Abstract
Introduction:
In critically ill children, inappropriate urinary catheter (UC) utilization is associated with increased morbidity, including catheter-associated urinary tract infections (CAUTIs). Checklists are effective for reducing medical errors, but there is little data on their impact on device utilization in pediatric critical care. In this study, we evaluated UC utilization trends and CAUTI rate after implementing a daily rounding checklist.
Methods:
A retrospective review of our checklist database from 2006 through 2016 was performed. The study setting was a 36-bed pediatric intensive care unit in a quaternary-care pediatric hospital. Interventions included the “Daily QI Checklist” in 2006, ongoing education regarding device necessity, and a CAUTI prevention bundle in 2013. UC utilization and duration were assessed via auto-correlated time series models and Cochran-Armitage tests for trend. Changes in CAUTI rate were assessed via Poisson regression.
Results:
UC utilization decreased from 30% of patient-days in 2006 to 18% in 2016 (P < 0.0001, Cochran-Armitage trend test), while duration of UC use (median, 2.0 days; interquartile range, 1–4) did not change over time (P = 0.18). CAUTI rate declined from 9.49/1,000 UC-days in 2009 to 1.04 in 2016 (P = 0.0047).
Conclusions:
Implementation of the checklist coincided with a sustained 40% reduction in UC utilization. The trend may be explained by a combination of more appropriate selection of patients for catheterization and improved timeliness of UC discontinuation. We also observed an 89% decline in CAUTI rate that occurred after stabilization of UC utilization. These findings underscore the potential impact of a checklist on incorporating best practices into daily care of critically ill children.
INTRODUCTION
Infection of the urinary tract represents a major contributor to hospital-associated morbidity, comprising 32% of all health care–associated infections (HAIs) and 13% of deaths due to health care–associated infections.1 The greatest risk factor for urinary tract infection in the hospital setting is the presence of a urinary catheter (UC),2 with 70–80% of urinary tract infections attributable to indwelling UCs.3 In turn, catheter-associated tract urinary infections (CAUTIs) markedly increase duration of hospitalization, cost, and mortality risk.4,5 Despite their prevalence and impact, 65–70% of CAUTIs are preventable.6 The Centers for Medicare and Medicaid Services does not reimburse hospitals for CAUTIs,7 a significant position, given that CAUTIs cost inpatient hospital services approximately $400 million in 2002 according to a Centers for Disease Control (CDC) analysis.8 As a result of the cost, morbidity, and potential for prevention of CAUTIs, there is significant financial and ethical impetus for health care institutions to reduce CAUTI rates.
A variety of methods of reducing CAUTIs have been reported and evaluated in the literature. A series of Cochrane reviews found that specialized drainage systems and antimicrobial catheter materials are not sufficient to significantly reduce CAUTI rates.9,10 Indeed, the CDC recommends against using complex urinary drainage systems and only recommends using antimicrobial-impregnated catheters as a second-line measure after implementing other CAUTI reduction strategies.11 The CDC also strongly recommends avoiding unnecessary UC placement and decreasing the duration that the UC is left in place. Unfortunately, unnecessary initiation of UCs is common, with as many as half of UCs being placed without an appropriate indication.12 In addition, prolonged catheterization results in the formation of pathogenic biofilms on the catheter surface, which serve as a nidus for infection.13,14 CAUTI rates rise linearly with increasing duration of catheterization.15 Conversely, decreasing inappropriate UC placement and duration not only reduces CAUTIs, but also reduces other forms of morbidity associated with UC use including urethral stricture, mobility impairment, gross hematuria, and urine leakage.16,17
Like other medical errors, inappropriate or prolonged urinary catheterizations are avoidable events sensitive to quality improvement interventions. Checklists are an effective tool for safeguarding against preventable medical errors and for improving compliance with best practices in a variety of settings.18,19 For example, a 2014 review by Simpson et al.20 found that the use of bundled interventions including a checklist was associated with lower rates of central-line–related bloodstream infections in critically adults.20 In another intensive care study, Berenholtz et al.21 estimated that the use of a checklist prevented 43 catheter-related bloodstream infections, 8 deaths, and $1.95 million in additional costs per year.21 Checklists have also been shown to reduce UC utilization and CAUTIs specifically. A study evaluating the effect of a UC indication checklist in an emergency department found that the introduction of the checklist coincided with a 75% reduction in the number of UCs placed over a 5-year period and a 22% decrease in inappropriate UC placement.22 In an adult critical care setting, another study investigating the effect of a checklist on the use of indwelling UCs demonstrated a significant reduction in the duration of catheterization and occurrences of CAUTIs.23 A similar study demonstrated that implementation of a daily rounding checklist in an adult intensive care unit reduced UC utilization by 15%.24
Although the vast majority of studies on UC utilization have focused on adults, CAUTIs are also an important source of morbidity in hospitalized children. Indeed, CAUTI rates in both critical care and general inpatient settings are similar for adult and pediatric populations.25 The few pediatric investigations that have been carried out are promising. A small study investigating the impact of a daily safety checklist in a pediatric intensive care unit (PICU) reported a significant reduction in UC-days over a 21-month period.26 Additionally, 2 studies investigating the impact of comprehensive quality improvement programs reported drops in CAUTI rate from 23.3 to 5.8/1,000 and 5.41 to 2.49/1,000 catheter-days in pediatric critical care and hospital-wide settings, respectively.27,28 The present study sought to add to this body of evidence by assessing trends in UC utilization and CAUTI rate after implementing a daily multi-professional verbalized checklist in a high-volume, quaternary-care PICU.
METHODS
Study Setting
The study was approved by the Institutional Review Board of Children’s Healthcare of Atlanta (CHOA) and took place in the PICU of Children’s Healthcare of Atlanta at Egleston, an academic, quaternary-care hospital. The PICU is a 36-bed combined medical/surgical (noncardiac) unit housed within the 272-bed pediatric hospital. It is equipped to care for children from 0 to 21 years of age with standard critical illnesses in addition to continuous renal replacement therapy, organ transplantation, and extracorporeal life support. Patients are rounded on each morning by a multidisciplinary team including a pediatric critical care medicine attending, fellow, resident, nurse, respiratory therapist, nutritionist, and pharmacist.
Intervention
A PICU Daily QI Checklist was developed in 2006 (Fig. 1). The checklist was developed internally via collaboration between physician and nursing leadership. It was initially on paper and subsequently was converted to a Microsoft Access database available to the attendings and fellows. It is completed during daily morning rounds by a physician member of the multidisciplinary team at the conclusion of bedside rounding, before moving to the next patient. For every patient in the PICU, the physician, usually a fellow, verbally reviews the checklist with each patient’s nurse while the full team is still present. Responses to the checklist are based on the status of the patient at rounding time, not on what was planned for the day. For example, a sedation holiday was marked “yes” only if it was performed within the previous 24 hours; a device was marked present only if it was still in place at the time of rounding. The PICU Daily QI Checklist was implemented in January 2006. In August 2009, the PICU began tracking CAUTIs in a separate database. In January 2013, the critical care department joined the Solutions for Patient Safety. As part of this collaborative, efforts to cultivate a nurse-driven, “one is not zero” culture were implemented in addition to a formal urinary tract infection prevention bundle. Components of the bundle include aseptic insertion technique, avoiding unnecessary utilization, maintaining a closed drainage system, perineal hygiene, bag placement below level of bladder with unobstructed flow, removal when no longer needed, and catheter securement. As these changes were made, the tracking of device-days transformed into daily discussion of device necessity aimed to reduce device utilization where appropriate.
Fig. 1.
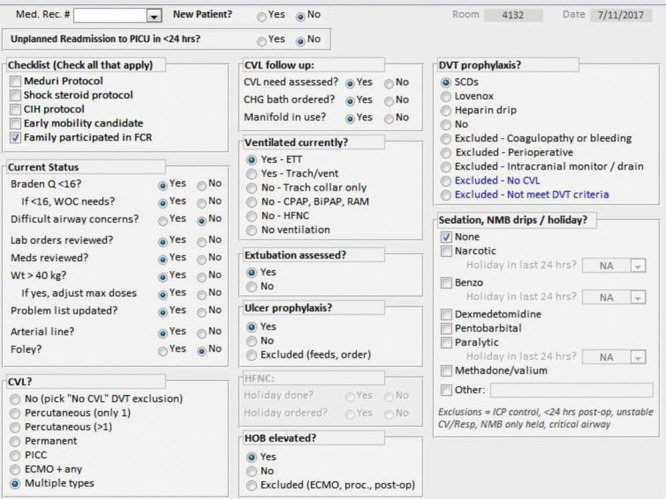
PICU daily rounding checklist.
Study Design and Outcomes
A retrospective review of our checklist database from April 2006 through December 2016 was performed. PICU patients were selected if they had a UC at any point in their PICU stay. Device utilization ratio was calculated as UC-day per patient-day. Duration of catheterization was expressed as median UC-days per patient admission. To control for variability in admission time, duration was also assessed as the ratio of UC-days to length of stay (LOS). CAUTI rate was calculated as the number of infections per 1,000 catheter-days.
Statistical Analysis
Descriptive statistics including proportions and medians [interquartile range (IQR)] were used to characterize the study population. Median descriptive statistics were reported instead of means due to nonnormal distributions and large variances in estimates. Cochran-Armitage trend tests were used to assess changes in overall UC catheter utilization, measured as the proportions of device days per total patient days. Trends in UC duration (median device days per patient’s admission and ratio of device days to total LOS per patient admission) were evaluated with auto-correlated time series models using monthly intervals. Similar methods were used to assess trends in checklist compliance. Compliance for the UC portion of the checklist was determined by dividing the number of patients for whom a response to the “Foley?” item (Fig. 1) was recorded by the total number of patients in the database. Compliance data for the CAUTI bundle was not available. In addition to Pediatric Risk of Mortality (PRISM) and Pediatric Index of Mortality 2 (PIM2) scores, which were only available for years 2011 to 2015, illness severity was approximated by percentage of ventilation days. Durbin-Watson statistics for autocorrelation were checked, and model residuals were assessed for normality. To examine overall CAUTI rate differences across years, rates were modeled using a Poisson model with year as a categorical predictor. Rate differences between different years were assessed by looking at differences in least-squares mean rates. A statistical process control chart (u-chart) created with QI Macros was also plotted to examine variation in the monthly CAUTI rate following implementation of the bundle. Standard definitions for determining centerline shifts and upper and lower control limits were used. All statistical testing was conducted using SAS 9.4 (Cary, N.C.). Significance was assessed at the 0.05 level.
RESULTS
UC data from 22,644 admissions (16,316 unique patients) with 94,398 patient-days from April 2006 to December 2016 were included in the analyses. UC utilization decreased from 30% (1,379 device days per 4,640 patient days) in 2006 to 18% (1,900 device days per 10,762 patient days) in 2016 (Table 1; Fig. 2). This statistically significant decline (Cochran-Armitage Trend Test: Z = -24.36, P < 0.0001) was sustained throughout the study period. Key interventions are annotated in Figure 2. Among 5,906 admissions who had a UC at any point during their PICU course, median (25th to 75th) UC days was 2.0 (1–4) and the average %UC-days/LOS was 60.5%. Duration of catheterization (Table 1) for admissions with a UC, expressed as median UC-days per admission or as the average % UC-days/LOS per admission, did not change over time (autoregressive models: β-estimate for month = 0.0001, P = 0.18; β = 0.001, P = 0.07, respectively). There were a total of 38,719 ventilation days, with 41% of patient days ventilated on average per total annual patient days; this did not change over time. Checklist compliance remained above 98% for the entire study period and did not change over time (Table 1). From 2011 to 2015, PIM2 scores (median, 0.98% in 2011 Q1 and 0.78% in 2015 Q4, P = 0.07) decreased slightly, although not significantly, whereas PRISM scores did not significantly change (0.41% in 2011 Q1 to 0.45% 2015 Q4; P = 0.78). CAUTI rate per 1,000 UC-days declined significantly from 9.49/1,000 UC days in 2009 to 1.04 in 2016 (P = 0.0047). Using August 2009 through December 2013 as the baseline period, there was a significant decrease in the monthly CAUTI rate in January 2015, 2 years after CAUTI bundle implementation (5.05/1,000 UC-days before January 2015 to 1.05/1,000 UC-days, Fig. 3).
Table 1.
Summary of Patient UC Utilization and Duration Over Time
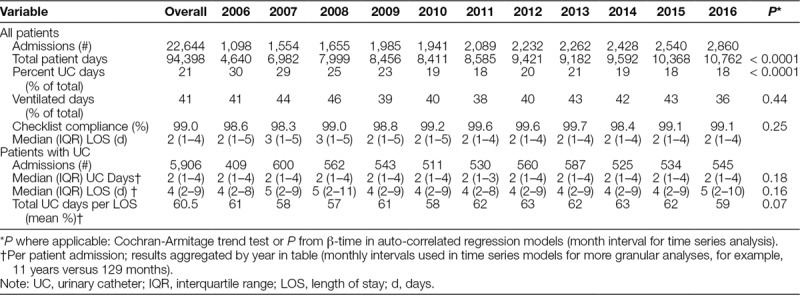
Fig. 2.
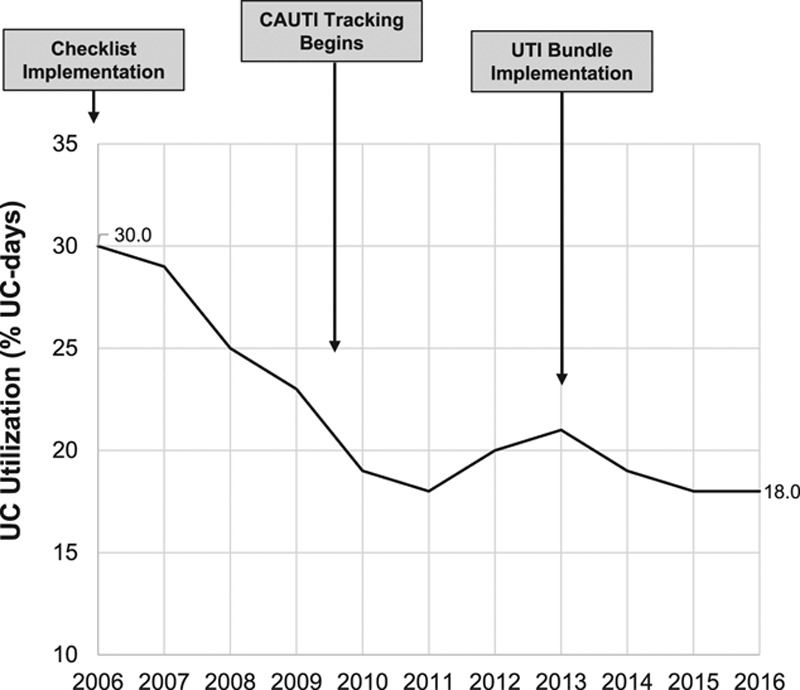
UC utilization and interventions over time. Cochran-Armitage Trend Test: Z = -24.36, P < 0.0001.
Fig. 3.
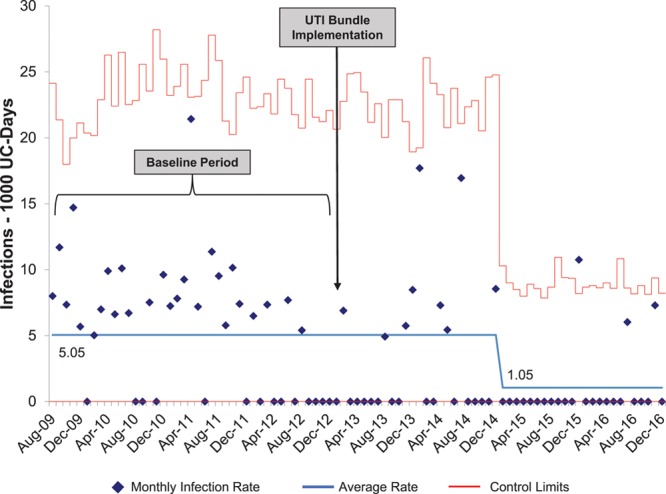
Control chart of CAUTIs over time. After the baseline period of August 2009 through December 2012, a change in mean CAUTI rate (centerline shift) occurred in January 2015 according to standard statistical process control rules.
DISCUSSION
Prolonged and/or unnecessary use of UCs represents a major source of morbidity in hospitalized patients, including in critically ill children.29 Daily rounding checklists are simple and effective tools for reducing medical errors and improving patient outcomes. Several studies have demonstrated the utility of checklists in the reduction of UC utilization in adult populations. However, there is a relative paucity of data in pediatric patients, especially in the intensive care setting. In the present study, we evaluated changes in UC utilization and CAUTI rate following the implementation of a PICU Daily QI Checklist (Fig. 1).
We observed a 40% reduction in UC utilization within the first 5 years following introduction of the checklist. This reduction was sustained for the remainder of the study, suggestive of more appropriate patient selection and timely removal. Over the same period, PICU volume significantly increased yet PRISM scores, PIM2 scores, and utilization rates for mechanical ventilation did not change (Table 1), effectively ruling out changes in illness severity as a driver of the decline in utilization. In addition, the introduction of safety culture-change efforts and the CAUTI prevention bundle in 2013 occurred after UC utilization had stabilized, making them unlikely contributors to utilization reduction. Interestingly, median duration of catheterization remained unchanged over time. Of the admissions with a UC, the percentage with short (younger than 2 days) and long (older than 5 days) catheterizations also did not change over time (P-trend = 0.28, P-trend = 0.55, respectively), indicating that the distribution of durations also remain unchanged. One explanation for this finding is that with daily directed attentiveness to UC necessity, unwarranted device initiation has been minimized for children admitted to the PICU. Understandably, there is a population of critically ill children for whom UC is necessary to aid in management and that is reflected in the finding that patients who did have an UC while in the PICU also had a longer median LOS (Table 1).
We also examined whether the decline in UC utilization was related to changes in CAUTI rate, which is associated with prolonged hospitalization, increased cost, and increased mortality in critically ill children.4,30 From 2009, when data became available, to 2016, there was an 89% decline CAUTI rate. This decline occurred after the UC utilization rate had reached its nadir, suggesting that the low utilization rate may haven a precondition for the reduction in CAUTI rate but was not the main driver. Instead, as illustrated in Figure 3, there was a shift in the mean CAUTI rate following CAUTI bundle implementation, implying that the bundle was a driving factor. The long latency between bundle implementation and change in the centerline suggests that other UC management practices initiated over this time period, such as disinfecting port protectors and establishing that urine cultures would be performed only if the urinalysis is consistent with infection, also contributed to the reduction in CAUTI rate.
A major strength of this study is that the longitudinal nature of our database allowed us to analyze a large dataset: 94,398 patient-days among 22,644 admissions. By comparison, a previous study assessing the effect of a checklist on device utilization in a PICU included only 4,001 patient-days among 660 admissions.26 Observations in our PICU are consistent with the previous study’s finding that UC utilization declined by 26% following the introduction of the checklist. Additionally, we have shown that this decrease has been sustained during the observed study period.
Our checklist has a number of features that may have contributed to its success. First, it was developed specifically for our practice setting, taking into account the needs of our PICU. Second, the checklist was integrated into morning rounds, rather than requiring a separate encounter. Consequently, it did not appreciably increase provider workload or require a data collector. Third, we used the checklist to spark a discussion regarding whether a UC was still appropriate for the patient, whether the catheter could be removed and, if not that day, what milestones should be achieved to reconsider removal. This experience is corroborated by prior investigations documenting improvement in attention to patient safety following quality improvement checklist implementation.31 Finally, we employed computerized physician prompting, a technique shown to both increase checklist compliance and decrease patient morbidity.32
Limitations of this study include the lack of a preintervention period before checklist implementation, which makes it impossible to attribute a causal relationship between the checklist and UC utilization. It is plausible that the reduction in catheter use is a consequence of larger trend in the intensive care unit. Additionally, CAUTI data were not available until 2009, so we were unable to assess the association between the initial decline in UC utilization and CAUTI rate. Finally, the study was conducted at a large, academic, quaternary care center and therefore may not be generalizable to all intensive care settings.
In conclusion, implementation and daily use of a PICU rounding checklist coincided with a significant reduction in UC utilization. This reduction, along with other quality improvement interventions including an infection prevention bundle, was followed by a significant decline in CAUTI rate. Taken together, these findings highlight the potential value of checklists in the incorporation of best practices into daily care of critically ill children and underscore the need for further evaluation of checklists in this vulnerable population.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online May 18, 2018
To Cite: Siegel BI, Figueroa J, Stockwell JA. Impact of a Daily Rounding Checklist to Reduce Urinary Catheter Utilization and Infection in a Pediatric Intensive Care Unit. Pediatr Qual Saf 2018;3:078.
Presented at AAP Critical Care Colloquium, September 15, 2017, Chicago, Ill.
REFERENCES
- 1.Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redder JD, Leth RA, Møller JK. Analysing risk factors for urinary tract infection based on automated monitoring of hospital-acquired infection. J Hosp Infect. 2016;92:397–400.. doi:10.1016/j.jhin.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Weber DJ, Sickbert-Bennett EE, Gould CV, et al. Incidence of catheter-associated and non-catheter-associated urinary tract infections in a healthcare system. Infect Control Hosp Epidemiol. 2011;32:822–823.. doi:10.1086/661107. [DOI] [PubMed] [Google Scholar]
- 4.Goudie A, Dynan L, Brady PW, et al. Costs of venous thromboembolism, catheter-associated urinary tract infection, and pressure ulcer. Pediatrics. 2015;136:432–439.. [DOI] [PubMed] [Google Scholar]
- 5.Platt R, Polk BF, Murdock B, et al. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307:637–642.. [DOI] [PubMed] [Google Scholar]
- 6.Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32:101–114.. [DOI] [PubMed] [Google Scholar]
- 7.Saint S, Meddings JA, Calfee D, et al. Catheter-associated urinary tract infection and the Medicare rule changes. Ann Intern Med. 2009;150:877–884.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Cdc. 2009;(March):13. Available at doi:http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed 13 June 2016. [Google Scholar]
- 9.Hagen S, Sinclair L, Cross S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2010:CD004012 doi:10.1002/14651858.CD004012.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Lam TBL, Omar MI, Fisher E, et al. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev. 2014;9:CD004013 doi:10.1002/14651858.CD004013.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Healthc Infect Control Pract Advis Comm. 2009:1–67.. doi:10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 12.Meddings J, Rogers MA, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23:277–289.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank DN, Wilson SS, St Amand AL, et al. Culture-independent microbiological analysis of foley urinary catheter biofilms. PLoS One. 2009;4:e7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin Proc. 1999;74:131–136.. [DOI] [PubMed] [Google Scholar]
- 15.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70.. [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth JM, Rogers MA, Krein SL, et al. Determining the noninfectious complications of indwelling urethral catheters: a systematic review and meta-analysis. Ann Intern Med. 2013;159:401–410.. [DOI] [PubMed] [Google Scholar]
- 17.Crouzet J, Bertrand X, Venier AG, et al. Control of the duration of urinary catheterization: impact on catheter-associated urinary tract infection. J Hosp Infect. 2007;67:253–257.. [DOI] [PubMed] [Google Scholar]
- 18.Byrnes MC, Schuerer DJ, Schallom ME, et al. Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence-based intensive care unit practices. Crit Care Med. 2009;37:2775–2781.. [DOI] [PubMed] [Google Scholar]
- 19.Haynes AB, Weiser TG, Berry WR, et al. ; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499.. [DOI] [PubMed] [Google Scholar]
- 20.Simpson CD, Hawes J, James AG, et al. Use of bundled interventions, including a checklist to promote compliance with aseptic technique, to reduce catheter-related bloodstream infections in the intensive care unit. Paediatr Child Health. 2014;19:e20–e23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–2020.. [DOI] [PubMed] [Google Scholar]
- 22.Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35:589–593.. [DOI] [PubMed] [Google Scholar]
- 23.Elpern EH, Killeen K, Ketchem A, et al. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18:535–41; quiz 542.. [DOI] [PubMed] [Google Scholar]
- 24.Carlos WG, Patel DG, Vannostrand KM, et al. Intensive care unit rounding checklist implementation. Effect of accountability measures on physician compliance. Ann Am Thorac Soc. 2015;12:533–538.. [DOI] [PubMed] [Google Scholar]
- 25.Dudeck MA, Edwards JR, Allen-Bridson K, et al. National healthcare safety network report, data summary for 2013, device-associated module. Am J Infect Control. 2015;43:206–221.. doi:10.1016/j.ajic.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarrago R, Nowak JE, Leonard CS, et al. Reductions in invasive device use and care costs after institution of a daily safety checklist in a pediatric critical care unit. Jt Comm J Qual Patient Saf. 2014;40:270–278.. [DOI] [PubMed] [Google Scholar]
- 27.Esteban E, Ferrer R, Urrea M, et al. The impact of a quality improvement intervention to reduce nosocomial infections in a PICU. Pediatr Crit Care Med. 2013;14:525–532.. [DOI] [PubMed] [Google Scholar]
- 28.Davis KF, Colebaugh AM, Eithun BL, et al. Reducing catheter-associated urinary tract infections: a quality-improvement initiative. Pediatrics. 2014;134:e857–e864.. [DOI] [PubMed] [Google Scholar]
- 29.Larsen GY, Donaldson AE, Parker HB, et al. Preventable harm occurring to critically ill children. Pediatr Crit Care Med. 2007;8:331–336.. [DOI] [PubMed] [Google Scholar]
- 30.Samraj R, Stalets E, Butcher J, et al. The impact of Catheter-Associated Urinary Tract Infection (CA-UTI) in critically ill children in the pediatric intensive care unit. J Pediatr Intensive Care. 2015;5:007–011.. doi:10.1055/s-0035-1568148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson SQ, Peterson DA, O’Brien-Ladner AR. Development and implementation of an ICU quality improvement checklist. AACN Adv Crit Care. 2007;18:183–189.. [DOI] [PubMed] [Google Scholar]
- 32.Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med. 2011;184:680–686.. [DOI] [PMC free article] [PubMed] [Google Scholar]


