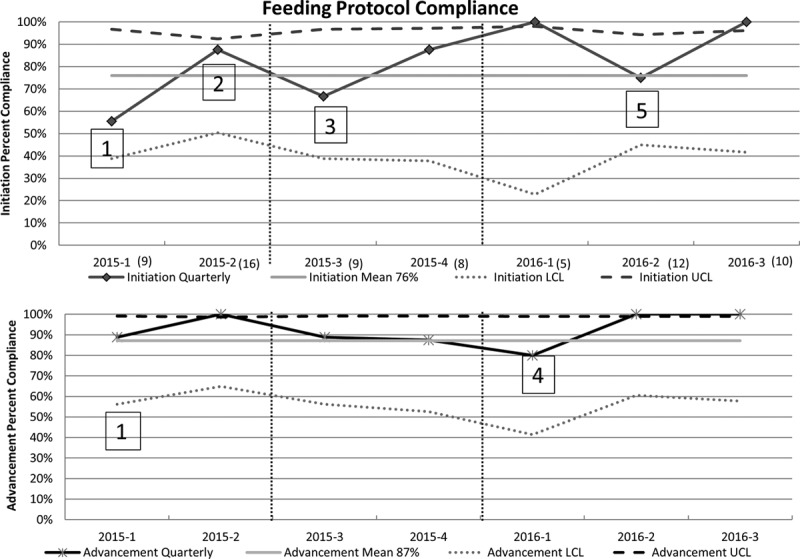
p chart showing initiation compliance and advancement compliance over time. Years are divided into 4 quarters with 3 months in each quarter and amount of patients screened in parentheses. LCL, lower control limit a: education reinforced amount to feed during open sternum vs. closed sternum b: education reinforced how to increase feeds by weight in milliliters every 6 hours to reach 135 ml/kg. UCL, upper control limit. 1Education to providers regarding inclusion criteria and definition of hemodynamic stability.2Education to providers regarding nothing by mouth status before and after chest closure and amount of feeds with an open chest. 3Amount of feeds aligned with high risk protocol causing confusion and requiring education. 4Re-education regarding advancement. 5Re-education regarding initiation of feeds in patients with primary chest closure verses delayed sternal closure.
The early study PDSAs were focused on widespread education to the providers regarding inclusion criteria, definition of pivotal elements of the protocol such as hemodynamic stability, criteria for advancement and which patients require swallow studies. The compliance to the protocol early in the study showed overall compliance of 70% showing deficits particularly in the initiation phase with only 55% compliance. Initial intervention to improve compliance included education regarding duration of not feeding by mouth before and after chest closure procedure and amount of feeds while the chest was open. With this intervention, there was improvement in compliance to 85% in the initiation phase of the protocol. However, there was a decline in the third quarter of 2015 to 66% with significant impact once the protocol was aligned to mirror the established high-risk protocol designed for single ventricle patients. The adjustment included providing feeds at 1 ml/h instead of 1 ml/kg/h while the chest was open. With education at the bedside and posters throughout the unit, there was steady improvement in initiation compliance to 85%. The advancement phase of the protocol had steady high compliance around 90%. However, there was a decline in the advancement compliance in the first quarter of 2016. The compliance significantly improved after reeducation to providers about regimen of feeds to increase by 1 ml/kg every 6 hours. We saw a steady improvement in compliance in both the initiation and advancement, reaching an overall compliance of 90% at the end of the study.
Balance Measures
CVL days increased in the postintervention period from a median of 5.5–6.5 days. However, complications including CLABSIs did not increase. There were no differences among discharge weights for age Z-score, readmissions up to 90 days, or the median number of abdominal radiographs per patient (Table 3).
DISCUSSION
The study found that implementation of a feeding protocol for CCHD undergoing complex biventricular repair resulted in improved consistency with methods in which neonates were fed enterally and an increase in the number of neonates fed enterally preoperatively. It also shows a reduction in the time to reach full volume goal feeds postoperatively, and a decrease in the postoperative use of TPN. The protocol was not associated with an increase in adverse events or unintended consequences.
Our protocol and study design is unique as we have focused on patients with biventricular physiology who underwent CPB and were occasionally hemodynamically unstable. Current published literature focuses on feeding protocols in neonates with single ventricles or mixed lesions including single and biventricular physiology. Some of the studies emphasize caloric delivery while others focus on standardization and decrease practice variation among providers.8 One major difference compared with other prior studies such as Simsic et al.8 is every patient in our inclusion cohort underwent CPB.8 Newcombe and Fry-Bowers2 excluded patients with hemodynamic instability defined as patients on Milrinone infusions or inotropic drips. We included the majority of these patients in our study.2
The National Pediatric Cardiology Quality Improvement Collaboration identified variability in feeding practices as a contributor to poor growth in single ventricle infants and encouraged the use of feeding algorithms to reduce provider inconsistency.12 This variability is a result of controversy regarding the safety of enteral feeding CHD patients preoperatively. There has historically been hesitation to feed these patients before surgery secondary to the concern for the risk of development of NEC.14 This risk of NEC is higher among single ventricle physiology patients.15–17 On the contrary, other studies have shown preoperative enteral feeding can be associated with decreased risk of prolonged feeding difficulties postoperatively in some populations and18 delaying enteral feeding may contribute to increased gut abnormalities and even delayed postnatal intestinal development, maturation, and motility problems.19 These data suggest the importance of enterally feeding hemodynamically stable patients preoperatively. The National Pediatric Cardiology Quality Improvement Collaboration also recommends enteral feeding in patients on PGE infusions and those who have an UAC.12 By instituting these same practices of a feeding protocol in the biventricular repair neonatal population, we showed a significant increase in preoperative feeding, without an increased incidence of NEC and despite PGE infusion and the presence of UAC.
One of the benefits of this protocol is the significantly increased preoperative speech therapy involvement. The goal of speech intervention was to facilitate long-term feeding success. The proportion of patients discharged home on full oral feeds did not improve. Literature has shown that tube feeding when nutritional requirements cannot be met orally is supported as safe and effective and results in shorter LOS and less risk of malnutrition.20,21 Neonates with CHD face significant barriers to successful oral feeding by hospital discharge22 and enteral feeding guidelines focus on hemodynamic stability and nutrition goals without regard to developmental milestones necessary to support oral feeds. Future longitudinal research will be necessary to analyze long-term follow-up in these patients regarding the association of earlier introduction of enteral feeding and duration of supplemental tube feedings.
Postoperative nutrition is important in this population, and malnutrition has been associated with morbidities such as increased LOS and increased mortality.3,4 We were able to decrease the time to reach goal feeds of 135 ml/kg/d by adherence to the feeding protocol. This feeding goal is not representative of caloric intake but was required before increasing caloric density and was representative of about 75% of the infant’s caloric need. This predetermined feeding goal allowed for monitoring of compliance with the protocol, which would then ensure enteral caloric delivery.
The role of parenteral nutrition to reach goal nutrient delivery and the time to initiate TPN when EN is insufficient remains unknown.23 However, TPN should be used for supplementation in infants who are severely malnourished, have poor enteral feed tolerance or are at risk of nutritional deterioration.24 We show that by use of a feeding protocol, TPN therapy was significantly reduced in the postintervention group, without adversely affecting weight for age Z-score at discharge. Several studies in children demonstrate clinical superiority of decreased TPN use in the intensive care unit.25 Patients have fewer new infections regardless of CVL duration, decreased cost, and shorter LOS.25–28 The American Society for Parenteral and Enteral Nutrition clinical guidelines state that EN should be the preferred mode of nutrient provision for critically ill children if tolerated.25 The concerns surrounding the timing and use of TPN in critically ill children can be clarified by the institution of clear guidelines outlining hemodynamic criteria for enteral feeding tolerance and timelines for initiation and discontinuation of TPN.23
Contrary to other studies, our postintervention group had increased hospital LOS. This increase is most likely attributable to the significantly increased preoperative wait period. This was identified upon further evaluation within our institution as most common among patients with a diagnosis of transposition of the great arteries, which comprised a fair majority (35%) of our defined moderate risk population. There has since been an initiative to decrease the preoperative wait time in these patients. There also was a gap of 1 year between preintervention and postintervention period during which there was a transition from 1 surgeon to another.
A major success of the current feeding protocol was the overall improvement in protocol adherence to 90% at the end of the study from an earlier adherence rate of 70%. Compliance is not always measured and reported in the literature, and yet it is difficult to assume outcome association with protocols without measured compliance. Close attention was paid to interim analysis to investigate when compliance began to decrease at certain intervals. One decline was attributed to delays in advancement when each feeding change required a new order. Therefore, we initiated a 1-click order than allowed for nursing controlled advancement without physician orders. Protocol deviations were due to clinical conditions such as fluid overload as providers deviated from the protocol to restrict total fluid intake. A second common reason for protocol deviation was the presence of postoperative high-output chylothorax. Once this occurred, patients were placed on a separate feeding algorithm. To further improve compliance in the future, we have made simplifications and modifications to our current feeding protocol to eliminate the use of TPN postoperatively altogether unless a patient has significant delays in initiation of EN. This change was initiated after results revealed that even though the use of TPN was significantly decreased in the postintervention group, this did not affect weight at discharge.
LIMITATIONS
This study has important limitations as a single center study with a unique medical record system. The 1-year period between pre- and postintervention groups was purposeful by design because the protocol development had begun, and premature implementation could introduce potential bias in the physician’s decisions before full implementation. However, the data from the postintervention period still may reflect change in practice that preceded the formal rollout of the protocol. This is especially true for data regarding the preoperative feeding rates where this part of the protocol was not formalized until several months into the postintervention period. The protocol was designed to target a specific fluid goal of 135 ml/kg/d as a marker of progression through the protocol rather than caloric goals necessary for weight gain. This target poses challenges in the interpretation of weight-related outcomes. It is unclear as to whether the increased LOS in the postintervention period was attributable to the adherence to this feeding protocol or secondary to the increased preoperative LOS and the complexity of the patient population. However, we cannot completely dismiss this as an unintended consequence, and this will be further evaluated by an internal institutional review. Lastly, despite limiting our analysis to infants with biventricular repairs, this is still a very heterogeneous population.
CONCLUSIONS
The implementation of a feeding protocol for neonates undergoing biventricular repair was associated with higher rates of preoperative enteral feeding and decreased time to goal enteral volume and utilization of TPN postoperatively. This collaborative effort from a multidisciplinary team improved standardization of care.
ACKNOWLEDGMENTS
The authors thank Randall Smout, MS, for providing analytical support and Annie Brazell, NP, for help in implementing the PDSA cycles.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online May 18, 2018
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Furlong-Dillard J, Neary A, Marietta J, Jones C, Jeffers G, Gakenheimer L, Puchalski M, Eckauser A, Delgado-Corcoran C. Evaluating the Impact of a Feeding Protocol in Neonates before and after Biventricular Cardiac Surgery: A Quality Improvement Project. Pediatr Qual Saf 2018;3:080.
REFERENCES
- 1.Owens JL, Musa N. Nutrition support after neonatal cardiac surgery. Nutr Clin Pract. 2009;24:242–249.. [DOI] [PubMed] [Google Scholar]
- 2.Newcombe J, Fry-Bowers E. A post-operative feeding protocol to improve outcomes for neonates with critical congenital heart disease. J Pediatr Nurs. 2017;35:139–143.. [DOI] [PubMed] [Google Scholar]
- 3.Wong JJ, Cheifetz IM, Ong C, et al. Nutrition support for children undergoing congenital heart surgeries: a narrative review. World J Pediatr Congenit Heart Surg. 2015;6:443–454.. [DOI] [PubMed] [Google Scholar]
- 4.Wang KS, Ford HR, Upperman JS. Metabolic response to stress in the neonate who has surgery. Neoreviews. 2006;7:410–418.. [Google Scholar]
- 5.Alten JA, Rhodes LA, Tabbutt S, et al. Perioperative feeding management of neonates with CHD: analysis of the Pediatric Cardiac Critical Care Consortium (PC4) registry. Cardiol Young. 2015;25:1593–1601.. [DOI] [PubMed] [Google Scholar]
- 6.Scahill CJ, Graham EM, Atz AM, et al. Preoperative feeding neonates with cardiac disease. World J Pediatr Congenit Heart Surg. 2017;8:62–68.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimura S, Miyazu M, Yoshizawa S, et al. Efficacy of an enteral feeding protocol for providing nutritional support after paediatric cardiac surgery. Anaesth Intensive Care. 2015;43:587–593.. [DOI] [PubMed] [Google Scholar]
- 8.Simsic JM, Carpenito KR, Kirchner K, et al. Reducing variation in feeding newborns with congenital heart disease. Congenit Heart Dis. 2017;12:275–281.. [DOI] [PubMed] [Google Scholar]
- 9.Madhok AB, Ojamaa K, Haridas V, et al. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–413.. [DOI] [PubMed] [Google Scholar]
- 10.Moellinger A, Torsch S, Abernathy S, et al. Postoperative feeding protocol improves outcomes following arterial switch operation. Poster Presented at: 27th Annual Southeast Pediatric Cardiovascular Society Conference;2014 Sept 5–6; Durham, N.C. [Google Scholar]
- 11.Heart Center Feeding Algorithm: Infant > 2.5 kg. Available at https://lane.stanford.edu/portals/cvicu.html. Accessed December 28, 2017.
- 12.Slicker J, Hehir DA, Horsley M, et al. ; Feeding Work Group of the National Pediatric Cardiology Quality Improvement Collaborative. Nutrition algorithms for infants with hypoplastic left heart syndrome; birth through the first interstage period. Congenit Heart Dis. 2013;8:89–102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards or QUality Improvement Reporting Excellence) revised publication guidelines from a detailed consensus process. J Contin Educ Nurs. 2015;46:501–507.. [DOI] [PubMed] [Google Scholar]
- 14.Scahill CJ, Graham EM, Atz AM, et al. Preoperative feeding neonates with cardiac disease. World J Pediatr Congenit Heart Surg. 2017;8:62–68.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker KC, Hornik CP, Cotten CM, et al. Necrotizing enterocolitis in infants with ductal-dependent congenital heart disease. Am J Perinatol. 2015;32:633–638.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElhinney DB, Hedrick HL, Bush DM, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106:1080–1087.. [DOI] [PubMed] [Google Scholar]
- 17.Giannone PJ, Luce WA, Nankervis CA, et al. Necrotizing enterocolitis in neonates with congenital heart disease. Life Sci. 2008;82:341–347.. [DOI] [PubMed] [Google Scholar]
- 18.Coker-Bolt P, Jarrard C, Woodard F, Merrill P. The effects of oral motor stimulation on feeding behaviors of infants born with univentricle anatomy. J Pediatr Nurs. 2013;28:64–71.. [DOI] [PubMed] [Google Scholar]
- 19.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007;85:629S–634S.. [DOI] [PubMed] [Google Scholar]
- 20.Kuwata S, Iwamoto Y, Ishido H, et al. Duodenal tube feeding: an alternative approach for effectively promoting weight gain in children with gastroesophageal reflux and congenital heart disease. Gastroenterol Res Pract. 2013;2013:181604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwalbe-Terilli CR, Hartman DH, Nagle ML, et al. Enteral feeding and caloric intake in neonates after cardiac surgery. Am J Crit Care. 2009;18:52–57.. [DOI] [PubMed] [Google Scholar]
- 22.Sables-Baus S, Kaufman J, Cook P, et al. Oral feeding outcomes in neonates with congenital cardiac disease undergoing cardiac surgery. Cardiol Young. 2012;22:42–48.. [DOI] [PubMed] [Google Scholar]
- 23.Vichayavilas P, Gist K, Kaufman J. More and sooner, but not necessarily better. J Thorac Dis. 2016;8:1877–1879.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cahill NE, Murch L, Jeejeebhoy K, et al. When early enteral feeding is not possible in critically ill patients: results of a multicenter observational study. JPEN J Parenter Enteral Nutr. 2011;35:160–168.. [DOI] [PubMed] [Google Scholar]
- 25.Fivez T, Kerklaan D, Mesotten D, et al. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374:1111–1122.. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca A, Burgermaster M, Larson E, et al. The relationship between total parenteral nutrition and central line-associated bloodstream infections. FASEB J. 2016;30:916–917.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutsogiannis J, Alberda C, Gramlich L, et al. Early use of supplemental parenteral nutrition in critically ill patients: results of an international multicenter observational study. Crit Care Med. 2011;39:2691–2699.. [DOI] [PubMed] [Google Scholar]
- 28.McClave SA, Martindale RG, Vanek VW, et al. ; A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009;33:277–316.. [DOI] [PubMed] [Google Scholar]


