Abstract
Background
Alport syndrome is a rare hereditary kidney disease manifested with progressive renal failure. Considerable variation exists in terms of disease progression among patients with Alport syndrome. Identification of patients at high risk of rapid progression remains an unmet need. Urinary epidermal growth factor (uEGF) has been shown to be independently associated with risk of progression to adverse kidney outcome in multiple independent adult chronic kidney disease (CKD) cohorts. In this study, we aim to assess if uEGF is associated with kidney impairment and its prognostic value for children with Alport syndrome.
Methods
One hundred and seventeen pediatric patients with Alport syndrome and 146 healthy children (3–18 years old) were included in this study. uEGF was measured in duplicates in baseline urine samples using ELISA (R&D) and concentration was normalized by urine creatinine (uEGF/Cr). In patients with longitudinal follow-up data (n = 38), progression was defined as deteriorated kidney function (CKD stage increase) during follow-up period (follow-up length is about 31 months in average). The association of baseline uEGF/Cr level with estimated glomerular filtration rate (eGFR) slope and Alport syndrome patients’ progression to a more advanced CKD stage during the follow-up period was used to evaluate the prognostic value of the marker.
Results
We found that uEGF/creatinine (uEGF/Cr) decreases with age in pediatric patients with Alport syndrome with a significantly faster rate than in healthy children of the same age group. uEGF/Cr is significantly correlated with eGFR (r = 0.75, p < 0.001), after adjustment for age. In 38 patients with longitudinal follow-up, we observed a significant correlation between uEGF/Cr and eGFR slope (r = 0.58, p < 0.001). Patients with lower uEGF/Cr level were at increased risk of progression to a higher CKD stage. uEGF/Cr was able to distinguish progressors from non-progressors with an AUC of 0.88, versus 0.77 by eGFR and 0.81 by 24-h urinary protein (24-h UP).
Conclusions
Our study suggests that uEGF/Cr is a promising biomarker for accelerated kidney function decline in pediatric patients with Alport syndrome. It may help to identify patients at high risk of progression for targeted clinical care and improve the patients’ stratification in interventional trials.
Keywords: Alport syndrome, Urinary epidermal growth factor (uEGF), Progression, Prognostic marker
Introduction
Alport syndrome is a hereditary kidney disease which manifests with hematuria, proteinuria, progressive renal failure, sensorineural deafness, or typical ocular changes, which are caused by mutations in the COL4A5, COL4A3, or COL4A4 genes [1]. In current clinical practice, the progression of Alport syndrome is determined by regular measurements of proteinuria and renal function. However, these parameters are not sensitive enough to predict the progression of Alport syndrome. Taking these shortcomings into consideration, additional biomarkers are urgently needed for better prediction of Alport syndrome progression.
Epidural growth factor (EGF) level has been shown to be regulated in kidney diseases [2, 3]. Urinary EGF (uEGF) is reported to be derived from the kidney and has been demonstrated to be downregulated in almost all rodent models of kidney diseases and many kinds of human renal diseases [4–12]. In 2015, Ju et al. reported that the addition of uEGF to creatinine ratio (uEGF/Cr) to standard clinical biomarkers [estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (ACR)] improved the prediction of chronic kidney disease (CKD) progression by following-up three independent diverse CKD cohorts [13]. Furthermore, in 2016, Betz et al. demonstrated that the lower uEGF/Cr at baseline dramatically increased the risk of rapid decline of renal function [14]. These studies further reinforce uEGF/Cr as a potential predictive marker for progressive renal diseases.
The additive value of uEGF/Cr to eGFR and ACR on prediction is likely due to its tissue specificity and the distinct pathogenic pathway it represents. EGF mRNA showed restricted expression in the kidney tubulointerstitium [13]. More specific localization has been reported in the thick ascending limb of Henle and distal tubules [15]. Kidney-restricted origination of uEGF minimized influences from extrarenal tissue/organs therefore can specifically reflect patients’ kidney function. In addition, uEGF is significantly inversely correlated with interstitial fibrosis and tubular atrophy (IF/TA) in patients with nephrotic syndrome [13]. This goes along with its mitogenic function and its reported role in enhancing tubular regeneration and repair [16].
Alport syndrome is well known as a kind of glomerular disease with the typical pathological changes of glomerular basement membrane (GBM) in the last decades. However, in recent years, a lot of studies have described that tubulointerstitial lesions in Alport syndrome, including tubulointerstitial infiltration by CXCR3-positive T cells in human Alport syndrome and interstitial fibrosis, tubule cell apoptosis, and tubular atrophy in animal models with Alport syndrome [17–20]. In addition, treatment of angiotensin-converting enzyme inhibitors (ACEI) in COL4A3 knockout mice delayed proteinuria and attenuated tubular injury and kidney fibrosis, but has no obvious function for amelioration of pathological changes of GBM [21, 22]. These studies indicated that tubular damage and interstitial fibrosis contribute to the progression of Alport syndrome.
Therefore, we hypothesized that uEGF, a non-invasive biomarker for tubular damage and interstitial fibrosis, can predict progression of Alport syndrome, in which tubulointerstitial impairment is a major contributor of kidney disease progression. In this study, we aimed to determine the prognostic value of uEGF on the progression of kidney impairment in children with Alport syndrome.
Materials and methods
Inclusion and exclusion criteria of subjects
Healthy children
The inclusion criteria were those (1) under the age of 18 and (2) whose routine urinalysis and urinary albumin creatinine ratio were normal. The exclusion criteria were those (1) with a history of chronic illness; (2) who suffer from infectious diseases (fever, cold, cough, diarrhea, etc.); and (3) have taken medication within the past 1 month.
Children with Alport syndrome
The inclusion criteria were those (1) under the age of 18 years old and (2) diagnosed with Alport syndrome by Peking University First Hospital on the basis of the presence of hematuria or a family history of renal diseases with at least one positive examination of GBM changes revealed by electron microscopy, or immunofluorescence of the collagen type IV, or genetic testing of COL4A3–5. The exclusion criteria were (1) unconfirmed diagnosis of Alport syndrome and (2) having other kidney diseases besides Alport syndrome.
Alport children included were grouped into four subgroups according to the level of proteinuria and eGFR that is hematuria, microalbuminuria, proteinuria, and renal dysfunction.
hematuria (24-h urinary protein (24-h UP) < 150 mg; microalbumin < 20 mg/l and eGFR ≥ 90 ml/min/1.73m2);
microalbuminuria (24-h UP < 150 mg; microalbumin ≥ 20 mg/l and eGFR ≥ 90 ml/min/1.73 m2);
proteinuria (24-h UP ≥ 150 mg and eGFR ≥ 90 ml/min/1.73 m2);
renal dysfunction (eGFR < 90 ml/min/1.73 m2)
Urine specimen collection and preparation
Spot urine samples were collected into disposable sterile urine cups from all the subjects included. Urine specimens were centrifuged at 4 °C at 4000 rpm/min for 15 min. The clear supernatants were transferred into new tubes which were immediately stored at − 80 °C. Urine specimens were diluted 200-fold and 250-fold for Alport syndrome and healthy control children respectively with calibrator diluent RD5E before measurements.
Measurements of uEGF and creatinine
Human EGF Quantikine ELISA kits (DEG00; R&D Systems) were used to measure EGF concentration in human urine. Urine creatinine concentration was measured by a Hitachi Automatic Biochemical Analyzer 7180. Log2 transformed uEGF/creatinine ratio was used for data analysis in this study. Fully validated ELISA assay was used for the measurement of uEGF. Three pre-measured samples with high, medium, or low concentrations were used as quality controls and were included in each plate to determine interplate variation. All samples, quality controls, and standards were run in duplicate. Intra-assay variation is less than 5% and inter-assay coefficients of variation were less than 10%.
Definition of Alport syndrome progression
The Alport syndrome progression was defined by two ways: one is using eGFR slope, the rate of eGFR change over the years, as a continuous variable (details in below the “Statistical analysis” section); the other one is CKD stage advance. Progressor was defined as patient whose CKD stage (by Kidney Disease Outcomes Quality Initiative (KDOQI) guideline) is higher at the end of observation than his/her CKD stage at baseline. The equation eGFR = 0.42 × height (cm)/serum creatinine (mg/dl) (ht/scr) was used to calculate the eGFR of children with Alport syndrome [23].
Determination of the cutoff of uEGF/Cr value
Alport syndrome children in respective age groups was separated into patients with “lower uEGF/Cr than healthy” and with “normal range uEGF/Cr” based on the cutoff value. In this study, the cutoff value was determined using lower 95% confidence interval (CI) of uEGF/Cr in healthy children in different age groups. Alport syndrome children whose uEGF/Cr were less than the cutoff value for the corresponding age group was defined as “lower uEGF/Cr than healthy,” whereas Alport syndrome children with “normal range uEGF/Cr” referred to those children whose uEGF/Cr concentration is higher than the cutoff value of the corresponding age group.
Statistical analysis
Estimated glomerular filtration rate slope determination was described previously [13]. In brief, patients with three or more GFR measurements during the follow-up period were included in fitting a linear mixed effect model GFR_ij = beta0 + beta1 × time_ij + b_0i + b_1i × time_ij + epsilon_ij to calculate eGFR slope percentage change (%) = (beta1 + b_1i) ∕ (beta0 + b_0i). Linear regression was applied to investigate the association between eGFR slope and uEGF/Cr, adjusted for age or baseline eGFR, respectively. Logistic regression and area under the curve (AUC) values from the ROC curves to compare nested models (www.inside-r.org/packages/cran/epicalc/docs/lrtest) were used to evaluate the classification power of the candidate marker. Statistical analysis was performed using R (www.R-project.org) and SPSS software (version 24). To compare skewed variables in multiple independent groups, the Kruskal-Wallis test was used. The Mann-Whitney test was applied to compare skewed distributed variables between two groups.
Results
uEGF/Cr decreases with age in healthy children
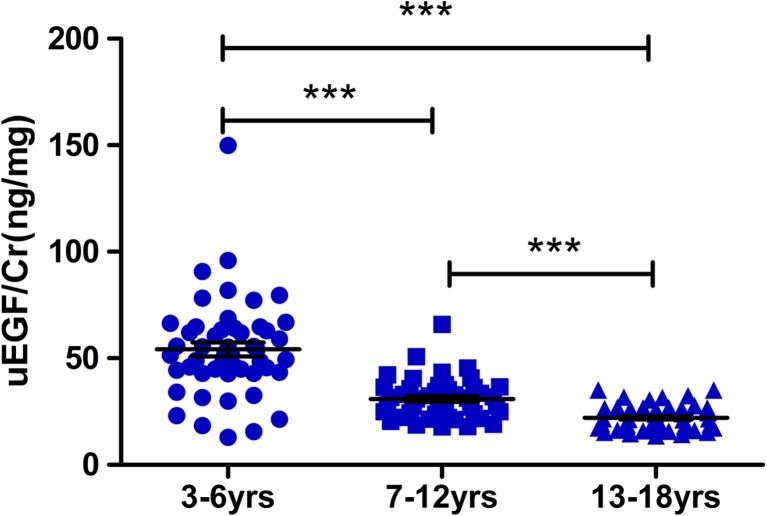
A total of 146 healthy children (3 to18 years old) were included in this study (Table 1). We divided these children into three age groups: pre-school age (3–6 years, N = 50), school age (7–12 years, N = 47), and adolescence (13–18 years, N = 49) and compared the concentration of uEGF/Cr in each group. A significant decrease in uEGF/Cr concentration was observed with increased age (Fig. 1). However, there was no significant difference in uEGF/Cr between males and females in pre-school age (p = 0.36), school age (p = 0.27), and adolescence (p = 0.12) groups, respectively.
Table 1.
Characteristics of the healthy children and children with Alport syndrome included in this study
| Characteristics | All healthy children | All children with Alport syndrome |
|---|---|---|
| Number | 146 | 117 |
| Median age (range) (years) | 9.5 (3–18) | 10 (3–17) |
| Male (%) | 74 (50.7%) | 99 (84.6%) |
| Mean height ± SD (cm) | 140.6 ± 25.4 | 138.4 ± 18.7 |
| Mean weight ± SD (kg) | 40.2 ± 21.5 | 34.7 ± 14.6 |
| Mean 24-h UP ± SD (g/24 h) | NA | 1.9 ± 2.3 |
| Mean eGFR ± SD (ml/min per 1.73 m2) | NA | 110.4 ± 51.1 |
| Genotype (89 of 117) | NA | XLAS (65/89) ARAS (24/89) |
eGFR estimated glomerular filtration rate, NA not available, UP urinary protein, XLAS X-linked Alport syndrome, ARAS autosomal recessive Alport syndrome
Fig. 1.
Urinary epidermal growth factor/creatinine (uEGF/Cr) levels in different age group of healthy children. uEGF/Cr of healthy children at 3–6 years (54.17 ± 22.84; N = 50) decreased 43% at 7–12 years (30.87 ± 9.37; N = 47) and 59% at 13–18 years (22.06 ± 5.78; N = 49).Triple asterisks denote p < 0.0001
uEGF/Cr is significantly lower in children with Alport syndrome compared to age-matched healthy controls
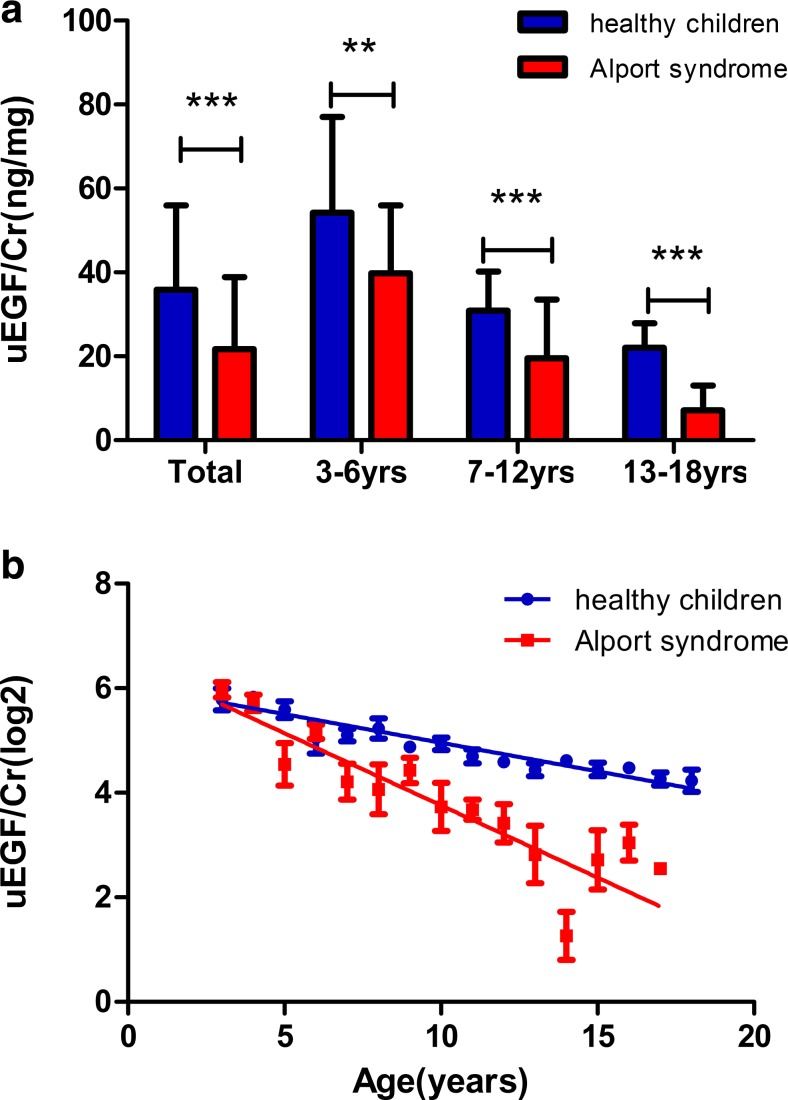
A total of 117 Alport children were included in this study (Table 1) from our Alport syndrome registry databank according to the inclusion criteria and exclusion criteria, which are described in detail in the “Materials and methods” section. Comparing the levels of uEGF/Cr in healthy children (N = 146) and Alport syndrome children (N = 117), the Alport syndrome children showed significantly lower uEGF/Cr compared to healthy children of the same age group. To adjust for age effect, we matched healthy children and Alport syndrome children in all three age groups as described above. Our results showed that uEGF/Cr levels of Alport syndrome children are significantly lower than those of healthy children in all three age groups (Fig. 2a).
Fig. 2.
Urinary epidermal growth factor/creatinine (uEGF/Cr) level in different age groups of healthy children and children with Alport syndrome (mean ± SD). a uEGF/Cr levels were lower in children with Alport syndrome (red column) in comparison with healthy children (blue column) overall and in each individual age group. Double asterisks represent p < 0.01; triple asterisks represent p < 0.0001. b uEGF/Cr levels decrease with age in healthy children (blue dots, N = 146) and children with Alport syndrome (red square, N = 117)
Furthermore, uEGF/Cr levels in both Alport syndrome children and healthy children showed a continuous reduction with increased age. uEGF/Cr level in Alport syndrome children, however, fell faster than did the level in healthy children from the same age group (Fig. 2b).
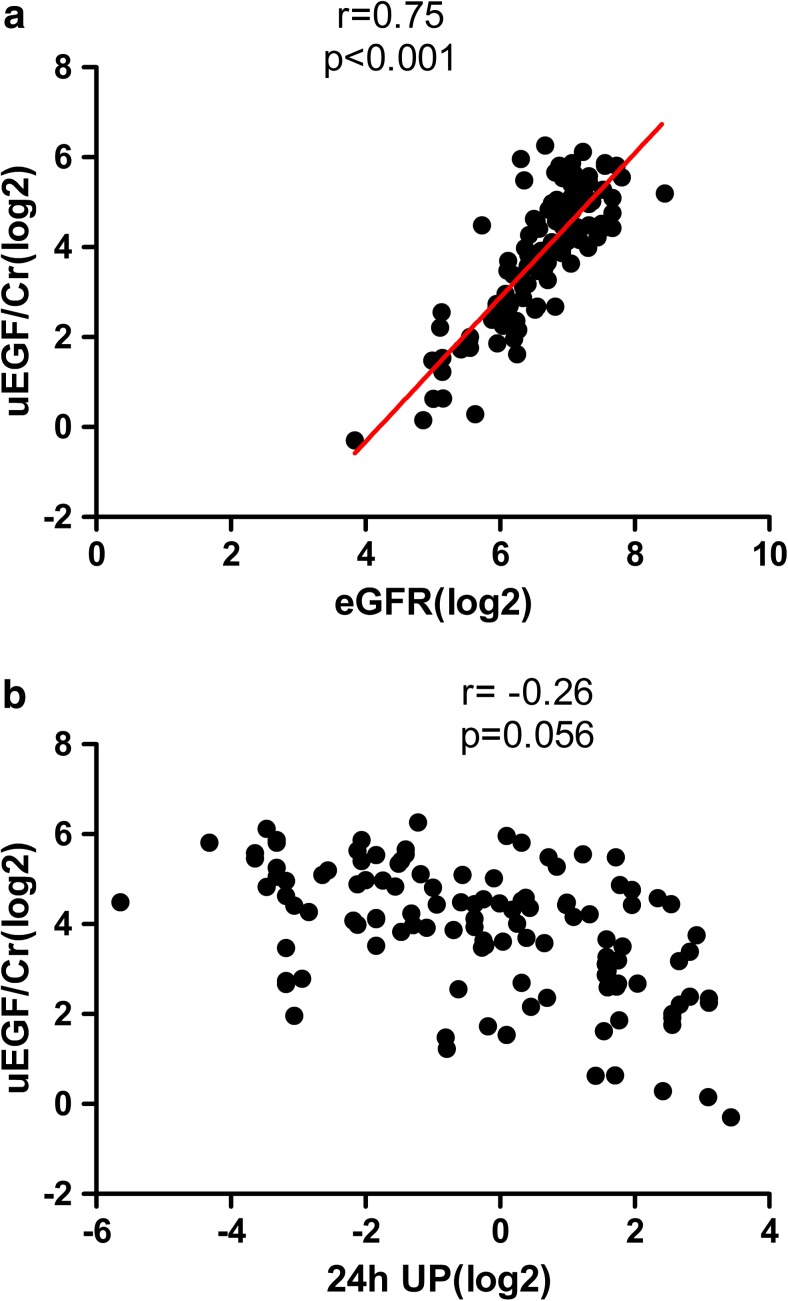
To investigate if uEGF/Cr level is associated with quantitative kidney function in Alport syndrome children, we analyzed the correlation of uEGF/Cr with eGFR and 24-h urinary protein (24-h UP). After adjustment for age, uEGF/Cr showed a tight and significant correlation with eGFR (r = 0.75, p < 0.001, Fig. 3a). The correlation between uEGF/Cr and 24-h UP is no more significant after adjusting for age (r = − 0.26, p = 0.06, Fig. 3b).
Fig. 3.
Correlation of one urinary epidermal growth factor/creatinine (uEGF/Cr) with kidney impairment, evaluated by estimated glomerular filtration rate (eGFR) and 24-h UP. a uEGF/Cr is significantly (p < 0.001) positively correlated with eGFR at the time of urine collection (N = 117). b uEGF/Cr showed a negative correlation with 24-h UP (N = 117), however, did not reach statistical significance
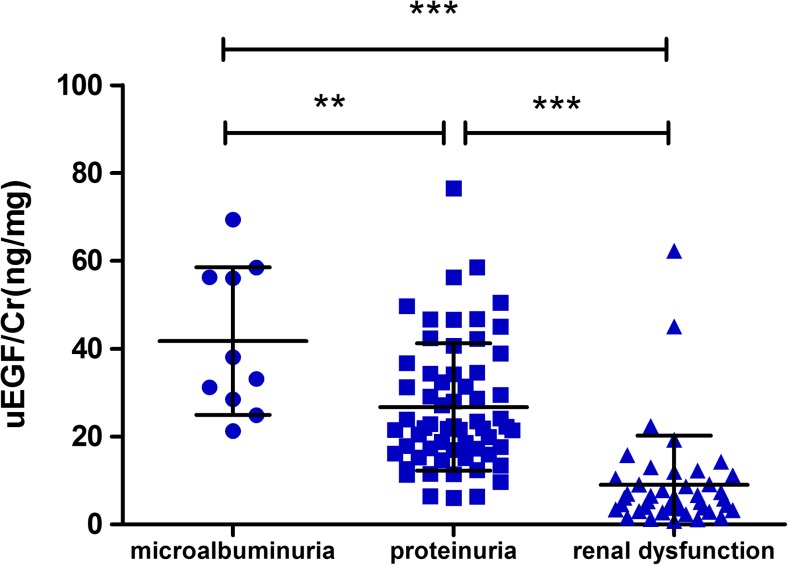
According to the level of proteinuria and eGFR, we divided children with Alport Syndrome into four subgroups, that is, hematuria, microalbuminuria, proteinuria, and renal dysfunction. Patients with proteinuria (24-h UP more than 150 mg but eGFR ≥ 90 ml/min/1.73m2) and renal dysfunction (eGFR < 90 ml/min/1.73m2) showed a significant reduction of uEGF/Cr compared to Alport children that are only microalbuminuria (Fig. 4). Children with hematuria were not included in this analysis due to the small samples size (N = 3) and large variation in this group.
Fig. 4.
The urinary epidermal growth factor/creatinine (uEGF/Cr) levels (mean ± SD) in children with Alport syndrome. Children were grouped based on their proteinuria levels and estimated glomerular filtration rate (eGFR). Patients with microalbuminuria (N = 10), proteinuria (N = 61), and renal dysfunction (N = 43) were included in this analysis. uEGF/Cr is 36 or 78% less in patients with proteinuria or renal dysfunction in comparison with patients with microalbuminuria, respectively. Double asterisks represent p = 0.008; triple asterisks represent p < 0.0001
uEGF/Cr and kidney disease progression in Alport syndrome
In Alport syndrome children with hematuria or microalbuminuria, we observed two distinct subgroups of patients based on uEGF/Cr level. We then asked if different uEGF/Cr levels are associated with future kidney disease progression and can therefore be used as an early prognostic marker.
To answer this question, we investigated those Alport syndrome children whose clinical follow-up data are available. In the 117 Alport syndrome patients, 38 children of school age or adolescence have been followed up for 30.58 ± 16.31 months.
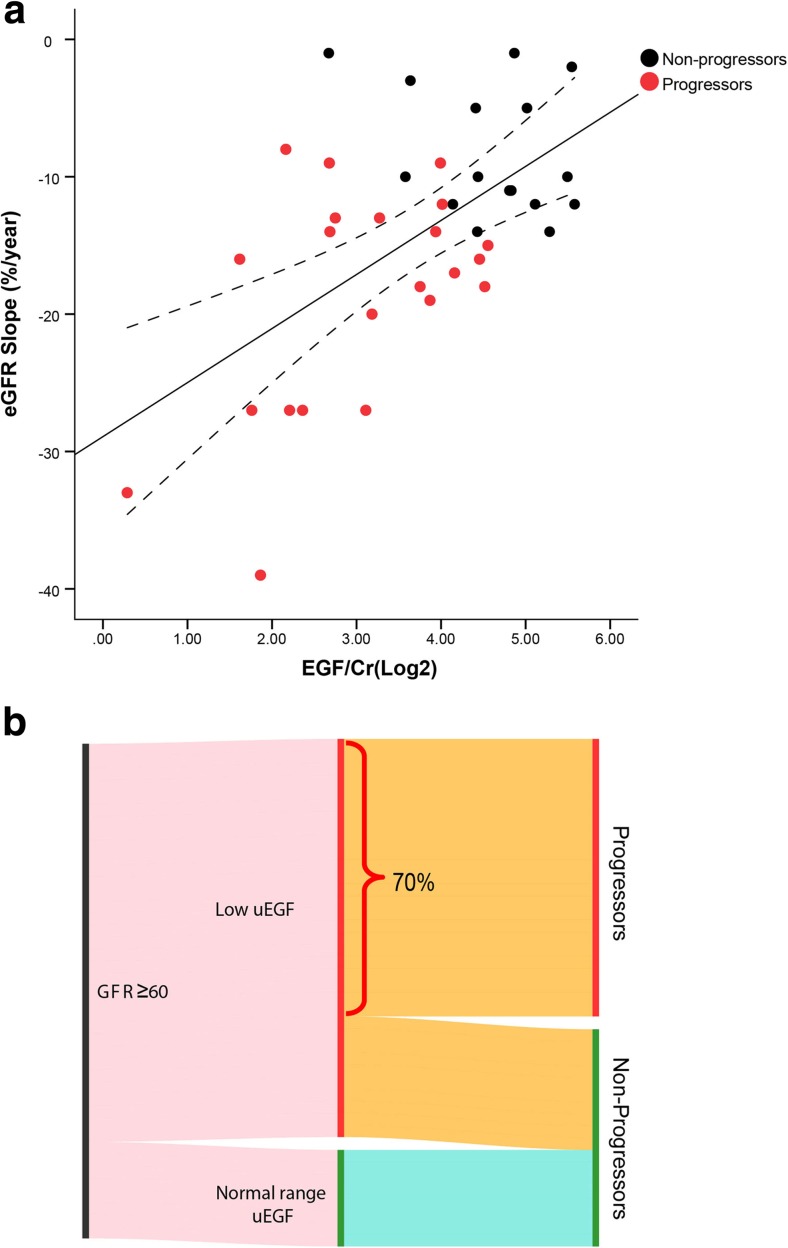
Kidney disease progression was defined in two different ways, first by eGFR slope, namely the rate of eGFR change over years. As demonstrated in Fig. 5a, uEGF/Cr is significantly and positively correlated with eGFR slope (r = 0.58, p < 0.001). The correlation remains significant after adjusting for age (adjusted r = 0.46, p < 0.01), 24-h UP (adjusted r = 0.46, p < 0.01), or baseline eGFR (adjusted r = 0.33, p < 0.05).
Fig. 5.
The level of one urinary epidermal growth factor/creatinine (uEGF/Cr) and kidney disease progression in patients with Alport syndrome. a uEGF/Cr is significantly and positively correlated with estimated glomerular filtration rate (eGFR) slope (r = 0.58, p < 0.001). The majority of progressors (red dots) had lower uEGF/Cr and steeper eGFR slope than non-progressors (black dots). b Thirty five children with Alport syndrome and eGFR above 60 were divided into “lower uEGF/Cr than healthy” (N = 27) and “normal range uEGF/Cr” (N = 8) groups based on cutoff values defined based on healthy children of the same age group, as described in the methods section. In patients with eGFR ≥ 60 but lower uEGF/Cr, 70% had progressed to more advanced chronic kidney disease (CKD) stages during follow-up, whereas in patients with normal range uEGF/Cr, none had progressed
Secondly, progression was also defined as patients progressed to a more advanced CKD stage (by KDOQI guideline) during the follow-up period when compared with baseline. “Progressors” referred to patients whose CKD stages at the end of observation were higher than that at baseline. “Non-progressors” were those patients whose CKD stages remained the same or lower than that at baseline. The clinical characteristics of “progressors” and “non-progressors” at baseline and the end of follow-up are depicted in Table 2. Significantly lower baseline uEGF/Cr and eGFR and significantly higher 24-h UP were observed at baseline between “progressors” and “non-progressors.. At the end of follow-up, eGFR remained to be significant whereas 24-h UP was no longer significant.
Table 2.
Basic clinical characteristics of progressors and non-progressors at baseline and the end of follow-up
| Progressors, N = 22 | Non-progressors, N = 16 | p value | |
|---|---|---|---|
| At baseline | |||
| uEGF/Cr (ng/mg) | 10.59 ± 6.83 | 27.83 ± 12.67 | 0.0001 |
| Age (years) | 11.18 ± 2.63 | 9.81 ± 2.32 | NS |
| eGFR (ml/min/1.73m2) | 99.48 ± 37.99 | 137.80 ± 38.52 | 0.004 |
| 24-h UP (g/24 h) | 3.41 ± 2.51b | 1.31 ± 1.23b | 0.02 |
| Genotype | XLAS (14/17) ARAS (3/17) | XLAS (11/13) ARAS (2/13) | NS |
| Baseline CKD stage | CKD1–2 (19/22) CKD3 (3/22) | CKD1–2 (16/16) CKD3 (0/16) | NS |
| At the end of follow-up | |||
| eGFR (ml/min/1.73m2) | 59.46 ± 25.63 | 113.50 ± 20.86 | < 0.0001 |
| 24-h UP (g/24 h) | 4.34 ± 3.78b | 1.89 ± 2.08b | NS |
| Time (months) a | 23.64 ± 13.28 | 24.75 ± 5.94 | NS |
Data are shown as mean ± SD; NS no significance, p > 0.05, XLAS X-linked Alport syndrome, ARAS autosomal recessive Alport syndrome, uEGF/Cr urinary epidermal growth factor/creatinine, eGFR estimated glomerular filtration rate, CKD chronic kidney disease
a“Time” indicates time to stage advance for progressors and time of follow-up for non-progressors
bThe value for 24-h UP was an average value of n = 16 and 14 patients respectively for progressors and non-progressors
To evaluate if males with X-linked Alport syndrome (XLAS) all progressed during the follow-up time, we investigated the 38 Alport patients with longitudinal follow-up. Twenty-three out of 38 patients were males with XLAS and 10 of 23 (43%) had not progressed during the follow-up time. Out of the 23 XLAS male patients, 13/17 (76%) with lower uEGF/Cr progressed and 6/6 (100%) XLAS male patients with normal range uEGF/Cr did not progress. There are only two XLAS female patients; after 2 years’ follow-up, one patient with lower uEGF/Cr progressed and the other one with normal range uEGF/Cr did not progress. Unfortunately, there are only five patients with ARAS and they all have lower uEGF/Cr; three out of five (60%) progressed.
In our study, among the 38 Alport patients with longitudinal follow-up, 30 patients have genetic analysis result available, 12 out of these 30 patients carry missense mutations and 18 carry nonsense mutations, deletion/insertion, or splicing mutations, referred to as “severe mutations.” We investigated the association between different types of mutations and kidney disease progression. We found that 6 out of the 12 patients (50%) in missense mutation group and 11 out of the18 patients (61%) in severe mutation group progressed to advanced CKD stages during the follow-up time. There is no significant difference between missense mutation and severe mutation subgroups (p = 0.71) in terms of disease progression.
To determine if patients with lower uEGF/Cr level are more likely to be a progressor, we defined a cutoff value of uEGF/Cr based on the lower 95% CI of uEGF/Cr in healthy children at different age groups to account for age-associated differences (28.12 and 20.40 ng/mg for children at school age and adolescence, respectively) as described in detail in the “Materials and methods” section. Using these cutoff values, 38 Alport syndrome children in respective age groups were divided into two groups: patients with “lower uEGF/Cr than healthy” (N = 30) and patients with “normal range uEGF/Cr” (N = 8). None of the Alport syndrome children with “normal range uEGF/Cr” progressed, whereas 73% of the Alport syndrome children with “lower uEGF/Cr than healthy” progressed to more advanced CKD stage at the end of follow-up compared to their baseline stage (Fig. 5b). Figure 5b demonstrated that for patients whose eGFR are over 60, uEGF/Cr can help to further stratify the patients: no progression has been observed in patients with normal range uEGF/Cr, whereas if patients have lower than healthy uEGF/Cr, despite the current “preserved” renal function, 70% of the patients (whose eGFR are over 60 but has lower uEGF/Cr) have proceeded to a more advanced stage in about 2 years of time.
Finally, the association of baseline uEGF/Cr with progression to more advanced stage was evaluated by classification power [comparing the area under receiver operating characteristic (ROC) curves of individual variables and the corresponding models] and survival analysis (time-to-event analysis). ROC analysis showed uEGF/Cr exhibits the highest area under the curve (AUC) of 0.88 (95% CI, 0.77 to 0.99), compared to 24-h UP [0.81 (0.67 to 0.95)] and eGFR [0.77 (0.62 to 0.92)]. The AUC for the base model (including eGFR and 24-h UP, adjusted for age and gender) is 0.86 (0.74 to 0.97); addition of uEGF/Cr to the base model increased AUC to 0.93 (0.85 to 0.99). Addition of uEGF/Cr resulted in a statistically improved model as evaluated by the likelihood test (p = 0.002).
Discussion
Patients with Alport syndrome show a large variation in rate of progression [24–26]. Genetic test may detect the causal mutations, however, cannot accurately predict if when and how fast the patients progress. As shown in our study, not all males with X-linked Alport syndrome progressed during the follow-up time. Due to lack of sensitive and specific prognostic biomarkers, patients at high risk of progression often miss the best time for treatment. To address this urgent need, we investigated if urinary EGF is associated with loss of kidney function and if it has prognostic value in patients with Alport syndrome, since urinary EGF has been reported previously as a prognostic marker in adult CKD progression. Our data showed that in 117 patients with a definitive diagnosis of Alport syndrome and no other kidney diseases, uEGF is highly and significantly correlated with baseline eGFR but not with 24-h proteinuria. These findings are consistent with previous findings in general CKD, IgAN, and nephrotic syndrome [13].
In a subgroup of patients whose follow-up data were available, we attempted to investigate the association of uEGF/Cr with progression of Alport syndrome. Due to small sample size and relatively short follow-up time, we defined “progression” as CKD stage advanced. This definition has been applied in multiple studies by independent groups and was considered acceptable [27–30]. We observed that uEGF/Cr levels at baseline in “progressors” were already significantly lower than those in “non-progressors.” Next, uEGF/Cr’s prognostic value was reflected by its significant correlation with eGFR slope (Fig. 5a). The progressors were clustered together by their low uEGF/Cr and steeper eGFR slope (Fig. 5a). While it is not surprising that steeper eGFR slope highly associated with progression to higher CKD stage, it is encouraging to observe that uEGF/Cr shows a high correlation with eGFR slope and progression. This result confirmed our previous finding in general adult CKD [13]. The association of uEGF/Cr with progression is likely due to the regenerative function of EGF [16]. Lower uEGF/Cr is not only an indication of current reduced kidney tubular mass thereby a good biomarker for cross-sectional kidney function, it also represents impaired regenerative function of the tubular cells, which is directly associated with progression. Finally, uEGF/Cr exhibited superior AUC compared to eGFR and proteinuria. Adding uEGF/Cr to the base model including eGFR and proteinuria resulted in a significant improvement of AUC. This is the first time, to the best of our knowledge, uEGF/Cr was reported to have additive prognostic value for CKD progression in patients with Alport syndrome.
A good biomarker represents the active mechanism underlying the pathogenesis of the disease. Alport syndrome is a well-known glomerular disease with typical pathological changes of the glomerular basement membrane. However, tubulointerstitial fibrosis, a hallmarker for kidney disease progression, has been reported in patients with Alport syndrome [21, 31]. IF/TA has been reported to be well represented by uEGF, a biomarker for tubulointerstitial damage [13]. This could explain the additive value of uEGF/Cr to eGFR and proteinuria, as the two standard clinical markers mainly reflect glomerular damage. If and how EGF is actively involved in Alport syndrome tubular damage repair and regeneration in Alport syndrome remains to be further studied.
It has been reported that there is a consistent downward trend of uEGF/Cr as a function of age from birth to 16 and from 20 to 79, respectively [32, 33]. Therefore, all analyses in our study have been adjusted for age as a significant confounding factor. Inevitably, the uEGF/Cr concentration in Alport syndrome children is an integrated outcome of the normal growth of the kidney and the kidney disease influence. Therefore, there is a need to evaluate age-associated uEGF/Cr reduction in healthy children to allow better correction of the confounding effect. We therefore started establishing a cohort of healthy children aged 3 to 18 years old. Using these healthy children as reference, we could demonstrate that uEGF/Cr levels of Alport syndrome children exhibited a much steeper trajectory over age compared to the reduction in healthy kids of the same age group (Fig. 2b).
The relevant next step would be to define an age-dependent normal range of uEGF/Cr. Once established, it will allow us to identify children with preserved renal function but may have lower than normal uEGF/Cr. As illustrated in Fig. 5b, 70% of Alport syndrome patients whose eGFR is above 60 but have lower uEGF/Cr (compared to healthy kids) progressed to a more advanced CKD stage in an average of about 30 months of follow-up time. Therefore, uEGF/Cr may contribute to the early identification of patients at high risk of kidney disease progression.
Our study has limitations. The small sample size (due to the rare disease nature) and short follow-up restricted us from performing standard time-to-event analysis using FDA approved kidney disease endpoint definition. Despite the small sample size, there were six patients who progressed to end-stage renal disease (ESRD) during the follow-up period. We evaluated the association of uEGF/Cr with time to ESRD using univariate Cox regression model and found that lower uEGF/Cr was significantly associated with increased risk of progression to ESRD (HR = 0.54, p = 0.001). However, due to the small event size, no multivariable model can be reliably applied; therefore, it remains to be studied if the association with time to ESRD is independent of eGFR or proteinuria. This is certainly worth further investigation in well-designed clinical studies with larger patient population. In addition, the clinical phenotype and outcomes of individuals with Alport syndrome (especially females with COL4A5 mutation, heterozygous autosomal mutation) are heterogeneous. It has been reported that 12% of females with COL4A5 mutation developed ESRD before the age of 40 years and there is no genotype-phenotype associations in the studied cohort [34]. Similar results have been reported by Yamamura et al., who analyzed female patients with X-linked Alport syndrome from 179 Japanese families and found that 15% of patients reached ESRD by the age of 40 [35]. Patients with autosomal-dominant Alport syndrome had a wide range of phenotypes from ESRD to isolated microhematuria [36, 37]. Therefore, identification of individuals with Alport syndrome, especially females with COL4A5 mutation and heterozygous autosomal mutation at high risk of rapid progression is indeed necessary for early and targeted treatment. From our current results, uEGF is likely a prognostic marker for risk evaluation in this population, which warrants further investigation in large study cohort.
Taken together, our data indicated that Alport syndrome children with a lower expression of uEGF/Cr were at higher risk of rapid progression, even if their eGFRs are still above 60. This finding supports the use of uEGF/Cr to improve early identification of patients at high risk of progression. In addition, this study provided additional data on uEGF/Cr in healthy children from age 3 to 18, contributed to the understanding of the expression of uEGF/Cr as a function of increasing age in healthy people. Prospective study includes more patients (particularly those who have low uEGF/Cr) and longer follow-up is required to validate the prognostic value of uEGF/Cr in children with Alport syndrome.
Acknowledgements
We acknowledge the support from Wei Li for data archival work and Yuan Wang and Jifan Guo for the urine collection.
Abbreviations
- 24-h UP
24-h urinary protein
- ACEI
Angiotensin-converting enzyme inhibitors
- ARAS
Autosomal recessive Alport syndrome
- CI
Confidence interval
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- GBM
Glomerular basement membrane
- IF/TA
Interstitial fibrosis/tubular atrophy
- ROC
Receiver operating characteristic
- uEGF
Urinary epidermal growth factor
- uEGF/Cr
Urinary epidermal growth factor/creatinine
- XLAS
X-linked Alport syndrome
Sources of funding
This work was supported by the National Key Research and Development Program of China: the registry study of rare diseases in children (No.2016YFC0901505), Beijing Key Laboratory of Molecular Diagnosis and Study on Pediatric Genetic Diseases (No. Z141107004414036). This work was partially supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (P30DK081943, to M.K.) and by the University of Michigan Health System (UMHS) and Peking University Health Science Center (PUHSC) Joint Institute for Translational and Clinical Research.
Conflict of interest
V.N., W.J, and M.K. hold a patent PCT/EP2014/073413 “Biomarkers and methods for progression prediction for chronic kidney disease”. The other authors declared no competing interests.
Ethics
The ethical committee of Peking University First Hospital approved the project and informed consents were obtained from all the subjects and their family members.
Contributor Information
Wenjun Ju, Phone: 734-615-0480, Email: wenjunj@med.umich.edu.
Jie Ding, Phone: 8610 8357 3238, Email: djnc_5855@126.com.
References
- 1.JKruegel J, Rubel D, Gross O. Alport syndrome-insights from basic and clinical research. Nat Rev Nephrol. 2013;9:170–178. doi: 10.1038/nrneph.2012.259. [DOI] [PubMed] [Google Scholar]
- 2.Dagogo-Jack S, Marshall SM, Kendall-Taylor P, Alberti KG. Urinary excretion of human epidermal growth factor in the various stages of diabetic nephropathy. Clin Endocrinol. 1989;31:167–173. doi: 10.1111/j.1365-2265.1989.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 3.Kok HM, Falke LL, Goldschmeding R, Nguyen TQ. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol. 2014;10:700–711. doi: 10.1038/nrneph.2014.184. [DOI] [PubMed] [Google Scholar]
- 4.Mattila AL, Viinikka L, Saario I, Perheentupa J. Human epidermal growth factor: renal production and absence from plasma. Regul Pept. 1988;23:89–93. doi: 10.1016/0167-0115(88)90424-7. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen PE, Hilchey SD, Nexo E, Poulsen SS, Quilley CP. Urinary epidermal growth factor is excreted from the rat isolated perfused kidney in the absence of plasma. J Endocrinol. 1993;139:227–234. doi: 10.1677/joe.0.1390227. [DOI] [PubMed] [Google Scholar]
- 6.Mathiesen ER, Nexo E, Hommel E, Parving HH. Reduced urinary excretion of epidermal growth factor in incipient and overt diabetic nephropathy. Diabet Med. 1989;6(2):121–126. doi: 10.1111/j.1464-5491.1989.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 7.Safirstein R, Zelent AZ, Price PM. Reduced renal prepro-epidermal growth factor mRNA and decreased EGF excretion in ARF. Kidney Int. 1989;36:810–815. doi: 10.1038/ki.1989.266. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri E, Gesualdo L, Petrarulo F, Schena FP. Urinary IL-6/EGF ratio: a useful prognostic marker for the progression of renal damage in IgA nephropathy. Kidney Int. 1996;50:1990–2001. doi: 10.1038/ki.1996.521. [DOI] [PubMed] [Google Scholar]
- 9.Tsau YK, Chen CH. Urinary epidermal growth factor excretion in children with chronic renal failure. Am J Nephrol. 1999;19:400–404. doi: 10.1159/000013485. [DOI] [PubMed] [Google Scholar]
- 10.Grandaliano G, Gesualdo L, Bartoli F, Ranieri E, Monno R, Leggio A, Paradies G, Caldarulo E, Infante B, Schena FP. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000;58:182–192. doi: 10.1046/j.1523-1755.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 11.Stangou M, Alexopoulos E, Papagianni A, Pantzaki A, Bantis C, Dovas S, Economidou D, Leontsini M, Memmos D. Urinary levels of epidermal growth factor, interleukin-6 and monocyte chemoattractant protein-1 may act as predictor markers of renal function outcome in immunoglobulin A nephropathy. Nephrology. 2009;14:613–620. doi: 10.1111/j.1440-1797.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- 12.Togashi Y, Miyamoto Y. Urinary cystatin C as a biomarker for diabetic nephropathy and its immunohistochemical localization in kidney in Zucker diabetic fatty (ZDF) rats. Exp Toxicol Pathol. 2013;65:615–622. doi: 10.1016/j.etp.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M, ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7:316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betz BB, Jenks SJ, Cronshaw AD, Lamont DJ, Cairns C, Manning JR, Goddard J, Webb DJ, Mullins JJ, Hughes J, McLachlan S, Strachan MWJ, Price JF, Conway BR. Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int. 2016;89:1125–1135. doi: 10.1016/j.kint.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Salido EC, Lakshmanan J, Fisher DA, Shapiro LJ, Barajas L. Expression of epidermal growth factor in the rat kidney. Histochemistry. 1991;96:65–72. doi: 10.1007/BF00266763. [DOI] [PubMed] [Google Scholar]
- 16.Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989;84:1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jedlicka J, Soleiman A, Draganovici D, Mandelbaum J, Ziegler U, Regele H, Wüthrich RP, Gross O, Anders HJ, Segerer S. Interstitial inflammation in Alport syndrome. Hum Pathol. 2010;41:582–593. doi: 10.1016/j.humpath.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M, Piaia A, Shenoy N, Kagan D, Gapp B, Kueng B, Weber D, Dietrich W, Ksiazek Progression of Alport kidney disease in Col4a3 knock out mice is independent of sex or macrophage depletion by clodronate treatment. PLoS One. 2015;10:e0141231. doi: 10.1371/journal.pone.0141231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benali SL, Lees GE, Nabity MB, Aricò A, Drigo M, Gallo E, Giantin M, Aresu L. X-linked hereditary nephropathy in Navasota dogs: clinical pathology, morphology, and gene expression during disease progression. Vet Pathol. 2016;53:803–812. doi: 10.1177/0300985815624494. [DOI] [PubMed] [Google Scholar]
- 21.Gross O, Beirowski B, Marie-Louise Koepke ML, Kuck J, Reiner M, Addicks K, Smyth N, Schulze-Lohoff E, Weber M. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 2003;63:438–446. doi: 10.1046/j.1523-1755.2003.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.Bae EH, Fang F, Williams VR, VR KA, Zhou X, Patel VB, Song X, John R, Oudit GY, Pei Y, Scholey JW. Murine recombinant angiotensin-converting enzyme 2 attenuates kidney injury in experimental Alport syndrome. Kidney Int. 2017;91:1347–1361. doi: 10.1016/j.kint.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Hari P, Biswas B, Pandey R, Kalaivani M, Kumar R, Bagga A. Updated height- and creatinine-based equation and its validation for estimation of glomerular filtration rate in children from developing countries. Clin Exp Nephrol. 2012;16(5):697–705. doi: 10.1007/s10157-012-0618-2. [DOI] [PubMed] [Google Scholar]
- 24.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Verellen C, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport syndrome: natural history in 195 families and genotype-phenotype correlations in males. J Am Soc Nephrol. 2000;11:649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 25.Hashimura Y, Nozu K, Kaito H, Nakanishi K, Fu XJ, Ohtsubo H, Hashimoto F, Oka M, Ninchoji T, Ishimori S, Morisada N, Matsunoshita N, Kamiyoshi N, Yoshikawa N, Iijima K. Milder clinical aspects of X-linked Alport syndrome in men positive for the collagen IV α5 chain. Kidney Int. 2014;85:1208–1213. doi: 10.1038/ki.2013.479. [DOI] [PubMed] [Google Scholar]
- 26.Temme J, Kramer A, Jager KJ, Lange K, Peters F, Müller GA, Kramar R, Heaf JG, Finne P, Palsson R, Reisæter AV, Hoitsma AJ, Metcalfe W, Postorino M, Zurriaga O, Santos JP, Ravani P, Jarraya F, Verrina E, Dekker FW, Gross O. Outcomes of male patients with Alport syndrome undergoing renal replacement therapy. Clin J Am Soc Nephrol. 2012;7:1969–1976. doi: 10.2215/CJN.02190312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamattina JC, Foley DP, Mezrich JD, Fernandez LA, Vidyasagar V, D’Alessandro AM, Musat AI, Samaniego-Picota MD, Pascual J, Alejandro MD, Leverson GE, Pirsch JD, Djamali A. Chronic kidney disease stage progression in liver transplant recipients. Clin J Am Soc Nephrol. 2011;6:1851–1857. doi: 10.2215/CJN.00650111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kukla A, Adulla M, Pascual J, Samaniego M, Nanovic L, Becker BN, Djamali A. CKD stage-to-stage progression in native and transplant kidney disease. Nephrol Dial Transplant. 2008;23:693–700. doi: 10.1093/ndt/gfm590. [DOI] [PubMed] [Google Scholar]
- 29.Lee CC, Sun CY, Wu IW, Wang SY, Wu MS. Metabolic syndrome loses its predictive power in late-stage chronic kidney disease progression—a paradoxical phenomenon. Clin Nephrol. 2011;75:141–149. doi: 10.5414/CNP75141. [DOI] [PubMed] [Google Scholar]
- 30.Marks A, Fluck N, Prescott GJ, Robertson LM, Simpson WG, Smith WC, Black C. Definitions of progression in chronic kidney disease—predictors and relationship to renal replacement therapy in a population cohort with a 6-year follow-up. Nephrol Dial Transplant. 2014;29:333–341. doi: 10.1093/ndt/gft393. [DOI] [PubMed] [Google Scholar]
- 31.Gross O, Perin L, Deltas C. Alport syndrome from bench to bedside: the potential of current treatment beyond RAAS blockade and the horizon of future therapies. Nephrol Dial Transplant. 2014;29:iv124–iv130. doi: 10.1093/ndt/gfu028. [DOI] [PubMed] [Google Scholar]
- 32.Mattila AL, Perheentupa J, Pesonen K, Viinikka L. Epidermal growth factor in human urine from birth to puberty. J Clin Endocrinol Metab. 1985;61:997–1000. doi: 10.1210/jcem-61-5-997. [DOI] [PubMed] [Google Scholar]
- 33.Uchihashi M, Hirata Y, Fujita T, Matsukura S. Age-related decrease of urinary excretion of human epidermal growth factor (hEGF) Life Sci. 1982;31:679–683. doi: 10.1016/0024-3205(82)90769-X. [DOI] [PubMed] [Google Scholar]
- 34.Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schröder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol. 2003;14:2603–2610. doi: 10.1097/01.ASN.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 35.Yamamura T, Nozu K, Fu XJ, Nozu Y, Ye MJ, Shono A, Yamanouchi S, Minamikawa S, Morisada N, Nakanishi K, Shima Y, Yoshikawa N, Ninchoji T, Morioka I, Kaito H, Iijima K. Natural history and genotype-phenotype correlation in female X-linked Alport syndrome. Kidney Int Rep. 2017;2:850–855. doi: 10.1016/j.ekir.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcocci E, Uliana V, Bruttini M, Artuso R, Silengo MC, Zerial M, Bergesio F, Amoroso A, Savoldi S, Pennesi M, Giachino D, Rombolà G, Fogazzi GB, Rosatelli C, Martinhago CD, Carmellini M, Mancini R, Di Costanzo G, Longo I, Renieri A, Mari F. Autosomal dominant Alport syndrome: molecular analysis of the COL4A4 gene and clinical outcome. Nephrol Dial Transplant. 2009;24:1464–1471. doi: 10.1093/ndt/gfn681. [DOI] [PubMed] [Google Scholar]
- 37.Pescucci C, Mari F, Longo I, Vogiatzi P, Caselli R, Scala E, Abaterusso C, Gusmano R, Seri M, Miglietti N, Bresin E, Renieri A. Autosomal-dominant Alport syndrome: natural history of a disease due to COL4A3 or COL4A4 gene. Kidney Int. 2004;65:1598–1603. doi: 10.1111/j.1523-1755.2004.00560.x. [DOI] [PubMed] [Google Scholar]